Probing the Dynamics of Li+ Ions on the Crystal Surface: A Solid-State NMR Study
Abstract
1. Introduction
2. Materials and Methods
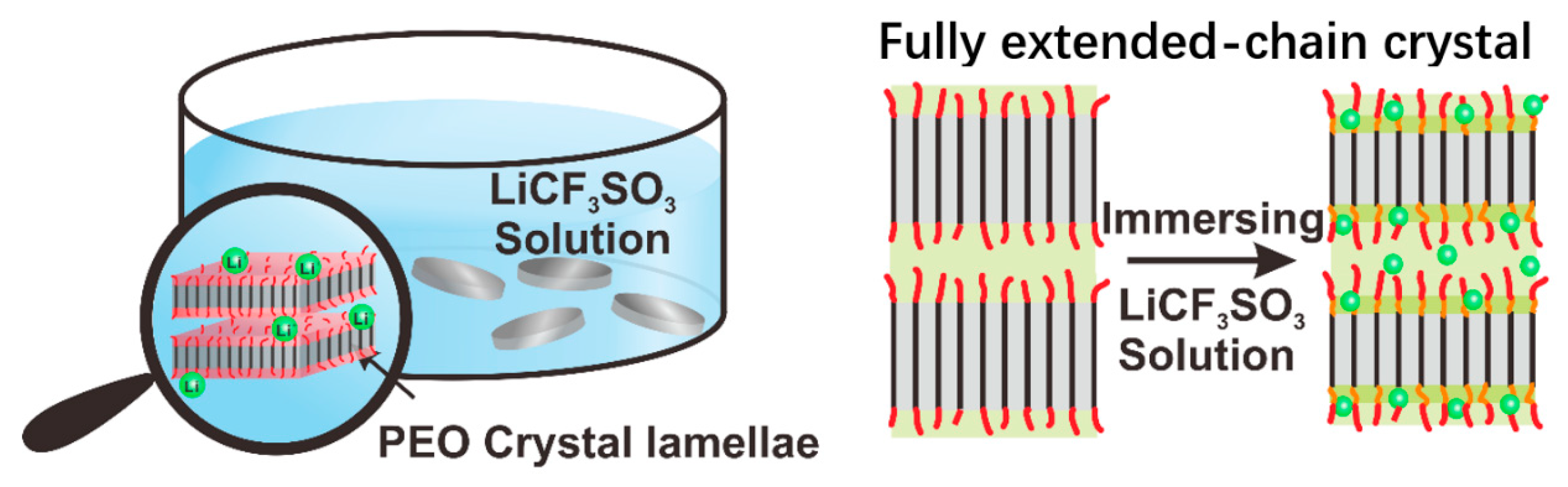
2.1. Sample Preparation
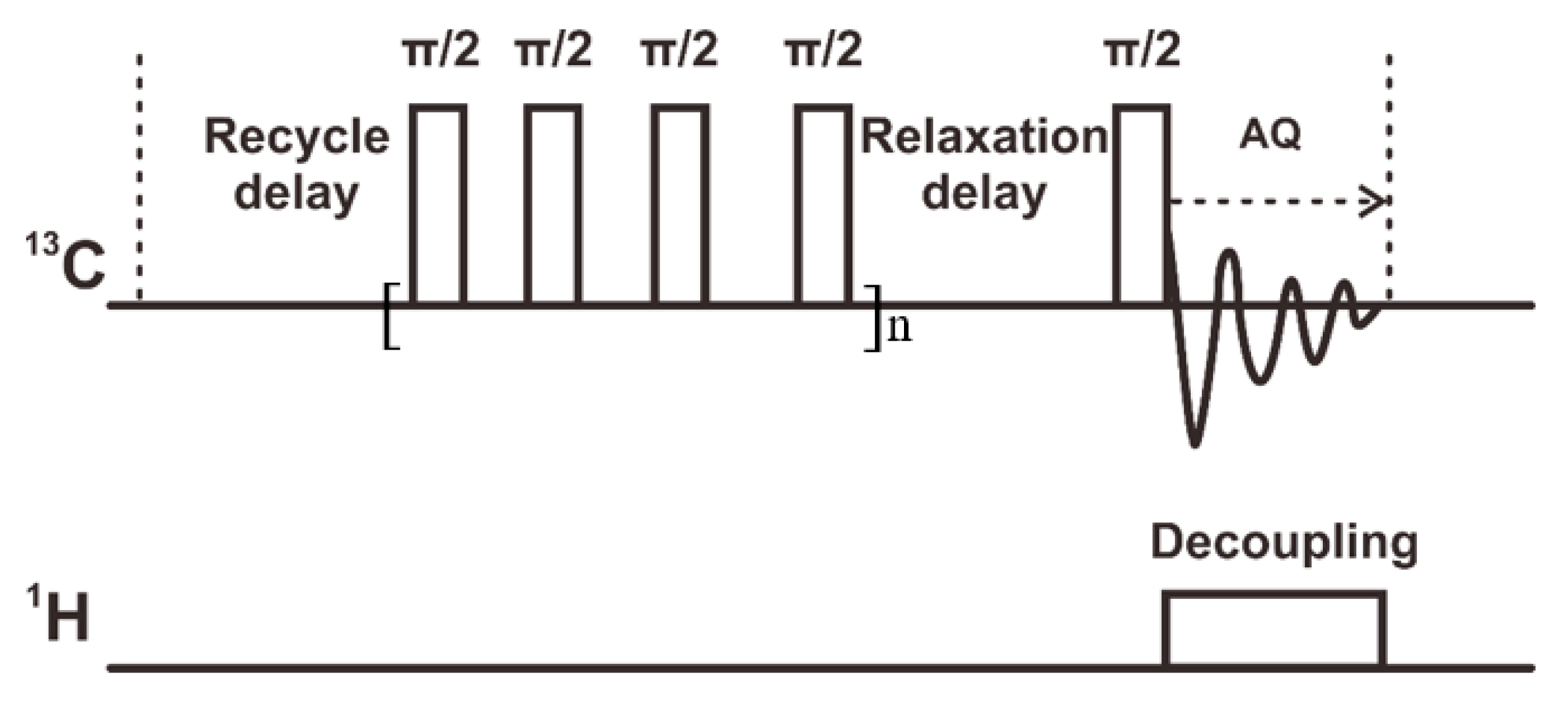
2.2. Solid-state NMR Experiments
2.3. X-ray Diffraction Measurements
2.4. Differential Scanning Calorimetry
2.5. Electrochemical Impedance Spectroscopy
3. Results and Discussion
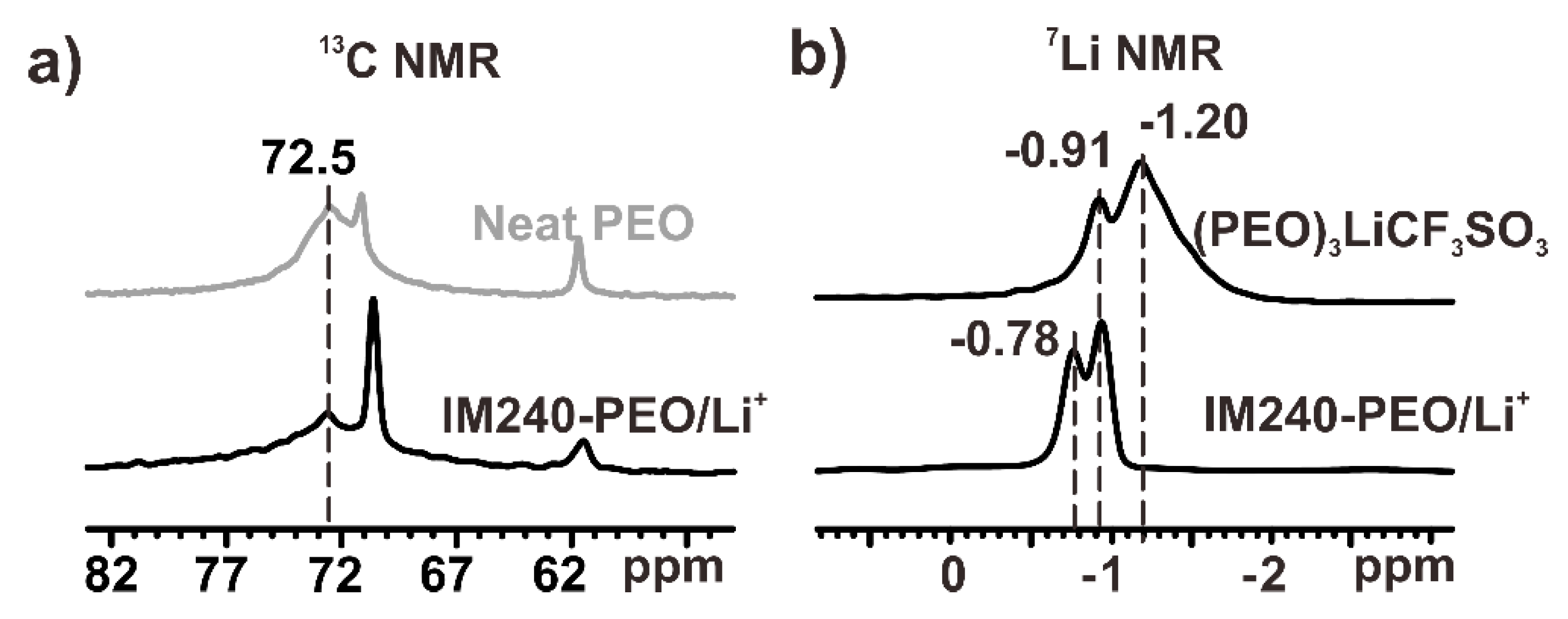
3.1. The Structure of IM-PEO/Li+
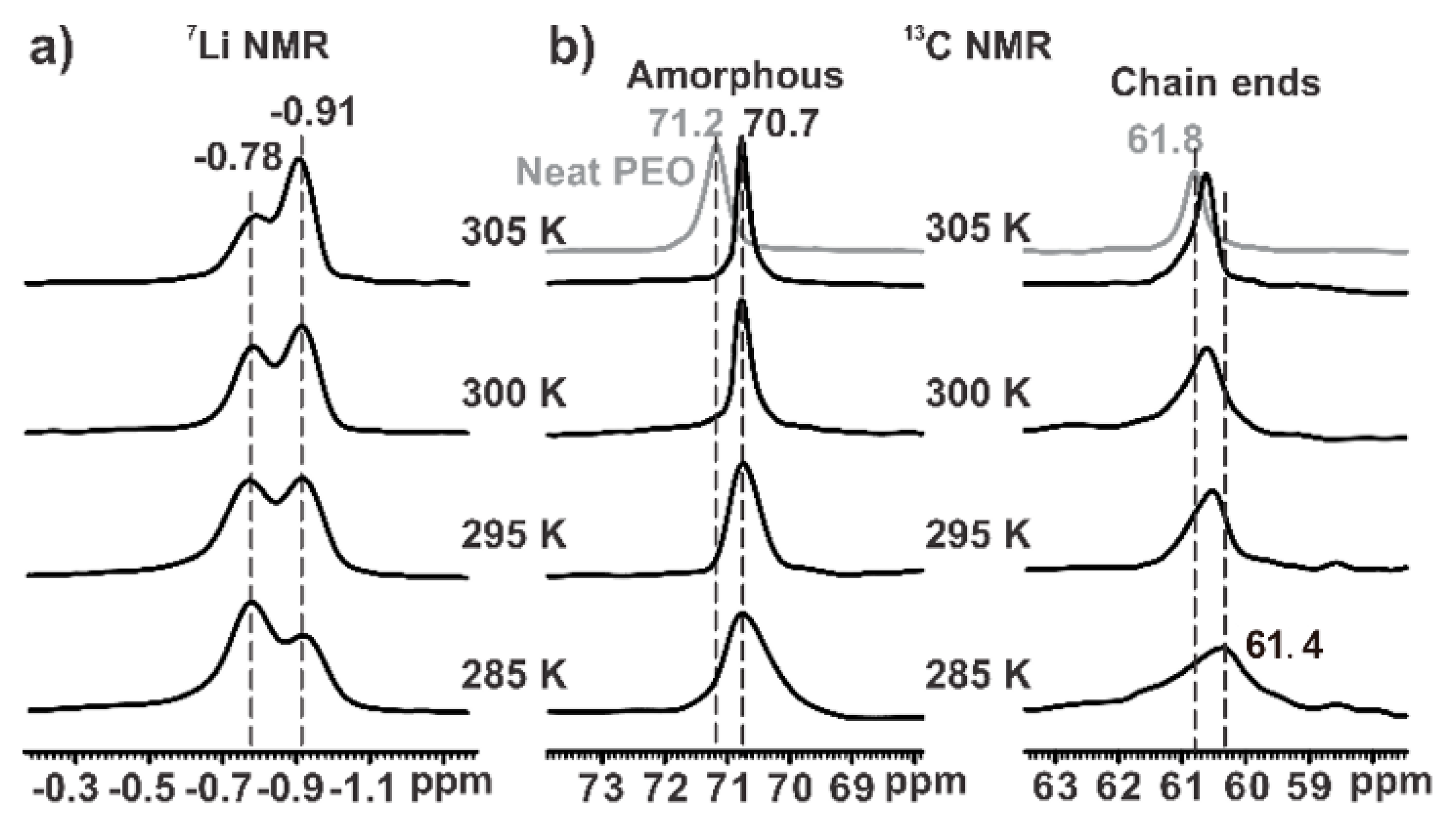
3.2. The Li+ Ions Around the Amorphous Chain Segments
3.3. The Li+ Ions Around the Chain Ends
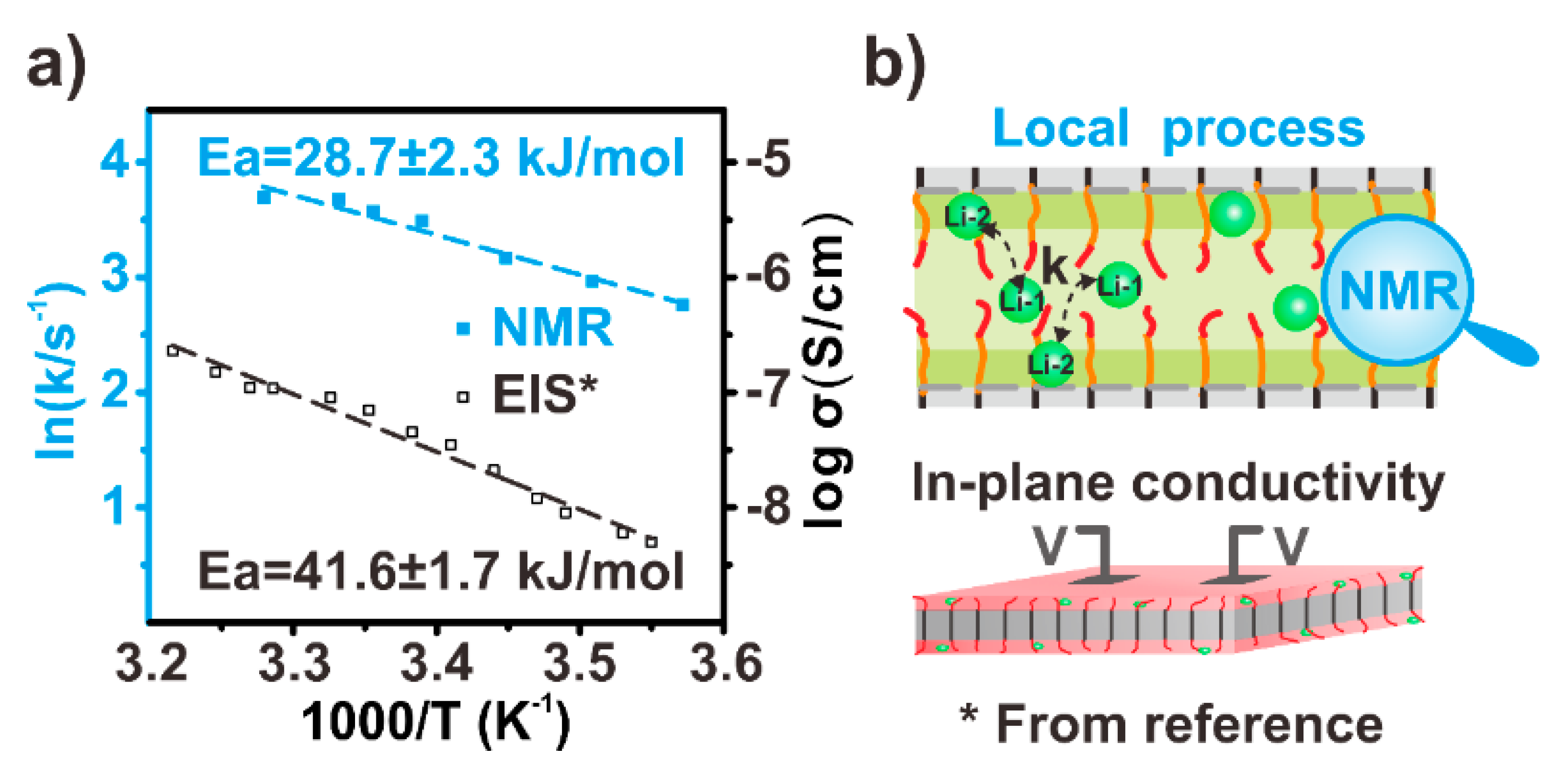
3.4. The Local Dynamics of Li+ Ions in IM240-PEO/Li+
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gadjourova, Z.; Andreev, Y.G.; Tunstall, D.P.; Bruce, P.G. Ionic conductivity in crystalline polymer electrolytes. Nature 2001, 412, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Smith, D.M.; Li, C.Y. Anisotropic ion transport in a poly(ethylene oxide)−LiClO4 solid state electrolyte templated by graphene oxide. Macromolecules 2015, 48, 4503–4510. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Shubha, N.; Zhu, H.; Forsyth, M.; Srinivasan, M. Study of lithium conducting single ion conductor based on polystyrene sulfonate for lithium battery application. Polymer 2016, 99, 748–755. [Google Scholar] [CrossRef]
- Tang, C.; Hackenberg, K.; Fu, Q.; Ajayan, P.M.; Ardebili, H. High ion conducting polymer nanocomposite electrolytes using hybrid nanofillers. Nano Lett. 2012, 12, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.; Chen, H.; Kuo, S.W.; Huang, C.F.; Chang, F.C. Investigating the effect of miscibility on the ionic conductivity of LiClO4/PEO/PCL ternary blends. Macromolecules 2004, 37, 8424–8430. [Google Scholar] [CrossRef]
- Berthier, C.; Gorecki, W.; Minier, M.; Armand, M.B.; Chabagno, J.M.; Rigaud, P. Microscopic investigation of ionic conductivity in alkali metal salts-poly(ethylene oxide) adducts. Solid State Ion. 1983, 11, 91–95. [Google Scholar] [CrossRef]
- Müller Plathe, F.; van Gunsteren, W.F. Computer simulation of a polymer electrolyte: Lithium iodide in amorphous poly(ethylene oxide). J. Chem. Phys. 1995, 103, 4745–4756. [Google Scholar] [CrossRef]
- Ríes, M.E.; Brereton, M.G.; Cruickshank, J.M.; Klein, P.G.; Ward, I.M. NMR Study of poly(ethylene oxide) complexes with LiCF3SO3. Macromolecules 1995, 28, 3282–3289. [Google Scholar] [CrossRef]
- Zhang, C.; Ainsworth, D.; Andreev, Y.G.; Bruce, P.G. Ionic conductivity in the solid glyme complexes [CH3O(CH2CH2O)nCH3]:LiAsF6 (n = 3, 4). J. Am. Chem. Soc. 2007, 129, 8700–8701. [Google Scholar] [CrossRef]
- Henderson, W.A.; Brooks, N.R.; Young, V.G., Jr. Single-crystal structures of polymer electrolytes. J. Am. Chem. Soc. 2003, 125, 12098–12099. [Google Scholar] [CrossRef] [PubMed]
- Miwa, Y.; Shimada, S.; Urakawa, O.; Nobukawa, S. Origin of high segmental mobility at chain ends of polystyrene. Macromolecules 2010, 43, 7192–7199. [Google Scholar] [CrossRef]
- Komlosh, M.E.; Callaghan, P.T. Segmental motion of entangled random coil polymers studied by pulsed gradient spin echo nuclear magnetic resonance. J. Chem. Phys. 1998, 109, 10053. [Google Scholar] [CrossRef]
- Vanhoorne, P.; Jéróme, R.; Teyssié, P.; Lauprétre, F. Direct NMR evidence for a local restriction in the segmental chain mobility of a model ionomer. Macromolecules 1994, 27, 2548–2552. [Google Scholar] [CrossRef]
- Von Meerwall, E.; Grigsby, J.; Tomich, D.; Van Antwerp, R. Effect of chain-end free volume on the diffusion of oligomers. J. Polym. Sci. Polym. Phys. Ed. 1982, 20, 1037–1053. [Google Scholar] [CrossRef]
- Ito, K.; Nishina, N.; Ohno, H. High lithium ionic conductivity of poly(ethylene oxide)s having sulfonate groups on their chain ends. J. Mater. Chem. 1997, 7, 1357–1362. [Google Scholar] [CrossRef]
- Jo, G.; Ahn, H.; Park, M.J. Simple route for tuning the morphology and conductivity of polymer electrolytes: One end functional group is enough. ACS Macro Lett. 2013, 2, 990–995. [Google Scholar] [CrossRef]
- Ito, K.; Ohno, H. Ionic conductivity of poly(ethylene oxide) having charges on the chain end. Solid State Ion. 1995, 79, 300–305. [Google Scholar] [CrossRef]
- Rhodes, C.P.; Frech, R. Local structures in crystalline and amorphous phases of diglyme-LiCF3SO3 and poly(ethylene oxide)-LiCF3SO3 systems: Implications for the mechanism of ionic transport. Macromolecules 2001, 34, 2660–2666. [Google Scholar] [CrossRef]
- Liivat, A.; Brandell, D.; Thomas, J.O. A molecular dynamics study of ion-conduction mechanisms in crystalline low-Mw LiPF6 PEO6. J. Mater. Chem. 2007, 17, 3938–3946. [Google Scholar] [CrossRef]
- Stöffler, H.; Zinkevich, T.; Yavuz, M.; Senyshyn, A.; Kulisch, J.; Hartmann, P.; Adermann, T.; Randau, S.; Richter, F.; Janek, J.; et al. Li+‑ion dynamics in β‑Li3PS4 observed by NMR: Local hopping and long-range transport. J. Phys. Chem. C 2018, 122, 15954–15965. [Google Scholar] [CrossRef]
- Fu, X.; Yang, G.; Wu, J.; Wang, J.; Chen, Q.; Yao, Y. Fast lithium-ion transportation in crystalline polymer electrolytes. ChemPhysChem 2018, 19, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Fullerton-Shirey, S.K.; Maranas, J.K. Effect of LiClO4 on the structure and mobility of PEO-based solid polymer electrolytes. Macromolecules 2009, 42, 2142–2156. [Google Scholar] [CrossRef]
- Kurz, R.; Achilles, A.; Chen, W.; Schäfer, M.; Seidlitz, A.; Golitsyn, Y.; Kressler, J.; Paul, W.; Hempel, G.; Miyoshi, T.; et al. Intracrystalline jump motion in poly(ethylene oxide) lamellae of variable thickness: A comparison of NMR methods. Macromolecules 2017, 50, 3890–3902. [Google Scholar] [CrossRef]
- Hong, Y.; Miyoshi, T. Solid-state NMR characterization of polymer chain structure and dynamics in polymer crystals. In Encyclopedia of Polymers and Composites; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Wang, S.; Hong, Y.; Yuan, S.; Chen, W.; Zhou, W.; Li, Z.; Wang, K.; Min, X.; Konishi, T.; Miyoshi, T. Chain trajectory, chain packing, and molecular dynamics of semicrystalline polymers as studied by solid-state NMR. Polymers 2018, 10, 775. [Google Scholar] [CrossRef] [PubMed]
- Marzantowicz, M.; Dygas, J.R.; Krok, F.; Florjańczyk, Z.; Zygadło-Monikowska, E. Influence of crystalline complexes on electrical properties of PEO:LiTFSI electrolyte. Electrochim. Acta 2007, 53, 1518–1526. [Google Scholar] [CrossRef]
- Karan, N.K.; Pradhan, D.K.; Thomas, R.; Natesan, B.; Katiyar, R.S. Solid polymer electrolytes based on polyethylene oxide and lithium trifluoro- methane sulfonate (PEO–LiCF3SO3): Ionic conductivity and dielectric relaxation. Solid State Ion. 2008, 179, 689–696. [Google Scholar] [CrossRef]
- Nan, C.W.; Fan, L.; Lin, Y.; Cai, Q. Enhanced ionic conductivity of polymer electrolytes containing nanocomposite SiO2 particles. Phys. Rev. Lett. 2003, 91, 26. [Google Scholar] [CrossRef]
- Fan, Li.; Wang, X.L.; Long, F.; Wang, X. Enhanced ionic conductivities in composite polymer electrolytes by using succinonitrile as a plasticizer. Solid State Ion. 2008, 179, 1772–1775. [Google Scholar] [CrossRef]
- Cheng, S.; Smith, D.M.; Li, C.Y. How does nanoscale crystalline structure affect ion transport in solid polymer electrolytes? Macromolecules 2014, 47, 3978–3986. [Google Scholar] [CrossRef]
- Cheng, S.Z.D.; Zhang, A.; Chen, J.H.; Heberer, D.P. Nonintegral and integral folding crystal growth in low-molecular mass poly (ethylene oxide) fractions. I. Isothermal lamellar thickening and thinning. J. Polym. Sci. Polym. Phys. 1991, 29, 287–297. [Google Scholar] [CrossRef]
- Cheng, S.Z.D.; Chen, J.; Zhang, A.; Heberer, D. Nonintegral and integral folding crystal growth in low-molecular mass poly (ethylene oxide) fractions. II. End-group effect: α, ω-methoxy-poly (ethylene oxide). J. Polym. Sci. Polym. Phys. 1991, 29, 299–310. [Google Scholar] [CrossRef]
- Cheng, S.Z.D.; Chen, J. Nonintegral and integral folding crystal growth in low-molecular mass poly (ethylene oxide) fractions, III. Linear crystal growth rates and crystal morphology. J. Polym. Sci. Polym. Phys. 1991, 29, 311–327. [Google Scholar]
- Spěváček, J.; Baldrian, J. Solid-state 13C NMR and SAXS characterization of the amorphous phase in low-molecular weight poly(ethylene oxide)s. Eur. Polym. J. 2008, 44, 4146–4150. [Google Scholar] [CrossRef]
- Wang, S.; Kang, J.; Jain, D.; Miyoshi, T. Application of NMR in polymer characterization. In Nuclear Magnetic Resonance; The Royel Society of Chemistry: Cambridge, UK, 2016; Volume 45, pp. 79–82. [Google Scholar]
- Miyoshi, T.; Takegoshi, K.; Hikichi, K. High-resolution solid-state 13C nuclear magnetic resonance study of a polymer complex: Poly(methacrylic acid)/poly(ethylene oxide). Polymer 1996, 37, 11–18. [Google Scholar] [CrossRef]
- Yang, L.; Heatley, F.; Blease, T.G.; Thompson, R. A study of the mechanism of the oxidative thermal degradation of poly(ethylene oxide) and poly(propylene oxide) using 1H- and 13C-NMR. Eur. Polym. J. 1996, 32, 535–547. [Google Scholar] [CrossRef]
- Frech, R.; Chintapalli, S.; Bruce, P.G.; Vincent, C.A. Crystalline and amorphous phases in the poly(ethylene oxide)-LiCF3SO3 system. Macromolecules 1999, 32, 808–813. [Google Scholar] [CrossRef]
- Frech, R.; Chintapalli, S.; Bruce, P.G.; Vincent, C.A. Structure of an amorphous polymer electrolyte poly(ethyleneoxide)3: LiCF3SO3. Chem. Commun. 1997, 2, 157–158. [Google Scholar] [CrossRef]
- Lightfoot, P.; Mehta, M.A.; Bruce, P.G. Crystal structure of the polymer electrolyte poly(ethylene oxide)3:LiCF3SO3. Science 1993, 5135, 883–885. [Google Scholar] [CrossRef]
- Levitt, M.H. Spin Dynamics: Basic Principles of NMR Spectroscopy; Wiley: New York, NY, USA, 2001; Chapter 19. [Google Scholar]
- Liu, S.; Imanishi, N.; Zhang, T.; Hirano, A.; Takeda, Y.; Yamamoto, O.; Yang, J. Effect of nano-silica filler in polymer electrolyte on Li dendrite formation in Li/poly(ethylene oxide)–Li(CF3SO2)2N/Li. J. Power Sources 2010, 195, 6847–6853. [Google Scholar] [CrossRef]
- Yang, L.Y.; Wei, D.X.; Xu, M.; Yao, Y.F.; Chen, Q. Transferring lithium ions in nanochannels: A PEO/Li+ solid polymer electrolyte design. Angew. Chem. Int. Ed. 2014, 53, 3631–3635. [Google Scholar] [CrossRef] [PubMed]


| Immersing time | IM105-PEO/Li+ | IM240-PEO/Li+ | IM390-PEO/Li+ |
|---|---|---|---|
| EO:Li+ in IM-PEO/Li+ | 10.8:1 | 5.9:1 | 5.4:1 |
| EO:Li+ in amorphous | 3.2:1 | 2.4:1 | 2.3:1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.-H.; Xia, T.; Chen, Q.; Yao, Y.-F. Probing the Dynamics of Li+ Ions on the Crystal Surface: A Solid-State NMR Study. Polymers 2020, 12, 391. https://doi.org/10.3390/polym12020391
Wang B-H, Xia T, Chen Q, Yao Y-F. Probing the Dynamics of Li+ Ions on the Crystal Surface: A Solid-State NMR Study. Polymers. 2020; 12(2):391. https://doi.org/10.3390/polym12020391
Chicago/Turabian StyleWang, Bi-Heng, Tian Xia, Qun Chen, and Ye-Feng Yao. 2020. "Probing the Dynamics of Li+ Ions on the Crystal Surface: A Solid-State NMR Study" Polymers 12, no. 2: 391. https://doi.org/10.3390/polym12020391
APA StyleWang, B.-H., Xia, T., Chen, Q., & Yao, Y.-F. (2020). Probing the Dynamics of Li+ Ions on the Crystal Surface: A Solid-State NMR Study. Polymers, 12(2), 391. https://doi.org/10.3390/polym12020391





