A Highly Efficient Ag Nanoparticle-Immobilized Alginate-g-Polyacrylonitrile Hybrid Photocatalyst for the Degradation of Nitrophenols
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of the PAN-g-Alg@Ag Nanocomposite Material
2.3. Analytical Techniques Used for Product Characterization
2.4. Photocatalytic Experiments
3. Results and Discussion
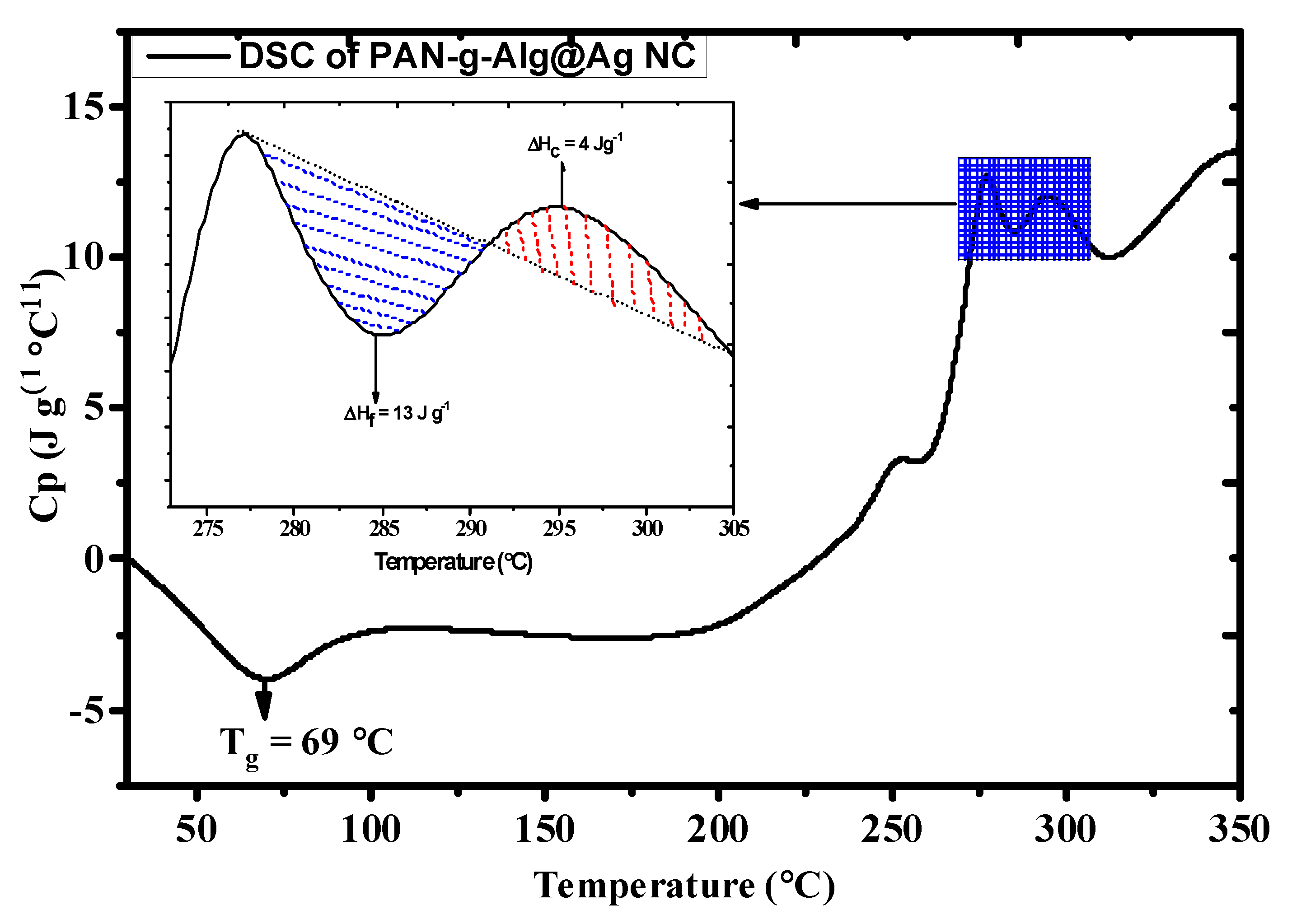
3.1. Characterization of the Synthesized Nanocomposite
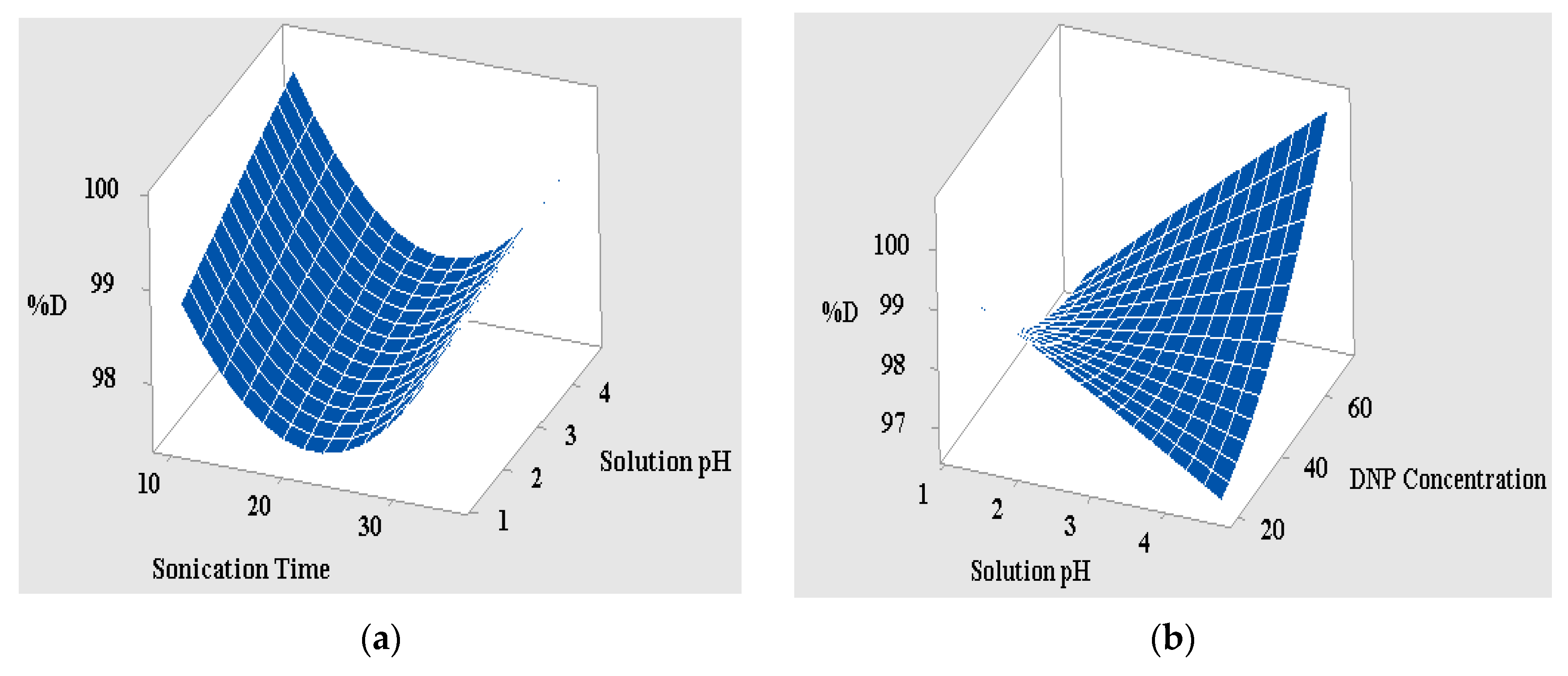
3.2. The RSM-Coupled Approach and Statistical Exploration
3.2.1. Analysis of Variance
3.2.2. Interpretation of the 3D Surface Data and Interaction Curves
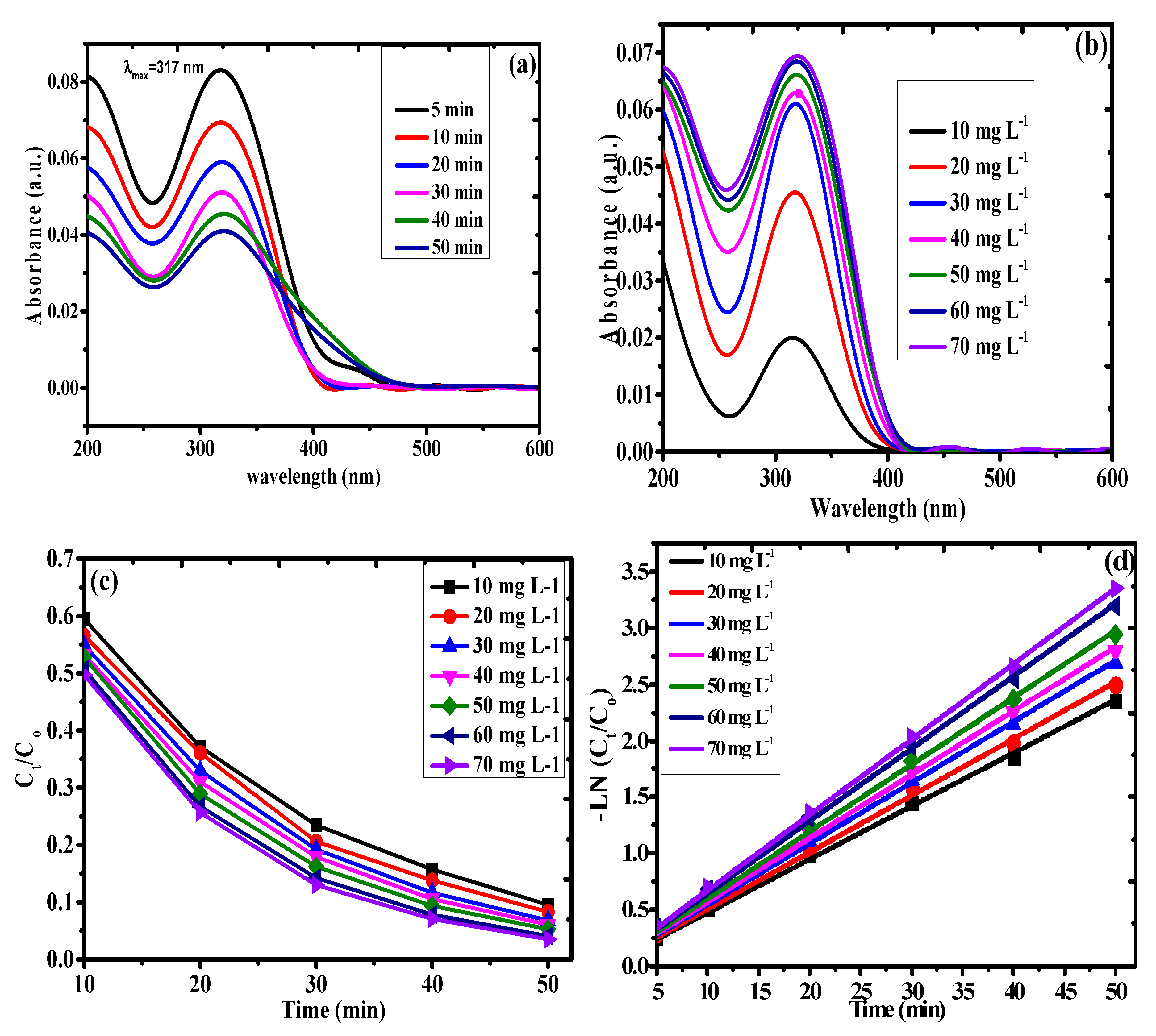
3.3. Kinetics of Photodegradation
3.4. Photocatalytic Activity of PAN-g-Alg@Ag NC and Its Individual Entities
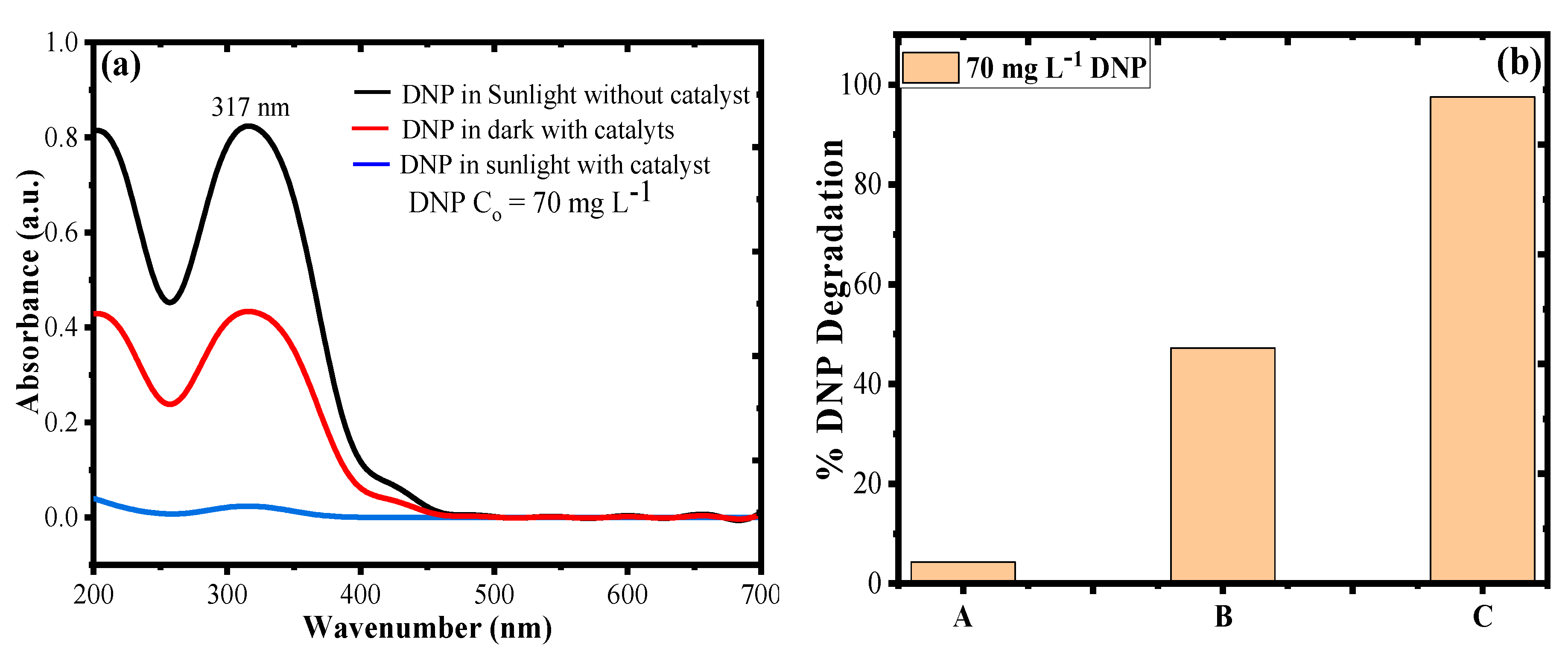
3.5. Photocatalytic Degradation of DNP in Dark and Sunlight
3.6. Effect of Electrolyte Concentration on DNP Degradation
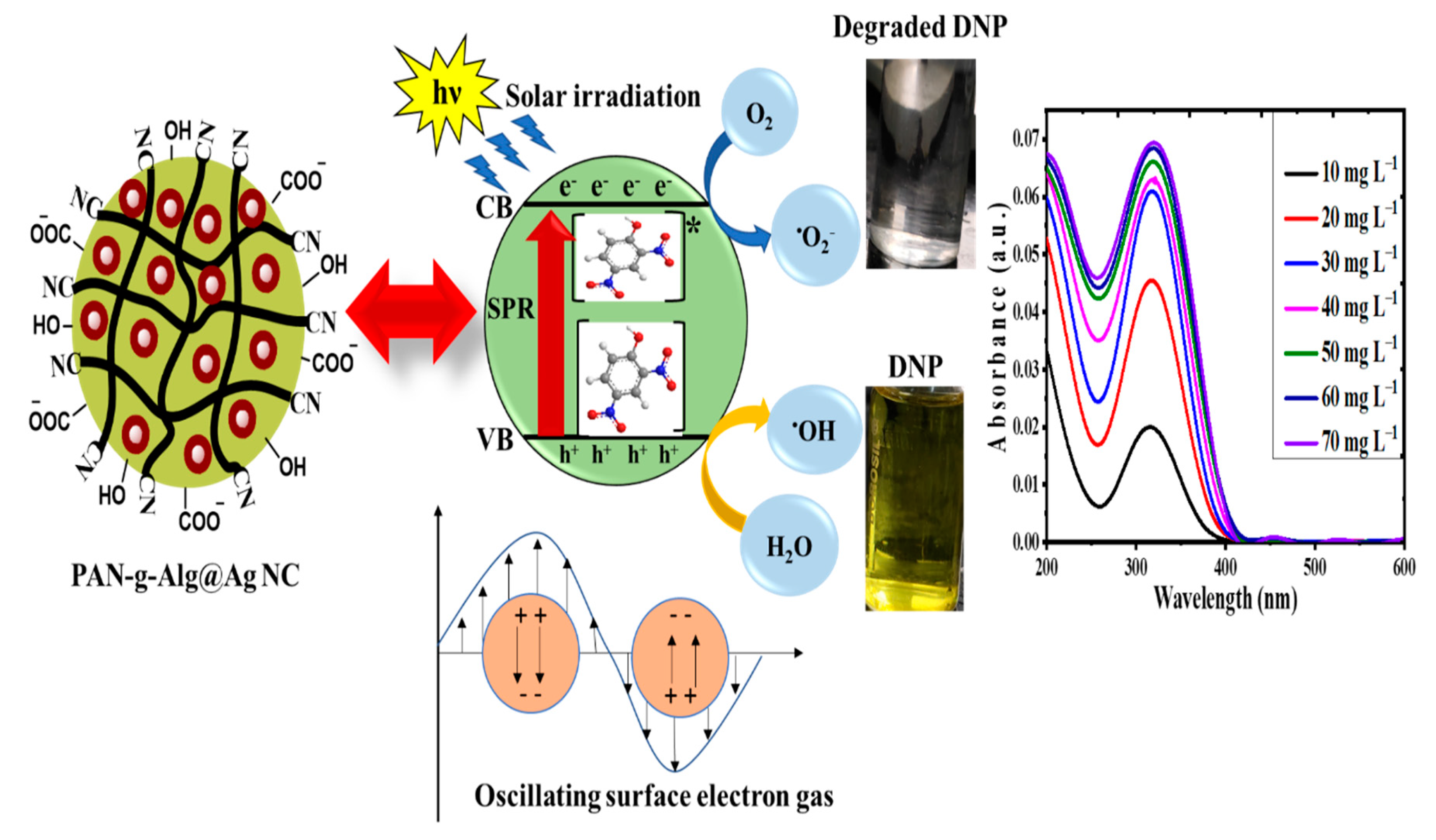
3.7. The Mechanism of Photodegradation
3.8. Regeneration and Reusability of PAN-g-Alg@Ag NC and Alg@Ag NPs
3.9. Comparison with Literature
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Alg | Alginate |
| PAN | Poly(acrylonitrile) |
| Alg@Ag NPs | Alginate functionalized Ag nanoparticles |
| DNP | 2, 4-Dinitrophenol |
| FTIR | Fourier-transform Infrared |
| SEM | Scanning Electron Microscope |
| TEM | Transmission Electron Microscope |
| XRD | X-ray Diffraction |
| EDX | Energy-dispersive X-ray spectroscopy |
| DSC | Differential Scanning Calorimetry |
| APS | Ammonium Persulfate |
| FAS | Ferrous Ammonium Sulfate |
References
- Bilal, M.; Adeel, M.; Rasheed, T.; Zhao, Y.; Iqbal, H.M.N. Emerging Contaminants of High Concern and Their Enzyme-Assisted Biodegradation—A Review. Environ. Int. 2019, 124, 336–353. [Google Scholar] [CrossRef]
- Rahmani, H.; Lakzian, A.; Karimi, A.; Halajnia, A. Efficient Removal of 2,4-Dinitrophenol from Synthetic Wastewater and Contaminated Soil Samples Using Free and Immobilized Laccases. J. Environ. Manag. 2020, 256, 109740. [Google Scholar] [CrossRef]
- Ahmad, R.; Hasan, I. Efficient Remediation of an Aquatic Environment Contaminated by Cr(VI) and 2,4-Dinitrophenol by XG-g-Polyaniline@ZnO Nanocomposite. J. Chem. Eng. Data 2017, 62, 1594–1607. [Google Scholar] [CrossRef]
- Zaharia, M.; Mihai, M.; Roman, T.; Zbancioc, G.; Pui, A.; Gradinaru, R.V.; Logigan, C.; Drochioiu, G. Unusual Ferrite Induced Photohydrolysis of Dinitrophenols to Nonaromatic and Nontoxic Derivatives. J. Photochem. Photobiol. A Chem. 2020, 394, 112497. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, S.; Kang, D.E.; Shin, J.Y.; Suh, H.; Kim, I. Synthesis of Multi-Amine Functionalized Hydrogel for Preparation of Noble Metal Nanoparticles: Utilization as Highly Active and Recyclable Catalysts in Reduction of Nitroaromatics. RSC Adv. 2013, 3, 4692–4703. [Google Scholar] [CrossRef]
- MirzaHedayat, B.; Noorisepehr, M.; Dehghanifard, E.; Esrafili, A.; Norozi, R. Evaluation of Photocatalytic Degradation of 2,4-Dinitrophenol from Synthetic Wastewater Using Fe3O4@SiO2@TiO2/R.GO Magnetic Nanoparticles. J. Mol. Liq. 2018, 264, 571–578. [Google Scholar]
- Hinrichs, R.Z.; Buczek, P.; Trivedi, J.J. Solar Absorption by Aerosol-Bound Nitrophenols Compared to Aqueous and Gaseous Nitrophenols. Environ. Sci. Technol. 2016, 50, 5661–5667. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Y.; Xia, X.; Wang, L. Popcorn Balls-like ZnFe2O4–ZrO2 Microsphere for Photocatalytic Degradation of 2,4-Dinitrophenol. Appl. Surf. Sci. 2017, 407, 470–478. [Google Scholar] [CrossRef]
- Wahid, A.; Asiri, A.M.; Rahman, M.M. One-Step Facile Synthesis of Nd2O3/ZnO Nanostructures for an Efficient Selective 2,4-Dinitrophenol Sensor Probe. Appl. Surf. Sci. 2019, 487, 1253–1261. [Google Scholar]
- Marimuthu, S.; Antonisamy, A.J.; Malayandi, S.; Rajendran, K.; Tsai, P.C.; Pugazhendhi, A.; Ponnusamy, V.K. Silver Nanoparticles in Dye Effluent Treatment: A Review on Synthesis, Treatment Methods, Mechanisms, Photocatalytic Degradation, Toxic Effects and Mitigation of Toxicity. J. Photochem. Photobiol. B Biol. 2020, 205, 111823. [Google Scholar] [CrossRef]
- Nariya, P.; Das, M.; Shukla, F.; Thakore, S. Synthesis of Magnetic Silver Cyclodextrin Nanocomposite as Catalyst for Reduction of Nitro Aromatics and Organic Dyes. J. Mol. Liq. 2020, 300, 112279. [Google Scholar] [CrossRef]
- Hasija, V.; Sudhaik, A.; Raizada, P.; Hosseini-Bandegharaei, A.; Singh, P. Carbon Quantum Dots Supported AgI/ZnO/Phosphorus Doped Graphitic Carbon Nitride as Z-Scheme Photocatalyst for Efficient Photodegradation of 2, 4-Dinitrophenol. J. Environ. Chem. Eng. 2019, 7, 103272. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Liu, C.; Ding, Y.; Zhang, S.; Zeng, Y.; Liu, Y.; Luo, S. A Bamboo-Inspired Hierarchical Nanoarchitecture of Ag/CuO/TiO2 Nanotube Array for Highly Photocatalytic Degradation of 2,4-Dinitrophenol. J. Hazard. Mater. 2016, 313, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Fiorenza, R.; Di Mauro, A.; Cantarella, M.; Privitera, V.; Impellizzeri, G. Selective Photodegradation of 2,4-D Pesticide from Water by Molecularly Imprinted TiO 2. J. Photochem. Photobiol. A Chem. 2019, 380, 111872. [Google Scholar] [CrossRef]
- Singh, P.; Sudhaik, A.; Raizada, P.; Shandilya, P.; Sharma, R.; Hosseini-Bandegharaei, A. Photocatalytic Performance and Quick Recovery of BiOI/Fe3O4 @graphene Oxide Ternary Photocatalyst for Photodegradation of 2,4-Dintirophenol under Visible Light. Mater. Today Chem. 2019, 12, 85–95. [Google Scholar] [CrossRef]
- Lamba, R.; Umar, A.; Mehta, S.K.; Kumar Kansal, S. Well-Crystalline Porous ZnO–SnO2 Nanosheets: An Effective Visible-Light Driven Photocatalyst and Highly Sensitive Smart Sensor Material. Talanta 2015, 131, 490–498. [Google Scholar] [CrossRef]
- Raizada, P.; Sudhaik, A.; Singh, P.; Shandilya, P.; Thakur, P.; Jung, H. Visible Light Assisted Photodegradation of 2,4-Dinitrophenol Using Ag2CO3 Loaded Phosphorus and Sulphur Co-Doped Graphitic Carbon Nitride Nanosheets in Simulated Wastewater. Arab. J. Chem. 2020, 13, 3196–3209. [Google Scholar] [CrossRef]
- Ganapathy Selvam, G.; Sivakumar, K. Phycosynthesis of Silver Nanoparticles and Photocatalytic Degradation of Methyl Orange Dye Using Silver (Ag) Nanoparticles Synthesized from Hypnea Musciformis (Wulfen) J.V. Lamouroux. Appl. Nanosci. 2015, 5, 617–622. [Google Scholar] [CrossRef] [Green Version]
- Jana, J.; Ganguly, M.; Pal, T. Enlightening Surface Plasmon Resonance Effect of Metal Nanoparticles for Practical Spectroscopic Application. RSC Adv. 2016, 6, 86174–86211. [Google Scholar] [CrossRef]
- Huang, S.; Xiao, Z.; Zhai, S.; Zhai, B.; Zhang, F.; An, Q. Fabrication of Highly-Stable Ag/CA@GTA Hydrogel Beads and Their Catalytic Application. RSC Adv. 2014, 4, 60460–60466. [Google Scholar] [CrossRef]
- Paques, J.P.; Van Der Linden, E.; Van Rijn, C.J.M.; Sagis, L.M.C. Preparation Methods of Alginate Nanoparticles. Adv. Colloid Interface Sci. 2014, 209, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, A.; Sharma, S.; Khuller, G.K. Inhalable Alginate Nanoparticles as Antitubercular Drug Carriers against Experimental Tuberculosis. Int. J. Antimicrob. Agents 2005, 26, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Nasser, W.S.; Osman, T.A.; Toprak, M.S.; Muhammed, M.; Uheida, A. Surface Modified of Polyacrylonitrile Nanofibers by TiO2/MWCNT for Photodegradation of Organic Dyes and Pharmaceutical Drugs under Visible Light Irradiation. Environ. Res. 2019, 179, 108788. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Hasan, I. Optimization of the Adsorption of Pb (II) from Aqueous Solution onto PAB Nanocomposite Using Response Surface Methodology. Environ. Nanotechnol. Monit. Manag. 2016, 6, 116–129. [Google Scholar] [CrossRef]
- Yang, H.Y.; Liu, J.; Wang, Y.X.; He, C.S.; Zhang, L.S.; Mu, Y.; Li, W.H. Bioelectrochemical Decolorization of a Reactive Diazo Dye: Kinetics, Optimization with a Response Surface Methodology, and Proposed Degradation Pathway. Bioelectrochemistry 2019, 128, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Samarbaf, S.; Tahmasebi Birgani, Y.; Yazdani, M.; Babaei, A.A. A Comparative Removal of Two Dyes from Aqueous Solution Using Modified Oak Waste Residues: Process Optimization Using Response Surface Methodology. J. Ind. Eng. Chem. 2019, 73, 67–77. [Google Scholar] [CrossRef]
- Hasan, I.; Bhatia, D.; Walia, S.; Singh, P. Removal of Malachite Green by Polyacrylamide-g-Chitosan γ-Fe2O3 Nanocomposite-an Application of Central Composite Design. Groundw. Sustain. Dev. 2020, 11, 100378. [Google Scholar] [CrossRef]
- Hasan, I.; Khan, R.A.; Alharbi, W.; Alharbi, K.H.; Abu Khanjer, M.; Alslame, A. Synthesis, Characterization and Photo-Catalytic Activity of Guar–Gum–:G–Aliginate@silver Bionanocomposite Material. RSC Adv. 2020, 10, 7898–7911. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.P.; Sharma, A.S.; Sharma, V.S.; Shimpi, N.G. Polyacrylonitrile Nanofibers Incorporating Silver-Decorated Graphitic Carbon Nitride for the Visible-Light-Activated Selective Oxidation of Styrene, Benzylic Methylene Groups, and Benzene. ACS Appl. Nano Mater. 2020, 3, 1922–1933. [Google Scholar] [CrossRef]
- Voo, W.P.; Lee, B.B.; Idris, A.; Islam, A.; Tey, B.T.; Chan, E.S. Production of Ultra-High Concentration Calcium Alginate Beads with Prolonged Dissolution Profile. RSC Adv. 2015, 5, 36687–36695. [Google Scholar] [CrossRef]
- Bhagyaraj, S.; Krupa, I. Alginate-Mediated Synthesis of Hetero-Shaped Silver Nanoparticles and Their Hydrogen Peroxide Sensing Ability. Molecules 2020, 25, 435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Lu, Z.; Wu, M.; Wu, Q.; Yang, J. Large-Scale Synthesis and Characterization of Magnetic Poly (Acrylic Acid) Nanogels via Miniemulsion Polymerization. RSC Adv. 2015, 5, 58889–58894. [Google Scholar] [CrossRef]
- Hebeish, A.A.; Ramadan, M.A.; Montaser, A.S.; Farag, A.M. Preparation, Characterization and Antibacterial Activity of Chitosan-g-Poly Acrylonitrile/Silver Nanocomposite. Int. J. Biol. Macromol. 2014, 68, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Rehan, M.; Nada, A.A.; Khattab, T.A.; Abdelwahed, N.A.M.; El-Kheir, A.A.A. Development of Multifunctional Polyacrylonitrile/Silver Nanocomposite Films: Antimicrobial Activity, Catalytic Activity, Electrical Conductivity, UV Protection and SERS-Active Sensor. J. Mater. Res. Technol. 2020, 9, 9380–9394. [Google Scholar] [CrossRef]
- Venkatesan, J.; Lee, J.Y.; Kang, D.S.; Anil, S.; Kim, S.K.; Shim, M.S.; Kim, D.G. Antimicrobial and Anticancer Activities of Porous Chitosan-Alginate Biosynthesized Silver Nanoparticles. Int. J. Biol. Macromol. 2017, 98, 515–525. [Google Scholar] [CrossRef]
- Scherrer, P. Estimation of the Size and Internal Structure of Colloidal Particles by Means of Rontgen Rays. Nachrichten von der Gesellschaft der Wissenschaften zu Göttingen 1918, 26, 98–100. [Google Scholar]
- Mehmood, A.; Murtaza, G.; Bhatti, T.M.; Kausar, R. Phyto-Mediated Synthesis of Silver Nanoparticles from Melia Azedarach, L. Leaf Extract: Characterization and Antibacterial Activity. Arab. J. Chem. 2017, 10, S3048–S3053. [Google Scholar] [CrossRef] [Green Version]
- Soltani, I.; Hraiech, S.; Horchani-Naifer, K.; Elhouichet, H.; Gelloz, B.; Férid, M. Growth of Silver Nanoparticles Stimulate Spectroscopic Properties of Er3+ Doped Phosphate Glasses: Heat Treatment Effect. J. Alloy. Compd. 2016, 686, 556–563. [Google Scholar] [CrossRef]
- Ghanavati Nasab, S.; Semnani, A.; Karimi, M.; Javaheran Yazd, M.; Cheshmekhezr, S. Synthesis of Ion-Imprinted Polymer-Decorated SBA-15 as a Selective and Efficient System for the Removal and Extraction of Cu (II) with Focus on Optimization by Response Surface Methodology. Analyst 2019, 144, 4596–4612. [Google Scholar] [CrossRef]
- Anfar, Z.; El Haouti, R.; Lhanafi, S.; Benafqir, M.; Azougarh, Y.; El Alem, N. Treated Digested Residue during Anaerobic Co-Digestion of Agri-Food Organic Waste: Methylene Blue Adsorption, Mechanism and CCD-RSM Design. J. Environ. Chem. Eng. 2017, 5, 5857–5867. [Google Scholar] [CrossRef]
- Wang, Z.; Ge, H.; Wang, X.; Ye, C.; Fan, S. Mono and Co-Immobilization of Imidazolium Ionic Liquids on Silica: Effects of the Substituted Groups on the Adsorption Behavior of 2,4-Dinitrophenol. RSC Adv. 2019, 9, 32425–32434. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Lai, C.; Huang, D.; Zeng, G.; Chen, L.; Qin, L.; Xu, P.; Cheng, M.; Huang, C.; Zhang, C.; et al. Preparation of Water-Compatible Molecularly Imprinted Thiol-Functionalized Activated Titanium Dioxide: Selective Adsorption and Efficient Photodegradation of 2, 4-Dinitrophenol in Aqueous Solution. J. Hazard. Mater. 2018, 346, 113–123. [Google Scholar] [PubMed]
- Shandilya, P.; Mittal, D.; Sudhaik, A.; Soni, M.; Raizada, P.; Saini, A.K.; Singh, P. GdVO4 Modified Fluorine Doped Graphene Nanosheets as Dispersed Photocatalyst for Mitigation of Phenolic Compounds in Aqueous Environment and Bacterial Disinfection. Sep. Purif. Technol. 2019, 210, 804–816. [Google Scholar] [CrossRef]
- Park, S.J.; Das, G.S.; Schütt, F.; Adelung, R.; Mishra, Y.K.; Tripathi, K.M.; Kim, T.Y. Visible-Light Photocatalysis by Carbon-Nano-Onion-Functionalized ZnO Tetrapods: Degradation of 2,4-Dinitrophenol and a Plant-Model-Based Ecological Assessment. NPG Asia Mater. 2019, 11, 1–13. [Google Scholar] [CrossRef]
- García Einschlag, F.S.; Lopez, J.; Carlos, L.; Capparelli, A.L.; Braun, A.M.; Oliveros, E. Evaluation of the Efficiency of Photodegradation of Nitroaromatics Applying the UV/H2O2 Technique. Environ. Sci. Technol. 2002, 36, 3936–3944. [Google Scholar] [CrossRef]
- Mavaei, M.; Chahardoli, A.; Shokoohinia, Y.; Khoshroo, A.; Fattahi, A. One-Step Synthesized Silver Nanoparticles Using Isoimperatorin: Evaluation of Photocatalytic, and Electrochemical Activities. Sci. Rep. 2020. [Google Scholar] [CrossRef] [Green Version]
- Mangalam, J.; Kumar, M.; Sharma, M.; Joshi, M. High Adsorptivity and Visible Light Assisted Photocatalytic Activity of Silver/Reduced Graphene Oxide (Ag/RGO) Nanocomposite for Wastewater Treatment. Nano Struct. Nano Objects 2019, 17, 58–66. [Google Scholar] [CrossRef]
- Roushani, M.; Mavaei, M.; Rajabi, H.R. Graphene Quantum Dots as Novel and Green Nano-Materials for the Visible-Light-Driven Photocatalytic Degradation of Cationic Dye. J. Mol. Catal. A Chem. 2015, 409, 102–109. [Google Scholar] [CrossRef]
- Silva, I.M.P.; Byzynski, G.; Ribeiro, C.; Longo, E. Different Dye Degradation Mechanisms for ZnO and ZnO Doped with N (ZnO:N). J. Mol. Catal. A Chem. 2016, 417, 89–100. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.Y.; Qi, H.X.; Ning, J.J.; Wang, J.F.; Ren, Z.G.; Lang, J.P. One silver(I)/tetraphosphine coordination polymer showing good catalytic performance in the photodegradation of nitroaromatics in aqueous solution. Appl. Catal. B Environ. 2015, 168, 98–104. [Google Scholar]
- Ma, J.; Zhang, L.Z.; Wang, Y.H.; Lei, S.L.; Luo, X.B.; Chen, S.H.; Zeng, G.S.; Zou, J.P.; Luo, S.L.; Au, C.T. Mechanism of 2,4-Dinitrophenol Photocatalytic Degradation by ζ-Bi2O3/Bi2MoO6 Composites under Solar and Visible Light Irradiation. Chem. Eng. J. 2014, 251, 371–380. [Google Scholar] [CrossRef]

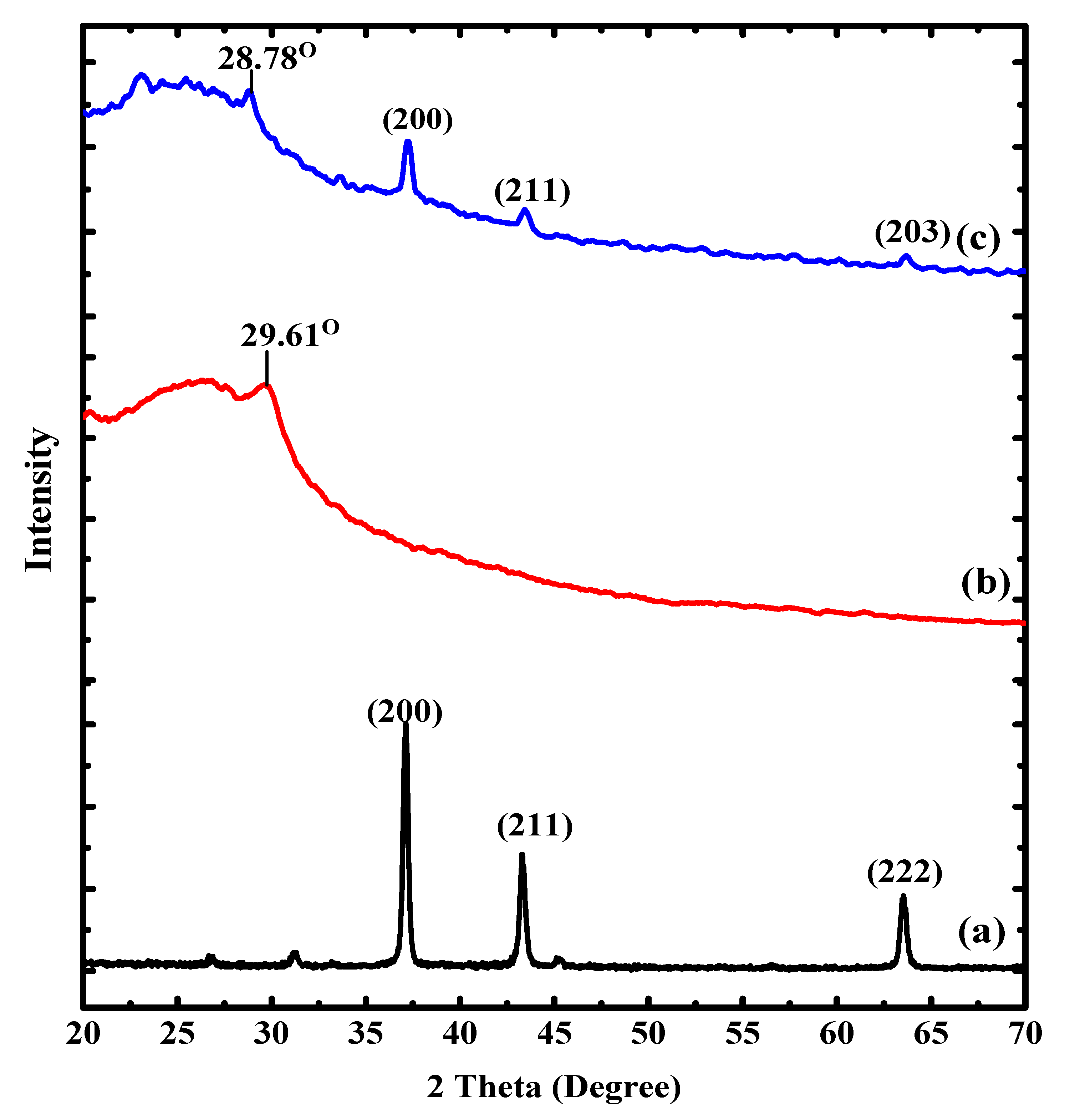
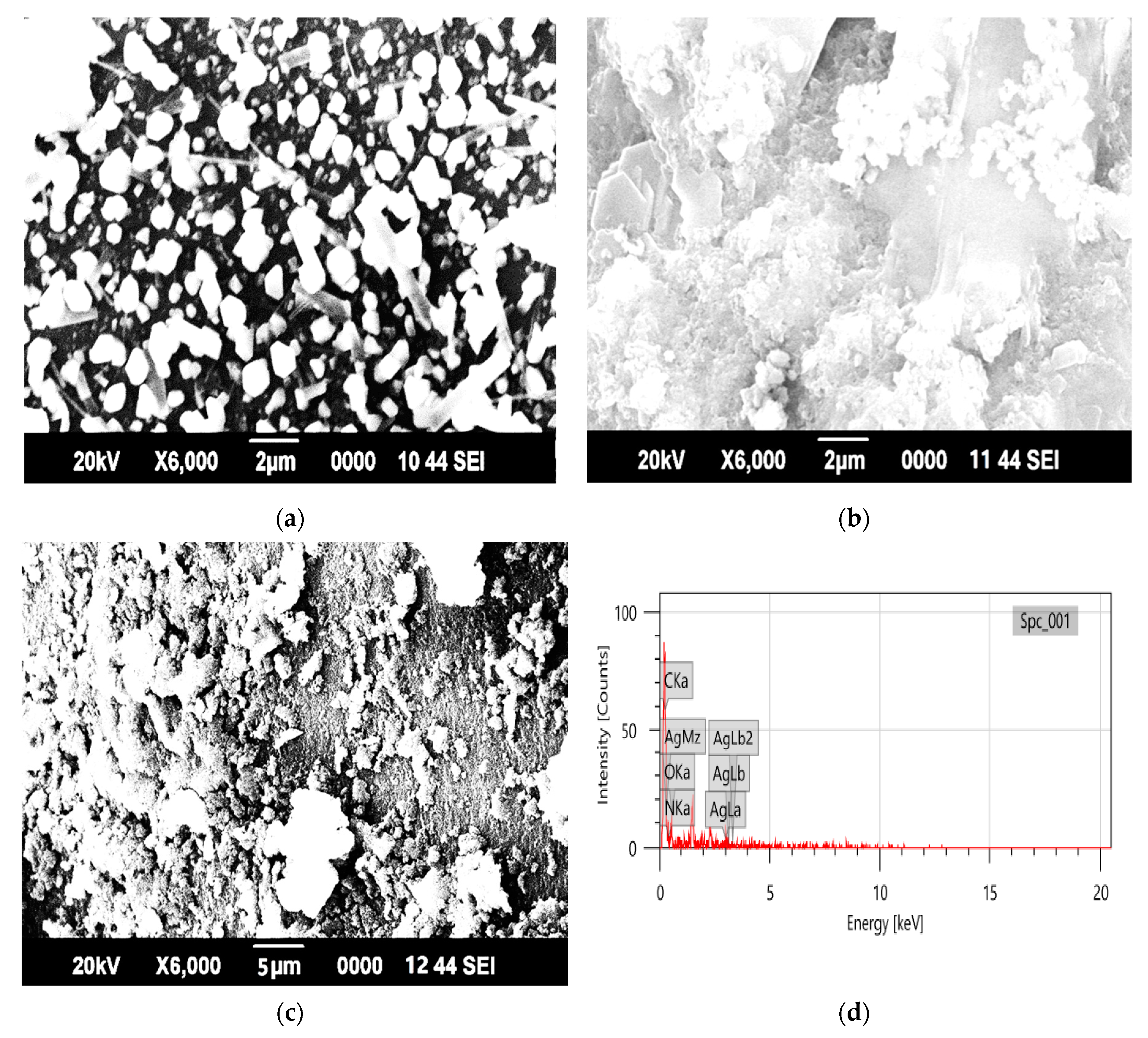
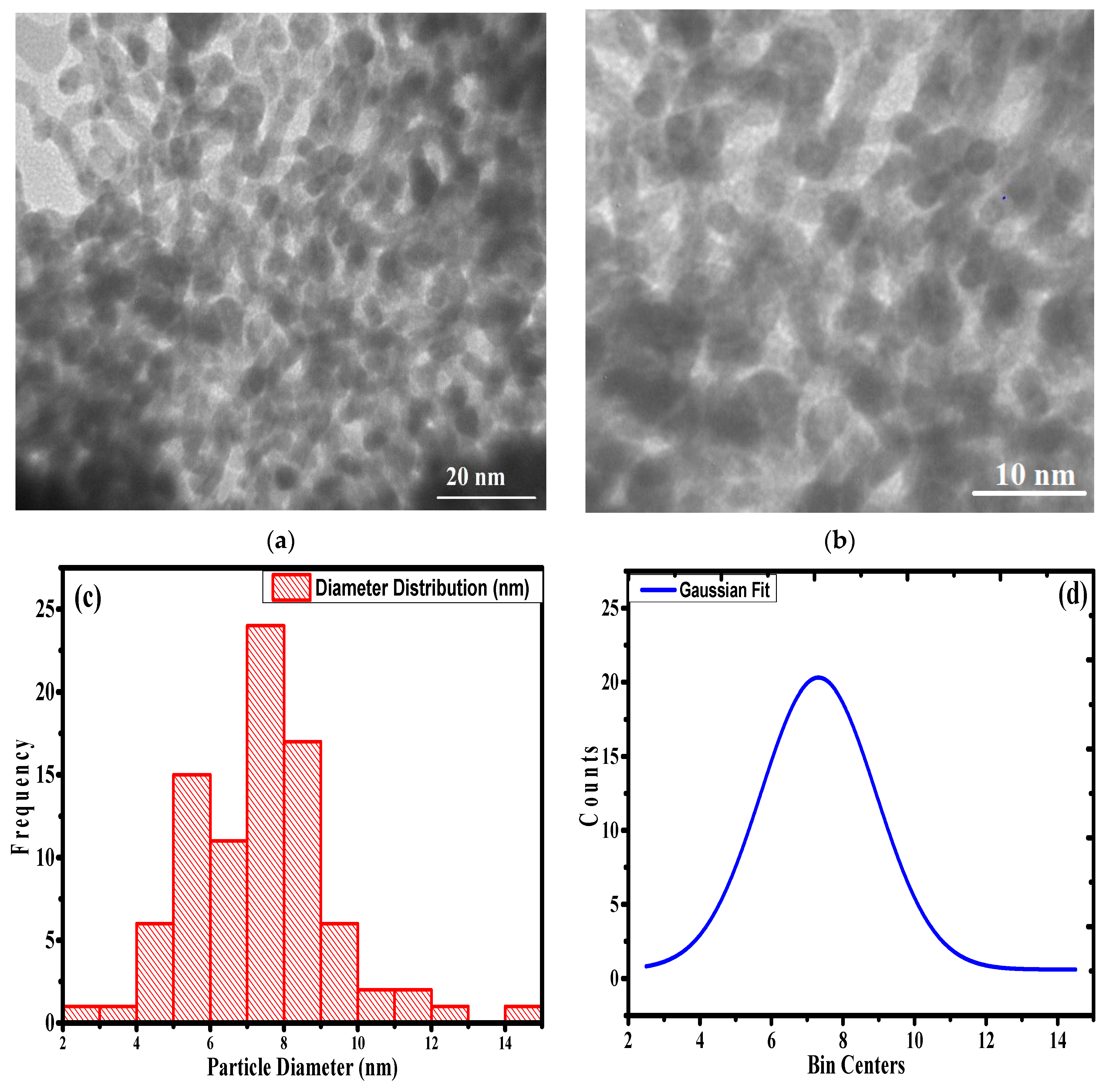
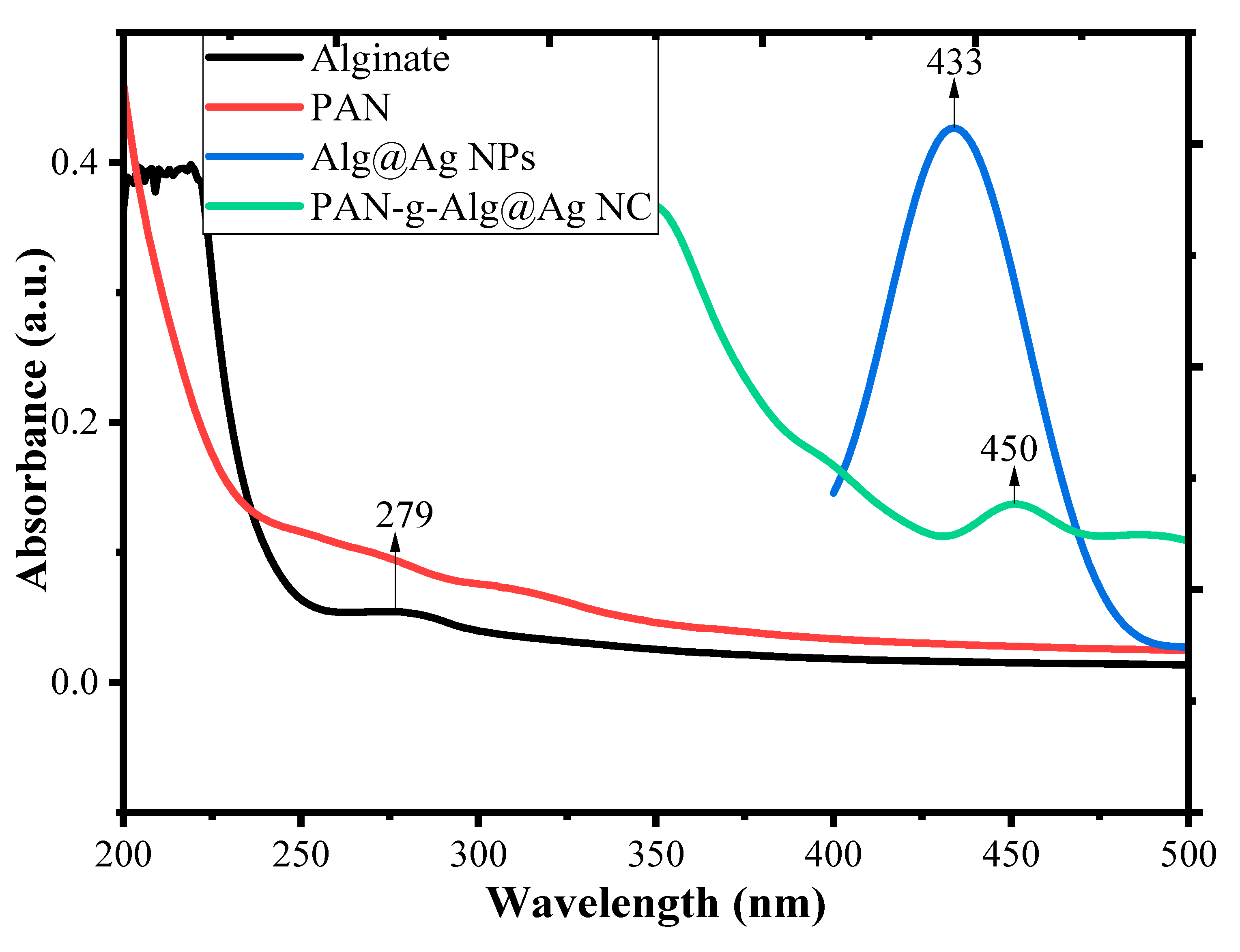


| Component | 2θ | FWHM (βhkl) | Interlayer Spacing (d200) (A°) | Size of Crystal (nm) at (200) | Dislocation Density (δ) × 1015 Lines (m−2) | % Crystallinity (%) |
|---|---|---|---|---|---|---|
| Alg@Ag NPs | 37.11 | 0.33 | 0.26 | 25.39 | 1.55 | 52 |
| PAN-g-Alg@Ag NC | 37.24 | 0.48 | 0.24 | 17.48 | 3.27 | 19 |
| Element | Mass % | Atom % | Binding Energy (KeV) |
|---|---|---|---|
| C | 24.03 | 29.13 | 0.26–0.29 |
| N | 36.21 | 37.64 | 0.44–0.46 |
| O | 35.95 | 32.72 | 0.52–0.54 |
| Ag | 3.80 | 0.51 | 3.00–3.20 |
| Source | DF | Adj SS | Adj MS | F Value | p > F Value |
|---|---|---|---|---|---|
| Model | 9 | 8.48 | 0.94 | 0.44 | 0.00 |
| A | 1 | 0.00 | 0.00 | 0.99 | 0.00 |
| B | 1 | 0.45 | 0.45 | 0.21 | 0.03 |
| C | 1 | 1.03 | 1.02 | 0.48 | 0.03 |
| A2 | 1 | 3.99 | 3.99 | 1.86 | 0.00 |
| B2 | 1 | 0.00 | 0.00 | 0.00 | 0.98 |
| C2 | 1 | 0.75 | 0.75 | 0.35 | 0.57 |
| A×B | 1 | 0.03 | 0.03 | 0.02 | 0.01 |
| A×C | 1 | 0.06 | 0.06 | 0.03 | 0.87 |
| B×C | 1 | 2.39 | 2.39 | 1.11 | 0.03 |
| Error | 10 | 21.51 | 2.15 |
| S.N. | Concentration (mg L−1) | Rate Constant (K) (min−1) | Half-Life (t1/2) (min) | R2 | SSE (×10−4) |
|---|---|---|---|---|---|
| 1 | 10 | 0.05 | 14.74 | 0.99 | 5.13 |
| 2 | 20 | 0.05 | 13.86 | 0.99 | 6.79 |
| 3 | 30 | 0.05 | 12.84 | 0.99 | 4.72 |
| 4 | 40 | 0.06 | 12.38 | 0.99 | 5.54 |
| 5 | 50 | 0.06 | 11.74 | 0.99 | 5.41 |
| 6 | 60 | 0.06 | 10.82 | 0.99 | 3.81 |
| 7 | 70 | 0.07 | 10.04 | 0.99 | 3.44 |
| Material/Photocatalyst | Irradiation Time (min) | pH | Kinetics | % Degradation of DNP | Reference |
|---|---|---|---|---|---|
| g-C3N4/AgI/ZnO/CQD | 120 min | 4 | Langmuir–Hinshelwood | 98% | [12] |
| Ag2CO3/PSGCN | 120 min | 4 | Pseudo-first order | 98% | [17] |
| CMIP-coated TiO2 | 240 min | 5 | Pseudo-first order | 76% | [43] |
| ZnFe2O4 | 15 min | 3 | Pseudo-first order | 82% | [4] |
| [Ag4(NO3)4(dpppda)]n | 300 min | 4 | Pseudo-zero order | 93% | [50] |
| ζ-Bi2O3/Bi2MoO6 | 100 min | 7 | Pseudo-first order | 86% | [51] |
| ZnFe2O4-ZrO2 | 60 min | 4 | Pseudo-first order | 90% | [8] |
| molecularly imprinted TiO2 | 240 min | 4 | Pseudo-first order | 74% | [14] |
| Ag/CuO/TiO2 | 80 min | 7 | Pseudo-first order | 99% | [13] |
| PAN–g–Alg@Ag | 35 min | 4.68 | Pseudo-first order | 99.46% | Present Study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, I.; Shekhar, C.; Alharbi, W.; Abu Khanjer, M.; Khan, R.A.; Alsalme, A. A Highly Efficient Ag Nanoparticle-Immobilized Alginate-g-Polyacrylonitrile Hybrid Photocatalyst for the Degradation of Nitrophenols. Polymers 2020, 12, 3049. https://doi.org/10.3390/polym12123049
Hasan I, Shekhar C, Alharbi W, Abu Khanjer M, Khan RA, Alsalme A. A Highly Efficient Ag Nanoparticle-Immobilized Alginate-g-Polyacrylonitrile Hybrid Photocatalyst for the Degradation of Nitrophenols. Polymers. 2020; 12(12):3049. https://doi.org/10.3390/polym12123049
Chicago/Turabian StyleHasan, Imran, Charu Shekhar, Walaa Alharbi, Maymonah Abu Khanjer, Rais Ahmad Khan, and Ali Alsalme. 2020. "A Highly Efficient Ag Nanoparticle-Immobilized Alginate-g-Polyacrylonitrile Hybrid Photocatalyst for the Degradation of Nitrophenols" Polymers 12, no. 12: 3049. https://doi.org/10.3390/polym12123049
APA StyleHasan, I., Shekhar, C., Alharbi, W., Abu Khanjer, M., Khan, R. A., & Alsalme, A. (2020). A Highly Efficient Ag Nanoparticle-Immobilized Alginate-g-Polyacrylonitrile Hybrid Photocatalyst for the Degradation of Nitrophenols. Polymers, 12(12), 3049. https://doi.org/10.3390/polym12123049






