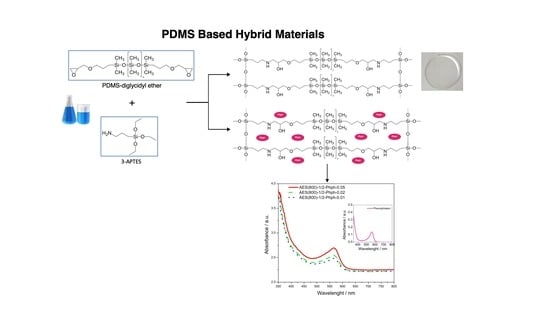
PDMS Based Hybrid Sol-Gel Materials for Sensing Applications in Alkaline Environments: Synthesis and Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
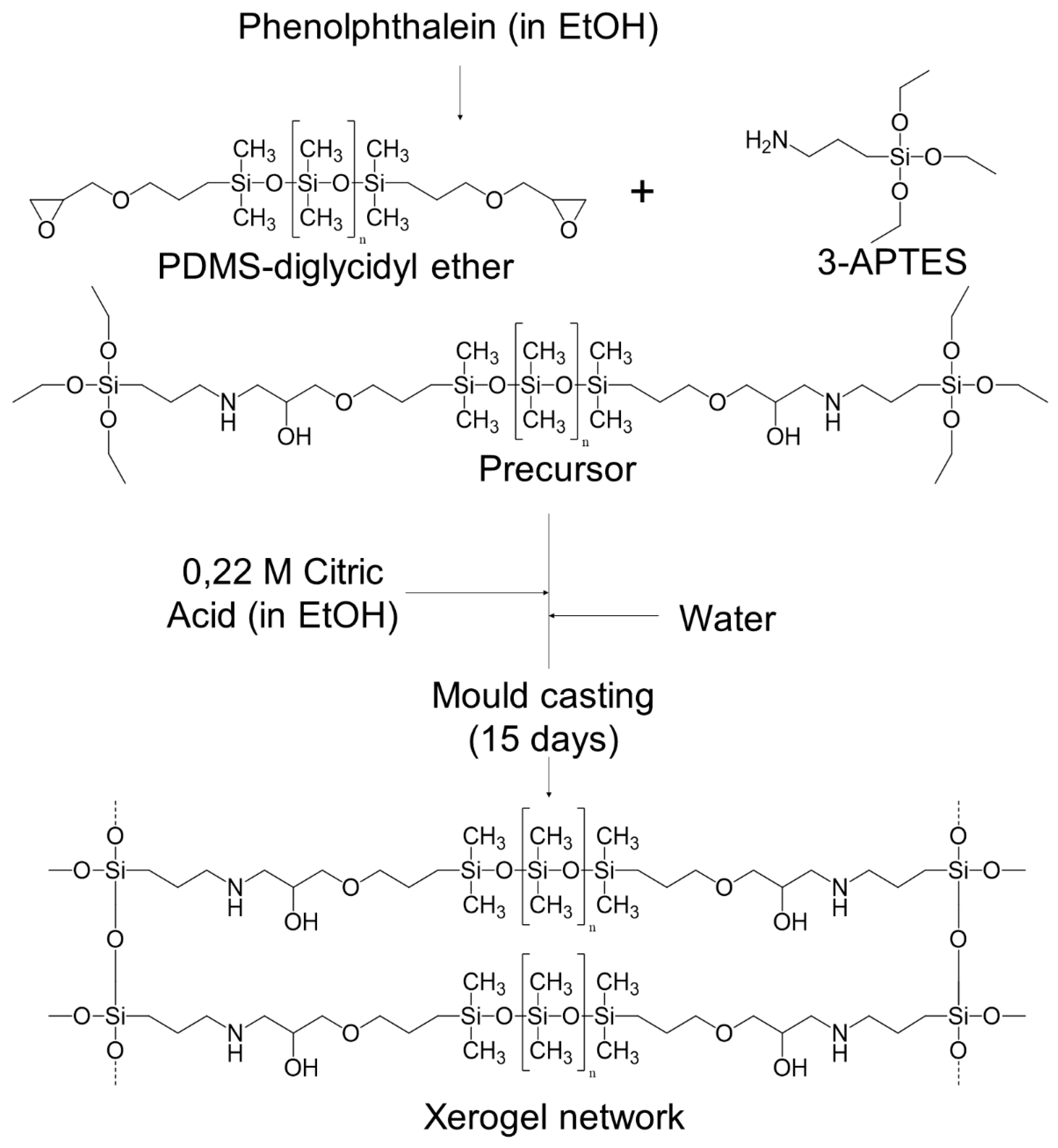
2.2. Synthesis of Organic-Inorganic Hybrid (OIH) Films
2.2.1. General Procedure
2.2.2. Phph Doped Films
2.3. Characterization of the OIH Films
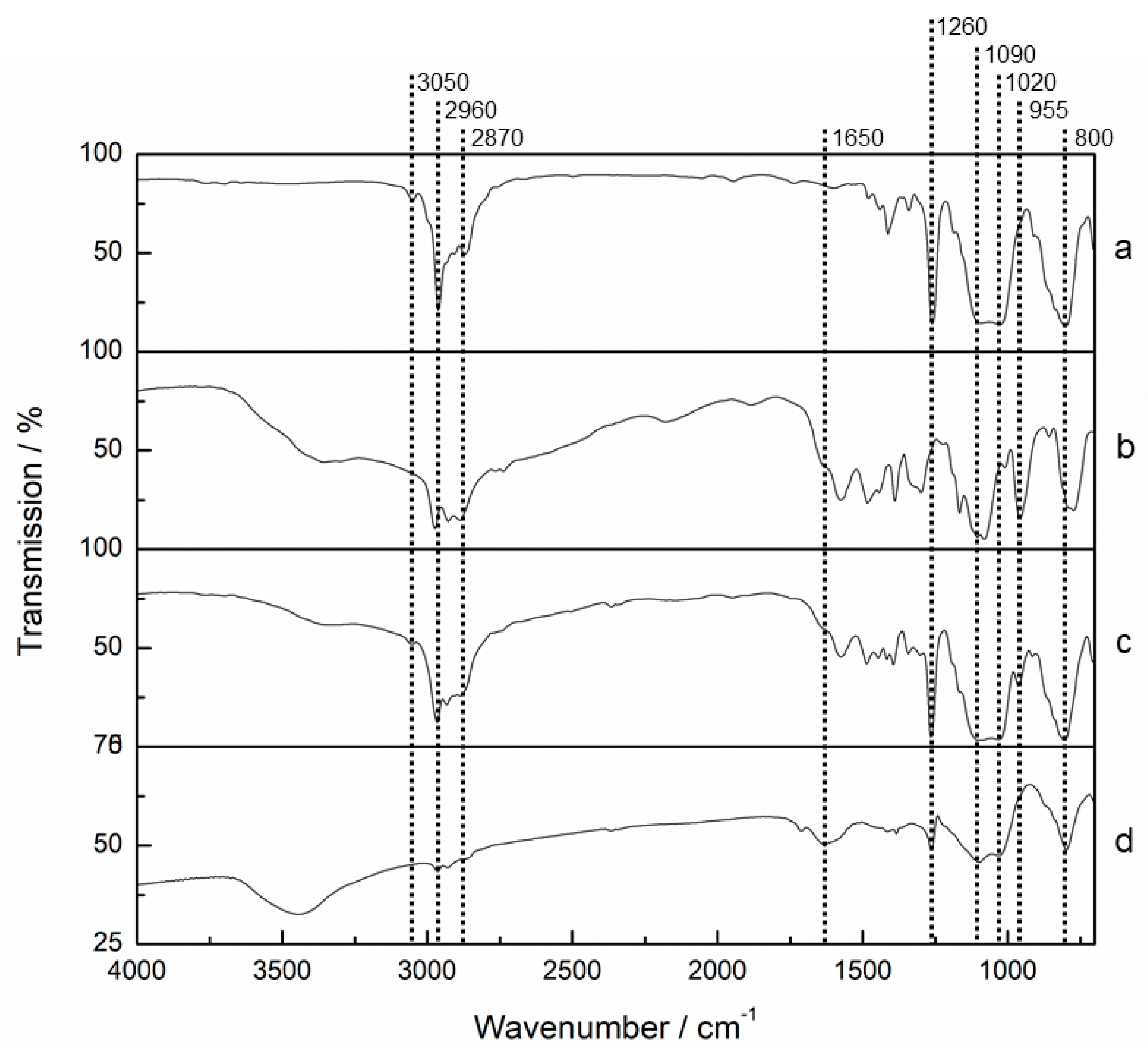
2.3.1. FTIR
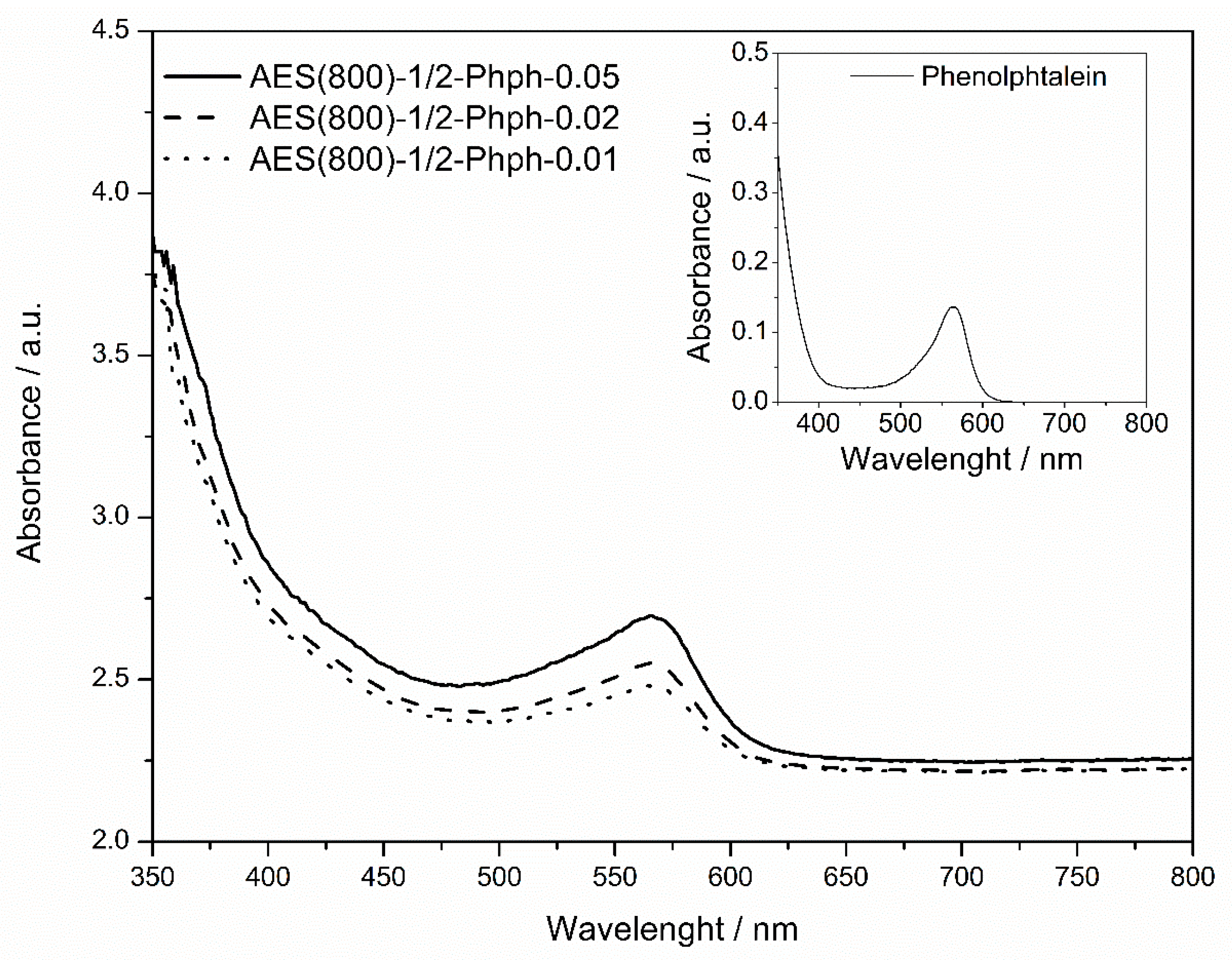
2.3.2. UV–Vis Spectroscopy
2.3.3. Electrochemical Impedance Spectroscopy (EIS)
2.3.4. TGA
3. Results and Discussion
3.1. FTIR Analysis
3.2. UV–Vis Analysis
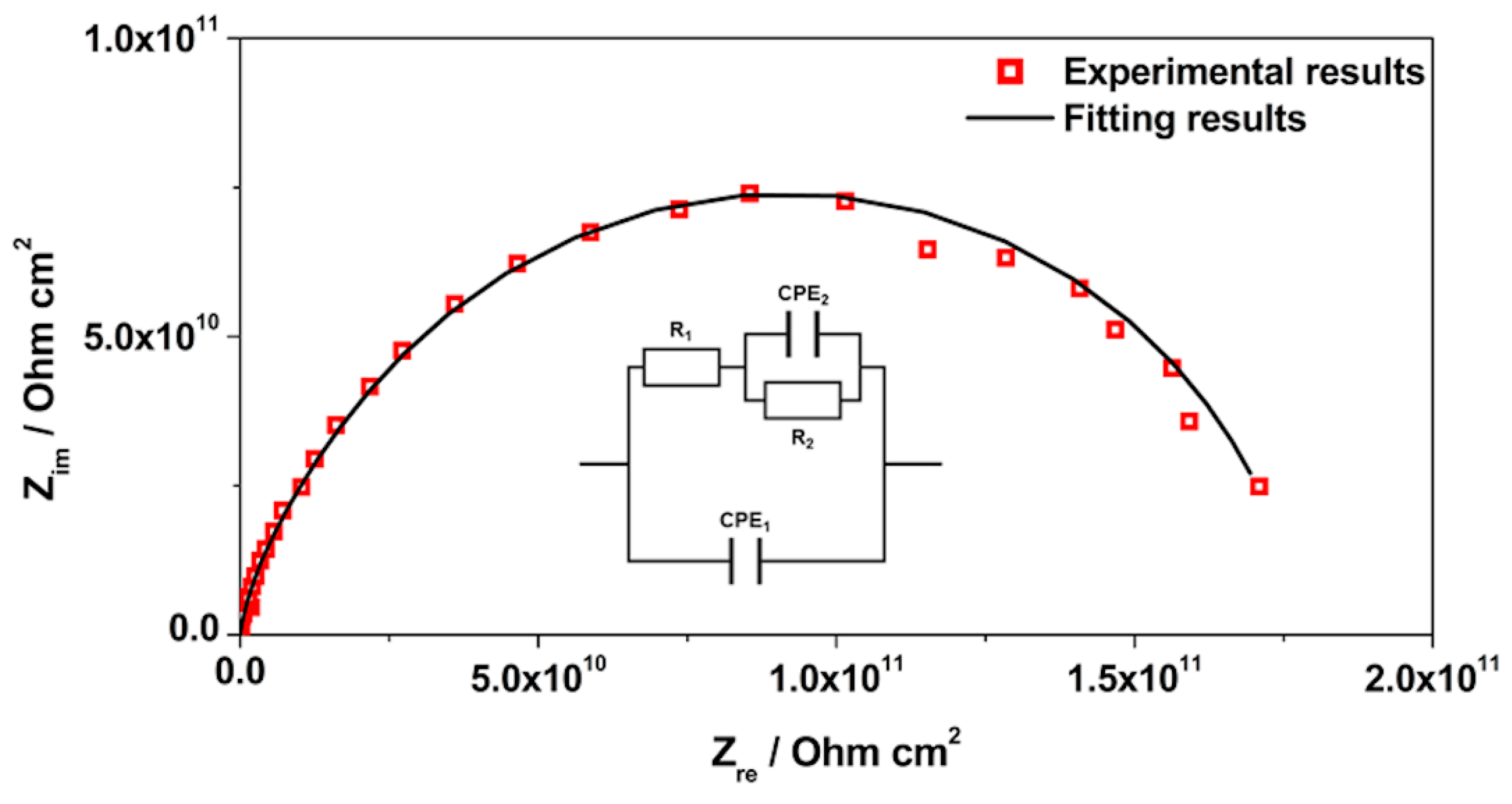
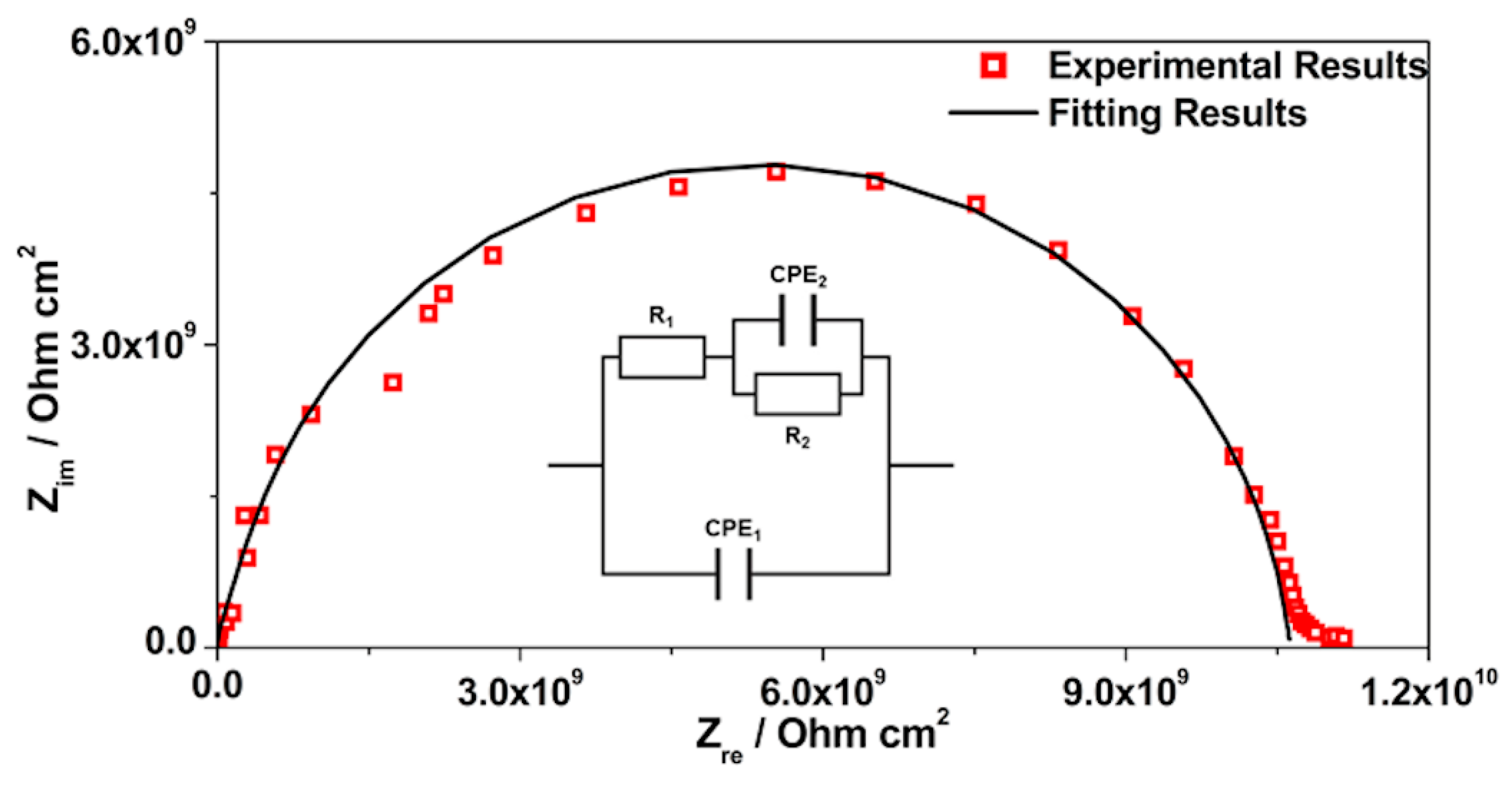
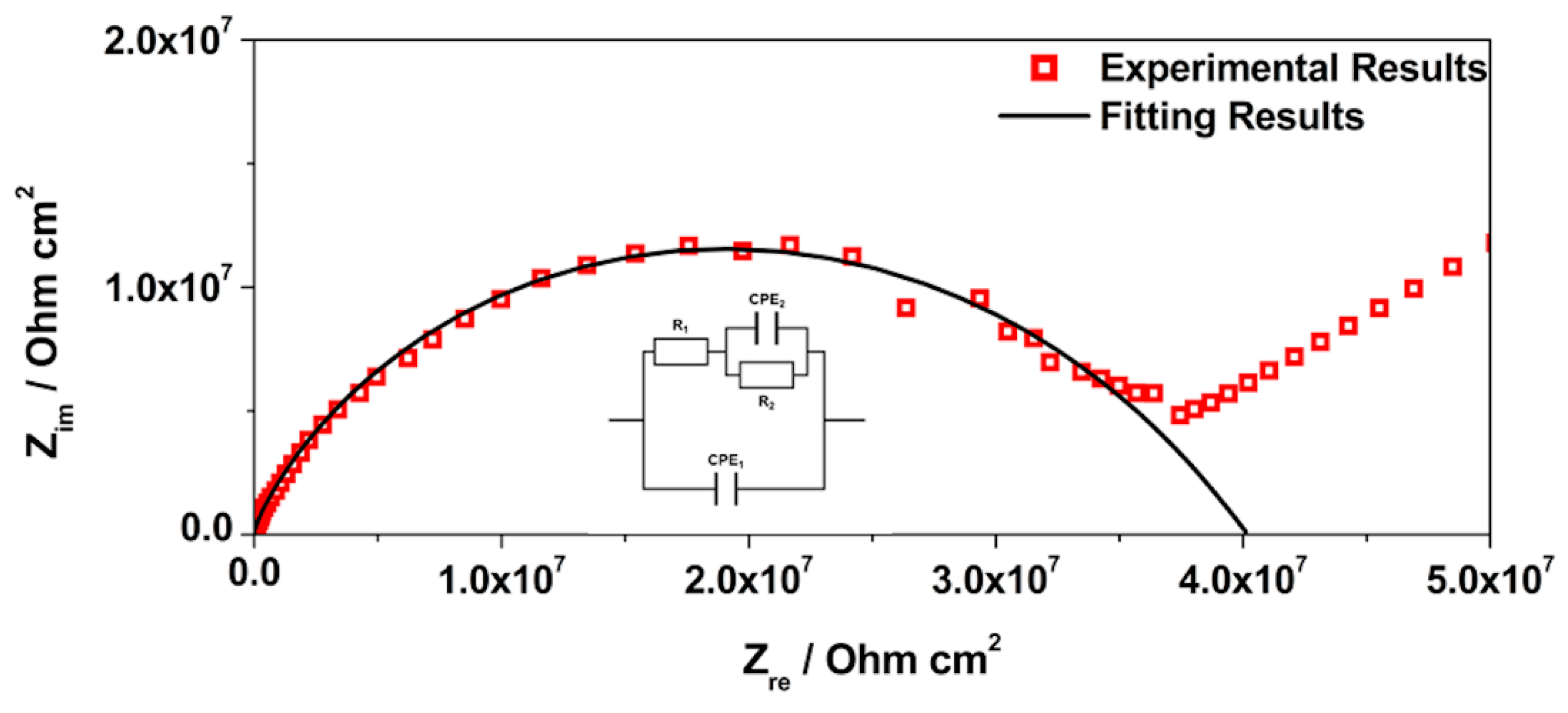
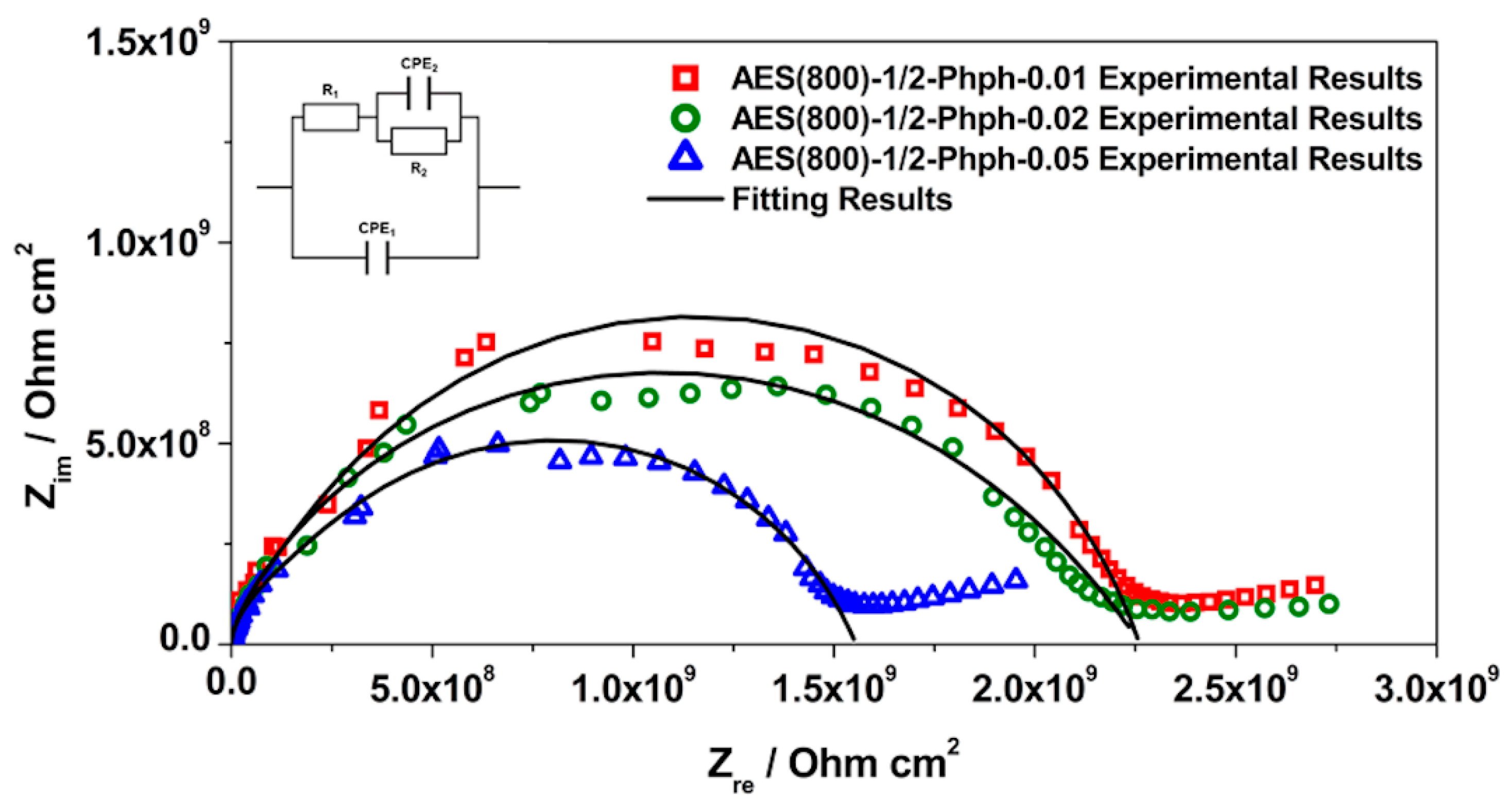
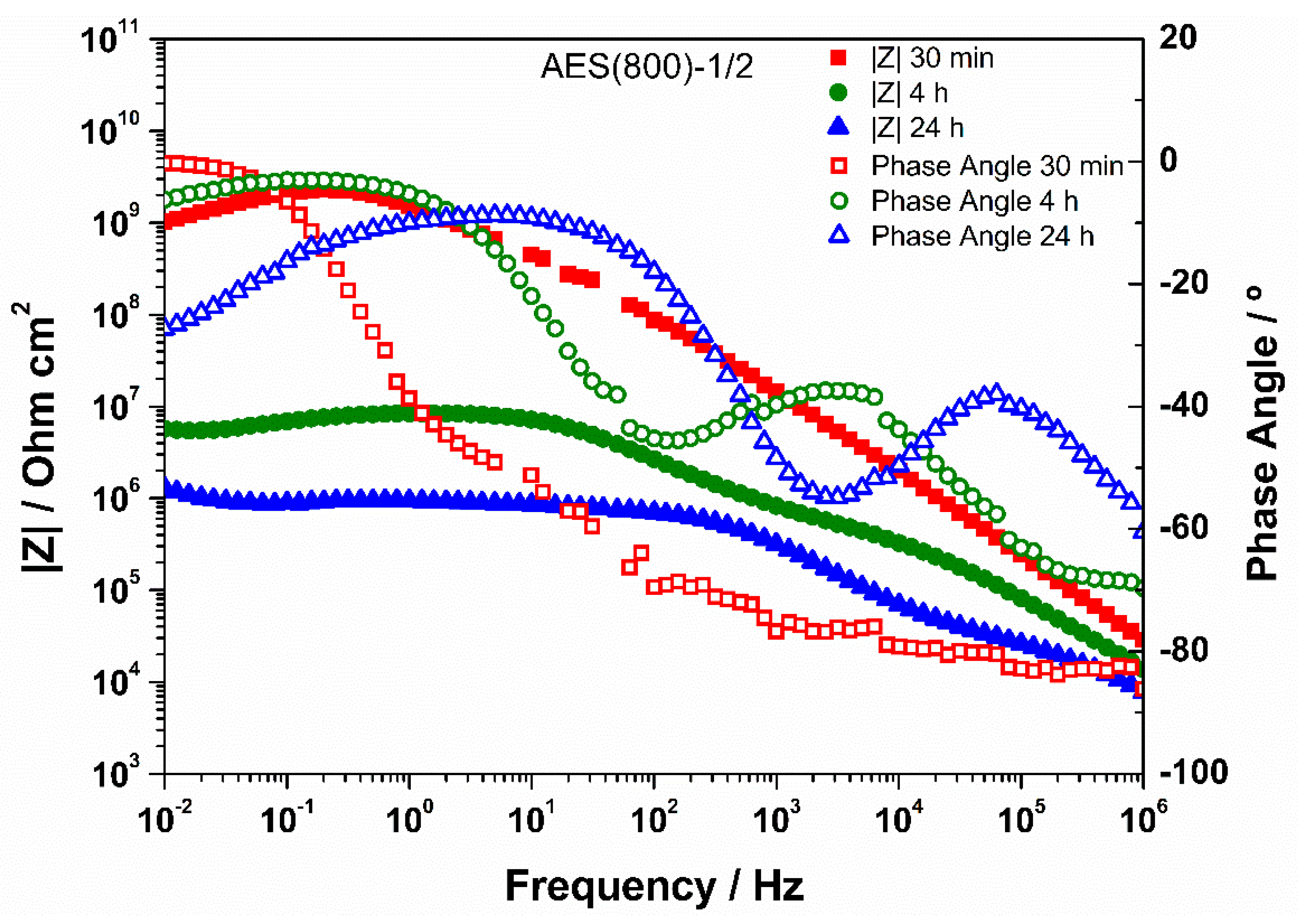
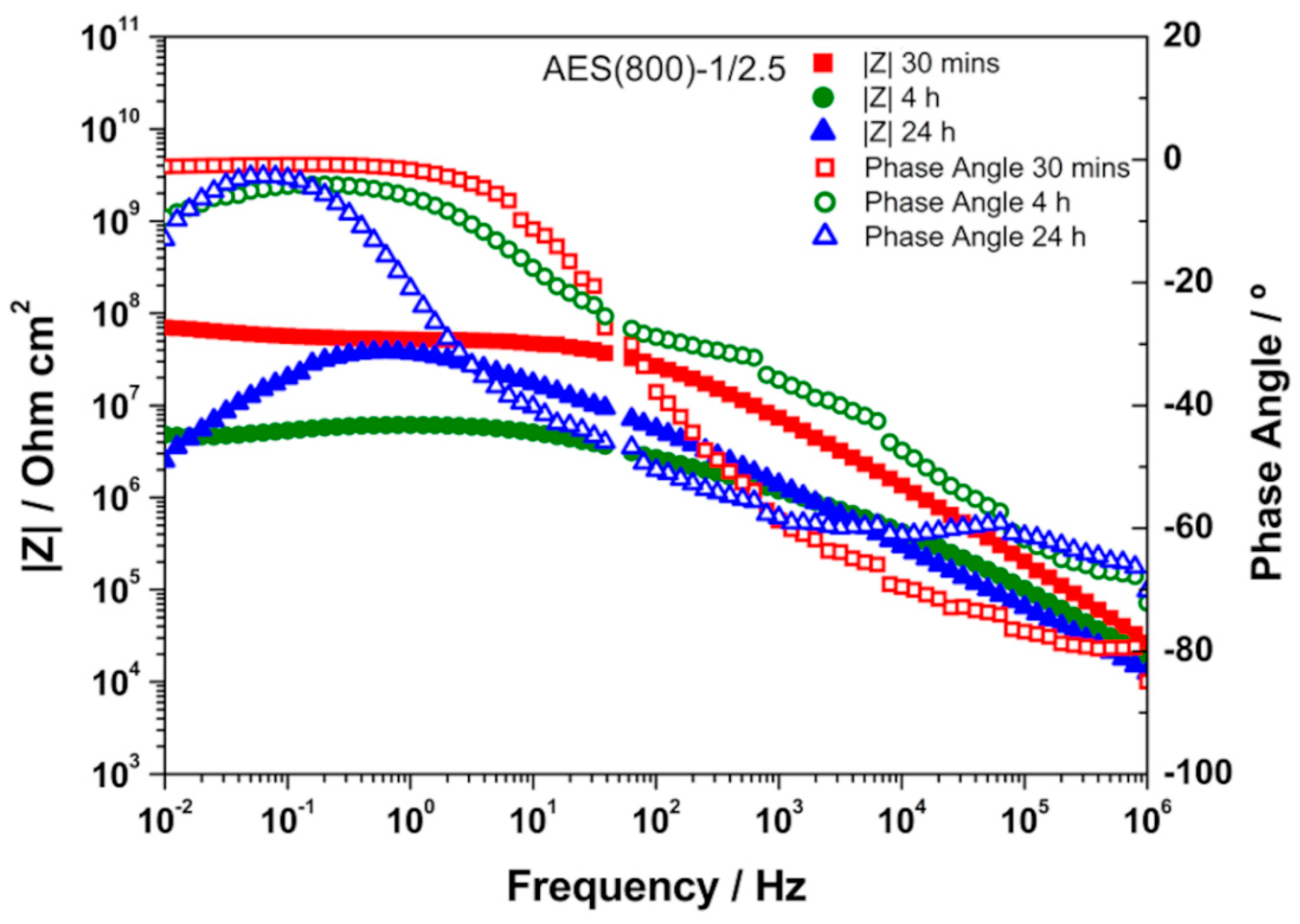
3.3. EIS Analysis
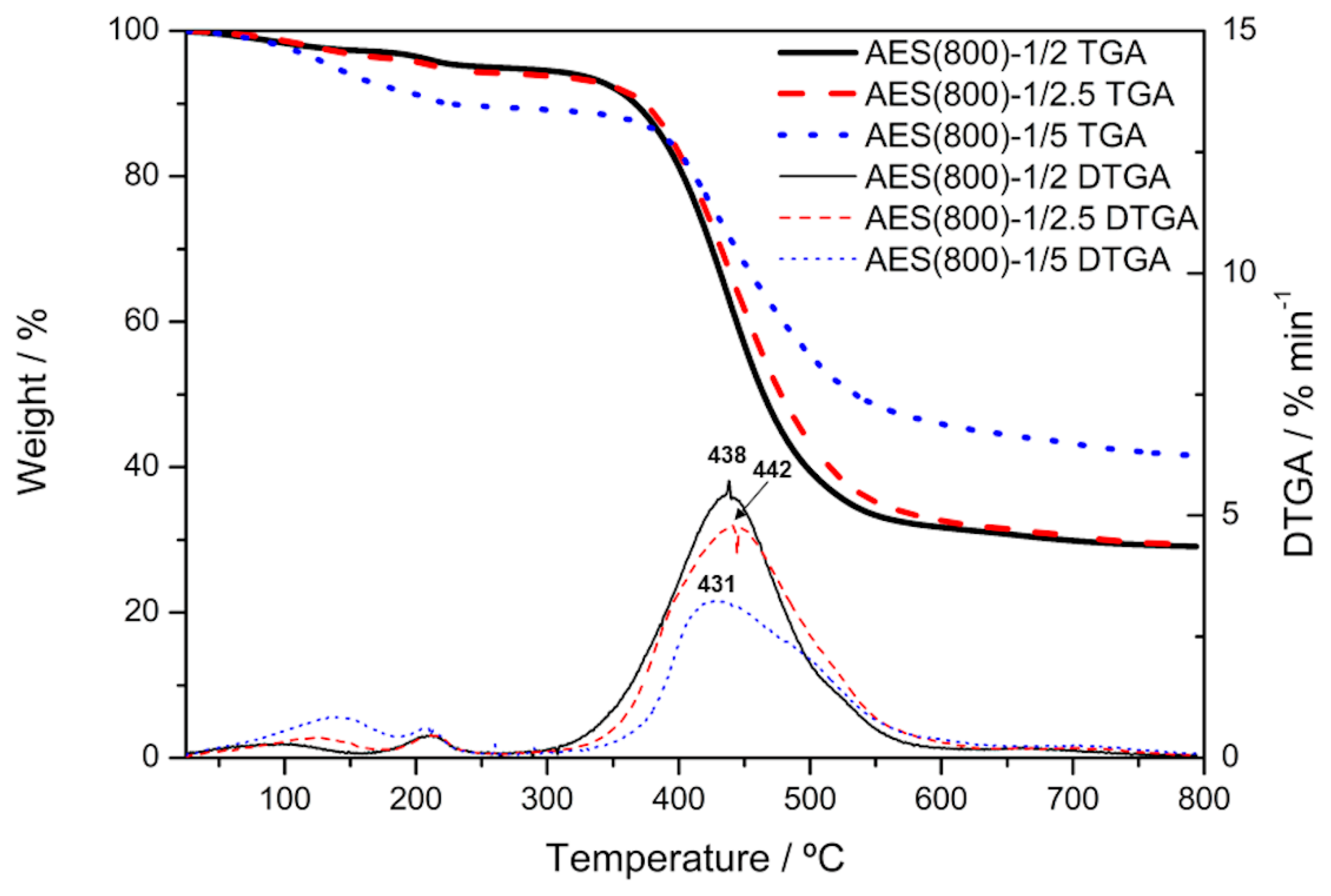
3.4. TGA Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schmidt, H. New Type of Non-Crystalline Solids between Inorganic and Organic Materials. J. Non Cryst. Solids 1985, 73, 681–691. [Google Scholar] [CrossRef]
- Mackenzie, J.D. Structures and Properties of Ormosils. J. Sol Gel Sci. Technol. 1994, 2, 81–86. [Google Scholar] [CrossRef]
- Figueira, R. Multifunctional and Smart Organic-Inorganic Hybrid Sol-Gel Coatings for Corrosion Protection Application. In Advances in Smart Coatings And Thin Films for Future Industrial and Biomedical Engineering Applications; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Figueira, R.B.; Fontinha, I.R.; Silva, C.J.R.; Pereira, E.V. Hybrid Sol-Gel Coatings: Smart and Green Materials for Corrosion Mitigation. Coatings 2016, 6, 12. [Google Scholar] [CrossRef]
- Figueira, R.B.; Silva, C.J.R. Application of Sol–Gel Method to Synthesize Organic–Inorganic Hybrid Coatings to Minimize Corrosion in Metallic Substrates. In Hybrid Organic-Inorganic Interfaces; John Wiley & Sons, Ltd.: New York, NY, USA, 2017; pp. 355–412. [Google Scholar] [CrossRef]
- Figueira, R.B.; Silva, C.J.R.; Pereira, E.V. Organic–Inorganic Hybrid Sol–Gel Coatings for Metal Corrosion Protection: A Review of Recent Progress. J. Coat. Technol. Res. 2015, 12, 1–35. [Google Scholar] [CrossRef]
- Figueira, R.B.; Silva, C.J.R.; Pereira, E.V. Hybrid Sol–Gel Coatings for Corrosion Protection of Galvanized Steel in Simulated Concrete Pore Solution. J. Coat. Technol. Res. 2016, 13, 355–373. [Google Scholar] [CrossRef]
- Boaretto, N.; Bittner, A.; Brinkmann, C.; Olsowski, B.-E.; Schulz, J.; Seyfried, M.; Vezzù, K.; Popall, M.; Di Noto, V. Highly Conducting 3D-Hybrid Polymer Electrolytes for Lithium Batteries Based on Siloxane Networks and Cross-Linked Organic Polar Interphases. Chem. Mater. 2014, 26, 6339–6350. [Google Scholar] [CrossRef]
- Mujahid, A.; Lieberzeit, P.A.; Dickert, F.L. Chemical Sensors Based on Molecularly Imprinted Sol-Gel Materials. Materials 2010, 3, 2196–2217. [Google Scholar] [CrossRef]
- Wright, J.; Sommerdijk, N.A.J.M. Sol-Gel Materials: Chemistry and Applications, 1st ed.; Gordon and Breach: London, UK, 2001. [Google Scholar]
- Ianculescu, A.C.; Vasilescu, C.A.; Crisan, M.; Raileanu, M.; Vasile, B.S.; Calugaru, M.; Crisan, D.; Dragan, N.; Curecheriu, L.; Mitoseriu, L. Formation Mechanism and Characteristics of Lanthanum-Doped BaTiO3 Powders and Ceramics Prepared by the Sol–Gel Process. Mater. Charact. 2015, 106, 195–207. [Google Scholar] [CrossRef]
- Kumar, P.; Yadav, A.K.; Joshi, A.G.; Bhattacharyya, D.; Jha, S.N.; Pandey, P.C. Influence of Li Co-Doping on Structural Property of Sol-Gel Derived Terbium Doped Zinc Oxide Nanoparticles. Mater. Charact. 2018, 142, 593–601. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Elsevier: Amsterdam, The Netherlands, 1990. [Google Scholar]
- Hench, L.L.; West, J.K. The Sol-Gel Process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Livage, J. Basic Principles of Sol-Gel Chemistry. In Sol-Gel Technologies for Glass Producers and Users; Aegerter, M., Mennig, M., Eds.; Springer: New York, NY, USA, 2004. [Google Scholar]
- Sakka, S.; Kozuka, H. Sol-Gel Processing. In Handbook of Sol-Gel Science and Technology; Springer Science & Business Media: Berlin, Germany, 2005. [Google Scholar]
- Su, H.-Y.; Chen, P.-L.; Lin, C.-S. Sol–Gel Coatings Doped with Organosilane and Cerium to Improve the Properties of Hot-Dip Galvanized Steel. Corros. Sci. 2016, 102, 63–71. [Google Scholar] [CrossRef]
- Tatsumisago, M.; Honjo, H.; Sakai, Y.; Minami, T. Proton-Conducting Silica-Gel Films Doped with a Variety of Electrolytes. Solid State Ion. 1994, 74, 105–108. [Google Scholar] [CrossRef]
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C.-M.; Mahapatra, C.; Kim, H.-W.; Knowles, J.C. Sol–Gel Based Materials for Biomedical Applications. Prog. Mater. Sci. 2016, 77, 1–79. [Google Scholar] [CrossRef]
- Nedeljko, P.; Turel, M.; Lobnik, A. Hybrid Sol-Gel Based Sensor Layers for Optical Determination of Biogenic Amines. Sens. Actuators B Chem. 2017, 246, 1066–1073. [Google Scholar] [CrossRef]
- Choodum, A.; Kanatharana, P.; Wongniramaikul, W.; NicDaeid, N. A Sol–Gel Colorimetric Sensor for Methamphetamine Detection. Sens. Actuators B Chem. 2015, 215, 553–560. [Google Scholar] [CrossRef]
- Hashem, S.G.; Elsaady, M.M.; Afify, H.G.; Omer, W.E.; Youssef, A.O.; El-Kemary, M.; Attia, M.S. Determination of Uric Acid in Serum Using an Optical Sensor Based on Binuclear Pd(II) 2-Pyrazinecarboxamide-Bipyridine Doped in a Sol Gel Matrix. Talanta 2019, 199, 89–96. [Google Scholar] [CrossRef]
- Wang, B.; Anslyn, E.V. Chemosensors: Principles, Strategies, and Applications; John Wiley & Sons, Ltd.: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Jerónimo, P.C.A.; Araújo, A.N.; Conceição, B.S.M.; Montenegro, M. Optical Sensors and Biosensors Based on Sol–Gel Films. Talanta 2007, 72, 13–27. [Google Scholar] [CrossRef]
- Nivens, D.A.; Zhang, Y.; Angel, S.M. A Fiber-Optic PH Sensor Prepared Using a Base-Catalyzed Organo-Silica Sol–Gel. Anal. Chim. Acta 1998, 376, 235–245. [Google Scholar] [CrossRef]
- Nivens, D.A.; Schiza, M.V.; Angel, S.M. Multilayer Sol–Gel Membranes for Optical Sensing Applications: Single Layer PH and Dual Layer CO2 and NH3 Sensors. Talanta 2002, 58, 543–550. [Google Scholar] [CrossRef]
- Riehle, N.; Thude, S.; Götz, T.; Kandelbauer, A.; Thanos, S.; Tovar, G.E.M.; Lorenz, G. Influence of PDMS Molecular Weight on Transparency and Mechanical Properties of Soft Polysiloxane-Urea-Elastomers for Intraocular Lens Application. Eur. Polym. J. 2018, 101, 190–201. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, J.; Guo, H.; Xu, T.; Li, Q.; Wen, C.; Sui, X.; Lin, C.; Zhang, J.; Zhang, L. Slime-Resistant Marine Anti-Biofouling Coating with PVP-Based Copolymer in PDMS Matrix. Chem. Eng. Sci. 2019, 207, 790–798. [Google Scholar] [CrossRef]
- Romo-Uribe, A.; Santiago-Santiago, K.; Reyes-Mayer, A.; Aguilar-Franco, M. Functional PDMS Enhanced Strain at Fracture and Toughness of DGEBA Epoxy Resin. Eur. Polym. J. 2017, 89, 101–118. [Google Scholar] [CrossRef]
- Eduok, U.; Faye, O.; Szpunar, J. Recent Developments and Applications of Protective Silicone Coatings: A Review of PDMS Functional Materials. Prog. Org. Coat. 2017, 111, 124–163. [Google Scholar] [CrossRef]
- Zhu, D.; Handschuh-Wang, S.; Zhou, X. Recent Progress in Fabrication and Application of Polydimethylsiloxane Sponges. J. Mater. Chem. A 2017, 5, 16467–16497. [Google Scholar] [CrossRef]
- Fujii, T. PDMS-Based Microfluidic Devices for Biomedical Applications. Microelectron. Eng. 2002, 61–62, 907–914. [Google Scholar] [CrossRef]
- Tamayo, A.; Téllez, L.; Rubio, J.; Rubio, F.; Oteo, J.L. Effect of Reaction Conditions on Surface Properties of TEOS–TBOT–PDMS Hybrid Materials. J. Sol Gel Sci. Technol. 2010, 55, 94–104. [Google Scholar] [CrossRef]
- Xu, F.; Li, D. Effect of the Addition of Hydroxyl-Terminated Polydimethylsiloxane to TEOS-Based Stone Protective Materials. J. Sol Gel Sci. Technol. 2013, 65, 212–219. [Google Scholar] [CrossRef]
- Dirè, S. Sol-Gel Derived Polysiloxane-Oxide Hybrid Materials: Extent of Phase Interaction. J. Sol Gel Sci. Technol. 2003, 26, 285–290. [Google Scholar] [CrossRef]
- Wolf, M.P.; Salieb-Beugelaar, G.B.; Hunziker, P. PDMS with Designer Functionalities—Properties, Modifications Strategies, and Applications. Prog. Polym. Sci. 2018, 83, 97–134. [Google Scholar] [CrossRef]
- Gao, H.; Hu, H.; Zhao, Y.; Li, J.; Lei, M.; Zhang, Y. Highly-Sensitive Optical Fiber Temperature Sensors Based on PDMS/Silica Hybrid Fiber Structures. Sens. Actuators Phys. 2018, 284, 22–27. [Google Scholar] [CrossRef]
- Wang, D.; Sheng, B.; Peng, L.; Huang, Y.; Ni, Z. Flexible and Optical Fiber Sensors Composited by Graphene and PDMS for Motion Detection. Polymers 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Taheri, S. A Review on Five Key Sensors for Monitoring of Concrete Structures. Constr. Build. Mater. 2019, 204, 492–509. [Google Scholar] [CrossRef]
- Figueira, R.B.; Sousa, R.; Coelho, L.; Azenha, M.; de Almeida, J.M.; Jorge, P.A.S.; Silva, C.J.R. Alkali-Silica Reaction in Concrete: Mechanisms, Mitigation and Test Methods. Constr. Build. Mater. 2019, 222, 903–931. [Google Scholar] [CrossRef]
- Winter, N.B. Understanding Cement: An Introduction to Cement Production, Cement Hydration and Deleterious Processes in Concrete; Microanalysis Consultants: St. Ives, UK, 2012. [Google Scholar]
- Parrott, L.J. A Review of Carbonation in Reinforced Concrete: A Review Carried out by C & CA under a BRE Contract; Cement and Concrete Association: Wexham Springs: Liverpool, UK, 1987. [Google Scholar]
- Behnood, A.; Van Tittelboom, K.; De Belie, N. Methods for Measuring PH in Concrete: A Review. Constr. Build. Mater. 2016, 105, 176–188. [Google Scholar] [CrossRef]
- Figueira, R.B. Electrochemical Sensors for Monitoring the Corrosion Conditions of Reinforced Concrete Structures: A Review. Appl. Sci. 2017, 7, 1157. [Google Scholar] [CrossRef]
- Plusquellec, G.; Geiker, M.R.; Lindgård, J.; Duchesne, J.; Fournier, B.; De Weerdt, K. Determination of the PH and the Free Alkali Metal Content in the Pore Solution of Concrete: Review and Experimental Comparison. Cem. Concr. Res. 2017, 96, 13–26. [Google Scholar] [CrossRef]
- Ferreira, M.; Castro-Camus, E.; Ottaway, D.; López-Higuera, J.; Feng, X.; Jin, W.; Jeong, Y.; Picqué, N.; Tong, L.; Reinhard, B.; et al. Roadmap on Optical Sensors. J. Opt. 2017, 19, 083001. [Google Scholar] [CrossRef]
- Leung, C.K.Y. Fiber Optic Sensors in Concrete: The Future? NDT E Int. 2001, 34, 85–94. [Google Scholar] [CrossRef]
- Dhouib, M.; Conciatori, D.; Sorelli, L. Optical Fiber Chloride Sensor for Health Monitoring of Structures in Cold Regions. Cold Reg. Eng. 2019, 391–397. [Google Scholar] [CrossRef]
- Basheer, P.A.M.; Grattan, K.; Sun, T.; Long, A.; Mcpolin, D.O.; Xie, W. Fiber Optic Chemical Sensor Systems for Monitoring PH Changes in Concrete. Proc. SPIE Int. Soc. Opt. Eng. 2004, 5586. [Google Scholar] [CrossRef]
- Mcpolin, D.O.; Basheer, P.A.M.; Grattan, K.; Long, A.; Sun, T.; Xie, W. Preliminary Development and Evaluation of Fibre Optic Chemical Sensors. J. Mater. Civ. Eng. 2011, 23, 1200–1210. [Google Scholar] [CrossRef]
- Sugama, T.; Brothers, L.E.; Weber, L. Acid-Resistant Polydimethylsiloxane Additive for Geothermal Well Cement in 150°C H2SO4 Solution. Adv. Cem. Res. 2003, 15, 35–44. [Google Scholar] [CrossRef]
- Sidek, O.; Afzal, M.H.B. A Review Paper on Fiber-Optic Sensors and Application of PDMS Materials for Enhanced Performance. In Proceedings of the 2011 IEEE Symposium on Business, Engineering and Industrial Applications (ISBEIA), Langkawi, Malaysia, 25–28 September 2011; pp. 458–463. [Google Scholar] [CrossRef]
- Sidek, O.; Kabir, S.; Afzal, M.H.B. Fiber Optic-Based Sensing Approach for Corrosion Detection. In Proceedings of the PIERS Proceedings, Suzhou, China, 12–16 September 2011. [Google Scholar]
- Park, C.; Han, Y.; Joo, K.-I.; Lee, Y.W.; Kang, S.-W.; Kim, H.-R. Optical Detection of Volatile Organic Compounds Using Selective Tensile Effects of a Polymer-Coated Fiber Bragg Grating. Opt. Express 2010, 18, 24753–24761. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Shee, Y.G.; Yap, B.K.; Adikan, F.R.M. Fiber Bragg Grating Based Sensing System: Early Corrosion Detection for Structural Health Monitoring. Sens. Actuators Phys. 2016, 246, 123–128. [Google Scholar] [CrossRef]
- Choi, J.-I.; Lee, Y.; Kim, Y.Y.; Lee, B.Y. Image-Processing Technique to Detect Carbonation Regions of Concrete Sprayed with a Phenolphthalein Solution. Constr. Build. Mater. 2017, 154, 451–461. [Google Scholar] [CrossRef]
- Figueira, R.B.; Callone, E.; Silva, C.J.R.; Pereira, E.V.; Dirè, S. Hybrid Coatings Enriched with Tetraethoxysilane for Corrosion Mitigation of Hot-Dip Galvanized Steel in Chloride Contaminated Simulated Concrete Pore Solutions. Materials 2017, 10, 306. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; Alonso, M.C.; Cecílio, P.; Montemor, M.F.; Andrade, C. Electrochemical and Analytical Assessment of Galvanized Steel Reinforcement Pre-Treated with Ce and La Salts under Alkaline Media. Cem. Concr. Compos. 2006, 28, 256–266. [Google Scholar] [CrossRef]
- Recio, F.; Alonso, C.; Gaillet, L.; Sánchez Moreno, M. Hydrogen Embrittlement Risk of High Strength Galvanized Steel in Contact with Alkaline Media. Corros. Sci. 2011, 53, 2853–2860. [Google Scholar] [CrossRef]
- Sakka, S.; Almeida, R.M. Characterization and Properties of Sol-Gel Materials and Products. In Handbook of Sol-Gel Science and Technology; Springer Science & Business Media: Berlin, Germany, 2005. [Google Scholar]
- Arkles, B.; Larson, G. Silicon Compounds: Silanes & Silicones, 3rd ed.; Gelest Inc.: Morrisville, PA, USA, 2013. [Google Scholar]
- Cai, D.; Neyer, A.; Kuckuk, R.; Heise, H.M. Raman, Mid-Infrared, near-Infrared and Ultraviolet–Visible Spectroscopy of PDMS Silicone Rubber for Characterization of Polymer Optical Waveguide Materials. J. Mol. Struct. 2010, 976, 274–281. [Google Scholar] [CrossRef]
- Macdonald, J.R. Impedance Spectroscopy Emphasizing Solid Materials and Analysis; John Wiley and Sons, Ltd.: New York, NY, USA, 1987. [Google Scholar]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; John Wiley and Sons, Ltd.: New York, NY, USA, 2008. [Google Scholar]
- Barsoukov, E.; Macdonald, J.R. (Eds.) Impedance Spectroscopy: Theory, Experiment, and Applications, 3rd ed.; Wiley: Hoboken, NJ, USA, 2018. [Google Scholar]
- Orazem, M.E.; Tribollet, B. The Electrochemical Society Series. In Electrochemical Impedance Spectroscopy; John Wiley & Sons, Ltd.: New York, NY, USA, 2017; pp. I–III. [Google Scholar] [CrossRef]
- Jorcin, J.-B.; Orazem, M.E.; Pébère, N.; Tribollet, B. CPE Analysis by Local Electrochemical Impedance Spectroscopy. Electrochimica Acta 2006, 51, 1473–1479. [Google Scholar] [CrossRef]
- Figueira, R.B.; Silva, C.J.R.; Pereira, E.V.; Salta, M.M. Alcohol-Aminosilicate Hybrid Coatings for Corrosion Protection of Galvanized Steel in Mortar. J. Electrochem. Soc. 2014, 161, C349–C362. [Google Scholar] [CrossRef]
- Qian, M.; Mcintosh Soutar, A.; Tan, X.H.; Zeng, X.T.; Wijesinghe, S.L. Two-Part Epoxy-Siloxane Hybrid Corrosion Protection Coatings for Carbon Steel. Thin Solid Films 2009, 517, 5237–5242. [Google Scholar] [CrossRef]
- Moreira, S.D.F.C.; Silva, C.J.R.; Prado, L.A.S.A.; Costa, M.F.M.; Boev, V.I.; Martín-Sánchez, J.; Gomes, M.J.M. Development of New High Transparent Hybrid Organic–Inorganic Monoliths with Surface Engraved Diffraction Pattern. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 492–499. [Google Scholar] [CrossRef]
- Thomas, T.H.; Kendrick, T.C. Thermal Analysis of Polydimethylsiloxanes. I. Thermal Degradation in Controlled Atmospheres. J. Polym. Sci. Part A 2 Polym. Phys. 1969, 7, 537–549. [Google Scholar] [CrossRef]
- Ručigaj, A.; Krajnc, M.; Sebenik, U. Kinetic Study of Thermal Degradation of Polydimethylsiloxane: The Effect of Molecular Weight on Thermal Stability in Inert Atmosphere. Polym. Sci. 2017, 3. [Google Scholar] [CrossRef]
- Gonzalez, J.; Iglio, R.; Barillaro, G.; Duce, C.; Tiné, M. Structural and Thermoanalytical Characterization of 3D Porous PDMS Foam Materials: The Effect of Impurities Derived from a Sugar Templating Process. Polymers 2018, 10, 616. [Google Scholar] [CrossRef]
- Taylor, H.F.W.; Famy, C.; Scrivener, K.L. Delayed Ettringite Formation. Cem. Concr. Res. 2001, 31, 683–693. [Google Scholar] [CrossRef]


| OIH Films | PDMS:APTES Ratio | Phph:PDMS Ratio |
|---|---|---|
| AES(800)-1/2 | 1:2 | - |
| AES(800)-1/2.5 | 1:2.5 | - |
| AES(800)-1/5 | 1:5 | - |
| AES(800)-1/2-Phph-0.01 | 1:2 | 1:100 |
| AES(800)-1/2-Phph-0.02 | 1:2 | 1:50 |
| AES(800)-1/2-Phph-0.05 | 1:2 | 1:20 |
| Phph | Ethanol | AES(800)-1/2-Phph-0.01 | AES(800)-1/2-Phph-0.02 | AES(800)-1/2-Phph-0.05 |
|---|---|---|---|---|
| Absorbance (566 nm) * | 0.136 | 0.411 | 0.482 | 0.627 |
| [Phph]/mol g−1 | 1.27 × 10−7 | 8.05 × 10−6 | 1.48 × 10−5 | 2.62 × 10−5 |
| Optical path/cm | 1.000 | 1.131 ** | 0.919 ** | 1.017 ** |
| Absorption coefficient/104 g mol−1 cm−1 | 107.08 | 4.52 | 3.55 | 2.35 |
| Sample | CPE1/Sα Ω−1 cm−2 | α1 | R1/Ω cm2 | CPE2/Sα Ω−1 cm−2 | α2 | R2/Ω cm2 | Rsample/Ω cm2 | χ2 |
|---|---|---|---|---|---|---|---|---|
| AES(800)-1/2 | 9.27 × 10−12 | 0.900 | 9.00 × 1010 | 8.12 × 10−12 | 0.900 | 8.84 × 1010 | 1.78 × 1011 | 2.24 × 10−1 |
| (1.54%) | (0.25%) | (0.01%) | (2.75%) | (0.51%) | (2.20%) | |||
| AES(800)-1/2.5 | 6.45 × 10−12 | 0.975 | 1.47 × 109 | 5.35 × 10−12 | 0.881 | 9.16 × 109 | 1.06 × 1010 | 4.81 × 10−3 |
| (2.17%) | (0.19%) | (13.10%) | (8.36%) | (2.90%) | (2.61%) | |||
| AES(800)-1/5 | 2.46 × 10−11 | 0.910 | 2.10 × 106 | 5.28 × 10−10 | 0.575 | 3.81 × 107 | 4.02 × 107 | 3.58 × 10−4 |
| (±13.19%) | (±0.97%) | (±9.90%) | (±10.64%) | (±2.57%) | (±3.00%) | |||
| AES(800)-1/2-Phph-0.01 | 6.60 × 10−12 | 0.961 | 3.17 × 108 | 2.97 × 10−11 | 0.748 | 1.94 × 109 | 2.26 × 109 | 2.61 × 10−3 |
| (±2.73%) | (±0.23%) | (±7.96%) | (±7.11%) | (±2.56%) | (±2.24%) | |||
| AES(800)-1/2-Phph-0.02 | 7.61 × 10−12 | 0.964 | 1.94 × 108 | 6.47 × 10−10 | 0.597 | 2.07 × 109 | 2.26 × 109 | 1.88 × 10−3 |
| (±3.26%) | (±0.27%) | (±15.50%) | (±5.95%) | (±3.01%) | (±2.63%) | |||
| AES(800)-1/2-Phph-0.05 | 8.32 × 10−12 | 0.954 | 2.82 × 108 | 5.99 × 10−11 | 0.713 | 1.27 × 109 | 1.55 × 109 | 1.31 × 10−3 |
| (±2.61%) | (±0.00%) | (±7.40%) | (±7.96%) | (±3.34%) | (±3.01%) |
| Films | T5 | Tmax |
|---|---|---|
| AES(800)-1/2 | 256 | 438 |
| AES(800)-1/2.5 | 213 | 442 |
| AES(800)-1/5 | 135 | 431 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, R.P.C.L.; Ferreira, B.; Azenha, M.; Costa, S.P.G.; Silva, C.J.R.; Figueira, R.B. PDMS Based Hybrid Sol-Gel Materials for Sensing Applications in Alkaline Environments: Synthesis and Characterization. Polymers 2020, 12, 371. https://doi.org/10.3390/polym12020371
Sousa RPCL, Ferreira B, Azenha M, Costa SPG, Silva CJR, Figueira RB. PDMS Based Hybrid Sol-Gel Materials for Sensing Applications in Alkaline Environments: Synthesis and Characterization. Polymers. 2020; 12(2):371. https://doi.org/10.3390/polym12020371
Chicago/Turabian StyleSousa, Rui P. C. L., Bárbara Ferreira, Miguel Azenha, Susana P. G. Costa, Carlos J. R. Silva, and Rita B. Figueira. 2020. "PDMS Based Hybrid Sol-Gel Materials for Sensing Applications in Alkaline Environments: Synthesis and Characterization" Polymers 12, no. 2: 371. https://doi.org/10.3390/polym12020371
APA StyleSousa, R. P. C. L., Ferreira, B., Azenha, M., Costa, S. P. G., Silva, C. J. R., & Figueira, R. B. (2020). PDMS Based Hybrid Sol-Gel Materials for Sensing Applications in Alkaline Environments: Synthesis and Characterization. Polymers, 12(2), 371. https://doi.org/10.3390/polym12020371