Synthesis and Properties of Water-Based Acrylic Adhesives with a Variable Ratio of 2-Ethylhexyl Acrylate and n-Butyl Acrylate for Application in Glass Bottle Labels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Emulsion Polymerization
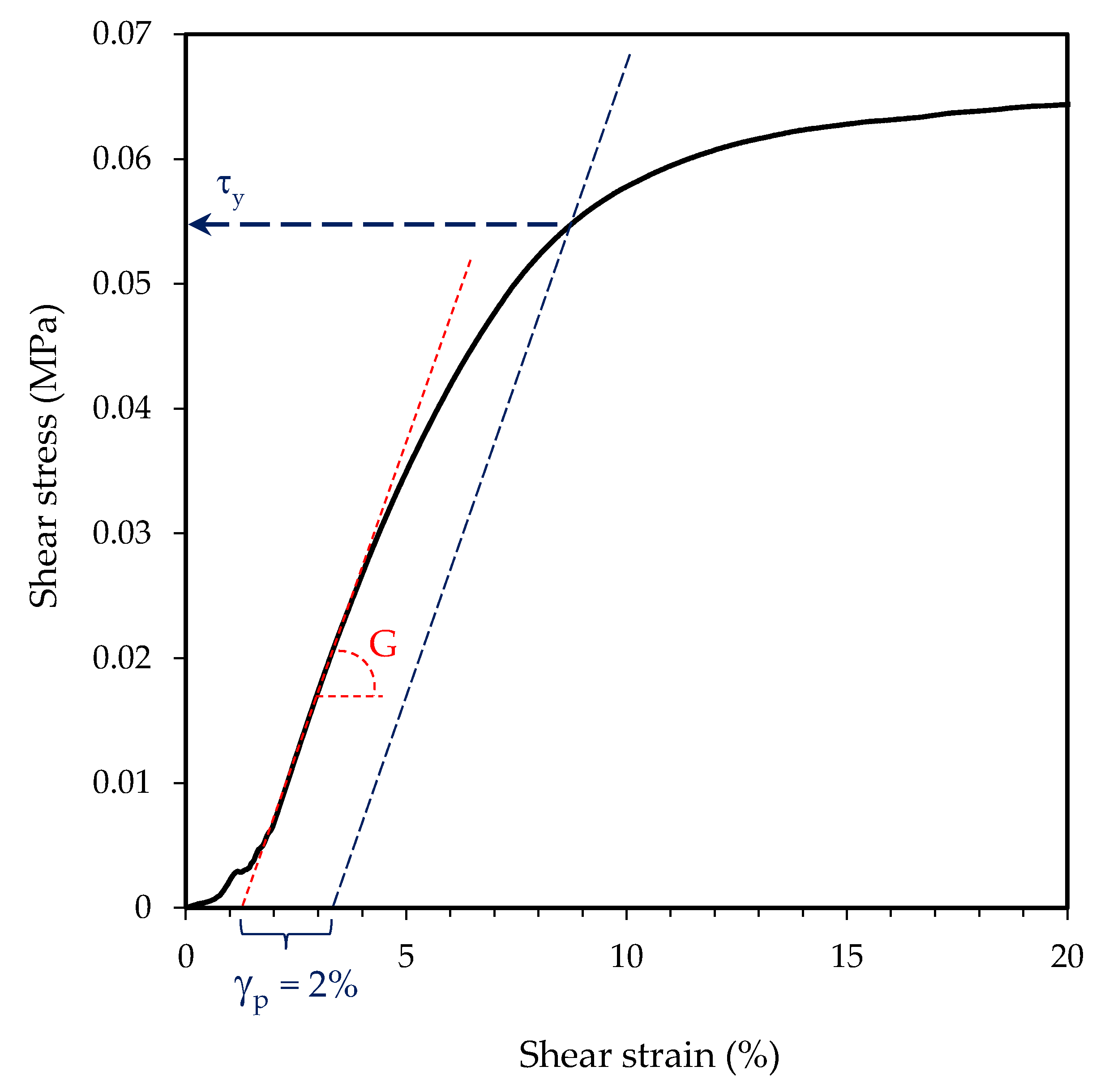
2.3. Latex Characterization
2.4. Adhesion Tests
2.5. Water Resistance Tests
3. Results and Discussion
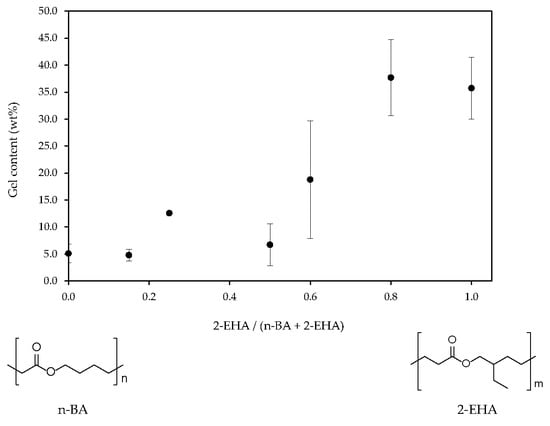
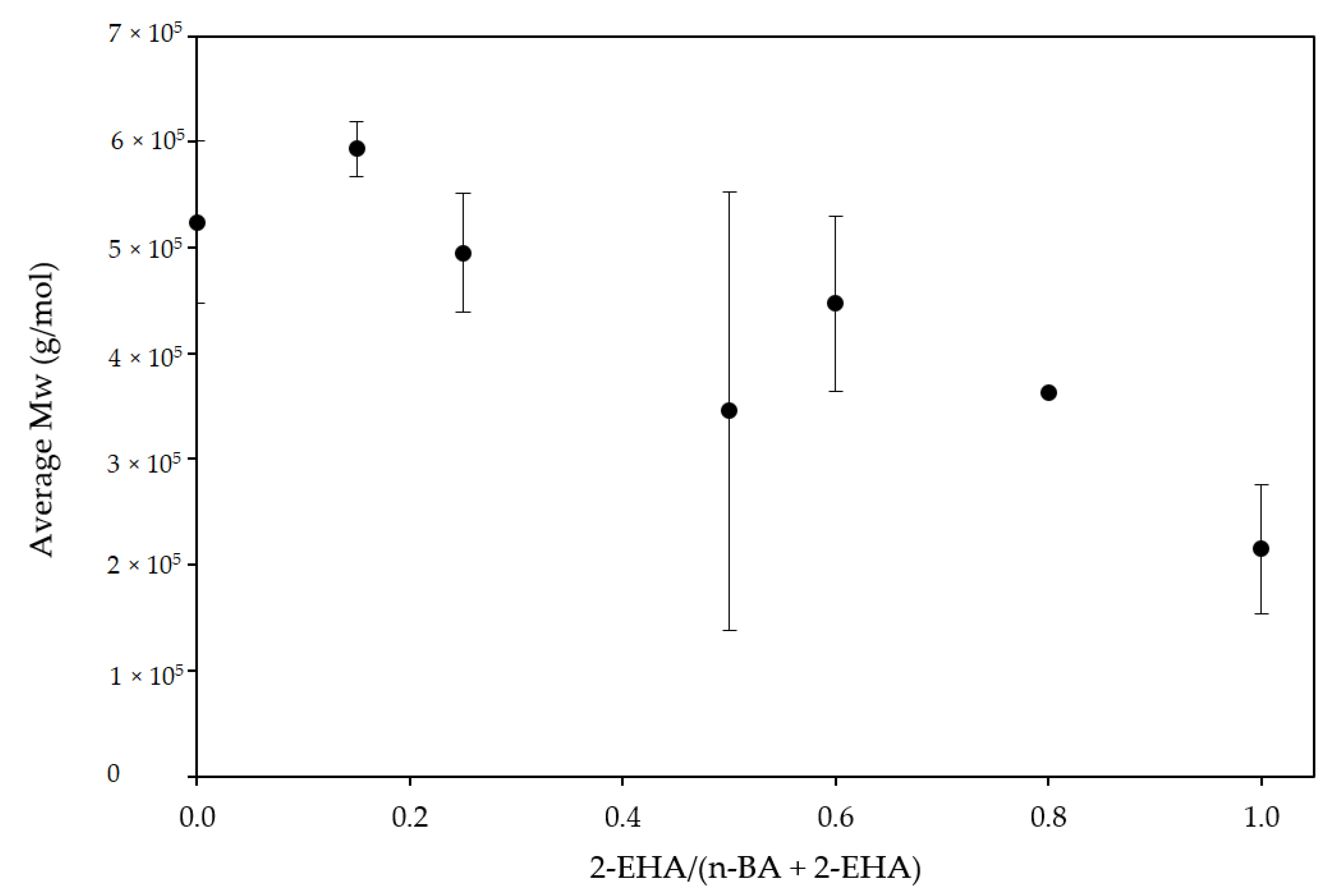
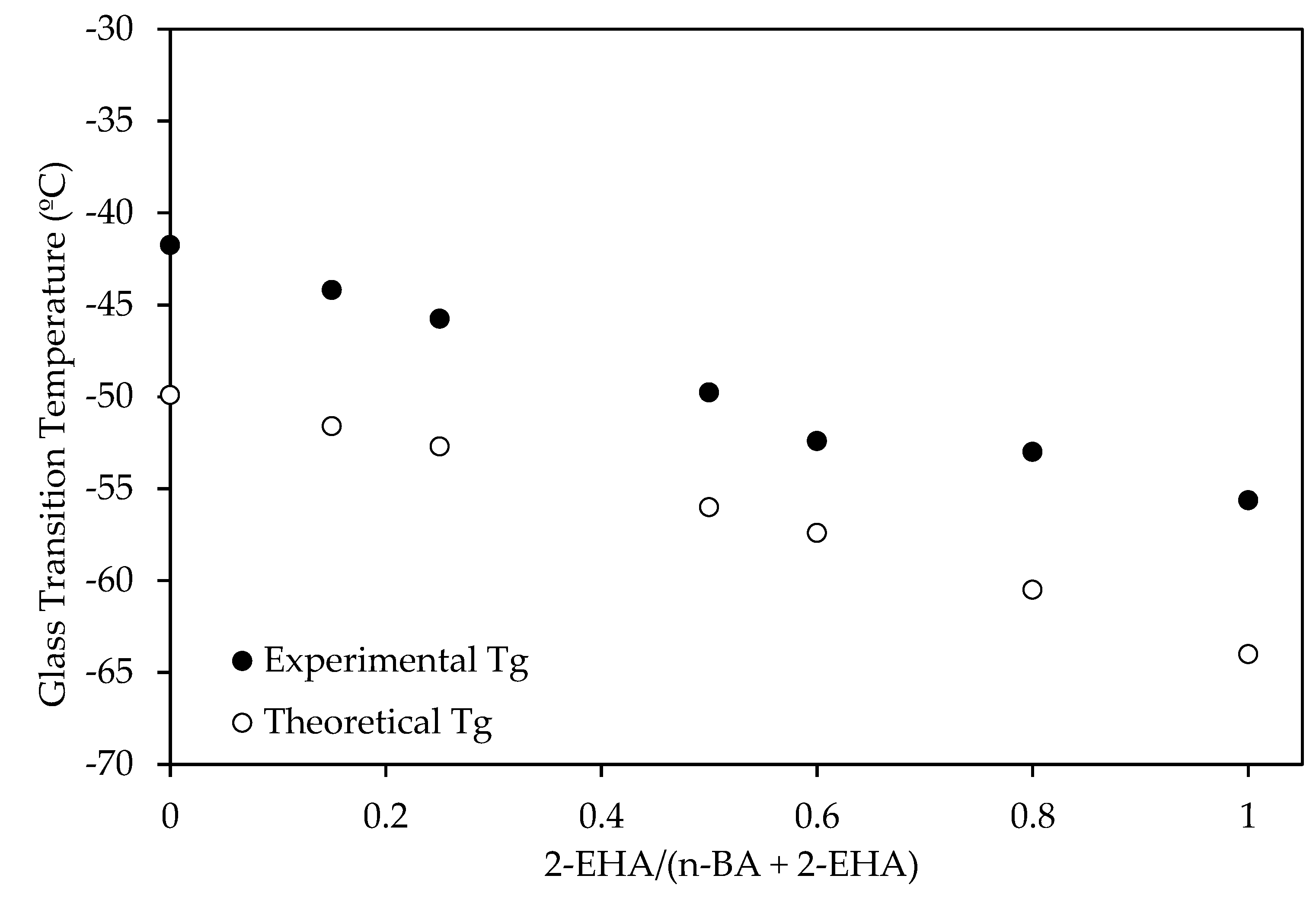
3.1. Physical-Chemical Properties
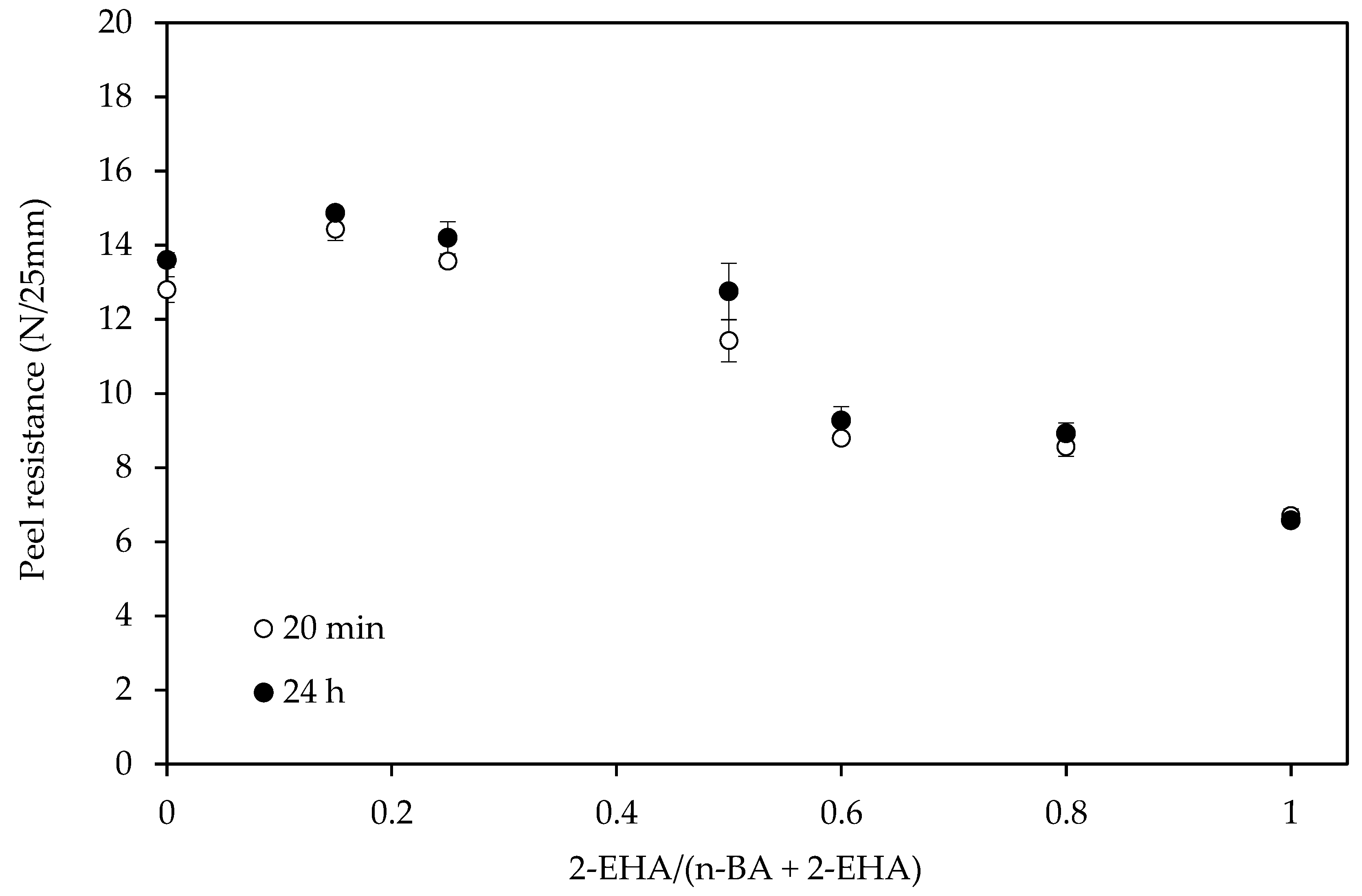
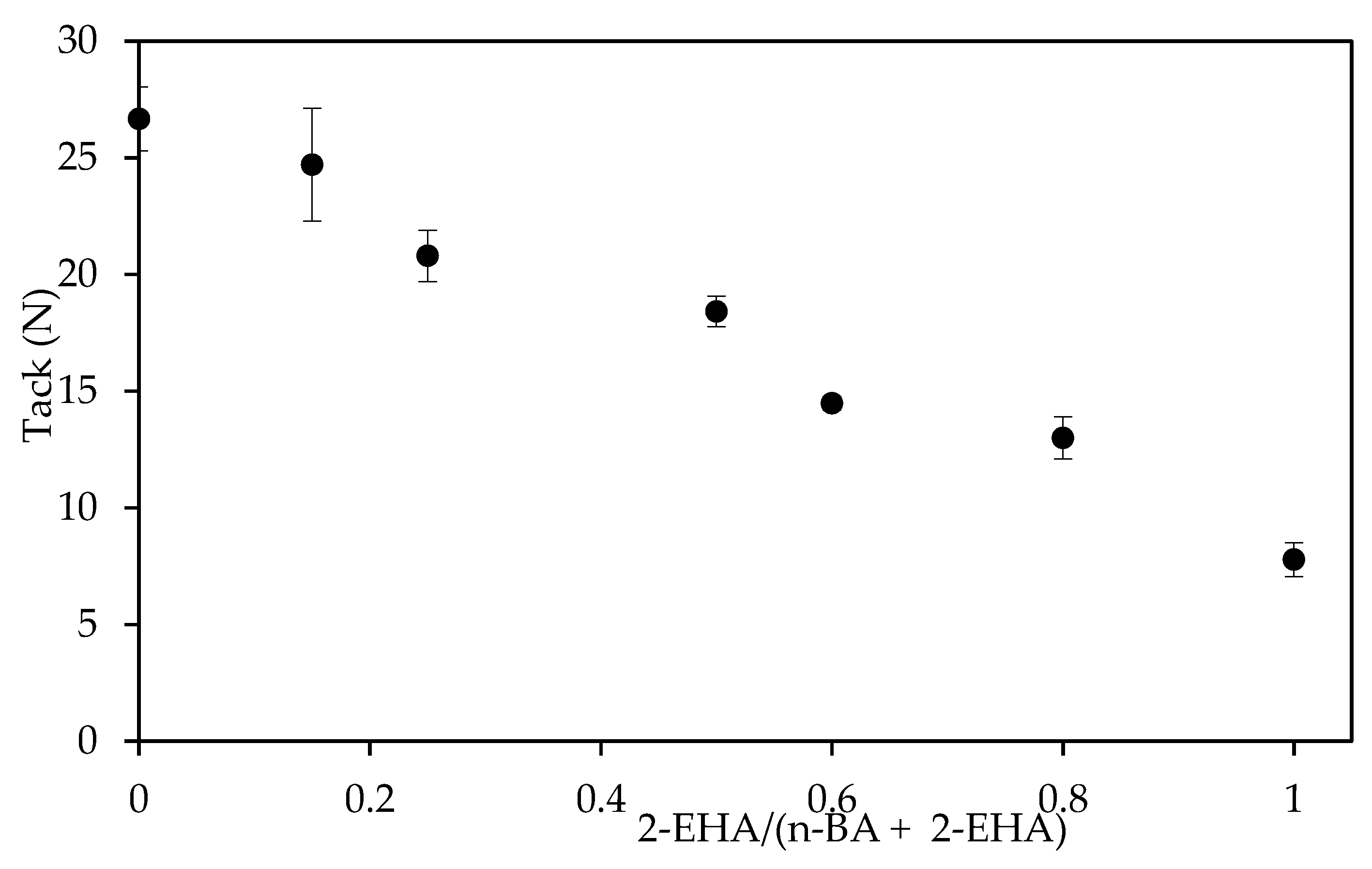
3.2. Adhesive Properties
3.3. Water Resistance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Broadbridge, P.; Chan, D.; Fowkes, N.; Fulford, G. Wet gum labeling of wine bottles. Proc. 1996 Math. Ind. Study Group 1996, 1, 103–113. [Google Scholar]
- Petrie, E. Adhesives Families. In Handbook of Adhesives and Sealants; McGraw-Hill Education: New York, NY, USA, 2004; pp. 410–411. [Google Scholar]
- Creton, C. Pressure-sensitive adhesives: An introductory course. MRS Bull. 2003, 28, 434–439. [Google Scholar] [CrossRef]
- Petrie, E. Adhesive Classification. In Handbook of Adhesives and Sealants; McGraw-Hill Education: New York, NY, USA, 2004; pp. 301–306. [Google Scholar]
- Penzel, E.; Ballard, N.; Asua, J.M. Polyacrylates. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 7–9. [Google Scholar]
- Aguirreurreta, Z.; Dimmer, J.-A.; Willerich, I.; De La Cal, J.C.; Leiza, J.R. Water Whitening Reduction in Waterborne Pressure-Sensitive Adhesives Produced with Polymerizable Surfactants. Macromol. Mater. Eng. 2015, 9, 925–936. [Google Scholar] [CrossRef]
- Moghbeli, M.R.; Zamir, S.M.; Molaee, B. Resultant synergism in the shear resistance of acrylic pressure-sensitive adhesives prepared by emulsion polymerization ofn-butyl acrylate/2-ethyl hexyl acrylate/acrylic acid. J. Appl. Polym. Sci. 2008, 108, 606–613. [Google Scholar] [CrossRef]
- López, B.L.; Murillo, E.; Hess, M. Synthesis and characterization of a pressure-sensitive adhesive based on an isobutyl acrylate/2-ethylhexyl acrylate copolymer. e-Polymers 2014, 4, 1–10. [Google Scholar] [CrossRef][Green Version]
- Klein, A.; Eric, S. Daniels Formulation Components. In Emulsion Polymerization and Emulsion Polymers; John Wiley & Sons: Hoboken, NJ, USA, 1998; Volume 36, pp. 208–234. [Google Scholar]
- Ismail, H.; Ahmad, Z.; Yew, F.W. Effect of monomer composition on adhesive performance for waterborne acrylic pressure-sensitive adhesives. J. Phys. Sci. 2011, 22, 51–63. [Google Scholar]
- Tobing, S.D.; Klein, A. Molecular parameters and their relation to the adhesive performance of emulsion acrylic pressure-sensitive adhesives. II. Effect of crosslinking. J. Appl. Polym. Sci. 2001, 79, 2558–2564. [Google Scholar] [CrossRef]
- Gower, M.D.; Shanks, R.A. The effect of varied monomer composition on adhesive performance and peeling master curves for acrylic pressure-sensitive adhesives. J. Appl. Polym. Sci. 2004, 93, 2909–2917. [Google Scholar] [CrossRef]
- Alarcia, F.; de la Cal, J.C.; Asua, J.M. Continuous production of specialty waterborne adhesives: Tuning the adhesive performance. Chem. Eng. J. 2006, 122, 117–126. [Google Scholar] [CrossRef]
- Brostow, W.; Chiu, R.; Kalogeras, I.M.; Vassilikou-Dova, A. Prediction of glass transition temperatures: Binary blends and copolymers. Mater. Lett. 2008, 62, 3152–3155. [Google Scholar] [CrossRef]
- FINAT Technical Committee FINAT Test Method no. 18 Dynamic Shear. In FINAT Technical Handbook; FINAT: The Hague, The Netherlands, 2001; Volume 11, pp. 42–44.
- ASTM International. Standard Test Method for Peel Adhesion of Pressure-Sensitive Tape 1; ASTM International: West Conshohocken, PA, USA, 2003; Volume 4. [Google Scholar]
- FINAT Test Method no. 9 ‘Loop’ tack measurement FINAT Technical Handbook. In FINAT Technical Handbook; FINAT: The Hague, The Netherlands, 2001; Volume 7, pp. 22–24.
- Pressure Sensitive Tape Council. Loop Tack Measurements. In Norm PSTC-16; Oakbrook Terrace, IL, USA, 2007; pp. 1–8. [Google Scholar]
- Richard, K. Trysk Print Solutions. In 5 Keys to Labels That Sell; Trysk Print Solutions: Seattle, WA, USA, 2014. [Google Scholar]
- Karyu, N.; Noda, M.; Fujii, S.; Nakamura, Y.; Urahama, Y. Effect of adhesive thickness on the wettability and deformability of polyacrylic pressure-sensitive adhesives during probe tack test. J. Appl. Polym. Sci. 2016, 133, 1–11. [Google Scholar] [CrossRef]
- Ahmad, N.M.; Heatley, F.; Lovell, P.A. Chain Transfer to Polymer in Free-Radical Solution Polymerization of n -Butyl Acrylate Studied by NMR Spectroscopy. Macromolecules 2002, 31, 2822–2827. [Google Scholar] [CrossRef]
- Alarcia, F.; De La Cal, J.C.; Asua, J.M. Scheduling the production of a portfolio of emulsion polymers in a single CSTR. Ind. Eng. Chem. Res. 2006, 45, 3711–3717. [Google Scholar] [CrossRef]
- Plessis, C.; Arzamendi, G.; Leiza, J.R.; Schoonbrood, H.A.S.; Charmot, D.; Asua, J.M. Decrease in effective acrylate propagation rate constants caused by intramolecular chain transfer. Macromolecules 2000, 33, 4–7. [Google Scholar] [CrossRef]
- Ballard, N.; Hamzehlou, S.; Asua, J.M. Intermolecular Transfer to Polymer in the Radical Polymerization of n-Butyl Acrylate. Macromolecules 2016, 49, 5418–5426. [Google Scholar] [CrossRef]
- Ballard, N.; Asua, J.M. Radical polymerization of acrylic monomers: An overview. Prog. Polym. Sci. 2018, 79, 40–60. [Google Scholar] [CrossRef]
- Plessis, C.; Arzamendi, G.; Leiza, J.R.; Schoonbrood, H.A.S.; Charmot, D.; Asua, J.M. Seeded semibatch emulsion polymerization of n-butyl acrylate. Kinetics and structural properties. Macromolecules 2000, 33, 5041–5047. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation; John Wiley & Sons: Hoboken, NJ, USA, 2004; pp. 1–23. [Google Scholar]
- Belaidi, O.; Bouchaour, T.; Maschke, U. Accuracy and Applicability of the New Exchange Correlation Functionals for Reproduction of the Infrared Spectra of Butyl Acrylate and Butyl Methacrylate Molecules. Org. Chem. Int. 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Belaidi, O.; Bouchaour, T.; Maschke, U. Molecular Structure and Vibrational Spectra of 2-Ethylhexyl Acrylate by Density Functional Theory Calculations. Org. Chem. Int. 2013, 2013, 1–14. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, T.H.; Park, Y.I.; Noh, S.M.; Kim, J.C. Effect of the n-butyl acrylate/2-ethylhexyl acrylate weight ratio on the performances of waterborne core–shell PSAs. J. Ind. Eng. Chem. 2017, 53, 111–118. [Google Scholar] [CrossRef]
- Beatty, M.W.; Swartz, M.L.; Moore, B.K.; Phillips, R.W.; Roberts, T.A. Effect of crosslinking agent content, monomer functionality, and repeat unit chemistry on properties of unfilled resins. J. Biomed. Mater. Res. 1993, 27, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Satas, D. Handbook of Pressure Sensitive Adhesive Technology, 2nd ed.; Van Nostrand Reinhold: New York, NY, USA, 1989; ISBN 978-1-4757-0868-4. [Google Scholar]







| Sample | n-BA | 2-EHA | AA |
|---|---|---|---|
| E0 | 97.5 | 0.0 | 2.5 |
| E15 | 82.9 | 14.6 | 2.5 |
| E25 | 73.1 | 24.4 | 2.5 |
| E50 | 48.8 | 48.8 | 2.5 |
| E60 | 39.0 | 58.5 | 2.5 |
| E80 | 19.5 | 78.0 | 2.5 |
| E100 | 0.0 | 97.5 | 2.5 |
| Sample | Average Particle size (nm) | Viscosity (cP) |
|---|---|---|
| E0 | 191 ± 1 | 95 ± 2 |
| E15 | 191 ± 1 | 110 ± 11 |
| E25 | 221 ± 3 | 103 ± 16 |
| E50 | 175 ± 1 | 163 ± 17 |
| E60 | 185 ± 2 | 148 ± 3 |
| E80 | 189 ± 1 | 153 ± 6 |
| E100 | 186 ± 1 | 250 ± 15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Márquez, I.; Alarcia, F.; Velasco, J.I. Synthesis and Properties of Water-Based Acrylic Adhesives with a Variable Ratio of 2-Ethylhexyl Acrylate and n-Butyl Acrylate for Application in Glass Bottle Labels. Polymers 2020, 12, 428. https://doi.org/10.3390/polym12020428
Márquez I, Alarcia F, Velasco JI. Synthesis and Properties of Water-Based Acrylic Adhesives with a Variable Ratio of 2-Ethylhexyl Acrylate and n-Butyl Acrylate for Application in Glass Bottle Labels. Polymers. 2020; 12(2):428. https://doi.org/10.3390/polym12020428
Chicago/Turabian StyleMárquez, Irene, Felipe Alarcia, and José Ignacio Velasco. 2020. "Synthesis and Properties of Water-Based Acrylic Adhesives with a Variable Ratio of 2-Ethylhexyl Acrylate and n-Butyl Acrylate for Application in Glass Bottle Labels" Polymers 12, no. 2: 428. https://doi.org/10.3390/polym12020428
APA StyleMárquez, I., Alarcia, F., & Velasco, J. I. (2020). Synthesis and Properties of Water-Based Acrylic Adhesives with a Variable Ratio of 2-Ethylhexyl Acrylate and n-Butyl Acrylate for Application in Glass Bottle Labels. Polymers, 12(2), 428. https://doi.org/10.3390/polym12020428