Utilization of Water-Soluble Aminoethylamino–β–Cyclodextrin in the Pfitzinger Reaction—Catalyzed to the Synthesis of Diversely Functionalized Quinaldine
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
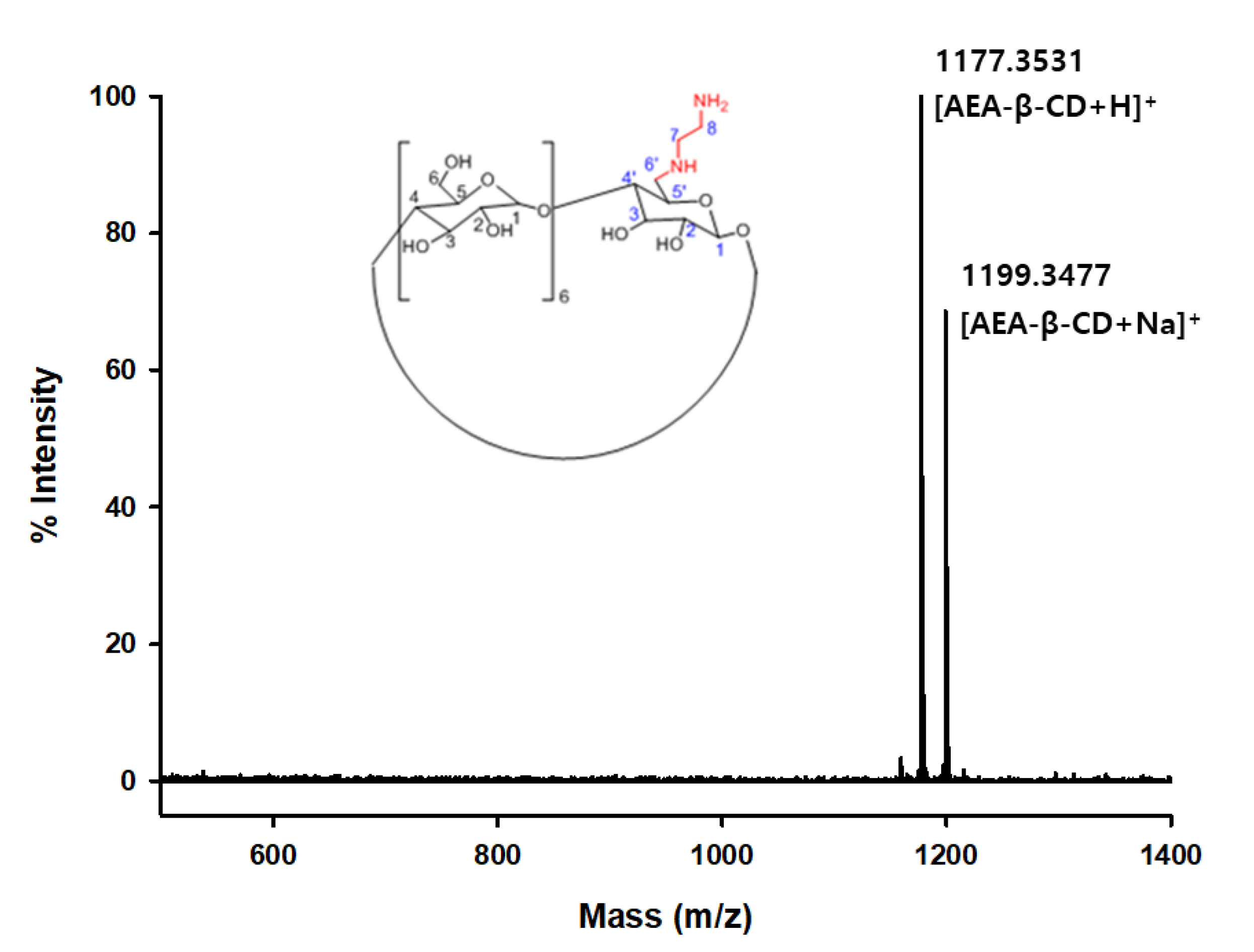
2.2. Synthesis of mono-6-deoxy-6-aminoethylamino-β-cyclodextrin (AEA–β–CD)
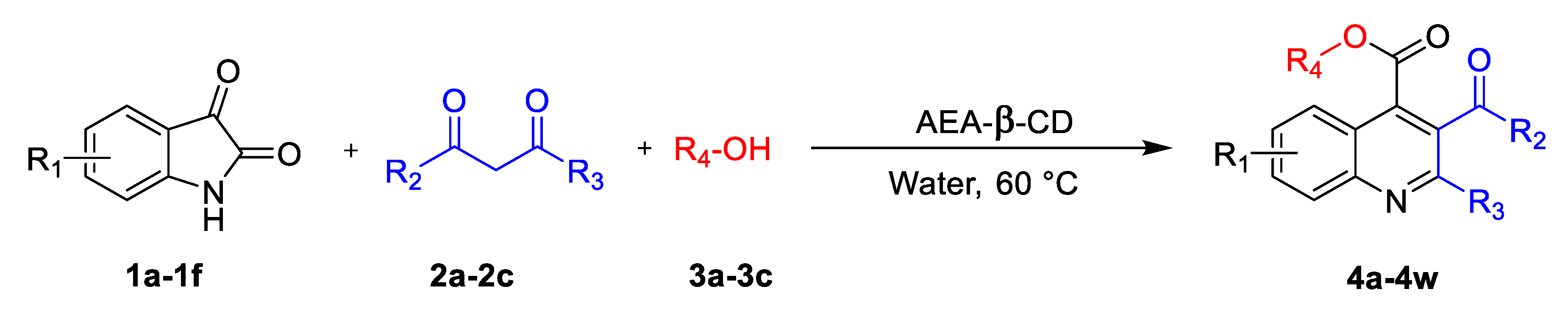
2.3. General Procedure for the Synthesis of Quinaldine
2.4. Matrix Assisted Laser Desorption/Ionization-Time of Flight (MALDI–TOF) Mass Spectrometry
2.5. Nuclear Magnetic Resonance (NMR) Spectroscopy
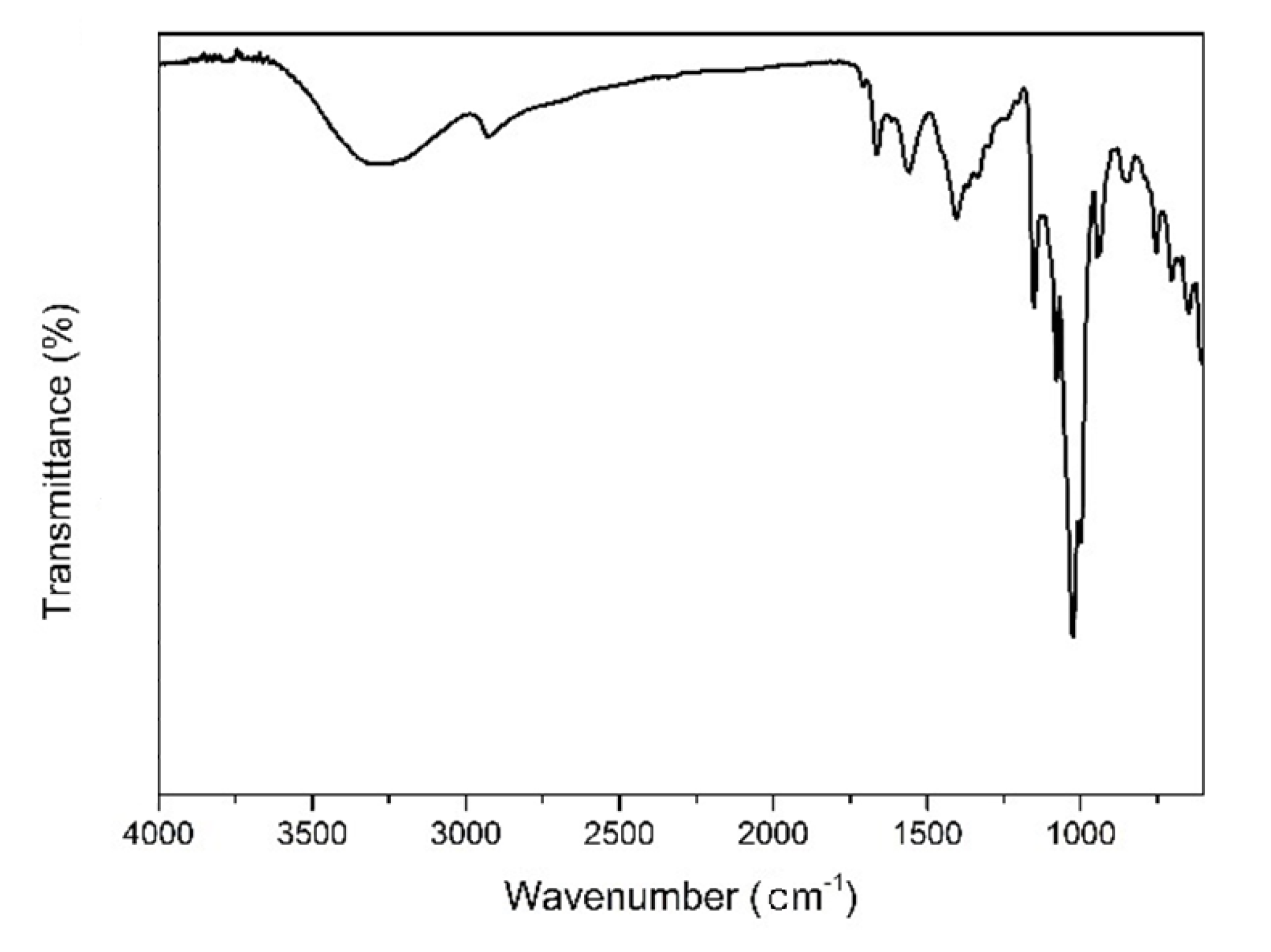
2.6. Fourier Transform-Infrared (FT-IR) Spectroscopy
2.7. Rotating Frame Nuclear Overhauser Spectroscopy (ROESY)
2.8. Field Emission Scanning Electron Microscopy (FE-SEM)
2.9. Differential Scanning Calorimetry (DSC)
2.10. Phase Solubility Analysis
3. Results and Discussion
3.1. Characterization of AEA–β–CD
3.2. Optimization of Reaction Conditions with AEA–β–CD as a Catalyst
3.3. Optimization of Reaction Conditions for Synthesis of Quinaldine Derivatives
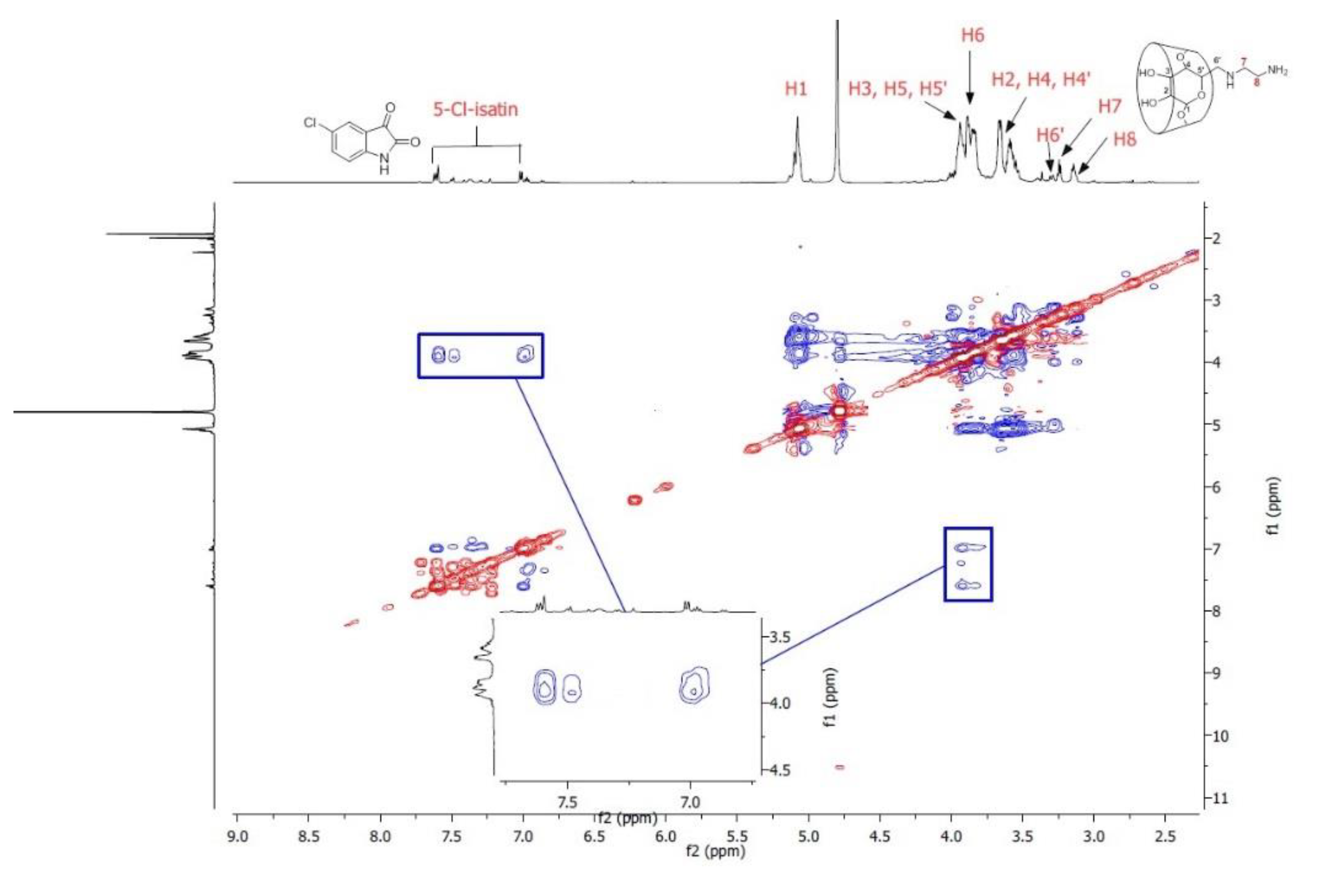
3.4. ROESY Spectroscopy of AEA-β-CD/CI Inclusion Complexes
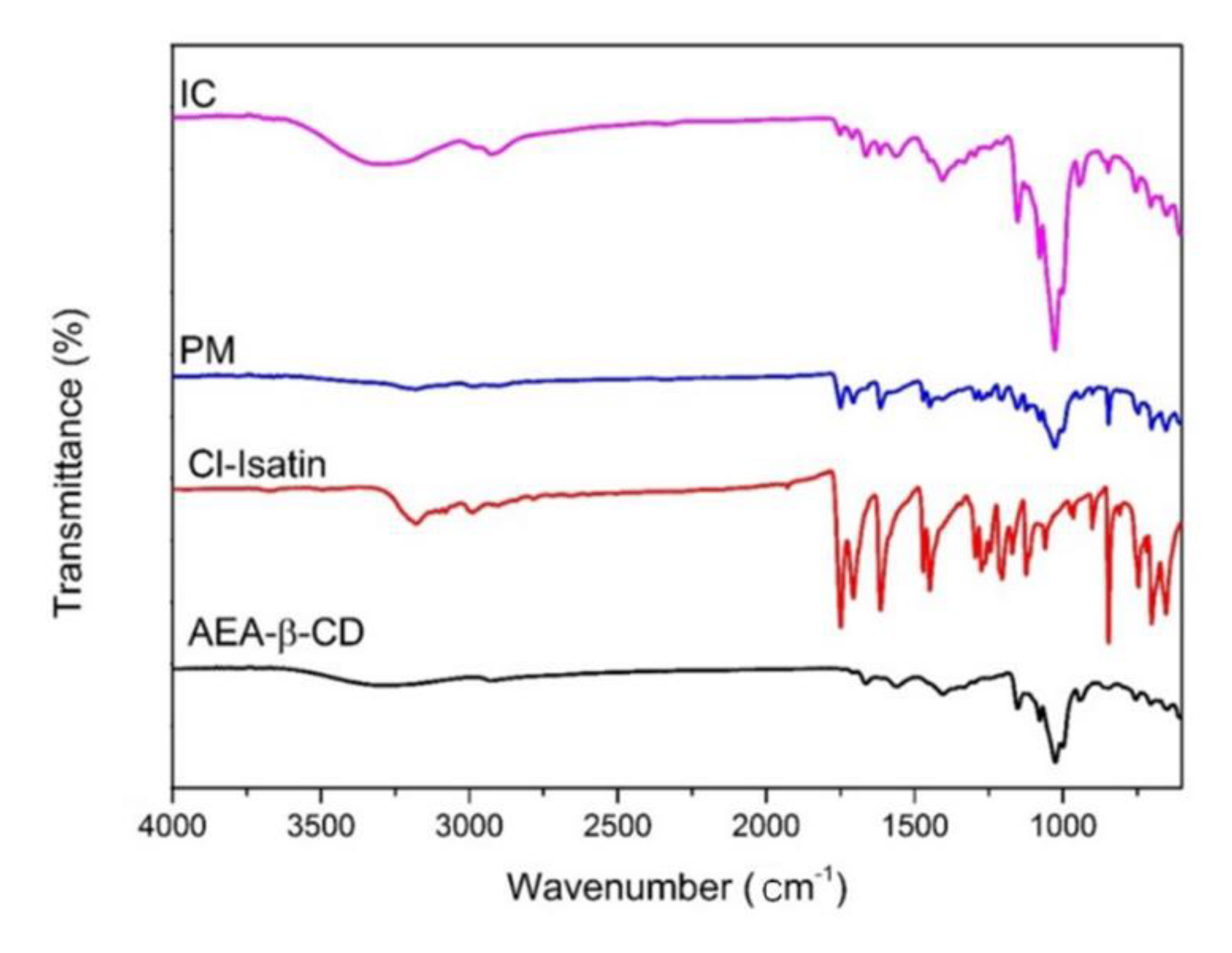
3.5. Fourier Transform Infrared (FT-IR) Spectroscopic Analysis
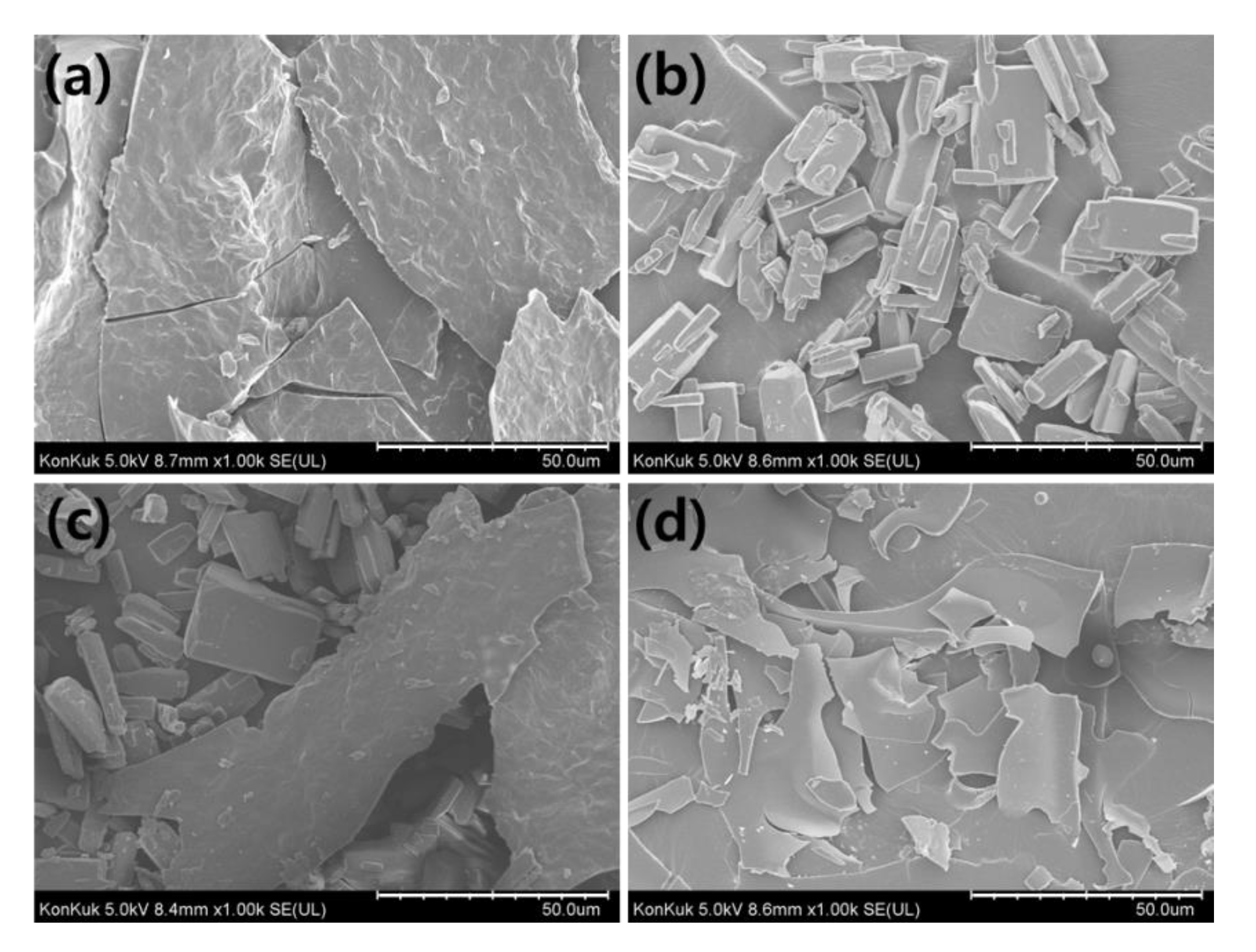
3.6. Field Emission Scanning Electron Microscopy (FE-SEM) Analysis
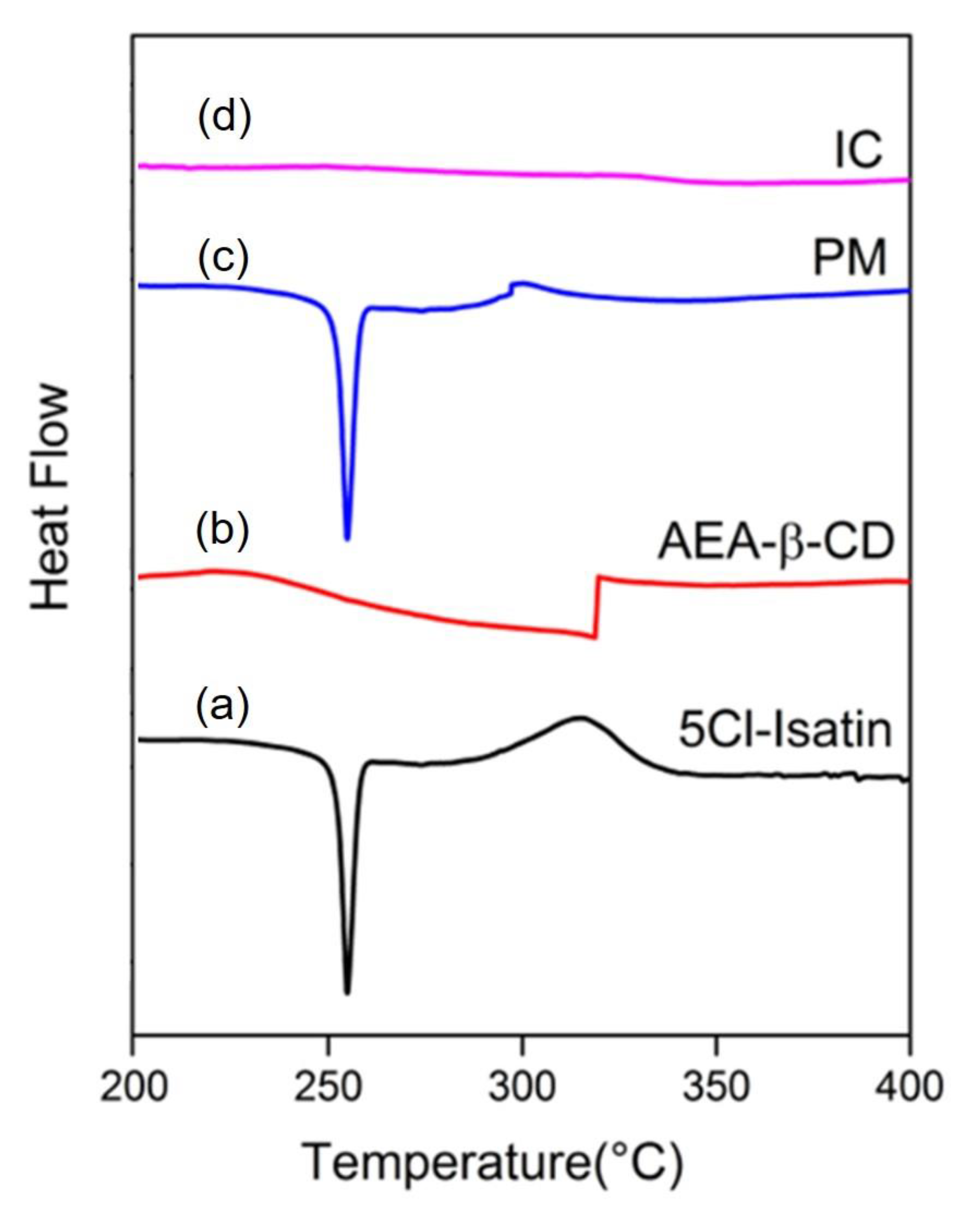
3.7. Differential Scanning Calorimetry (DSC) Analysis
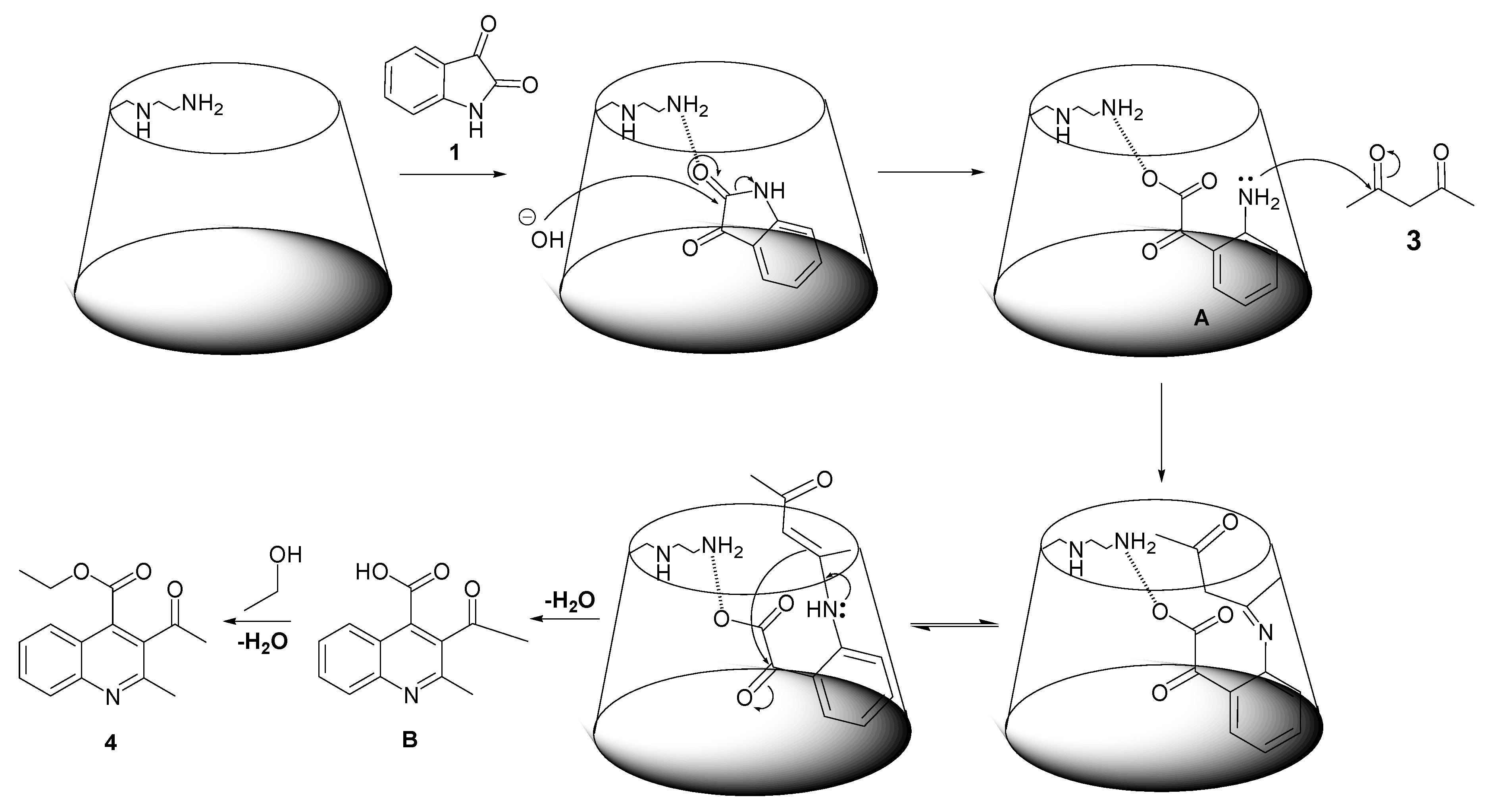
3.8. Synthetic Mechanism of Diversely-Substituted Quinaldine Derivatives with AEA–β–CD as a Catalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Michael, J. Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep. 2008, 25, 166–187. [Google Scholar] [CrossRef] [PubMed]
- Keri, R.S.; Patil, S.A. Quinoline: A promising antitubercular target. Biomed. Pharmacother. 2014, 68, 1161–1175. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, K.A.; Loughlin, W.A.; Young, D.J. Quinolines as chemotherapeutic agents for leishmaniasis. Mini Rev. Med. Chem. 2013, 13, 730–743. [Google Scholar] [CrossRef] [PubMed]
- Zajdel, P.; Partyka, A.; Marciniec, K.; Bojarski, A.J.; Pawlowski, M.; Wesolowska, A. Quinoline-and isoquinoline-sulfonamide analogs of aripiprazole: Novel antipsychotic agents? Future Med. 2014, 6, 57–75. [Google Scholar] [CrossRef] [PubMed]
- Vlahopoulos, S.; Critselis, E.; Voutsas, I.F.; Perez, S.A.; Moschovi, M.; Baxevanis, C.N.; Chrousos, G.P. New use for old drugs? Prospective targets of chloroquines in cancer therapy. Curr. Drug Targ. 2014, 15, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Pal, M. Quinolines: A new hope against inflammation. Drug Discov. Today 2013, 18, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Musiol, R. Quinoline-based HIV integrase inhibitors. Curr. Pharm. Design. 2013, 19, 1835–1849. [Google Scholar] [CrossRef]
- Musiol, R.; Serda, M.; Hensel-Bielowka, S.; Polanski, J. Quinoline-based antifungals. Curr. Med. 2010, 17, 1960–1973. [Google Scholar] [CrossRef]
- Chou, L.C.; Chen, C.T.; Lee, J.C.; Way, T.D.; Huang, C.H.; Huang, S.M.; Chien, D.S. Synthesis and preclinical evaluations of 2-(2-fluorophenyl)-6, 7-methylenedioxyquinolin-4-one monosodium phosphate (CHM-1− P-Na) as a potent antitumor agent. J. Med. Chem. 2010, 53, 1616–1626. [Google Scholar] [CrossRef]
- Chou, L.C.; Tsai, M.T.; Hsu, M.H.; Wang, S.H.; Way, T.D.; Huang, C.H.; Huang, L.J. Design, synthesis, and preclinical evaluation of new 5, 6-(or 6, 7-) disubstituted-2-(fluorophenyl) quinolin-4-one derivatives as potent antitumor agents. J. Med. Chem. 2010, 53, 8047–8058. [Google Scholar] [CrossRef]
- Bharate, J.B.; Vishwakarma, R.A.; Bharate, S.B. Metal-free domino one-pot protocols for quinoline synthesis. RSC Adv. 2015, 5, 42020–42053. [Google Scholar] [CrossRef]
- Khan, A.R.; Forgo, P.; Stine, K.J.; D’Souza, V.T. Methods for selective modifications of cyclodextrins. Chem. Rev. 1998, 98, 1977–1996. [Google Scholar] [CrossRef] [PubMed]
- Shinde, V.V.; Jeong, D.; Joo, S.W.; Cho, E.; Jung, S. Mono-6-deoxy-6-aminopropylamino-β-cyclodextrin as a supramolecular catalyst for the synthesis of indolyl 1H-pyrrole via one-pot four component reaction in water. Catal. Com. 2018, 103, 83–87. [Google Scholar] [CrossRef]
- Ren, Y.; Yang, B.; Liao, X. Merging supramolecular catalysis and aminocatalysis: Amino-appended β-cyclodextrins (ACDs) as efficient and recyclable supramolecular catalysts for the synthesis of tetraketones. RSC Adv. 2016, 6, 22034–22042. [Google Scholar] [CrossRef]
- Ren, Y.; Yang, B.; Liao, X. The amino side chains do matter: Chemoselectivity in the one-pot three-component synthesis of 2-amino-4 H-chromenes by supramolecular catalysis with amino-appended β-cyclodextrins (ACDs) in water. Catal. Sci. Technol. 2016, 6, 4283–4293. [Google Scholar] [CrossRef]
- Shinde, V.V.; Jeong, D.; Jung, S. An amino-chain modified β-cyclodextrin: A Supramolecular Ligand for Pd(OAc)2 acceleration in suzuki–miyaura coupling reactions in water. Catalysts 2019, 9, 111. [Google Scholar] [CrossRef]
- Shinde, V.V.; Jeong, D.; Jung, S. Supramolecular aminocatalysis via inclusion complex: Amino-doped β-cyclodextrin as an efficient supramolecular catalyst for the synthesis of chromeno pyrimido [1, 2-b] indazol in water. J. Ind. Eng. Chem. 2018, 68, 6–13. [Google Scholar] [CrossRef]
- Choi, J.M.; Lee, B.; Jeong, D.; Park, K.H.; Choi, E.J.; Jeon, Y.J.; Lee, I.S. Characterization and regulated naproxen release of hydroxypropyl cyclosophoraose-pullulan microspheres. J. Ind. Eng. Chem. 2017, 48, 108–118. [Google Scholar] [CrossRef]
- Higuchi, T.K.A.C. A phase solubility technique. Adv. Anal. Chem. Instrum. 1965, 4, 117–211. [Google Scholar]
- Zhang, J.; Ma, P.X. Cyclodextrin-based supramolecular systems for drug delivery: Recent progress and future perspective. Adv. Drug Deliv. Rev. 2013, 65, 1215–1233. [Google Scholar] [CrossRef]
- Saha, S.; Roy, A.; Roy, K.; Roy, M.N. Study to explore the mechanism to form inclusion complexes of β-cyclodextrin with vitamin molecules. Sci. Rep. 2016, 6, 35764. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Patel, M. Solid-state characterization and in vitro dissolution behavior of lorazepam: Hydroxypropyl-β-cyclodextrin inclusion complex. Drug. Discov. Ther. 2010, 4, 442–452. [Google Scholar] [PubMed]
- De Araujo, D.R.; Tsuneda, S.S.; Cereda, C.M.; Carvalho, F.D.G.; Preté, P.S.; Fernandes, S.A.; De FA Braga, A. Development and pharmacological evaluation of ropivacaine-2-hydroxypropyl-β-cyclodextrin inclusion complex. Eur. J. Pharm. Sci. 2008, 33, 60–71. [Google Scholar] [CrossRef] [PubMed]

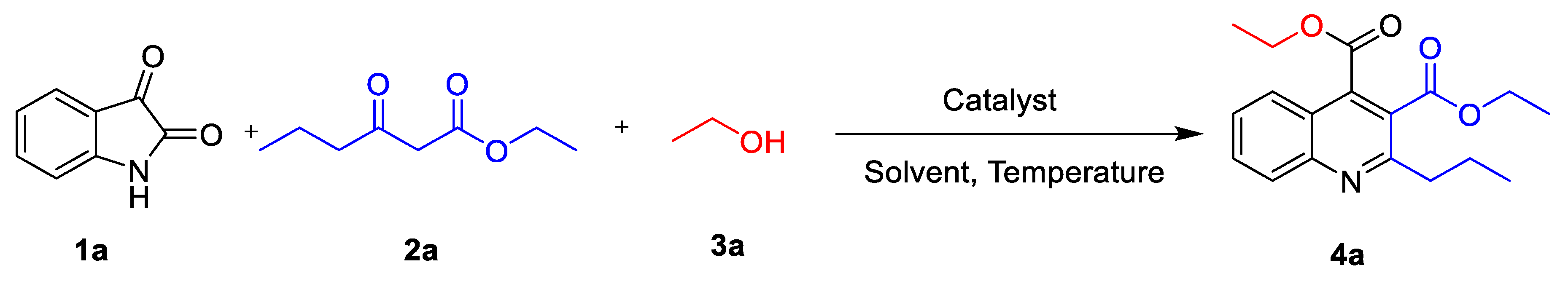
| Entry | Catalyst (mol %) | Solvent | Temperature (°C) | Time (h) | Yield b (%) |
|---|---|---|---|---|---|
| 1 | - | Water | RT | 16 | NR c |
| 2 | β-CD (1) | Water | RT | 13 | NR c |
| 3 | β-CD (1) | Water | 80 | 8 | 15 |
| 4 | AEA-β-CD (1) | Water | 50 | 7 | 51 |
| 5 | Ethylenediamine (1) | Water | 50 | 8 | 27 |
| 6 | K2CO3 (1) | Water | 50 | 9 | 16 |
| 7 | KOH (1) | Water | 50 | 6 | 33 |
| 8 | NaOH (1) | Water | 50 | 5 | 39 |
| 9 | Piperidine (1) | Water | 50 | 10 | 17 |
| 10 | AEA–β–CD (0.5) | Water | 50 | 7 | 11 |
| 11 | AEA–β–CD (1.5) | Water | 50 | 7 | 51 |
| 12 | AEA–β–CD (1) | DMSO | 50 | 9 | 28 |
| 13 | AEA–β–CD (1) | DMF | 50 | 6 | 41 |
| 14 | AEA–β–CD (1) | THF | 50 | 7 | 32 |
| 15 | AEA–β–CD (1) | DCM | 50 | 10 | 22 |
| 16 | AEA–β–CD (1) | Water | 40 | 10 | 32 |
| 17 d | AEA–β–CD (1) | Water | 60 | 3 | 87, 87, 85, 85 |
| 18 | AEA–β–CD (1) | Water | 80 | 3 | 87 |
| 19 | AEA–β–CD (1), Adamantane carboxylic acid (1) | Water | 60 | 3 | NR c |
| Temperature (°C) | S0 | Slope | Kc(M−1) |
|---|---|---|---|
| 80 | 2.4421 | 0.3576 | 227.9406 |
| 60 | 2.5155 | 0.3756 | 239.1361 |
| 50 | 3.4329 | 0.3244 | 139.8711 |
| Entry | Isatin (1) | 1,3-dicarbonyl (2) | Alcohol (3) | Product | Time (h) | Yield b |
|---|---|---|---|---|---|---|
| 1 | Isatin | ethyl 3-oxohexanoate | ethanol | 4a | 3 | 87 |
| 2 | 5-Bromoisatin | ethyl 3-oxohexanoate | ethanol | 4b | 3 | 89 |
| 3 | 5-Methoxyisatin | ethyl 3-oxohexanoate | ethanol | 4c | 2 | 90 |
| 4 | 5-Fluoroisatin | ethyl 3-oxohexanoate | ethanol | 4d | 3 | 86 |
| 5 | 5-Nitroisatin | ethyl 3-oxohexanoate | ethanol | 4e | 4 | 77 |
| 6 | Isatin | ethyl 3-oxobutanoate | ethanol | 4f | 2 | 92 |
| 7 | 5-Chloroisatin | ethyl 3-oxobutanoate | ethanol | 4g | 2 | 91 |
| 8 | 5-Methoxyisatin | ethyl 3-oxobutanoate | ethanol | 4h | 2 | 92 |
| 9 | 5-Fluoroisatin | ethyl 3-oxobutanoate | ethanol | 4i | 2 | 90 |
| 10 | 5-Bromoisatin | ethyl 3-oxobutanoate | ethanol | 4j | 2 | 90 |
| 11 | 5-Fluoroisatin | pentane-2,4-dione | ethanol | 4k | 3 | 92 |
| 12 | 5-Isatin | pentane-2,4-dione | ethanol | 4l | 3 | 92 |
| 13 | 5-Bromoisatin | pentane-2,4-dione | ethanol | 4m | 3 | 90 |
| 14 | 5-Chloroisatin | pentane-2,4-dione | ethanol | 4n | 4 | 89 |
| 15 | Isatin | pentane-2,4-dione | Isopropanol | 4o | 4 | 91 |
| 16 | 5-Fluoroisatin | pentane-2,4-dione | Isopropanol | 4p | 4 | 90 |
| 17 | 5-Methoxyisatin | pentane-2,4-dione | Isopropanol | 4q | 3 | 87 |
| 18 | 7-Fluoroisatin | pentane-2,4-dione | Isopropanol | 4r | 4 | 69 |
| 19 | Isatin | pentane-2,4-dione | Butanol | 4s | 5 | 84 |
| 20 | 5-Bromoisatin | pentane-2,4-dione | Butanol | 4t | 5 | 86 |
| 21 | 5-Fluoroisatin | pentane-2,4-dione | Butanol | 4u | 5 | 83 |
| 22 | 5-Chloroisatin | pentane-2,4-dione | Butanol | 4v | 5 | 85 |
| 23 | 5-Methoxyisatin | pentane-2,4-dione | Butanol | 4w | 5 | 85 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Shinde, V.V.; Jeong, D.; Jung, S. Utilization of Water-Soluble Aminoethylamino–β–Cyclodextrin in the Pfitzinger Reaction—Catalyzed to the Synthesis of Diversely Functionalized Quinaldine. Polymers 2020, 12, 393. https://doi.org/10.3390/polym12020393
Kim Y, Shinde VV, Jeong D, Jung S. Utilization of Water-Soluble Aminoethylamino–β–Cyclodextrin in the Pfitzinger Reaction—Catalyzed to the Synthesis of Diversely Functionalized Quinaldine. Polymers. 2020; 12(2):393. https://doi.org/10.3390/polym12020393
Chicago/Turabian StyleKim, Yohan, Vijay Vilas Shinde, Daham Jeong, and Seunho Jung. 2020. "Utilization of Water-Soluble Aminoethylamino–β–Cyclodextrin in the Pfitzinger Reaction—Catalyzed to the Synthesis of Diversely Functionalized Quinaldine" Polymers 12, no. 2: 393. https://doi.org/10.3390/polym12020393
APA StyleKim, Y., Shinde, V. V., Jeong, D., & Jung, S. (2020). Utilization of Water-Soluble Aminoethylamino–β–Cyclodextrin in the Pfitzinger Reaction—Catalyzed to the Synthesis of Diversely Functionalized Quinaldine. Polymers, 12(2), 393. https://doi.org/10.3390/polym12020393






