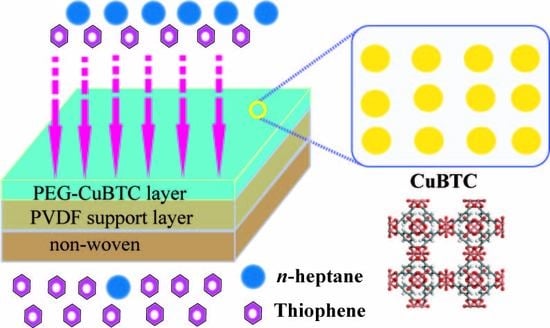
Improved Desulfurization Performance of Polyethyleneglycol Membrane by Incorporating Metal Organic Framework CuBTC
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Membrane Preparation
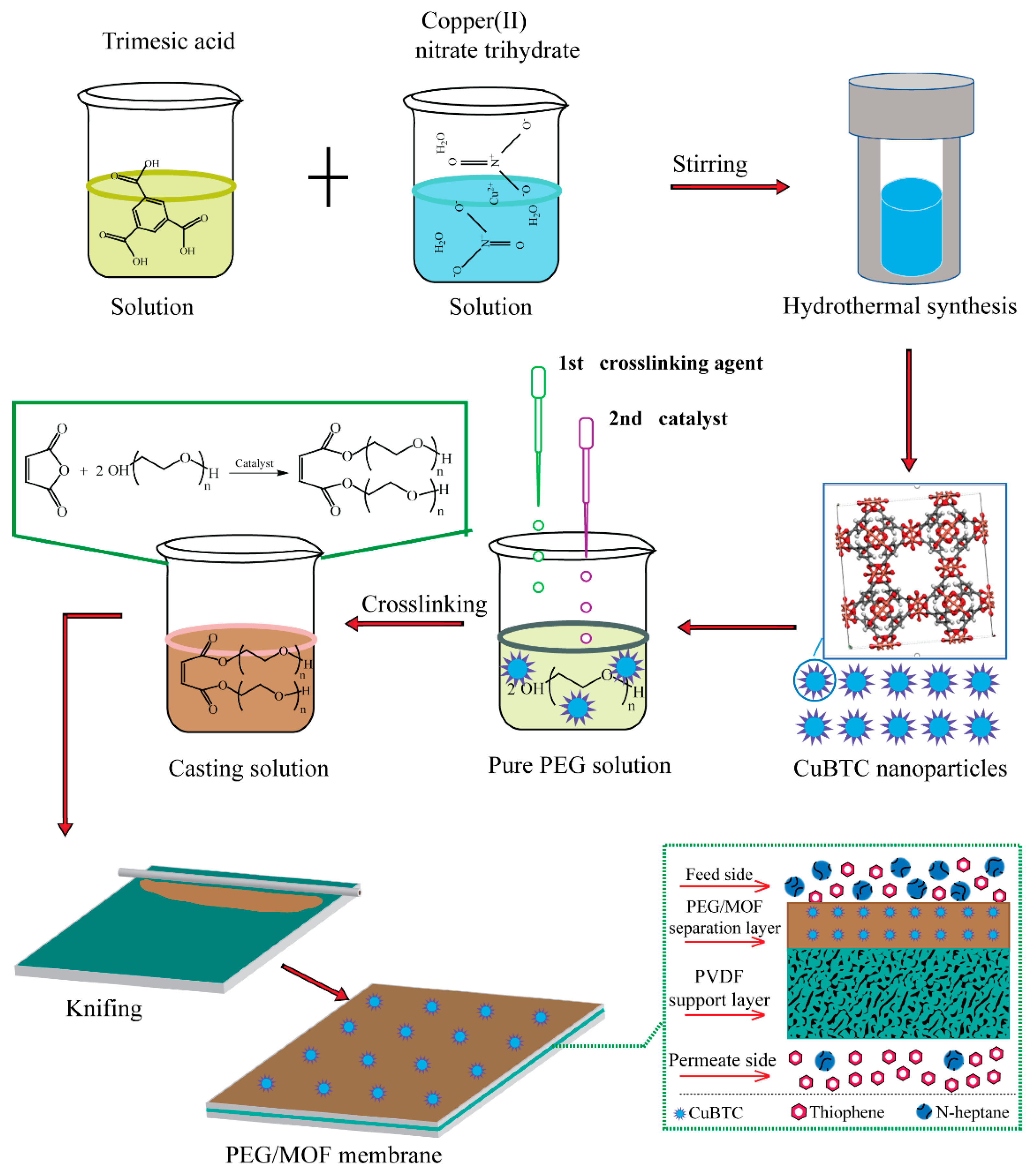
2.2.1. Synthesis of CuBTC Particles
2.2.2. Preparation of CuBTC-filled PEG MMMs
2.3. Characterization of CuBTC and Membranes
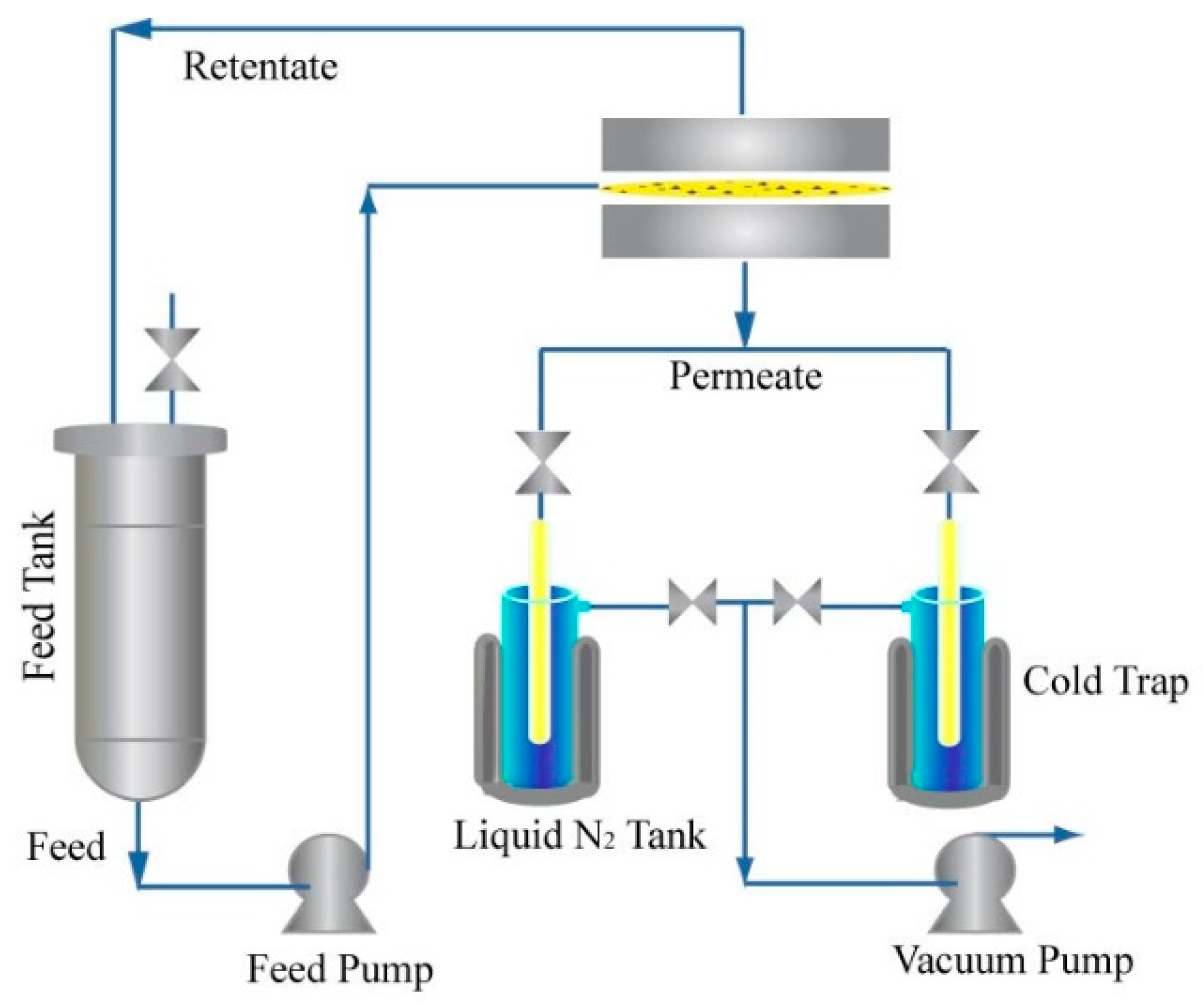
2.4. Pervaporation Experiments
3. Results and Discussions
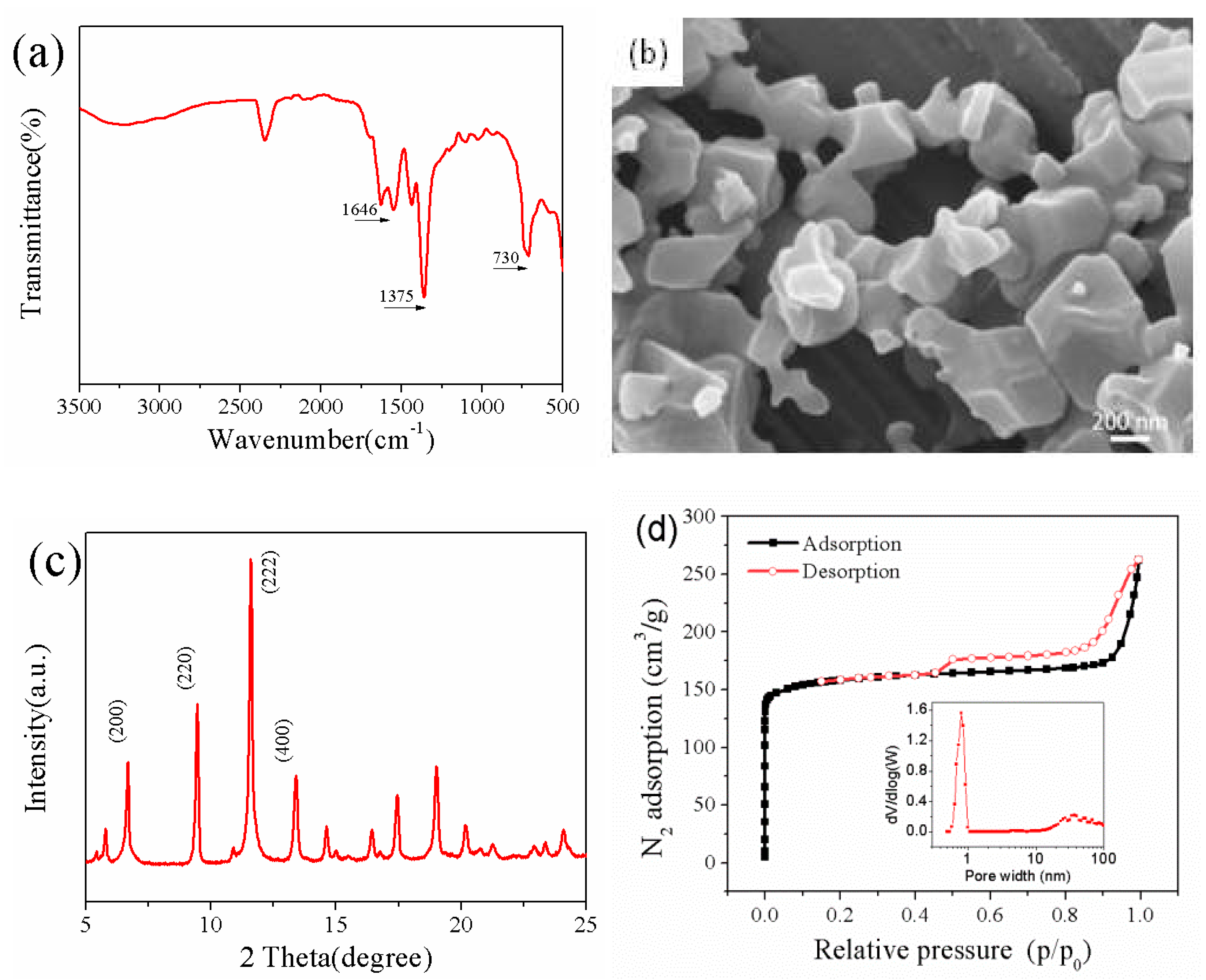
3.1. Characterization of CuBTC Particles
3.2. Characterization of CuBTC/PEG Hybrid Membranes
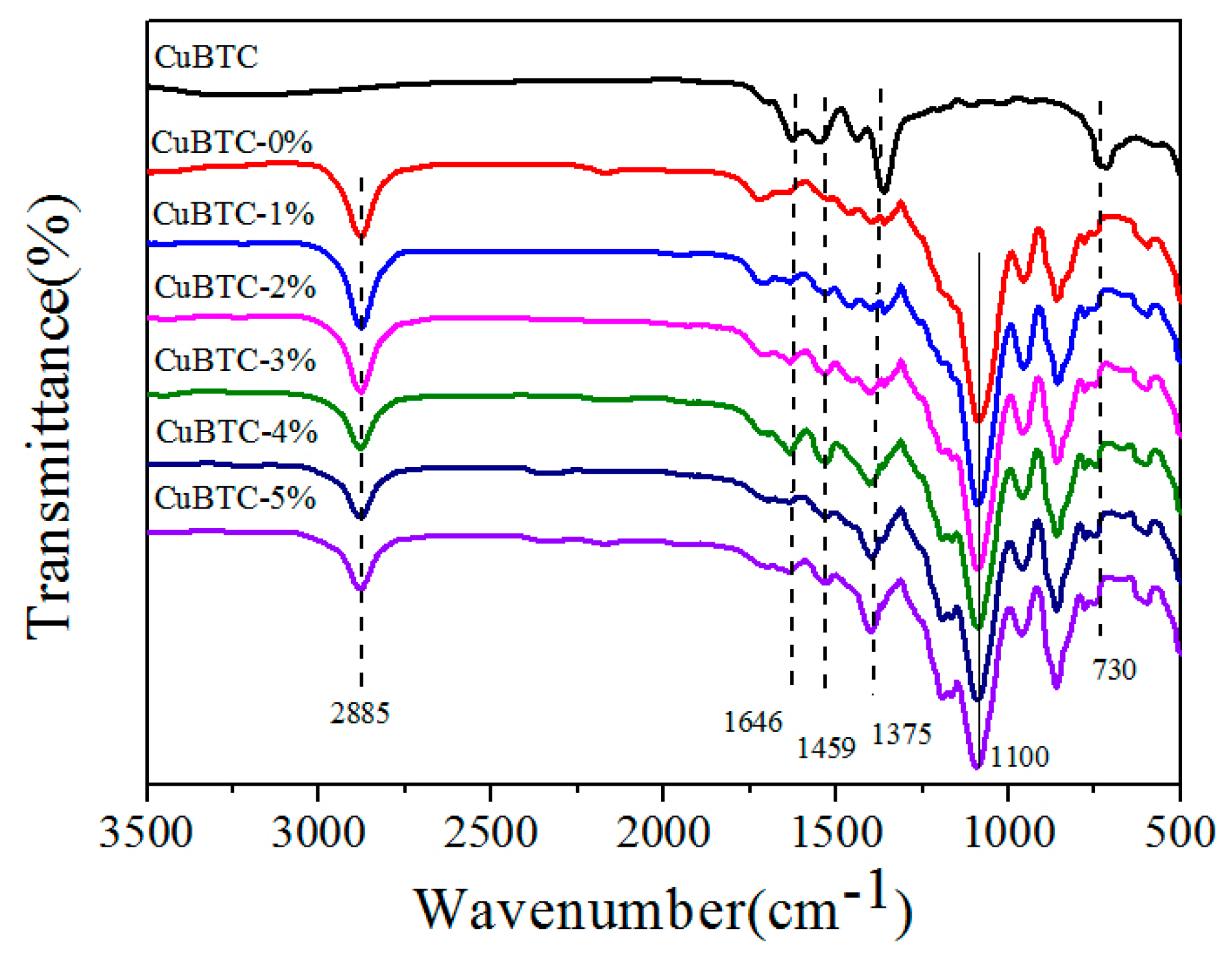
3.2.1. FT-IR Spectra of CuBTC/PEG Hybrid Membranes
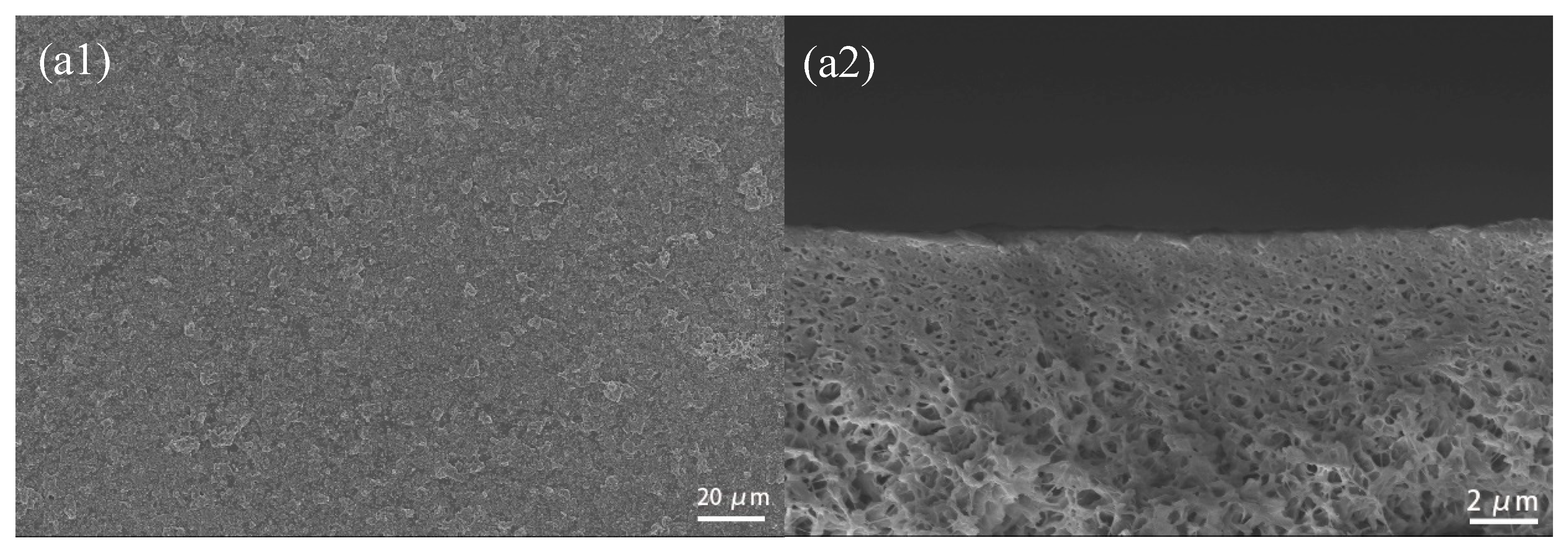
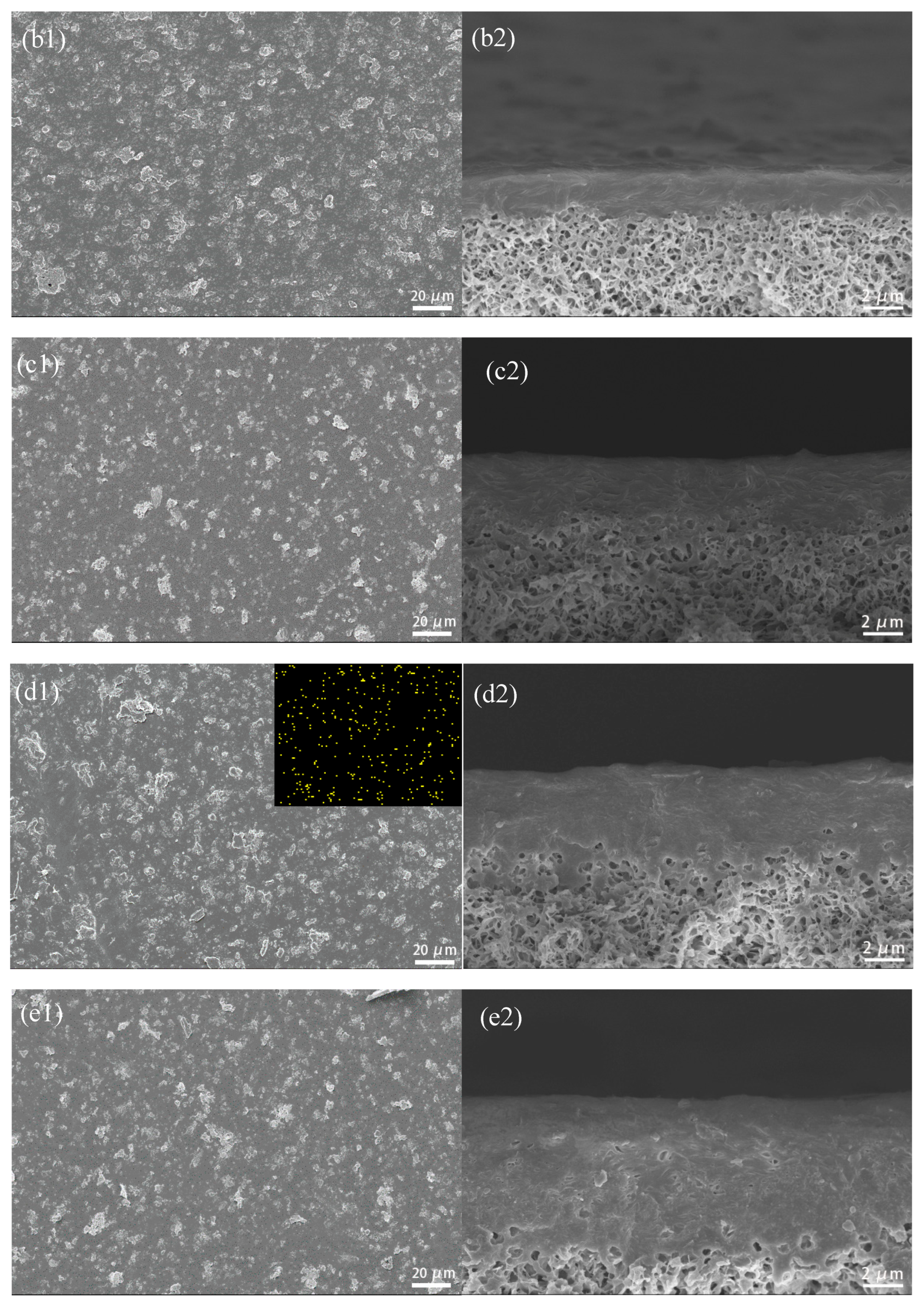
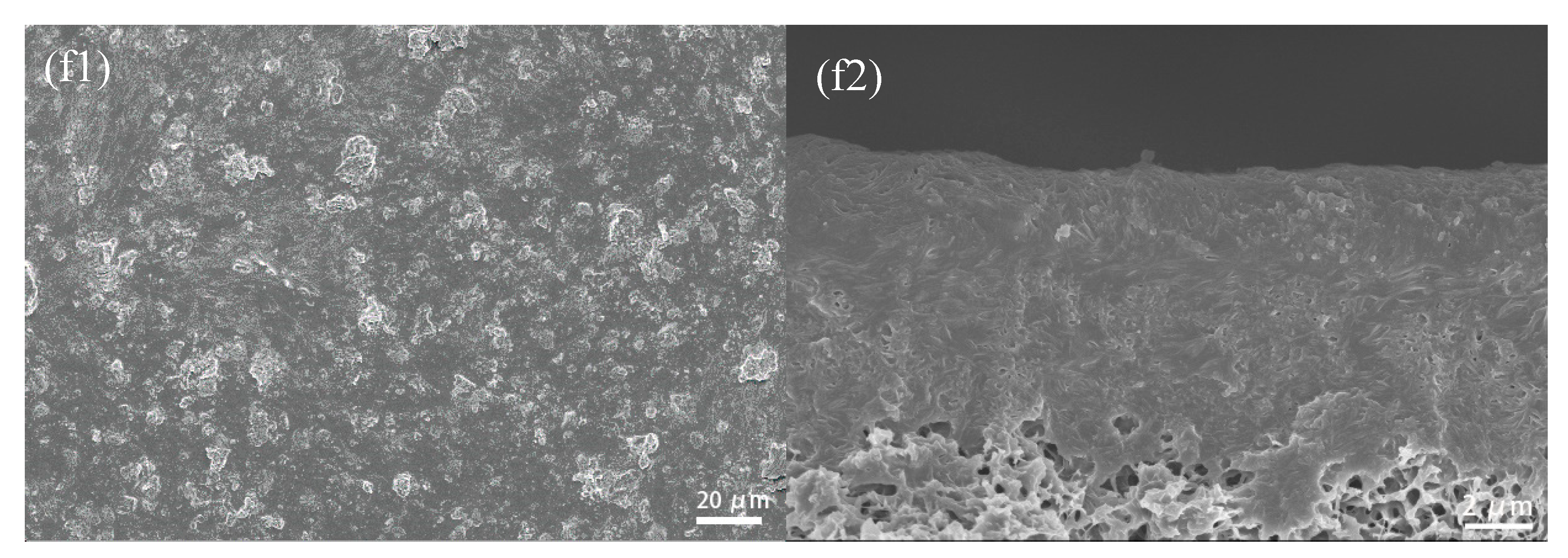
3.2.2. SEM Photographs of CuBTC-Filled PEG MMMs
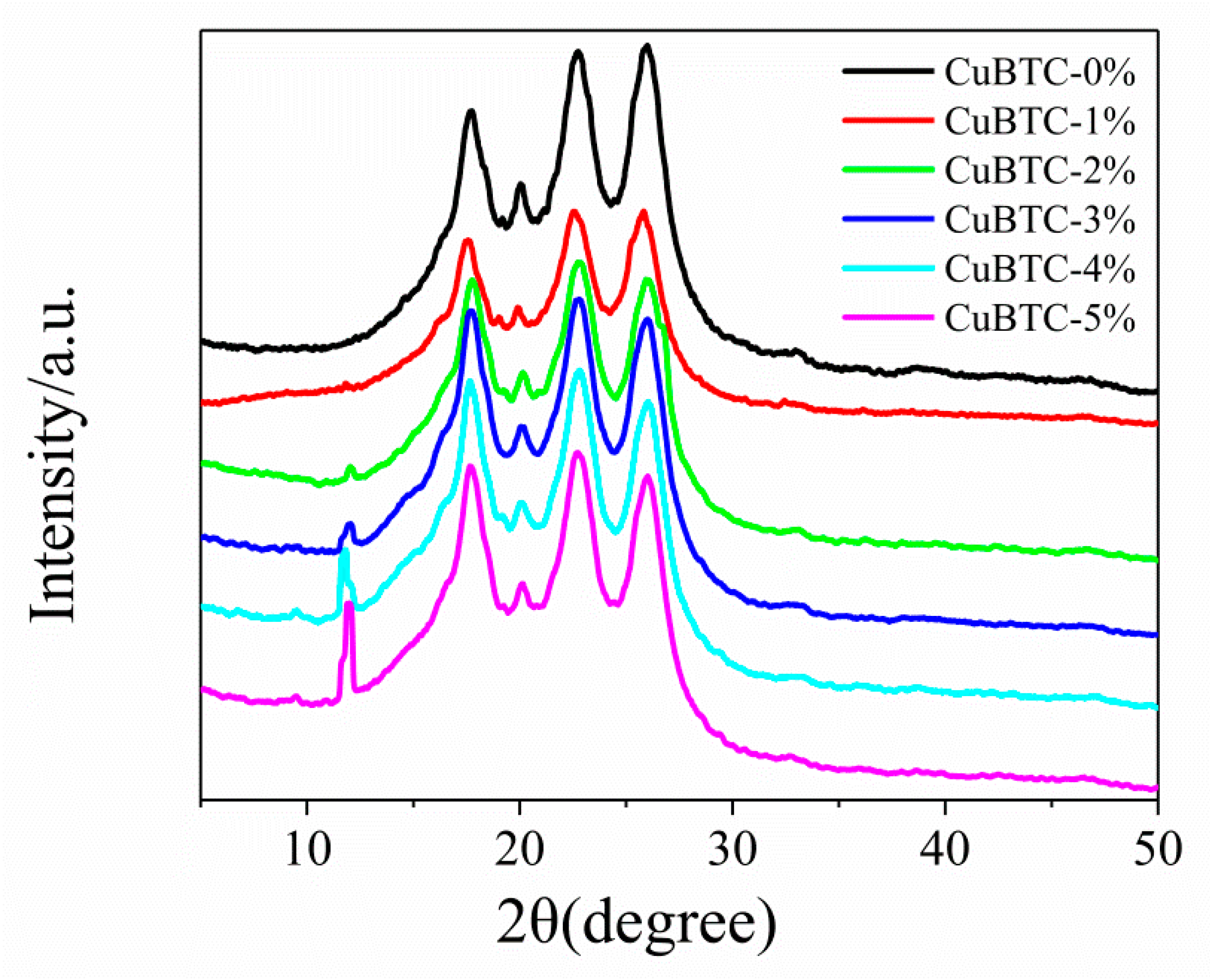
3.2.3. XRD Patterns of CuBTC-Filled PEG MMMs
3.3. Pervaporation Performances of CuBTC-Filled PEG MMMs
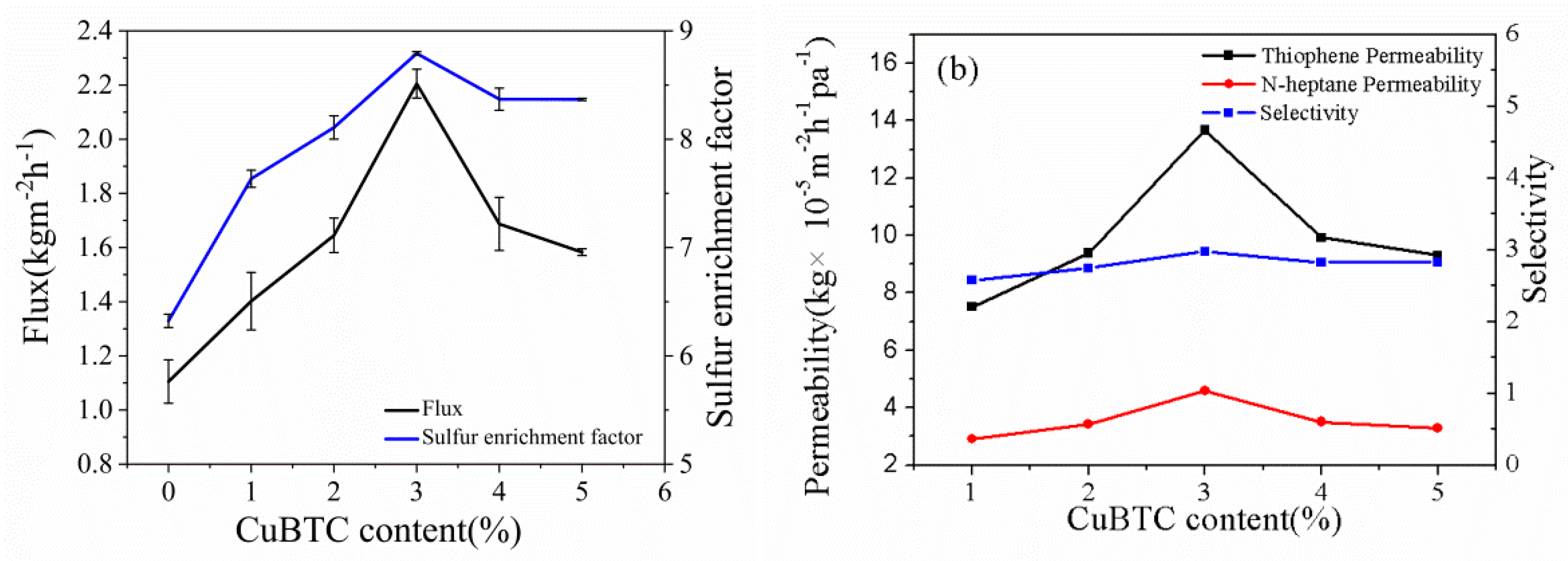
3.3.1. Effect of CuBTC Particle Content
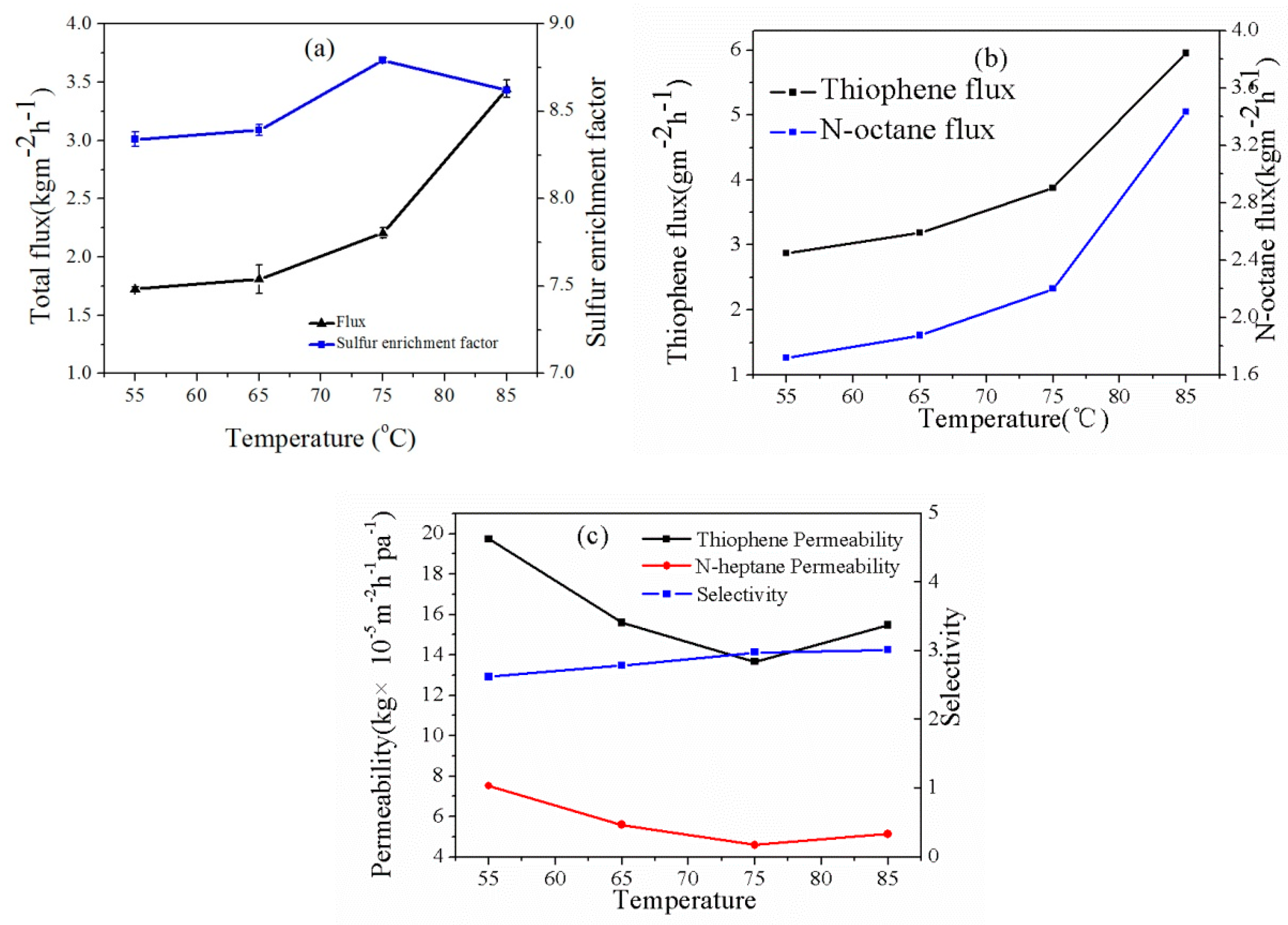
3.3.2. Effect of Feed Temperature
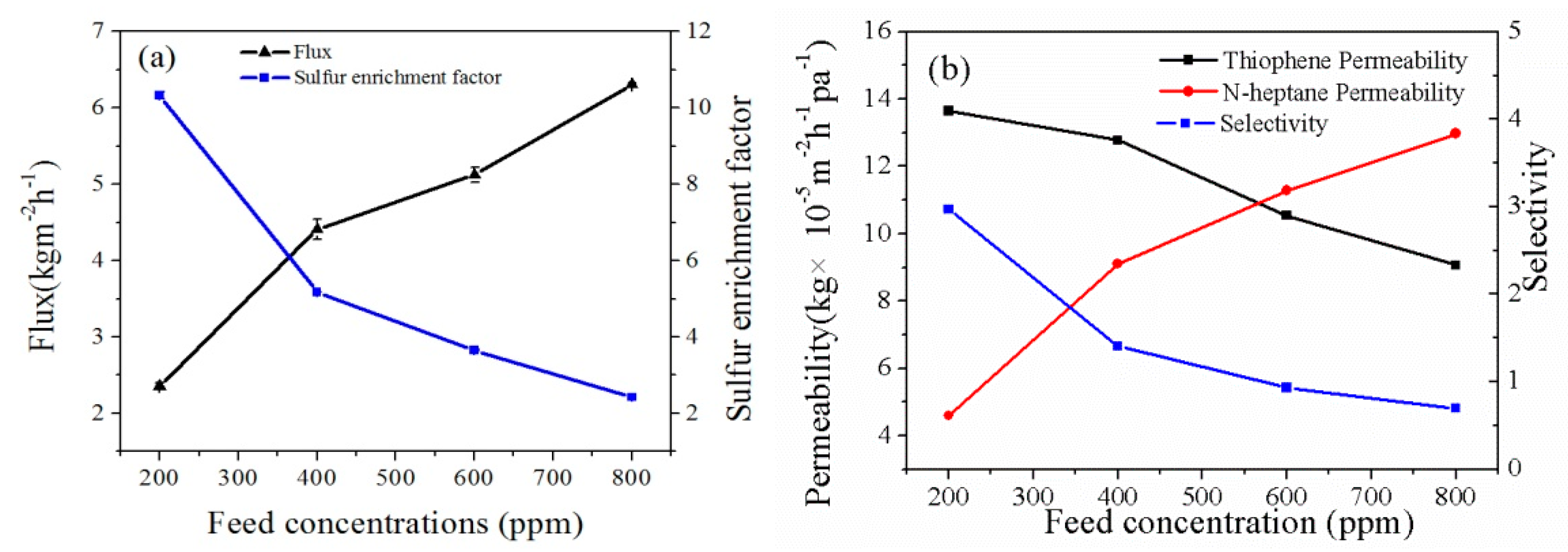
3.3.3. Effect of Feed Sulfur Content
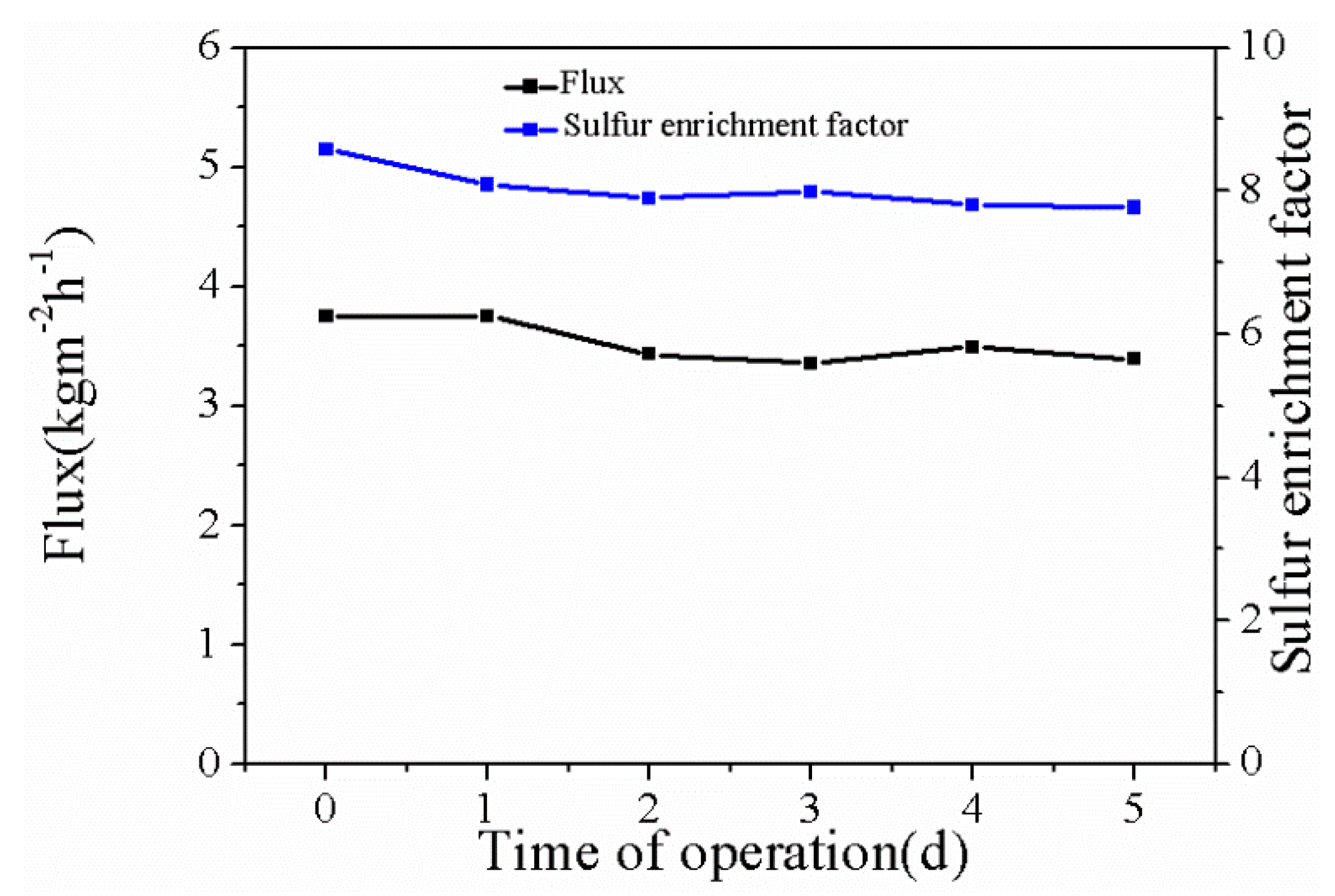
3.3.4. The Long-Term Stability of the Membranes
3.3.5. Comparison of Pervaporation Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stanislaus, A.; Marafi, A.; Rana, M.S. Recent advances in the science and technology of ultralow sulfur diesel (ULSD) production. Catal. Today 2010, 153, 1–68. [Google Scholar] [CrossRef]
- Li, W.L.; Liu, Q.F.; Xing, J.M.; Gao, H.S.; Xiong, X.C.; Li, Y.G.; Li, X.; Liu, H.Z. High-efficiency desulfurization by adsorption with mesoporous aluminosilicates. AIChE J. 2007, 49, 3263–3268. [Google Scholar] [CrossRef]
- Hao, L.W.; Su, T.; Hao, D.M.; Deng, C.L.; Ren, W.Z.; Lü, H.Y. Oxidative desulfurization of diesel fuel with caprolactam-based acidic deep eutectic solvents: Tailoring the reactivity of DESs by adjusting the composition. Chinese. J. Catal. 2018, 39, 1552–1559. [Google Scholar] [CrossRef]
- Ha, Y.; Guo, B.S.; Li, Y.H. Sensitivity and economic analysis of a catalytic distillation process for alkylation desulfurization of fluid catalytic cracking (FCC) gasoline. J. Chem. Technol. Biot. 2016, 91, 490–506. [Google Scholar] [CrossRef]
- Chang, J.H.; Kim, Y.J.; Lee, B.H.; Cho, K.S.; Ryu, H.W.; Chang, Y.K.; Chang, H.N. Production of a desulfurization biocatalyst by two-stage fermentation and its application for the treatment of model and diesel oils. Biotechnol. Prog. 2001, 17, 876–880. [Google Scholar] [CrossRef]
- Han, X.L.; Hu, T.T.; Wang, Y.; Chen, H.Y.; Wang, Y.Q.; Yao, R.Q.; Ma, X.X.; Li, J.D.; Li, X.F. A water-based mixing process for fabricating ZIF-8/PEG mixed matrix membranes with efficient desulfurization performance. Sep. Purif. Technol. 2019, 214, 61–66. [Google Scholar] [CrossRef]
- Şen, F.; Kahraman, M.V. Preparation and characterization of hybrid cationic hydroxyethyl cellulose/sodium alginate polyelectrolyte antimicrobial films. Polym. Adv. Technol. 2018, 29, 1895–1901. [Google Scholar] [CrossRef]
- Seo, K.; Sinha, K.; Novitskaya, E.; Graeve, O.A. Polyvinylpyrrolidone (PVP) effects on iron oxide nanoparticle formation. Mater. Lett. 2018, 215, 203–206. [Google Scholar] [CrossRef]
- Wang, H.N.; Wang, F.; Li, X.Q.; Peng, X.P.; Ci, Z.P.; Wang, Z.F. Preparation and performance investigation of polydimethylsiloxane microsphere/polyvinyl alcohol composite hydrogel. Mater. Lett. 2018, 228, 399–402. [Google Scholar] [CrossRef]
- Gao, J.J.; Zhu, S.; Dai, Y.F.; Xiong, C.Y.; Li, C.X.; Yang, W.M.; Jiang, X.M. Performance and mechanism for extractive desulfurization of fuel oil using modified polyethylene glycol. Fuel 2018, 233, 704–713. [Google Scholar] [CrossRef]
- Yuan, S.M.; Ma, C.C.; Chang, C.L.; Lin, Y.Y.; Teng, C.C. Preparation and morphological, electrical, and mechanical properties of polyimide-grafted MWCNT/polyimide composite. J. Polym. Sci. 2017, 45, 3349–3358. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, Y.; Li, H. Pervaporation and sorption behavior of zeolite-filled polyethylene glycol hybrid membranes for the removal of thiophene species. J. Colloid. Interf. Sci. 2010, 350, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Z.; Zhang, Y.M.; Li, J.D.; Chen, J. Preparation and characterization of PEG/PVDF composite membranes and effects of solvents on its pervaporation performance in heptane desulfurization. Desalin. Water Treat. 2012, 46, 1–11. [Google Scholar] [CrossRef]
- Yang, Z.J.; Zhang, W.; Wang, T.; Li, J.D. Improved thiophene solution selectivity by Cu2+, Pb2+ and Mn2+ ions in pervaporative poly[bis(p-methyl phenyl) phosphazene]desulfurization membrane. J. Membr. Sci. 2014, 454, 463–469. [Google Scholar] [CrossRef]
- Yu, S.N.; Jiang, Z.Y.; Li, W.D.; Mayta, J.Q.; Ding, H.; Song, Y.M.; Li, Z.; Dong, Z.W.; Pan, F.S.; Wang, B.Y.; et al. Elevated performance of hybrid membranes by incorporating metal organic framework CuBTC for pervaporative desulfurization of gasoline. Chem. Eng. Process. 2018, 123, 12–19. [Google Scholar] [CrossRef]
- Yu, S.N.; Jiang, Z.Y.; Ding, H.; Pan, F.S.; Wang, B.Y.; Yang, J.; Cao, X.Z. Elevated pervaporation performance of polysiloxane membrane using channels and active sites of metal organic framework CuBTC. J. Membr. Sci. 2015, 481, 73–81. [Google Scholar] [CrossRef]
- Hu, W.L.; Han, X.L.; Liu, L.L.; Zhang, X.; Xue, J.Q.; Wang, B.Y.; Zhang, P.; Cao, X.Z. PEG/PVDF Membranes for Separating Organosulphur Compounds from N-Heptane: Effect of PEG Molecular Weight. Can. J. Chem. Eng. 2016, 9999, 1–8. [Google Scholar]
- Schlichte, K.; Kratzke, T.; Kaskel, S. Improved synthesis, thermal stability and catalytic properties of the metal–organic framework compound Cu3(BTC)2. Micropor. Mesopor. Mat. 2004, 73, 81–88. [Google Scholar] [CrossRef]
- Al-Janabi, N.; Hill, P.; Torrente-Murciano, L.; Garforth, A.; Gorgojo, P.; Siperstein, F.; Fan, X.L. Mapping the Cu-BTC metal–organic framework (HKUST-1) stability envelope in the presence of water vapour for CO2 adsorption from flue gases. Chem. Eng. J. 2015, 281, 669–677. [Google Scholar] [CrossRef]
- Ameloot, R.; Gobechiya, E.; Uji-i, H.; Martens, J.A.; Hofkens, J.; Alaerts, L.; Sels, B.F.; De Vos, D.E. Direct patterning of oriented metal–organic framework crystals via control over crystallization kinetics in clear precursor solutions. Adv. Mater. 2010, 22, 2685–2688. [Google Scholar] [CrossRef]
- Silvestre, M.E.; Franzreb, M.; Weidler, P.G.; Shekhah, O.; Wöll, C. Magnetic cores with porous coatings: Growth of metal-organic frameworks on particles using liquid phase epitaxy. Adv. Funct. Mater. 2013, 23, 1210–1213. [Google Scholar] [CrossRef]
- Ma, X.; Peng, S.P.; Li, W.M.; Liu, H.D.; Chen, Y.F. Efficient removal of low concentration methyl mercaptan by HKUST-1 membrane constructed on porous alumina granules. CrysEngComm 2018, 20, 407–411. [Google Scholar] [CrossRef]
- Finocchio, E.; Cristiani, C.; Dotelli, G.; Stampino, P.G.; Zampori, L. Thermal evolution of PEG-based and BRIJ-based hybridorgano-inorganic materials. FT-IR studies. Vib. Spectrosc. 2014, 71, 47–56. [Google Scholar] [CrossRef]
- Zeng, Y.P.; Zhu, X.M.; Yuan, Y.; Zhang, X.B.; Ju, S.G. Molecular simulations for adsorption and separation of thiophene and benzene in Cu-BTC and IRMOF-1 metal-organic frameworks. Sep. Purif. Technol. 2012, 95, 149–156. [Google Scholar] [CrossRef]
- Wei, X.L.; Cheng, X.J.; Xie, C.G. Initiation of chain reaction in catalytic pyrolysis of n-Heptane over zeolite catalysts. Acta Petrolei Sinica. 2013, 29, 13–20. [Google Scholar]
- Han, X.L.; Sun, H.X.; Liu, L.L.; Wang, Y.Q.; He, G.H.; Li, J.D. Improved desulfurization performance of polydimethylsioxane membrane by incorporating metal organic framework CPO-27-Ni. Sep. Purif. Technol. 2019, 217, 86–94. [Google Scholar] [CrossRef]
- Kong, Y.; Lin, L.G.; Zhang, Y.Z.; Lu, F.W.; Xie, K.K.; Liu, R.K.; Guo, L.; Shao, S.; Yang, J.R.; Shi, D.Q. Studies on polyethylene glycol/polyethersulfone composite membranes for FCC gasoline desulphurization by pervaporation. Eur. Polym. J. 2008, 44, 3335–3343. [Google Scholar] [CrossRef]
- Lin, L.; Kong, Y.; Xie, K.; Lu, F.; Liu, R.; Guo, L.; Shao, S.; Yang, J.; Shi, D.; Zhang, Y. Polyethylene glycol/polyurethane blend membranes for gasoline desulphurization by pervaporation technique. Sep. Purif. Technol. 2008, 61, 293–300. [Google Scholar] [CrossRef]

| Membrane | Feed | C (μg/g) | T (°C) | Flux (kg/(m2·h) | Enrichment Factor | Reference |
|---|---|---|---|---|---|---|
| PEG-PES | FCC gasoline | 900 | 100 | 3.37 | 3.63 | [27] |
| PEG-PU | FCC gasoline | 1200 | 110 | 2.5 | 4.03 | [28] |
| PEG-CuY | FCC gasoline | 1190 | 110 | 3.19 | 2.95 | [12] |
| CuBTC-PeBAX | Thiophene/octane | 1300 | 70 | 16.45 | 4.04 | [15] |
| CuBTC-PDMS | Thiophene/n-octane | 1300 | 40 | 5.24 | 5.20 | [16] |
| CPO-27-Ni--PDMS | Thiophene/n-heptane | 200 | 45 | 5.92 | 4.05 | [26] |
| ZIF-8-PEG | Thiophene/n-heptane | 200 | 75 | 1.96 | 8.93 | [6] |
| CuBTC-PEG | Thiophene/n-heptane | 200 | 75 | 2.206 | 8.37 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, C.; Fan, X.; Han, X.; Li, J.; Vardhan, H. Improved Desulfurization Performance of Polyethyleneglycol Membrane by Incorporating Metal Organic Framework CuBTC. Polymers 2020, 12, 414. https://doi.org/10.3390/polym12020414
Cai C, Fan X, Han X, Li J, Vardhan H. Improved Desulfurization Performance of Polyethyleneglycol Membrane by Incorporating Metal Organic Framework CuBTC. Polymers. 2020; 12(2):414. https://doi.org/10.3390/polym12020414
Chicago/Turabian StyleCai, Caibin, Xiaotao Fan, Xiaolong Han, Jiding Li, and Harsh Vardhan. 2020. "Improved Desulfurization Performance of Polyethyleneglycol Membrane by Incorporating Metal Organic Framework CuBTC" Polymers 12, no. 2: 414. https://doi.org/10.3390/polym12020414
APA StyleCai, C., Fan, X., Han, X., Li, J., & Vardhan, H. (2020). Improved Desulfurization Performance of Polyethyleneglycol Membrane by Incorporating Metal Organic Framework CuBTC. Polymers, 12(2), 414. https://doi.org/10.3390/polym12020414