Chitosan Composites in Packaging Industry—Current Trends and Future Challenges
Abstract
1. Introduction
2. Application of Chitosan as a Coating
3. Chitosan Films for Food Packaging
3.1. Blends and Bilayers of Chitosan and Other Biopolymers
3.2. Nanocomposites
3.2.1. Montmorillonite
3.2.2. Cellulosic Nanofibers and Nanocrystals
3.2.3. Metal Oxides
3.3. Active Films of Chitosan
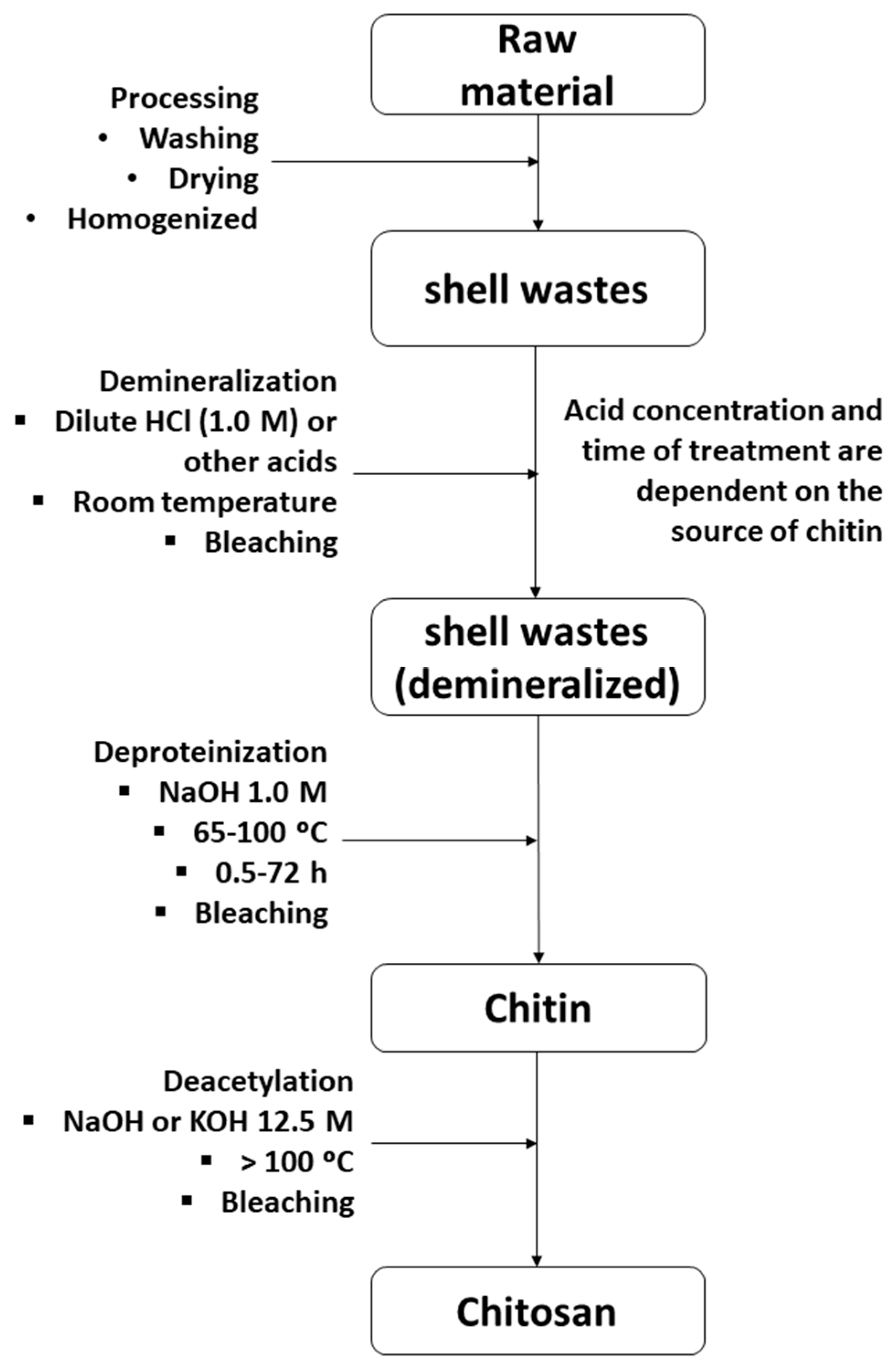
4. Novel Extraction Methods of Chitin and the Production of Chitosan
5. Biological Production of Chitin and Chitosan
6. Scale-Up Production
6.1. Thermoplastic Chitosan Films
6.2. Novel Green Solvents as Plasticizers for Thermomechanical Treatment of Chitosan—Deep Eutetic Solvents (DES)
7. Conclusions and Final Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Mujtaba, M.; Morsi, R.E.; Kerch, G.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Khawar, K.M. Current advancements in chitosan-based film production for food technology: A review. Int. J. Biol. Macromol. 2019, 121, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.G.L.; Ribeiro-Santos, R.; Rodrigues, P.F.; Otoni, C.G.; Duarte, M.P.; Coelhoso, I.M.; Fernando, A.L. Nanomaterial migration from composites into food matrices. In Composite Materials for Food Packaging; Cirillo, G., Kozlowski, M.A., Spizzirri, U.G., Eds.; Scrivener Publishing LLC: Beverly, MA, USA, 2018; p. 465. ISBN 9781119160205. [Google Scholar]
- Vasile, C. Polymeric Nanocomposites and Nanocoatings for Food Packaging: A Review. Materials 2018, 11, 1834. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.G.L.; Fernando, A.L.; Pires, J.R.A.; Rodrigues, P.F.; Lopes, A.A.S.; Fernandes, F.M.B. Physical properties of chitosan films incorporated with natural antioxidants. Ind. Crops Prod. 2017, 107, 565–572. [Google Scholar] [CrossRef]
- Kerch, G. Chitosan films and coatings prevent losses of fresh fruit nutritional quality: A review. Trends Food Sci. Technol. 2015, 46, 159–166. [Google Scholar] [CrossRef]
- Dehghani, S.; Hosseini, S.V.; Regenstein, J.M. Edible films and coatings in seafood preservation: A review. Food Chem. 2018, 240, 505–513. [Google Scholar] [CrossRef]
- Yuan, G.; Chen, X.; Li, D. Chitosan films and coatings containing essential oils: The antioxidant and antimicrobial activity, and application in food systems. Food Res. Int. 2016, 89, 117–128. [Google Scholar] [CrossRef]
- Ganiari, S.; Choulitoudi, E.; Oreopoulou, V. Edible and active films and coatings as carriers of natural antioxidants for lipid food. Trends Food Sci. Technol. 2017, 68, 70–82. [Google Scholar] [CrossRef]
- Sorrentino, A.; Gorrasi, G.; Vittoria, V. Potential perspectives of bio-nanocomposites for food packaging applications. Trends Food Sci. Technol. 2007, 18, 84–95. [Google Scholar] [CrossRef]
- Aranaz, I.; Mengíbar, M.; Harris, R. Functional characterization of chitin and chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar]
- Coma, V. Bioactive packaging technologies for extended shelf life of meat-based products. Meat Sci. 2008, 78, 90–103. [Google Scholar] [CrossRef]
- Dutta, P.K.; Tripathi, S.; Mehrotra, G.K.; Dutta, J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009, 114, 1173–1182. [Google Scholar] [CrossRef]
- Soares, N.F.F.; Pires, A.C.S.; Camilloto, G.P.; Santiago-Silva, P.; Espitia, P.J.P.; Silva, W.A. Recent patents on active packaging for food application. Recent Pat. Food. Nutr. Agric. 2009, 1, 171–178. [Google Scholar] [CrossRef]
- Ahmed, I.; Lin, H.; Zou, L.; Brody, A.L.; Li, Z.; Qazi, I.M.; Pavase, T.R.; Lv, L. A comprehensive review on the application of active packaging technologies to muscle foods. Food Control 2017, 82, 163–178. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Rodrigues, P.F.; Duarte, M.P.; Fernando, A.L. Antioxidant Migration Studies in Chitosan Films Incorporated with Plant Extracts. J. Renew. Mater. 2018, 6, 548–558. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Vieira, É.T.; Coelhoso, I.M.; Duarte, M.P.; Fernando, A.L. Activity of chitosan-montmorillonite bionanocomposites incorporated with rosemary essential oil: From in vitro assays to application in fresh poultry meat. Food Hydrocoll. 2019, 89, 241–252. [Google Scholar] [CrossRef]
- Pires, J.R.A.; de Souza, V.G.L.; Fernando, A.L. Chitosan/montmorillonite bionanocomposites incorporated with rosemary and ginger essential oil as packaging for fresh poultry meat. Food Packag. Shelf Life 2018, 17, 142–149. [Google Scholar] [CrossRef]
- Müller, K.; Bugnicourt, E.; Latorre, M.; Jorda, M.; Echegoyen Sanz, Y.; Lagaron, J.; Miesbauer, O.; Bianchin, A.; Hankin, S.; Bölz, U.; et al. Review on the Processing and Properties of Polymer Nanocomposites and Nanocoatings and Their Applications in the Packaging, Automotive and Solar Energy Fields. Nanomaterials 2017, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.L.; Morgado, C.M.A.; Marque, K.M.; Mattiuz, C.F.M.; Mattiuz, B. Pós-colheita de mangas ‘tommy atkins’ recobertas com quitosana. Rev. Bras. Frutic. 2011, 33, 337–343. [Google Scholar] [CrossRef]
- Chien, P.; Sheu, F.; Yang, F.H. Effects of edible chitosan coating on quality and shelf life of sliced mango fruit. J. Food Eng. 2007, 78, 225–229. [Google Scholar] [CrossRef]
- Petrou, S.; Tsiraki, M.; Giatrakou, V.; Savvaidis, I.N. Chitosan dipping or oregano oil treatments, singly or combined on modified atmosphere packaged chicken breast meat. Int. J. Food Microbiol. 2012, 156, 264–271. [Google Scholar] [CrossRef]
- Su, H.K.; Hong, K.N.; Prinyawiwatkul, W. Effect of molecular weight, type of chitosan, and chitosan solution pH on the shelf-life and quality of coated eggs. J. Food Sci. 2007, 72, 44–48. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Kamil, J.Y.V.A.; Shahidi, F. Chitosan as an Edible Invisible Film for Quality Preservation of Herring and Atlantic Cod. J. Agric. Food Chem. 2002, 50, 5167–5178. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.; Xie, J.; Zhang, L.; Sun, D.; Gong, D. Effects of chitosan coating on postharvest life and quality of guava (Psidium guajava L.) fruit during cold storage. Sci. Hortic. 2012, 144, 172–178. [Google Scholar] [CrossRef]
- Vieira, J.M.; Flores-lópez, M.L.; Jasso, D.; Rodríguez, D.; Sousa, M.C.; Vicente, A.A.; Martins, J.T. Postharvest Biology and Technology Effect of chitosan—Aloe vera coating on postharvest quality of blueberry (Vaccinium corymbosum) fruit. Postharvest Biol. Technol. 2016, 116, 88–97. [Google Scholar] [CrossRef]
- Alvarez, M.V.; Ponce, A.G.; Moreira, M.D.R. Antimicrobial efficiency of chitosan coating enriched with bioactive compounds to improve the safety of fresh cut broccoli. LWT Food Sci. Technol. 2013, 50, 78–87. [Google Scholar] [CrossRef]
- Sabaghi, M.; Maghsoudlou, Y.; Khomeiri, M.; Ziaiifar, A.M. Active edible coating from chitosan incorporating green tea extract as an antioxidant and antifungal on fresh walnut kernel. Postharvest Biol. Technol. 2015, 110, 224–228. [Google Scholar] [CrossRef]
- Xiao, C.; Zhu, L.; Luo, W.; Song, X.; Deng, Y. Combined action of pure oxygen pretreatment and chitosan coating incorporated with rosemary extracts on the quality of fresh-cut pears. Food Chem. 2010, 121, 1003–1009. [Google Scholar] [CrossRef]
- Nair, M.S.; Saxena, A.; Kaur, C. Effect of chitosan and alginate based coatings enriched with pomegranate peel extract to extend the postharvest quality of guava (Psidium guajava L.). Food Chem. 2018, 240, 245–252. [Google Scholar] [CrossRef]
- Goddard, J.M.; Hotchkiss, J.H. Polymer surface modification for the attachment of bioactive compounds. Prog. Polym. Sci. 2007, 32, 698–725. [Google Scholar] [CrossRef]
- Munteanu, B.S.; Aytac, Z.; Pricope, G.M.; Uyar, T.; Vasile, C. Polylactic acid (PLA)/Silver-NP/VitaminE bionanocomposite electrospun nanofibers with antibacterial and antioxidant activity. J. Nanopart. Res. 2014, 16, 2643. [Google Scholar] [CrossRef]
- Stoleru, E.; Munteanu, S.B.; Dumitriu, R.P.; Coroaba, A.; Drobotă, M.; Zemljic, L.F.; Pricope, G.M.; Vasile, C. Polyethylene materials with multifunctional surface properties by electrospraying chitosan/vitamin E formulation destined to biomedical and food packaging applications. Iran. Polym. J. 2016, 25, 295–307. [Google Scholar] [CrossRef]
- Stoleru, E.; Dumitriu, R.P.; Munteanu, B.S.; Zaharescu, T.; Tănase, E.E.; Mitelut, A.; Ailiesei, G.L.; Vasile, C. Novel procedure to enhance PLA surface properties by chitosan irreversible immobilization. Appl. Surf. Sci. 2016, 367, 407–417. [Google Scholar] [CrossRef]
- Pâslaru, E.; Fras Zemljic, L.; Bračič, M.; Vesel, A.; Petrinić, I.; Vasile, C. Stability of a chitosan layer deposited onto a polyethylene surface. J. Appl. Polym. Sci. 2013, 130, 2444–2457. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int. J. Biol. Macromol. 2014, 64, 353–367. [Google Scholar] [CrossRef]
- Xu, Y.X.; Kim, K.M.; Hanna, M.A.; Nag, D. Chitosan–starch composite film: Preparation and characterization. Ind. Crops Prod. 2005, 21, 185–192. [Google Scholar] [CrossRef]
- Jindal, M.; Kumar, V.; Rana, V.; Tiwary, A.K. An insight into the properties of Aegle marmelos pectin-chitosan cross-linked films. Int. J. Biol. Macromol. 2013, 52, 77–84. [Google Scholar] [CrossRef]
- Freitas, F.; Alves, V.D.; Reis, M.A.; Crespo, J.G.; Coelhoso, I.M. Microbial polysaccharide-based membranes: Current and future applications. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Ferreira, A.R.V.; Torres, C.A.V.; Freitas, F.; Sevrin, C.; Grandfils, C.; Reis, M.A.M.; Alves, V.D.; Coelhoso, I.M. Development and characterization of bilayer films of FucoPol and chitosan. Carbohydr. Polym. 2016, 147, 8–15. [Google Scholar] [CrossRef]
- Rivero, S.; García, M.A.; Pinotti, A. Composite and bi-layer films based on gelatin and chitosan. J. Food Eng. 2009, 90, 531–539. [Google Scholar] [CrossRef]
- Ferreira, C.O.; Nunes, C.A.; Delgadillo, I.; Lopes-da-Silva, J.A. Characterization of chitosan-whey protein films at acid pH. Food Res. Int. 2009, 42, 807–813. [Google Scholar] [CrossRef]
- Kurek, M.; Galus, S.; Debeaufort, F. Surface, mechanical and barrier properties of bio-based composite films based on chitosan and whey protein. Food Packag. Shelf Life 2014, 1, 56–67. [Google Scholar] [CrossRef]
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C 2013, 33, 1819–1841. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.G.L.; Fernando, A.L. Nanoparticles in food packaging: Biodegradability and potential migration to food—A review. Food Packag. Shelf Life 2016, 8, 63–70. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Rodrigues, P.F.; Lopes, A.A.S.; Fernandes, F.M.B.; Duarte, M.P.; Coelhoso, I.M.; Fernando, A.L. Bionanocomposites of chitosan/montmorillonite incorporated with Rosmarinus officinalis essential oil: Development and physical characterization. Food Packag. Shelf Life 2018, 16, 148–156. [Google Scholar] [CrossRef]
- Beigzadeh Ghelejlu, S.; Esmaiili, M.; Almasi, H. Characterization of chitosan-nanoclay bionanocomposite active films containing milk thistle extract. Int. J. Biol. Macromol. 2016, 86, 613–621. [Google Scholar] [CrossRef]
- Vlacha, M.; Giannakas, A.; Katapodis, P.; Stamatis, H.; Ladavos, A.; Barkoula, N.M. On the efficiency of oleic acid as plasticizer of chitosan/clay nanocomposites and its role on thermo-mechanical, barrier and antimicrobial properties—Comparison with glycerol. Food Hydrocoll. 2016, 57, 10–19. [Google Scholar] [CrossRef]
- Qin, Y.Y.; Zhang, Z.H.; Li, L.; Yuan, M.L.; Fan, J.; Zhao, T.R. Physio-mechanical properties of an active chitosan film incorporated with montmorillonite and natural antioxidants extracted from pomegranate rind. J. Food Sci. Technol. 2015, 52, 1471–1479. [Google Scholar] [CrossRef]
- Nouri, A.; Yaraki, M.T.; Ghorbanpour, M.; Agarwal, S.; Gupta, V.K. Enhanced Antibacterial effect of chitosan film using Montmorillonite/CuO nanocomposite. Int. J. Biol. Macromol. 2018, 109, 1219–1231. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Rodrigues, C.; Rodrigues, P.F.; Lopes, A.; Silva, R.J.; Caldeira, J.; Duarte, M.P.; Fernandes, F.B.; Coelhoso, I.M.; et al. Physical and Morphological Characterization of Chitosan/Montmorillonite Films Incorporated with Ginger Essential Oil. Coatings 2019, 9, 700. [Google Scholar] [CrossRef]
- Giannakas, A.; Vlacha, M.; Salmas, C.; Leontiou, A.; Katapodis, P.; Stamatis, H.; Barkoula, N.M.; Ladavos, A. Preparation, characterization, mechanical, barrier and antimicrobial properties of chitosan/PVOH/clay nanocomposites. Carbohydr. Polym. 2016, 140, 408–415. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.; Vieira, É.T.; Coelhoso, I.M.; Duarte, M.P.; Fernando, A.L. Shelf Life Assessment of Fresh Poultry Meat Packaged in Novel Bionanocomposite of Chitosan/Montmorillonite Incorporated with Ginger Essential Oil. Coatings 2018, 8, 177. [Google Scholar] [CrossRef]
- Celebi, H.; Kurt, A. Effects of processing on the properties of chitosan/cellulose nanocrystal films. Carbohydr. Polym. 2015, 133, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Wei, C.; Gong, Y.; Wang, S.; Ding, W. Mechanical and Water-Resistant Properties of Eco-Friendly Chitosan Membrane Reinforced with Cellulose Nanocrystals. Polymers 2019, 11, 166. [Google Scholar] [CrossRef] [PubMed]
- Abdul Khalil, H.P.S.; Saurabh, C.K.; Adnan, A.S.; Nurul Fazita, M.R.; Syakir, M.I.; Davoudpour, Y.; Rafatullah, M.; Abdullah, C.K.; Haafiz, M.K.M.; Dungani, R. A review on chitosan-cellulose blends and nanocellulose reinforced chitosan biocomposites: Properties and their applications. Carbohydr. Polym. 2016, 150, 216–226. [Google Scholar] [CrossRef]
- Pires, R.A.; Gomes, V.; De Souza, L.; Fernando, A.L. Production of Nanocellulose from Lignocellulosic Biomass Wastes: Prospects and Limitations. In Innovation, Engineering and Entrepreneurship; Springer: Cham, Switzerland, 2019; Volume 505, pp. 719–725. [Google Scholar] [CrossRef]
- Pires, J.R.A.; Souza, V.G.L.; Fernando, A.L. Valorization of energy crops as a source for nanocellulose production—Current knowledge and future prospects. Ind. Crops Prod. 2019, 140, 111642. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, X.; Zhao, R.; Guo, D.; Zhang, J. Preparation and properties of chitosan/guar gum/nanocrystalline cellulose nanocomposite films. Carbohydr. Polym. 2018, 197, 128–136. [Google Scholar] [CrossRef]
- Noorbakhsh-Soltani, S.M.; Zerafat, M.M.; Sabbaghi, S. A comparative study of gelatin and starch-based nano-composite films modified by nano-cellulose and chitosan for food packaging applications. Carbohydr. Polym. 2018, 189, 48–55. [Google Scholar] [CrossRef]
- Youssef, A.M.; Abou-Yousef, H.; El-Sayed, S.M.; Kamel, S. Mechanical and antibacterial properties of novel high performance chitosan/nanocomposite films. Int. J. Biol. Macromol. 2015, 76, 25–32. [Google Scholar] [CrossRef]
- Al-Naamani, L.; Dobretsov, S.; Dutta, J. Chitosan-zinc oxide nanoparticle composite coating for active food packaging applications. Innov. Food Sci. Emerg. Technol. 2016, 38, 231–237. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, G.; Wang, Y.; Zhao, Y.; Su, H.; Tan, T. Preparation of chitosan-TiO2 composite film with efficient antimicrobial activities under visible light for food packaging applications. Carbohydr. Polym. 2017, 169, 101–107. [Google Scholar] [CrossRef]
- Tian, F.; Chen, W.; Wu, C.E.; Kou, X.; Fan, G.; Li, T.; Wu, Z. Preservation of Ginkgo biloba seeds by coating with chitosan/nano-TiO2 and chitosan/nano-SiO2 films. Int. J. Biol. Macromol. 2019, 126, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Madani, M. Effect of Functionalized TiO2 on Mechanical, Thermal and Swelling Properties of Chitosan-Based Nanocomposite Films. Polym. Plast. Technol. Eng. 2015, 54, 1035–1042. [Google Scholar] [CrossRef]
- Yun, Y.H.; Yun, J.W.; Yoon, S.D.; Byun, H.S. Physical properties and photocatalytic activity of chitosan-based nanocomposites added titanium oxide nanoparticles. Macromol. Res. 2016, 24, 51–59. [Google Scholar] [CrossRef]
- De Silva, R.T.; Mantilaka, M.M.M.G.P.G.; Ratnayake, S.P.; Amaratunga, G.A.J.; de Silva, K.M.N. Nano-MgO reinforced chitosan nanocomposites for high performance packaging applications with improved mechanical, thermal and barrier properties. Carbohydr. Polym. 2017, 157, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Jahed, E.; Khaledabad, M.A.; Almasi, H.; Hasanzadeh, R. Physicochemical properties of Carum copticum essential oil loaded chitosan films containing organic nanoreinforcements. Carbohydr. Polym. 2017, 164, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Hafsa, J.; Smach, M.A.; Ben Khedher, M.R.; Charfeddine, B.; Limem, K.; Majdoub, H.; Rouatbi, S. Physical, antioxidant and antimicrobial properties of chitosan films containing Eucalyptus globulus essential oil. LWT Food Sci. Technol. 2016, 68, 356–364. [Google Scholar] [CrossRef]
- Perdones, A.; Escriche, I.; Chiralt, A.; Vargas, M. Effect of chitosan-lemon essential oil coatings on volatile profile of strawberries during storage. Food Chem. 2016, 197, 979–986. [Google Scholar] [CrossRef]
- Yuan, G.; Lv, H.; Yang, B.; Chen, X.; Sun, H. Physical properties, antioxidant and antimicrobial activity of chitosan films containing carvacrol and pomegranate peel extract. Molecules 2015, 20, 11034–11045. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Torlak, E.; Akın-Evingür, G.; Özen, İ.; Erim, F.B. Antimicrobial and physical properties of chitosan films incorporated with turmeric extract. Int. J. Biol. Macromol. 2017, 101, 882–888. [Google Scholar] [CrossRef]
- Quesada, J.; Sendra, E.; Navarro, C.; Sayas-Barberá, E. Antimicrobial Active Packaging including Chitosan Films with Thymus vulgaris L. Essential Oil for Ready-to-Eat Meat. Foods 2016, 5, 57. [Google Scholar] [CrossRef]
- Lekjing, S. A chitosan-based coating with or without clove oil extends the shelf life of cooked pork sausages in refrigerated storage. Meat Sci. 2016, 111, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Food applications of emulsion-based edible films and coatings. Trends Food Sci. Technol. 2015, 45, 273–283. [Google Scholar] [CrossRef]
- Hromiš, N.M.; Lazić, V.L.; Markov, S.L.; Vaštag, Ž.G.; Popović, S.Z.; Šuput, D.Z.; Džinić, N.R.; Velićanski, A.S.; Popović, L.M. Optimization of Chitosan Biofilm Properties by Addition of Caraway Essential Oil and Beeswax; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; Volume 158, ISBN 3812148536. [Google Scholar]
- Vargas, M.; Perdones, Á.; Chiralt, A.; Cháfer, M.; González-Martínez, C. Effect of homogenization conditions on physicochemical properties of chitosan-based film-forming dispersions and films. Food Hydrocoll. 2011, 25, 1158–1164. [Google Scholar] [CrossRef]
- Sanuja, S.; Agalya, A.; Umapathy, M.J. Synthesis and characterization of zinc oxide-neem oil-chitosan bionanocomposite for food packaging application. Int. J. Biol. Macromol. 2015, 74, 76–84. [Google Scholar] [CrossRef]
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S.M.H. Development and evaluation of a novel biodegradable film made from chitosan and cinnamon essential oil with low affinity toward water. Food Chem. 2010, 122, 161–166. [Google Scholar] [CrossRef]
- Antony, R.; Arun, T.; Manickam, S.T.D. A review on applications of chitosan-based Schiff bases. Int. J. Biol. Macromol. 2019, 129, 615–633. [Google Scholar] [CrossRef]
- Khoushab, F.; Yamabhai, M. Chitin research revisited. Mar. Drugs 2010, 8, 1988–2012. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- de Queiroz Antonino, R.; Lia Fook, B.; de Oliveira Lima, V.; de Farias Rached, R.; Lima, E.; da Silva Lima, R.; Peniche Covas, C.; Lia Fook, M. Preparation and Characterization of Chitosan Obtained from Shells of Shrimp (Litopenaeus vannamei Boone). Mar. Drugs 2017, 15, 141. [Google Scholar] [CrossRef]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Kjartansson, G.T.; Zivanovic, S.; Kristbergsson, K.; Weiss, J. Sonication-assisted extraction of chitin from North Atlantic shrimps (Pandalus borealis). J. Agric. Food Chem. 2006, 54, 5894–5902. [Google Scholar] [CrossRef] [PubMed]
- Baxter, S.R. Molecular Weight and Degree of Acetylation of Ultrasonicated Chitosan; University of Tennessee: Knoxville, TN, USA, 2004. [Google Scholar]
- Wu, T. Sonochemical and Hydrophobic Modification of Chitin and Chitosan; University of Tennessee: Knoxville, TN, USA, 2007. [Google Scholar]
- EL Knidri, H.; Dahmani, J.; Addaou, A.; Laajeb, A.; Lahsini, A. Rapid and efficient extraction of chitin and chitosan for scale-up production: Effect of process parameters on deacetylation degree and molecular weight. Int. J. Biol. Macromol. 2019, 139, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Zdanowicz, M.; Wilpiszewska, K.; Spychaj, T. Deep eutectic solvents for polysaccharides processing. A review. Carbohydr. Polym. 2018, 200, 361–380. [Google Scholar] [CrossRef]
- Sharma, M.; Mukesh, C.; Mondal, D.; Prasad, K. Dissolution of α-chitin in deep eutectic solvents. RSC Adv. 2013, 3, 18149–18155. [Google Scholar] [CrossRef]
- Zhu, P.; Gu, Z.; Hong, S.; Lian, H. One-pot production of chitin with high purity from lobster shells using choline chloride–malonic acid deep eutectic solvent. Carbohydr. Polym. 2017, 177, 217–223. [Google Scholar] [CrossRef]
- Ramírez-Wong, D.G.; Ramírez-Cardona, M.; Sánchez-Leija, R.J.; Rugerio, A.; Mauricio-Sánchez, R.A.; Hernández-Landaverde, M.A.; Carranza, A.; Pojman, J.A.; Garay-Tapia, A.M.; Prokhorov, E.; et al. Sustainable-solvent-induced polymorphism in chitin films. Green Chem. 2016, 18, 4303–4311. [Google Scholar] [CrossRef]
- Ozkan, A.; Rorrer, G.L. Effects of CO2 delivery on fatty acid and chitin nanofiber production during photobioreactor cultivation of the marine diatom Cyclotella sp. Algal Res. 2017, 26, 422–430. [Google Scholar] [CrossRef]
- Chiriboga, O.; Rorrer, G.L. Phosphate addition strategies for enhancing the co-production of lipid and chitin nanofibers during fed-batch cultivation of the diatom Cyclotella sp. Algal Res. 2019, 38, 101403. [Google Scholar] [CrossRef]
- Rorrer, G.; Antonio Torres, J.; Durst, R.; Kelly, C.; Gale, D.; Maddux, B.; Ozkan, A. The Potential of a Diatom-Based Photosynthetic Biorefinery for Biofuels and Valued Co-Products. Curr. Biotechnol. 2016, 5, 237–248. [Google Scholar] [CrossRef]
- Zhao, Y.; Park, R.D.; Muzzarelli, R.A.A. Chitin deacetylases: Properties and applications. Mar. Drugs 2010, 8, 24–46. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, G.S.; Kaur, S.; Brar, S.K.; Verma, M. Green synthesis approach: Extraction of chitosan from fungus mycelia. Crit. Rev. Biotechnol. 2013, 33, 379–403. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Gawad, K.M.; Hifney, A.F.; Fawzy, M.A.; Gomaa, M. Technology optimization of chitosan production from Aspergillus niger biomass and its functional activities. Food Hydrocoll. 2017, 63, 593–601. [Google Scholar] [CrossRef]
- Bilbao-Sainz, C.; Chiou, B.S.; Williams, T.; Wood, D.; Du, W.X.; Sedej, I.; Ban, Z.; Rodov, V.; Poverenov, E.; Vinokur, Y.; et al. Vitamin D-fortified chitosan films from mushroom waste. Carbohydr. Polym. 2017, 167, 97–104. [Google Scholar] [CrossRef]
- Vendruscolo, F.; Ninow, J.L. Apple pomace as a substrate for fungal chitosan production in an airlift bioreactor. Biocatal. Agric. Biotechnol. 2014, 3, 338–342. [Google Scholar] [CrossRef]
- Ghormade, V.; Pathan, E.K.; Deshpande, M.V. Can fungi compete with marine sources for chitosan production? Int. J. Biol. Macromol. 2017, 104, 1415–1421. [Google Scholar] [CrossRef]
- Ferreira, A.R.V.; Alves, V.D.; Coelhoso, I.M. Polysaccharide-based membranes in food packaging applications. Membranes 2016, 6, 22. [Google Scholar] [CrossRef]
- Epure, V.; Griffon, M.; Pollet, E.; Avérous, L. Structure and properties of glycerol-plasticized chitosan obtained by mechanical kneading. Carbohydr. Polym. 2011, 83, 947–952. [Google Scholar] [CrossRef]
- Galvis-Sánchez, A.C.; Castro, M.C.R.; Biernacki, K.; Gonçalves, M.P.; Souza, H.K.S. Natural deep eutectic solvents as green plasticizers for chitosan thermoplastic production with controlled/desired mechanical and barrier properties. Food Hydrocoll. 2018, 82, 478–489. [Google Scholar] [CrossRef]
- Matet, M.; Heuzey, M.C.; Pollet, E.; Ajji, A.; Avérous, L. Innovative thermoplastic chitosan obtained by thermo-mechanical mixing with polyol plasticizers. Carbohydr. Polym. 2013, 95, 241–251. [Google Scholar] [CrossRef]
- Matet, M.; Heuzey, M.C.; Ajji, A.; Sarazin, P. Plasticized chitosan/polyolefin films produced by extrusion. Carbohydr. Polym. 2015, 117, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Lopez, O.; Garcia, M.A.; Villar, M.A.; Gentili, A.; Rodriguez, M.S.; Albertengo, L. Thermo-compression of biodegradable thermoplastic corn starch films containing chitin and chitosan. LWT Food Sci. Technol. 2014, 57, 106–115. [Google Scholar] [CrossRef]
- Dang, K.M.; Yoksan, R. Development of thermoplastic starch blown film by incorporating plasticized chitosan. Carbohydr. Polym. 2015, 115, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Dang, K.M.; Yoksan, R. Morphological characteristics and barrier properties of thermoplastic starch/chitosan blown film. Carbohydr. Polym. 2016, 150, 40–47. [Google Scholar] [CrossRef]
- Mendes, J.F.; Paschoalin, R.T.; Carmona, V.B.; Sena Neto, A.R.; Marques, A.C.P.; Marconcini, J.M.; Mattoso, L.H.C.; Medeiros, E.S.; Oliveira, J.E. Biodegradable polymer blends based on corn starch and thermoplastic chitosan processed by extrusion. Carbohydr. Polym. 2016, 137, 452–458. [Google Scholar] [CrossRef]
- Galvis-Sánchez, A.C.; Sousa, A.M.M.; Hilliou, L.; Gonçalves, M.P.; Souza, H.K.S. Thermo-compression molding of chitosan with a deep eutectic mixture for biofilms development. Green Chem. 2016, 18, 1571–1580. [Google Scholar] [CrossRef]
- Almeida, C.M.R.; Magalhães, J.M.C.S.; Souza, H.K.S.; Gonçalves, M.P. The role of choline chloride-based deep eutectic solvent and curcumin on chitosan films properties. Food Hydrocoll. 2018, 81, 456–466. [Google Scholar] [CrossRef]
- Pereira, P.F.; Andrade, C.T. Optimized pH-responsive film based on a eutectic mixture-plasticized chitosan. Carbohydr. Polym. 2017, 165, 238–246. [Google Scholar] [CrossRef]

| Food Applied | Active Compound Incorporated | Storage Condition | Key Findings | Ref. |
|---|---|---|---|---|
| Mango (Tommy Atkins) | - | 23 °C | The coating delayed the ripening of semi-ripe mangoes stored at 23 °C. The level 1.5% provided better maintenance of physical chemical parameters assessed. | [19] |
| Sliced Mango | - | 6 °C | Chitosan coating retarded water loss and the drop in sensory quality, increasing the soluble solid content, titratable acidity and ascorbic acid content, and inhibiting the growth of microorganisms. | [20] |
| Chicken breast | 0.25% oregano essential oil (OEO) | 4 °C | The shelf-life of chicken fillets was extended using, either OEO singly, and/or chitosan, by approximately 6 (with only OEO) and more than 15 (chitosan coating with or without OEO) days. Treated chicken samples with the coatings were sensorially acceptable during the entire refrigerated storage period (21 days), not negatively influencing the taste of chicken samples | [21] |
| Egg | - | 25 °C | The coating created a protective barrier against the transfer of moisture and carbon dioxide through the eggshell, keeping a high Haugh unit and yolk index, while preserving it from Salmonella enteritidis. | [22] |
| Fresh fillets of Atlantic cod (Gadus morhua) and herring (Clupea harengus) | - | 4 °C | Potential of chitosan as a preservative coating in reducing or preventing moisture loss, lipid oxidation, and microbial growth | [23] |
| Guava (Psidium guajava L.) fruit | - | 11 °C | Fruits coated with 2.0% chitosan reduced weight and firmness loss, delayed changes in chlorophyll and malondialdehyde contents and soluble solids content, retarded the loss of vitamin C and the decrease of titratable acidity, during 12 days of storage, delaying ripening process. | [24] |
| Blueberry (Vaccinium corymbosum) fruit | Aloe vera extract | 5 °C | Microbiological growth and water loss levels were approximately reduced by 50% and 42%, respectively, in coated blueberries after 25 d compared to uncoated blueberries. The chitosan coatings with the extract have proven to have great potential in expanding the shelf- life of fruits. | [25] |
| Fresh cut broccoli | Bioactive compounds and essential oils | 5–7 °C | Pristine chitosan coating or enriched with bioactive compounds/essential oils resulted in a significant reduction in mesophilic and psychotropic counts. The enrichment with active compounds improved the antimicrobial action of chitosan. The application of these coatings did not introduce deleterious effects on the sensory attributes of broccoli. | [26] |
| Walnut kernels | Tea extract | Room temperature | Effective inhibition of lipid oxidation and fungal growth during storage of walnut kernels (18 weeks) with chitosan coating combined with green tea extract. No significant effect on sensory properties was observed during storage time. The results suggested that the active coating could prolong the shelf life of walnut kernels | [27] |
| Fresh-cut pears | Rosemary extracts | 20 °C | The study suggests that chitosan + rosemary extract coating have the potential to improve the quality of fresh-cut pears and extend the shelf-life, by reducing changes in pH, inhibiting polyphenol oxidase activity, weight loss, and retaining high firmness and soluble solid content. | [28] |
| Guava (Psidium guajava L.) fruit | Pomegranate peel extract | 10 °C | Samples coated with chitosan enriched with bioactive extract proved to be an effective treatment to maintain the overall fruit quality during 20 days at low temperature storage. | [29] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, V.G.L.; Pires, J.R.A.; Rodrigues, C.; Coelhoso, I.M.; Fernando, A.L. Chitosan Composites in Packaging Industry—Current Trends and Future Challenges. Polymers 2020, 12, 417. https://doi.org/10.3390/polym12020417
Souza VGL, Pires JRA, Rodrigues C, Coelhoso IM, Fernando AL. Chitosan Composites in Packaging Industry—Current Trends and Future Challenges. Polymers. 2020; 12(2):417. https://doi.org/10.3390/polym12020417
Chicago/Turabian StyleSouza, Victor G. L., João R. A. Pires, Carolina Rodrigues, Isabel M. Coelhoso, and Ana Luísa Fernando. 2020. "Chitosan Composites in Packaging Industry—Current Trends and Future Challenges" Polymers 12, no. 2: 417. https://doi.org/10.3390/polym12020417
APA StyleSouza, V. G. L., Pires, J. R. A., Rodrigues, C., Coelhoso, I. M., & Fernando, A. L. (2020). Chitosan Composites in Packaging Industry—Current Trends and Future Challenges. Polymers, 12(2), 417. https://doi.org/10.3390/polym12020417








