Figure 1.
External appearance of the obtained polymeric materials, containing 0%, 0.5%, 1.0%, 2.0% and 5.0% of birch bark extract (BBE) and received with the use of ultrasonic treatment (with US) and without the use of ultrasonic treatment (without US): (a) 0% BBE without US; (b) 0% BBE with US; (c) 0.5% BBE without US; (d) 0.5% BBE with US; (e) 1.0% BBE without US; (f) 1.0% BBE with US; (g) 2.0% BBE without US; (h) 2.0% BBE with US; (i) 5.0% BBE without US; (j) 5.0% BBE with US.
Figure 1.
External appearance of the obtained polymeric materials, containing 0%, 0.5%, 1.0%, 2.0% and 5.0% of birch bark extract (BBE) and received with the use of ultrasonic treatment (with US) and without the use of ultrasonic treatment (without US): (a) 0% BBE without US; (b) 0% BBE with US; (c) 0.5% BBE without US; (d) 0.5% BBE with US; (e) 1.0% BBE without US; (f) 1.0% BBE with US; (g) 2.0% BBE without US; (h) 2.0% BBE with US; (i) 5.0% BBE without US; (j) 5.0% BBE with US.
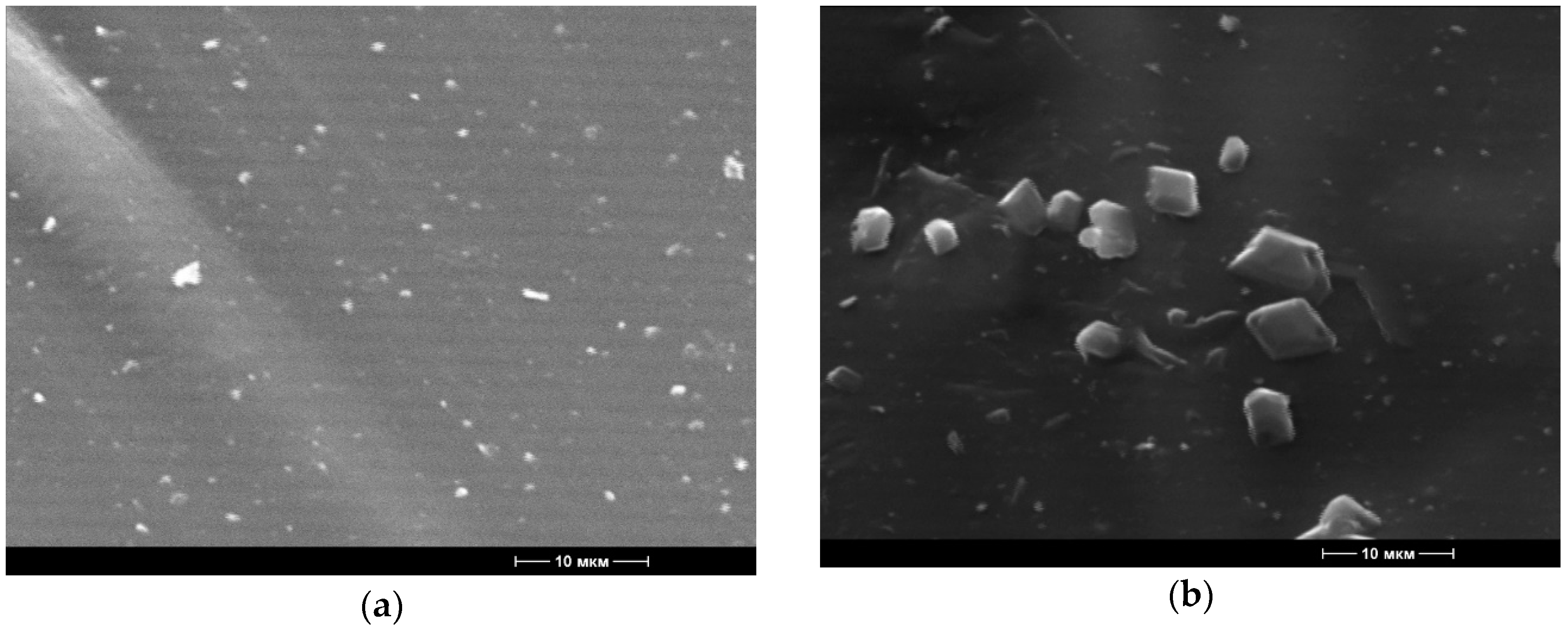
Figure 2.
Microphotographs of the surface structure of the materials (5000× magnification) based on polyethylene with 2.0% content of birch bark extract, received with the use of ultrasonic treatment (a) and without the use of ultrasonic treatment (b).
Figure 2.
Microphotographs of the surface structure of the materials (5000× magnification) based on polyethylene with 2.0% content of birch bark extract, received with the use of ultrasonic treatment (a) and without the use of ultrasonic treatment (b).
Figure 3.
Microphotographs of the surface structure of the materials (5000× magnification) based on polyethylene with 5.0% content of birch bark extract, received with the use of ultrasonic treatment (a) and without the use of ultrasonic treatment (b).
Figure 3.
Microphotographs of the surface structure of the materials (5000× magnification) based on polyethylene with 5.0% content of birch bark extract, received with the use of ultrasonic treatment (a) and without the use of ultrasonic treatment (b).
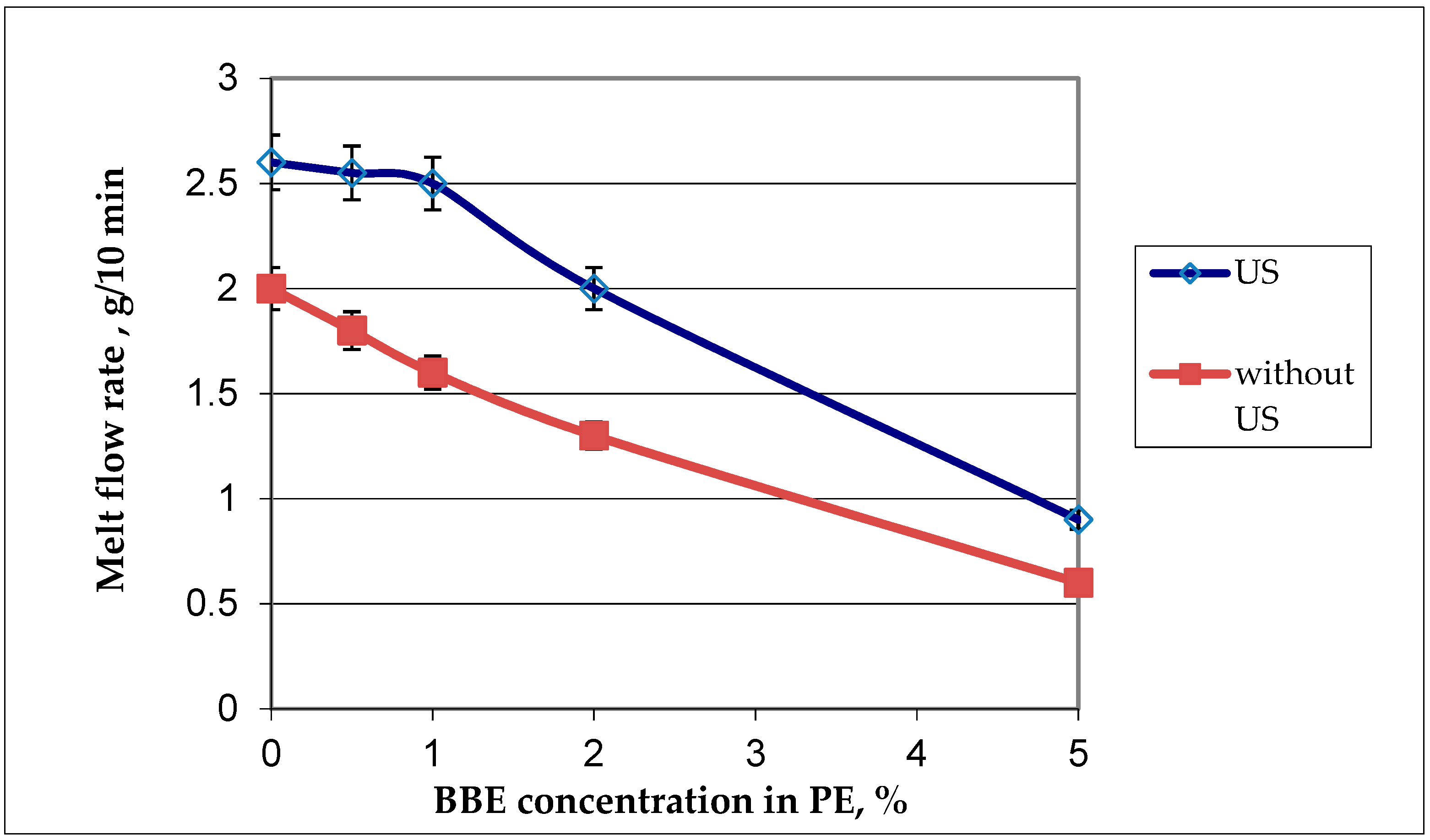
Figure 4.
Graphic dependance of the melt flow rate on the birch bark extract (BBE) content in polyethylene (PE) and on ulrasonic (US) treatment.
Figure 4.
Graphic dependance of the melt flow rate on the birch bark extract (BBE) content in polyethylene (PE) and on ulrasonic (US) treatment.
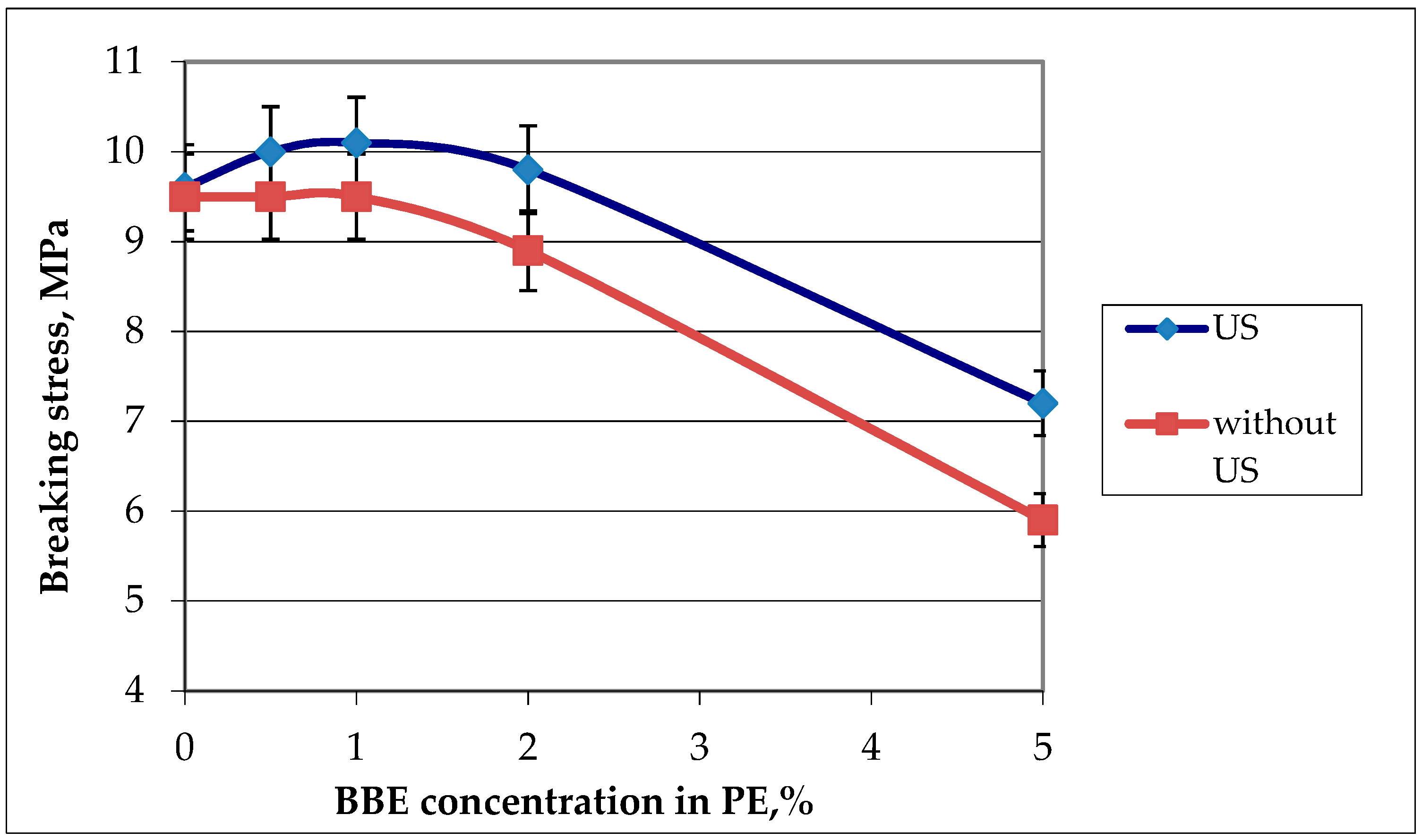
Figure 5.
Graphic dependence of the breaking stress on the birch bark extract (BBE) content in polyethylene (PE) and on ultrasonic (US) treatment.
Figure 5.
Graphic dependence of the breaking stress on the birch bark extract (BBE) content in polyethylene (PE) and on ultrasonic (US) treatment.
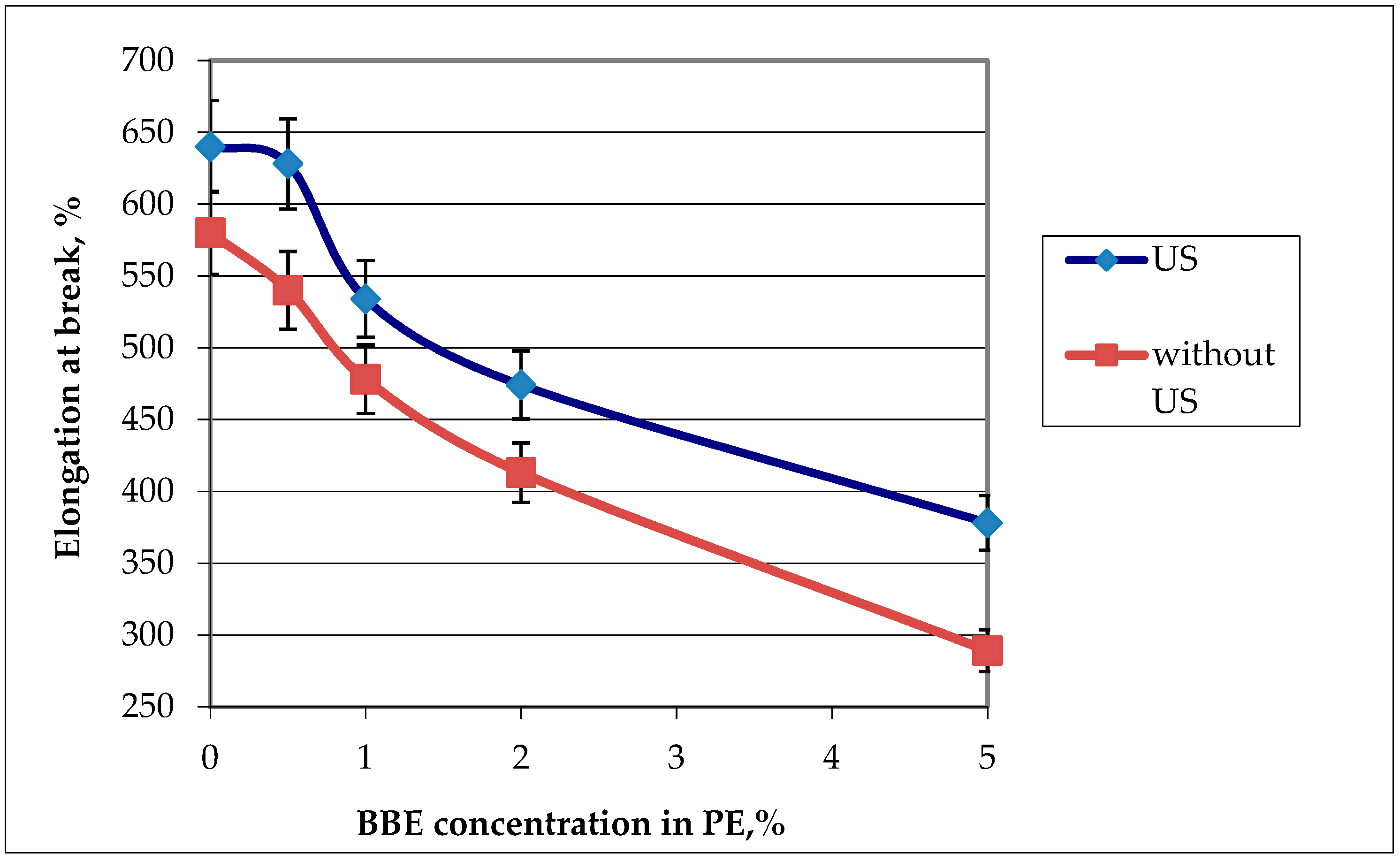
Figure 6.
Graphic dependence of the elongation at break on the birch bark extract (BBE) content in polyethylene (PE) and on ultrasonic (US) treatment.
Figure 6.
Graphic dependence of the elongation at break on the birch bark extract (BBE) content in polyethylene (PE) and on ultrasonic (US) treatment.
Figure 7.
External appearance of polyethylene without birch bark extract (a) and polyethylene with 1.0% content of birch bark extract (b) and the use of ultrasonic treatment after the incubation under the influence of C. albicans for 24 h.
Figure 7.
External appearance of polyethylene without birch bark extract (a) and polyethylene with 1.0% content of birch bark extract (b) and the use of ultrasonic treatment after the incubation under the influence of C. albicans for 24 h.
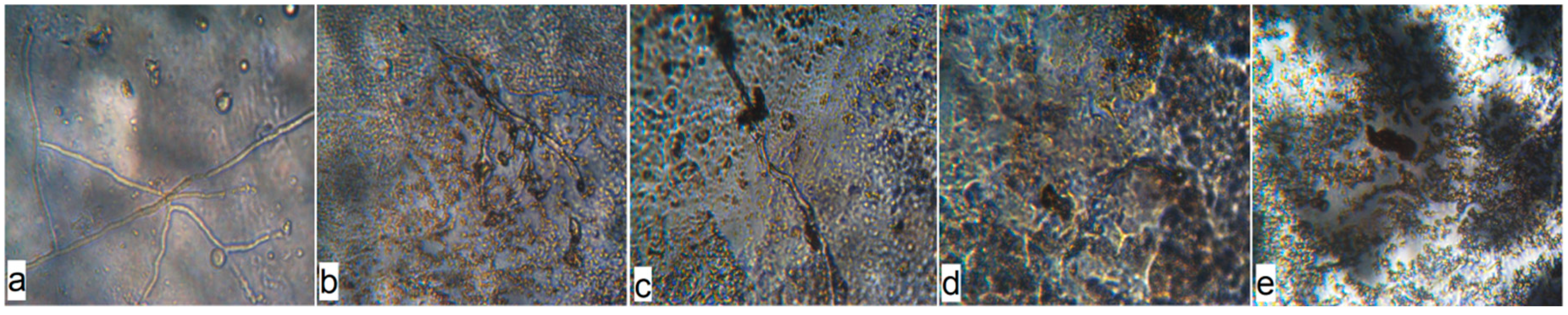
Figure 8.
Microphotographs of the surface of the inoculated polyethylene materials with birch bark extract and with the use of ultrasonic treatment on the 14th day of A. niger exposure (100× magnification): (a) PE 0% BBE ultrasound; (b) PE 0.5% BBE, ultrasound; (c) PE 1.0% BBE, ultrasound; (d) PE 2.0% BBE, ultrasound; (e) PE 5.0% BBE, ultrasound.
Figure 8.
Microphotographs of the surface of the inoculated polyethylene materials with birch bark extract and with the use of ultrasonic treatment on the 14th day of A. niger exposure (100× magnification): (a) PE 0% BBE ultrasound; (b) PE 0.5% BBE, ultrasound; (c) PE 1.0% BBE, ultrasound; (d) PE 2.0% BBE, ultrasound; (e) PE 5.0% BBE, ultrasound.
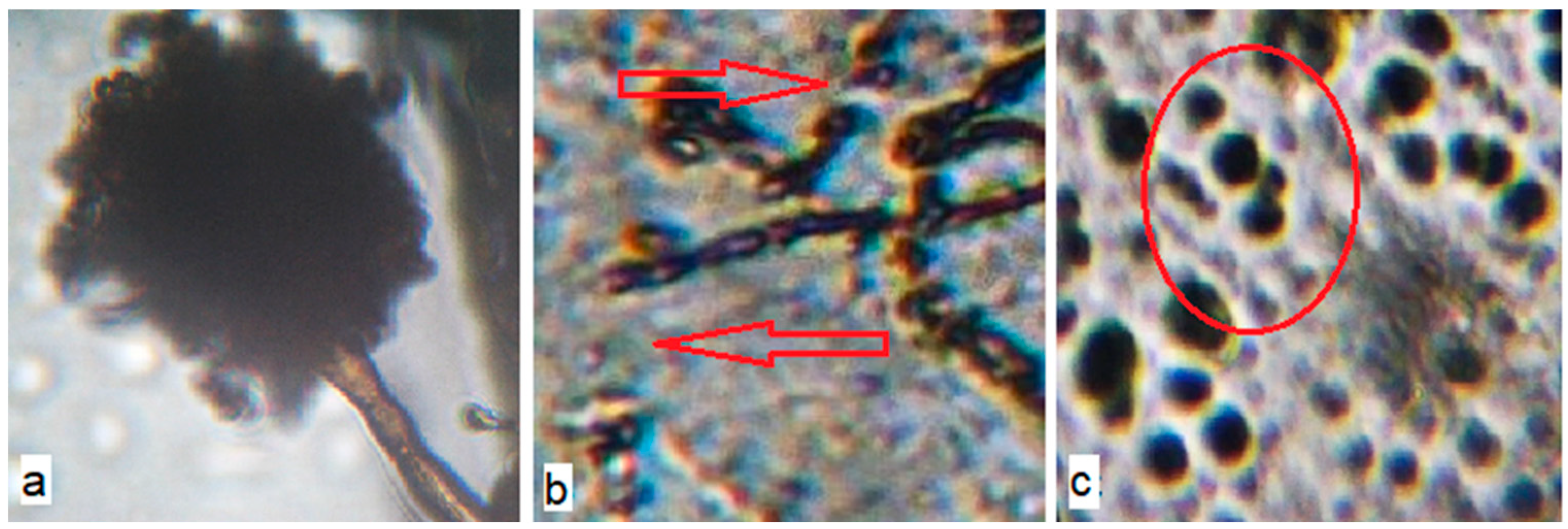
Figure 9.
Microphotographs of the surface of the inoculated polyethylene (PE) materials with birch bark extract (BBE) and with the use of ultrasonic treatment on the 14th day of the exposure of A. niger (400× magnification): (a) PE 0% BBE, ultrasound; (b) PE 0.5% BBE, ultrasound; (c) PE 2.0% BBE, ultrasound.
Figure 9.
Microphotographs of the surface of the inoculated polyethylene (PE) materials with birch bark extract (BBE) and with the use of ultrasonic treatment on the 14th day of the exposure of A. niger (400× magnification): (a) PE 0% BBE, ultrasound; (b) PE 0.5% BBE, ultrasound; (c) PE 2.0% BBE, ultrasound.
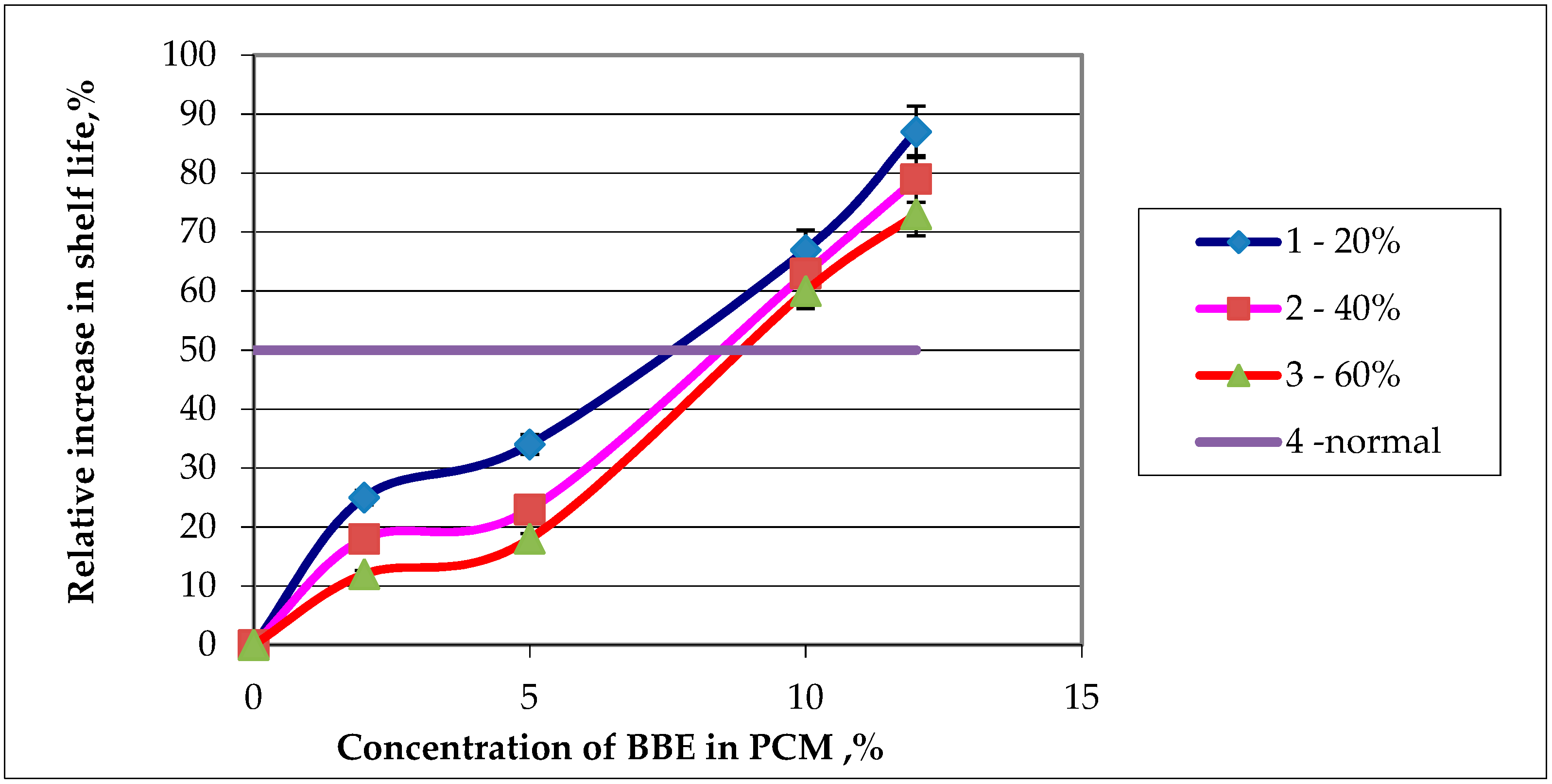
Figure 10.
Dependence of the relative increase in shelf life of the food product on the contents of birch bark extract (BBE) in composition material (PCM): (1—content of thermoplastic starch 20% in PCM; 2—content of thermoplastic starch 30% in PCM; 3—content of thermoplastic starch 60% in PCM; 4—desired value to increase product storage).
Figure 10.
Dependence of the relative increase in shelf life of the food product on the contents of birch bark extract (BBE) in composition material (PCM): (1—content of thermoplastic starch 20% in PCM; 2—content of thermoplastic starch 30% in PCM; 3—content of thermoplastic starch 60% in PCM; 4—desired value to increase product storage).
Table 1.
The main characteristics of polyethylene.
Table 1.
The main characteristics of polyethylene.
| Parameter | Value |
|---|
| Average molecular weight | 78,600 |
| Melting point, mp, °C | 105–108 |
| Density, ρ, g/cm3, ρ | 0.919–0.925 |
| Melt flow rate, g/10 min | 2–2.5 |
| Elongation at break, εp, % | 600–800 |
| Breaking stress, σp, MPa | 11–15 |
Table 2.
Temperature conditions for the processing of the polyethylene compositions in the extruder.
Table 2.
Temperature conditions for the processing of the polyethylene compositions in the extruder.
| Temperature in the Extruder by Zone, °C | Polymer Compositions 2 |
|---|
| Zone 1 1 | Zone 2 | Zone 3 | Zone 4 |
|---|
| 120 | 160 | 180 | 190 | PE with BBE |
| 120 | 150 | 150 | 160 | PE with BBE and starch |
Table 3.
Component composition of polymeric compositions based on polyethylene (PE) with birch bark extract (BBE).
Table 3.
Component composition of polymeric compositions based on polyethylene (PE) with birch bark extract (BBE).
| Polymer Composition | The Content of the Compositions, % |
|---|
| Polyethylene | Birch Bark Extract | Irganox 1010 |
|---|
| PE 0.5% BBE | 98.5 | 0.5 | 1 |
| PE 1.0% BBE | 98 | 1 | 1 |
| PE 2.0% BBE | 97 | 2 | 1 |
| PE 5.0% BBE | 94 | 5 | 1 |
| PE 0% BBE | 100 | 0 | 0 |
Table 4.
The component composition of the polymeric compositions based on polyethylene (PE) with birch bark extract (BBE) and thermoplastic starch.
Table 4.
The component composition of the polymeric compositions based on polyethylene (PE) with birch bark extract (BBE) and thermoplastic starch.
| Polymer Composition | The Content of the Compositions, % |
|---|
| Polyethylene with Irganox 1010 | Birch Bark Extract | Thermoplastic Starch |
|---|
| PC 20 BBE 0 | 80 | 0 | 20 |
| PC 40 BBE 0 | 60 | 0 | 40 |
| PC 60 BBE 0 | 40 | 0 | 60 |
| PC 20 BBE 2 | 78 | 2 | 20 |
| PC 40 BBE 2 | 58 | 2 | 40 |
| PC 60 BBE 2 | 38 | 2 | 60 |
| PC 20 BBE 5 | 75 | 5 | 20 |
| PC 40 BBE 5 | 55 | 5 | 40 |
| PC 60 BBE 5 | 35 | 5 | 60 |
| PC 20 BBE 8 | 72 | 8 | 20 |
| PC 40 BBE 8 | 52 | 8 | 40 |
| PC 60 BBE 8 | 32 | 8 | 60 |
| PC 20 BBE 12 | 68 | 12 | 20 |
| PC 40 BBE 12 | 48 | 12 | 40 |
| PC 60 BBE 12 | 28 | 12 | 60 |
Table 5.
Results of the visual assessment of the surface of polymeric materials based on polyethylene (PE) with birch bark extract (BBE) received with (with US) and without the use of ultrasonic (without US) treatment, inoculated for 24–48 h.
Table 5.
Results of the visual assessment of the surface of polymeric materials based on polyethylene (PE) with birch bark extract (BBE) received with (with US) and without the use of ultrasonic (without US) treatment, inoculated for 24–48 h.
| Polymer Composition | Visual Assessment |
|---|
| E. coli | C. albicans | P. commune |
|---|
| PE 0% BBE without US | Surface growth | Surface growth | Surface growth |
| PE 0% BBE with US | Surface growth | Surface growth | Surface growth |
| PE 0.5% BBE without US | Slow surface growth | Slow surface growth | Slow surface growth |
| PE 0.5% BBE with US | Slow surface growth | Slow surface growth | Slow surface growth |
| PE 1.0% BBE without US | Lack of surface growth | Lack of surface growth | Lack of surface growth |
| PE 1.0% BBE with US | Lack of surface growth | Lack of surface growth | Lack of surface growth |
| PE 2.0% BBE without US | Lack of surface growth | Lack of surface growth | Lack of surface growth |
| PE 2.0% BBE with US | Lack of surface growth | Lack of surface growth | Lack of surface growth |
| PE 5.0% BBE without US | Zone of inhibition 1.8 ± 0.2 mm | Zone of inhibition 1.5 ± 0.1 mm | Zone of inhibition 3.0 ± 0.5 mm |
| PE 5.0% BBE with US | Zone of inhibition 1.7 ± 0.2 mm | Zone of inhibition 1.5 ± 0.2 mm | Zone of inhibition 3.1 ± 0.5 mm |
Table 6.
Results of sanitary chemical research of extarcts of various model environments of materials based on polyethylene with 2.0% content of birch bark extract and the use of ultrasonic treatment.
Table 6.
Results of sanitary chemical research of extarcts of various model environments of materials based on polyethylene with 2.0% content of birch bark extract and the use of ultrasonic treatment.
| Name of Indicator, mg/dm3 | Permissible Norm | Citric Acid
2.0% | Lactic Acid
3.0% | Solution
NaCl 0.3% |
|---|
| Acetaldehyde | ≤0.2 | <0.05 | <0.05 | <0.05 |
| Ethyl acetate | ≤0.1 | <0.05 | <0.05 | <0.05 |
| Hexane | ≤0.1 | <0.05 | <0.05 | <0.05 |
| Heptane | ≤0.1 | <0.05 | <0.05 | <0.05 |
| Acetone | ≤0.1 | <0.05 | <0.05 | <0.05 |
| Formaldehyde | ≤0.1 | <0.025 | <0.025 | <0.025 |
| Methyl alcohol | ≤0.2 | 0.08 | 0.12 | 0.17 |
| Butyl alcohol | ≤0.5 | <0.05 | <0.05 | <0.05 |
| Isobutyl alcohol | ≤0.5 | <0.05 | <0.05 | <0.05 |
| Propyl alcohol | ≤0.1 | <0.05 | <0.05 | <0.05 |
| Isopropyl alcohol | ≤0.1 | <0.05 | <0.05 | <0.05 |
Table 7.
Change in the content of the quantity of mesophilic aerobic and facultative anaerobic microorganisms, CFU/g, in parts of poultry carcasses during storage.
Table 7.
Change in the content of the quantity of mesophilic aerobic and facultative anaerobic microorganisms, CFU/g, in parts of poultry carcasses during storage.
| Type of Packaging | Duration of Storage, days |
|---|
| 0 | 2 | 3 | 4 | 5 |
|---|
| PE 0% BBE US | <102 | (5.75 ± 0.30) × 102 | (7.40 ± 0.28) × 102 | (0.95 ± 0.20) × 103 | (2.11 ± 0.14) × 103 |
| PE 2% BBE US | <102 | (3.10 ± 0.22) × 102 | (5.50 ± 0.20) × 102 | (0.72 ± 0.18) × 103 | (0.90 ± 0.21) × 103 |
Table 8.
Dependance of the melt flow rate index (MRF) on the composition of polymeric compositions material, received with (with US) and without the use of ultrasonic treatment (without US).
Table 8.
Dependance of the melt flow rate index (MRF) on the composition of polymeric compositions material, received with (with US) and without the use of ultrasonic treatment (without US).
| Polymer Composition | Average Values MRF, g/10 min |
|---|
| with US | without US |
|---|
| PC 20 BBE 0 | 1.7 | 1.2 |
| PC 40 BBE 0 | 1.2 | 0.7 |
| PC 60 BBE 0 | 0.9 | 0.6 |
| PC 20 BBE 2 | 1.3 | 1.0 |
| PC 40 BBE 2 | 0.9 | 0.8 |
| PC 60 BBE 2 | 0.7 | 0.4 |
| PC 20 BBE 5 | 1.1 | 0.8 |
| PC 40 BBE 5 | 0.8 | 0.4 |
| PC 60 BBE 5 | 0.5 | 0.2 |
| PC 20 BBE 8 | 0.9 | 0.5 |
| PC 40 BBE 8 | 0.4 | 0.2 |
| PC 60 BBE 8 | 0.3 | 0.1 |
| PC 20 BBE 12 | 0.8 | 0.5 |
| PC 40 BBE 12 | 0.4 | 0.2 |
| PC 60 BBE 12 | 0.2 | 0.05 |
Table 9.
Physico-mechanical properties of composite polymeric films (PC) according to the content of birch bark extract (BBE) and the use of ultrasonic treatment (US).
Table 9.
Physico-mechanical properties of composite polymeric films (PC) according to the content of birch bark extract (BBE) and the use of ultrasonic treatment (US).
| Polymer Composition | Breaking Stress, MPa | Elongation at Break, % |
|---|
| with US | without US | with US | without US |
|---|
| PC 20 BBE 0 | 11 | 9.7 | 160 | 90 |
| PC 40 BBE 0 | 9.1 | 7.8 | 58 | 19 |
| PC 60 BBE 0 | 7.1 | 6.3 | 25 | 9 |
| PC 20 BBE 2 | 10.5 | 9.2 | 158 | 90 |
| PC 40 BBE 2 | 8.8 | 7.1 | 56 | 15 |
| PC 60 BBE 2 | 6.7 | 5.9 | 24 | 8 |
| PC 20 BBE 5 | 10.1 | 8.5 | 155 | 86 |
| PC 40 BBE 5 | 8.1 | 6.7 | 42 | 13 |
| PC 60 BBE 5 | 6.3 | 5.4 | 24 | 8 |
| PC 20 BBE 8 | 9.7 | 7.9 | 140 | 67 |
| PC 40 BBE 8 | 6.9 | 5.9 | 36 | 12 |
| PC 60 BBE 8 | 5.9 | 4.6 | 10 | 6 |
| PC 20 BBE 12 | 9.2 | 7.3 | 114 | 60 |
| PC 40 BBE 12 | 6.7 | 5.4 | 33 | 12 |
| PC 60 BBE 12 | 5.3 | 4.3 | 9 | 5 |
Table 10.
Change in relative elongation at break of composite polymeric materials (PC) containing birch bark extract (BBE), received with (with US) and without the use of ultrasonic treatment (without US).
Table 10.
Change in relative elongation at break of composite polymeric materials (PC) containing birch bark extract (BBE), received with (with US) and without the use of ultrasonic treatment (without US).
| Polymer Composition | Change in Elongation at Break after Composting for 6 months, % |
|---|
| with US | without US |
|---|
| PC 20 BBE 0 | 20–23 | 16–18 |
| PC 40 BBE 0 | 39–43 | 28–32 |
| PC 60 BBE 0 | 78–81 | 67–69 |
| PC 20 BBE 8 | 17–19 | 13–15 |
| PC 40 BBE 8 | 32–35 | 24–28 |
| PC 60 BBE 8 | 60–68 | 46–54 |