New Insight into the Mechanism of Drug Release from Poly(d,l-lactide) Film by Electron Paramagnetic Resonance
Abstract
:1. Introduction
2. Materials and Methods
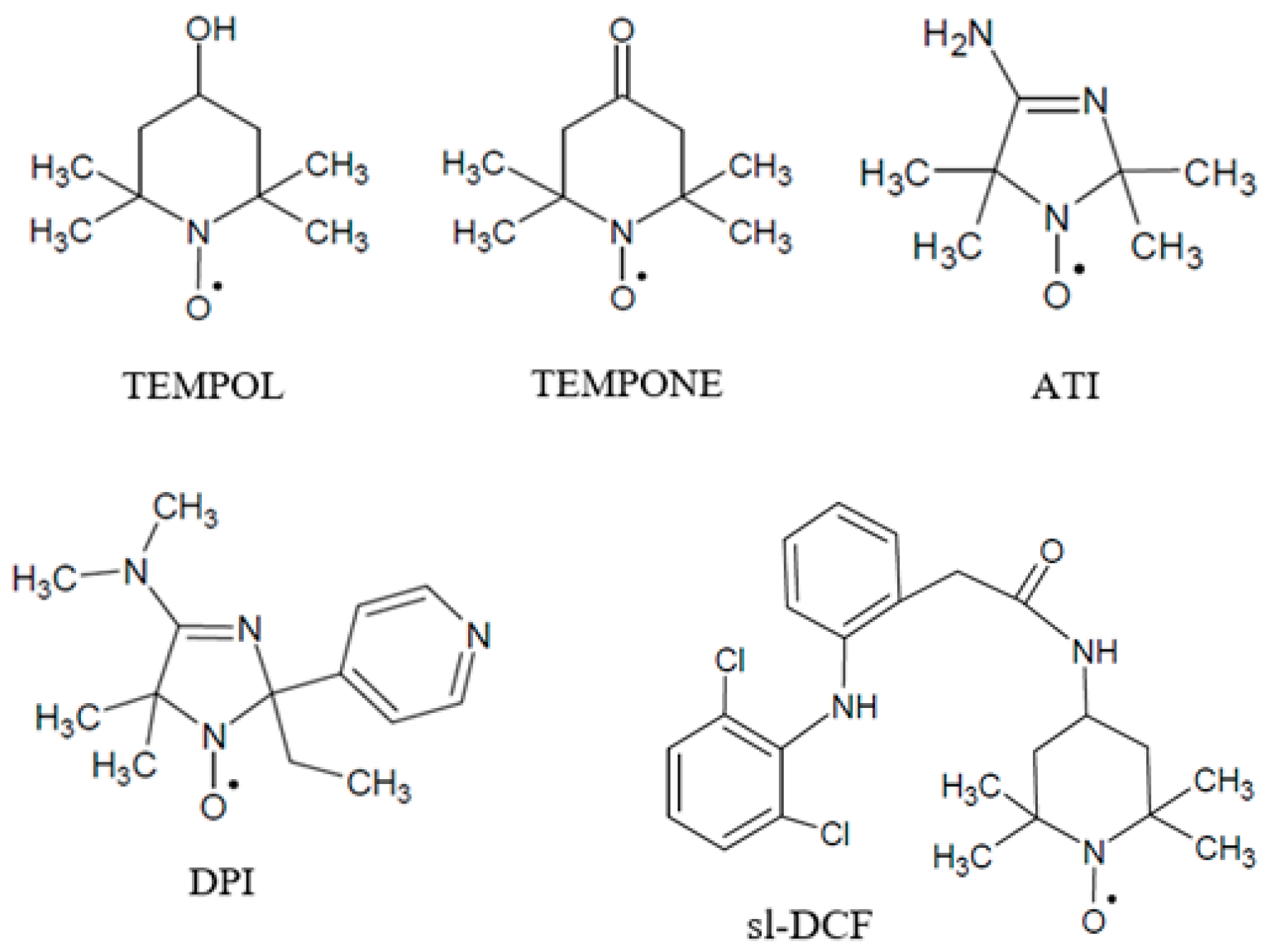
2.1. Materials and Substances
2.2. Samples Preparation
2.3. Release Experiments
2.4. EPR Spectroscopy
2.4.1. Spectra Recording
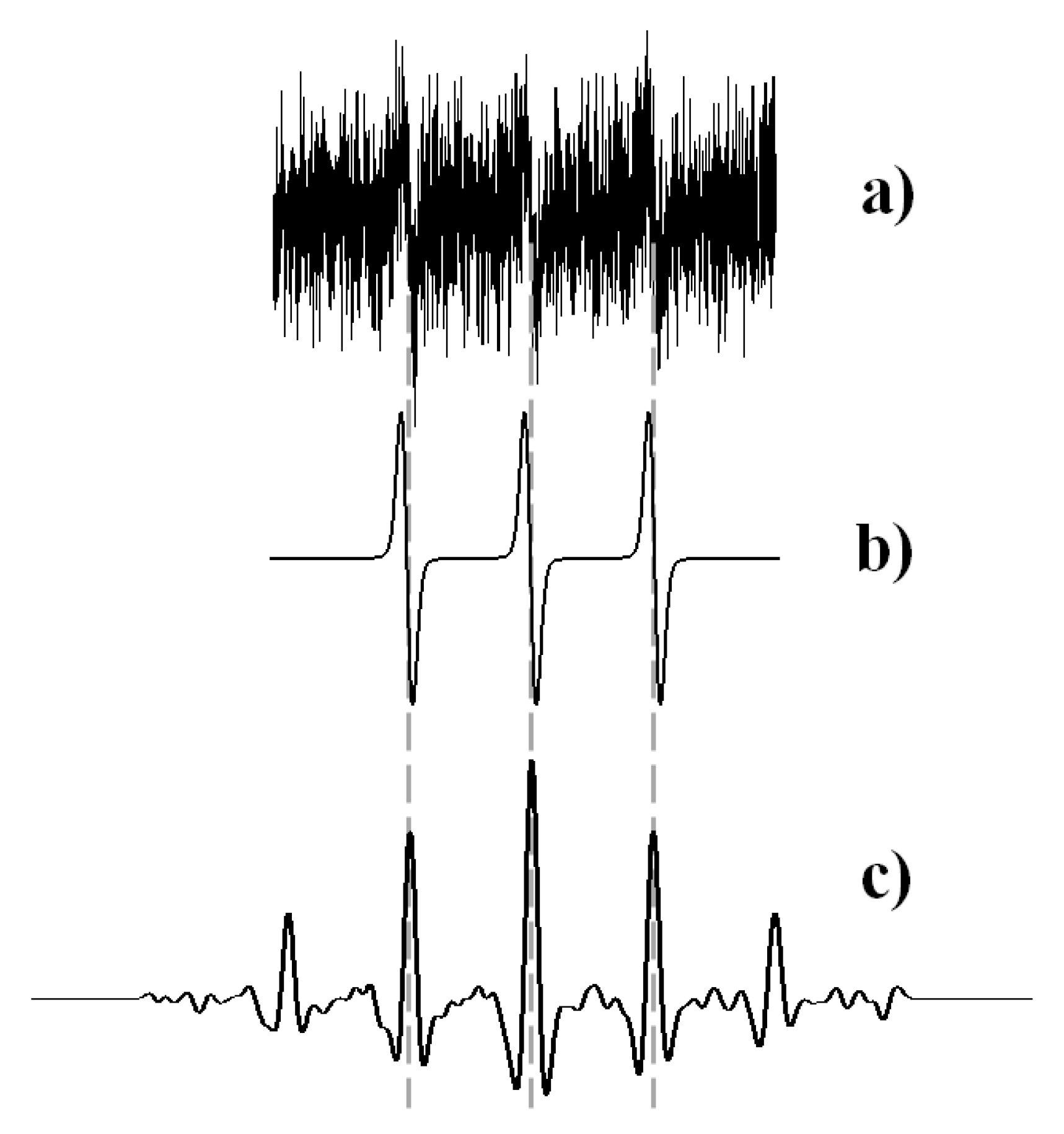
2.4.2. Numerical Analysis of EPR Spectra
3. Results and Discussion
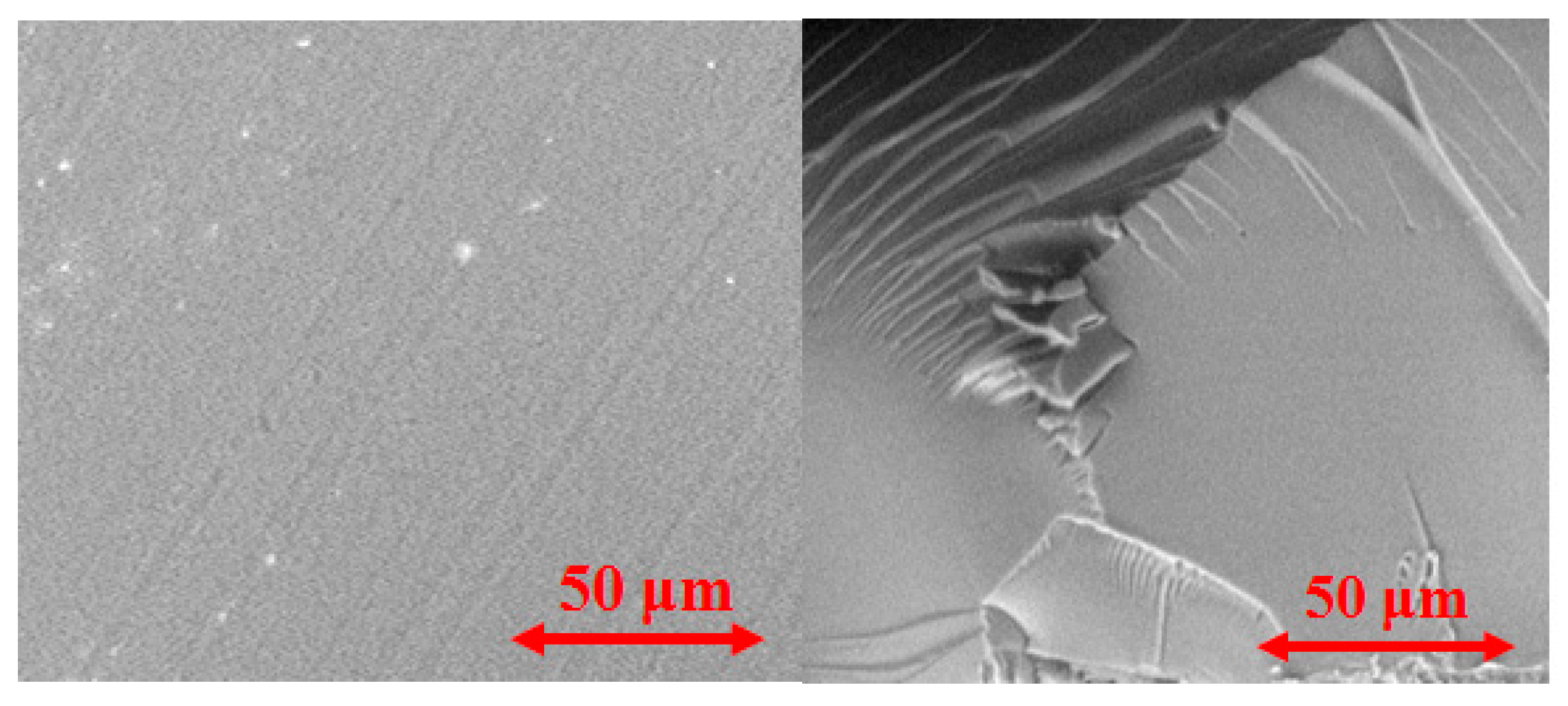
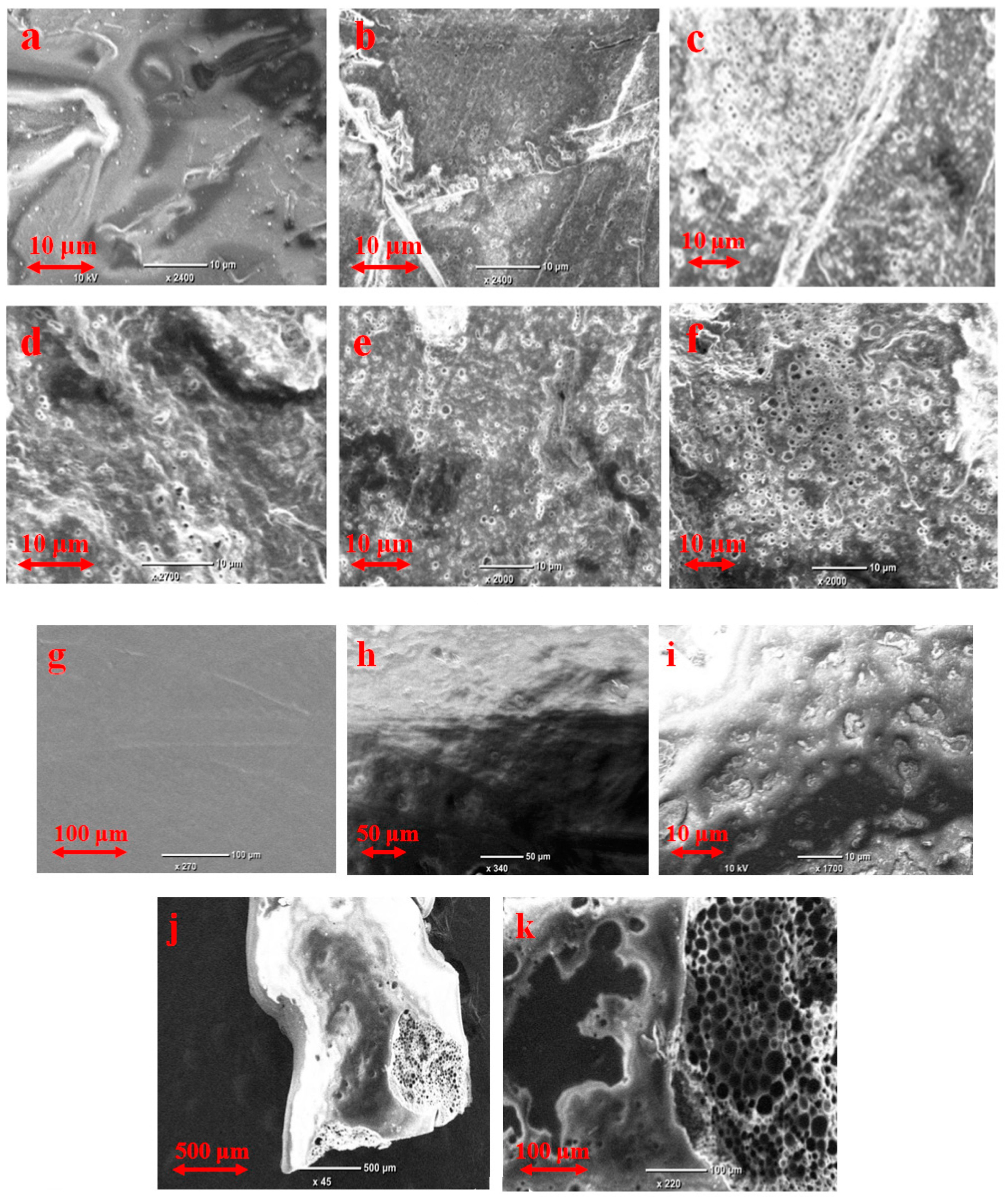
3.1. Physicochemical Characterization of the Samples
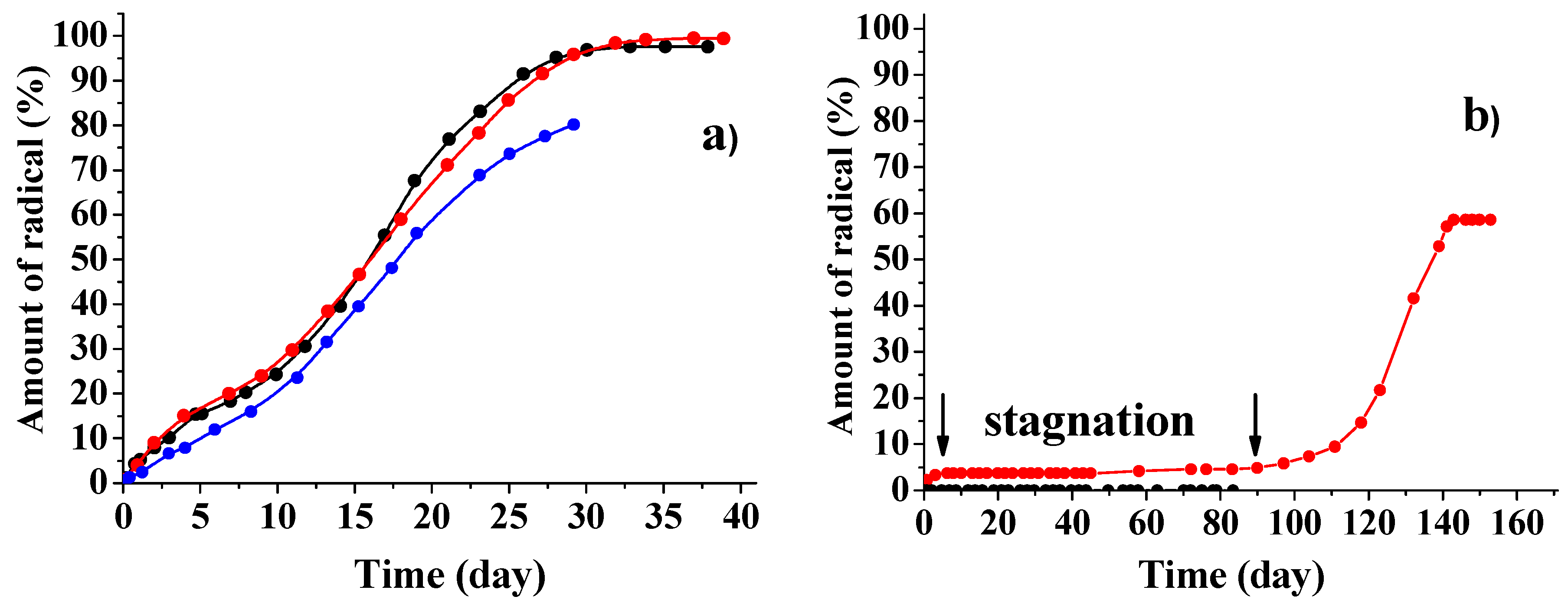
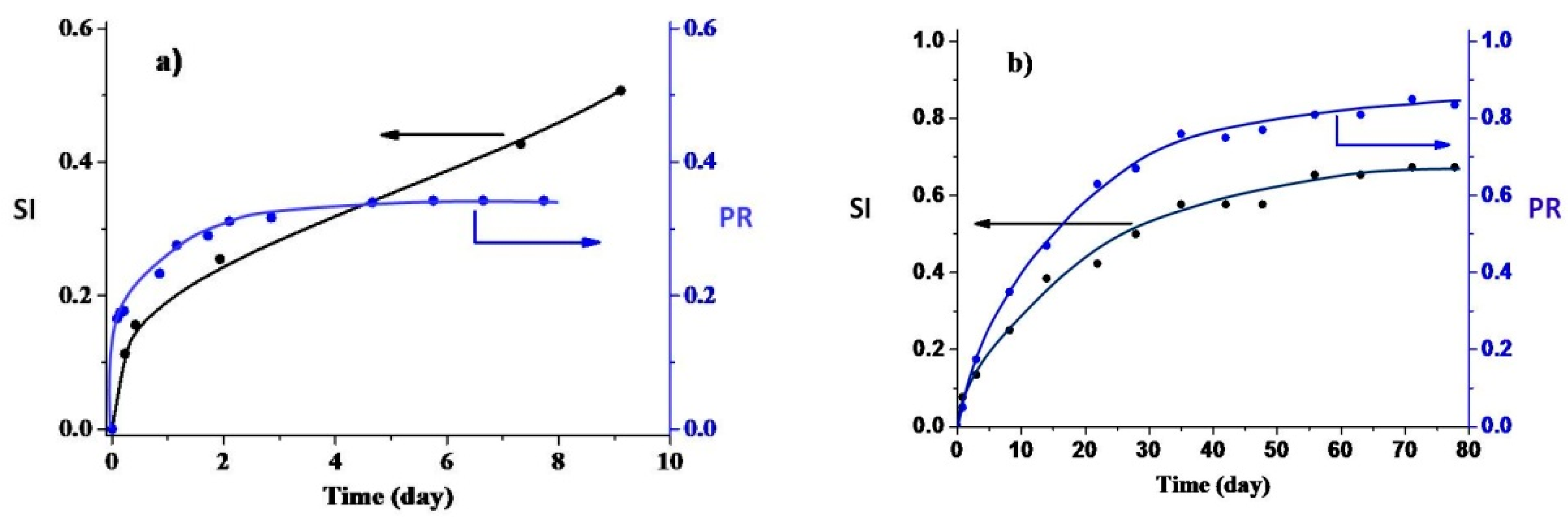
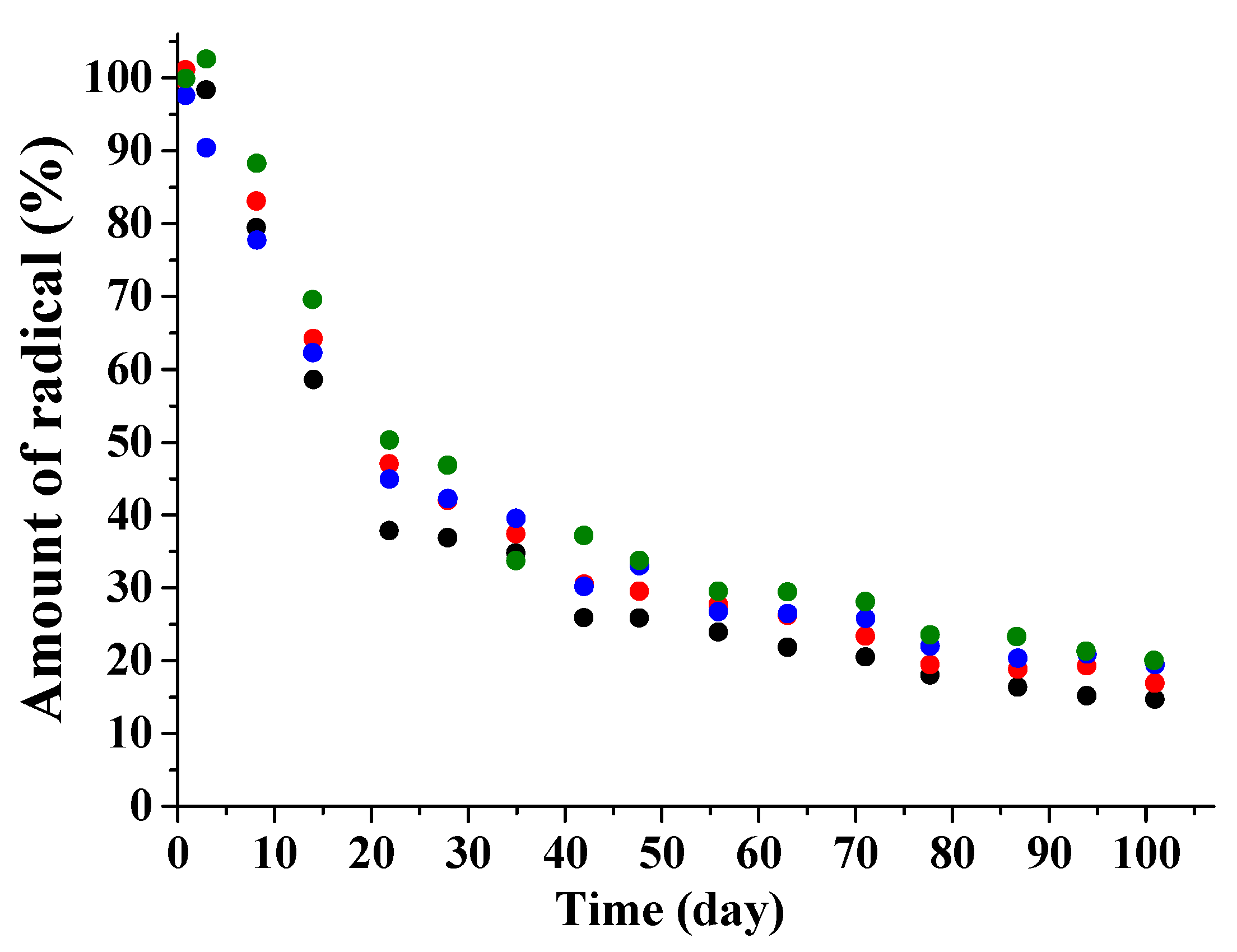
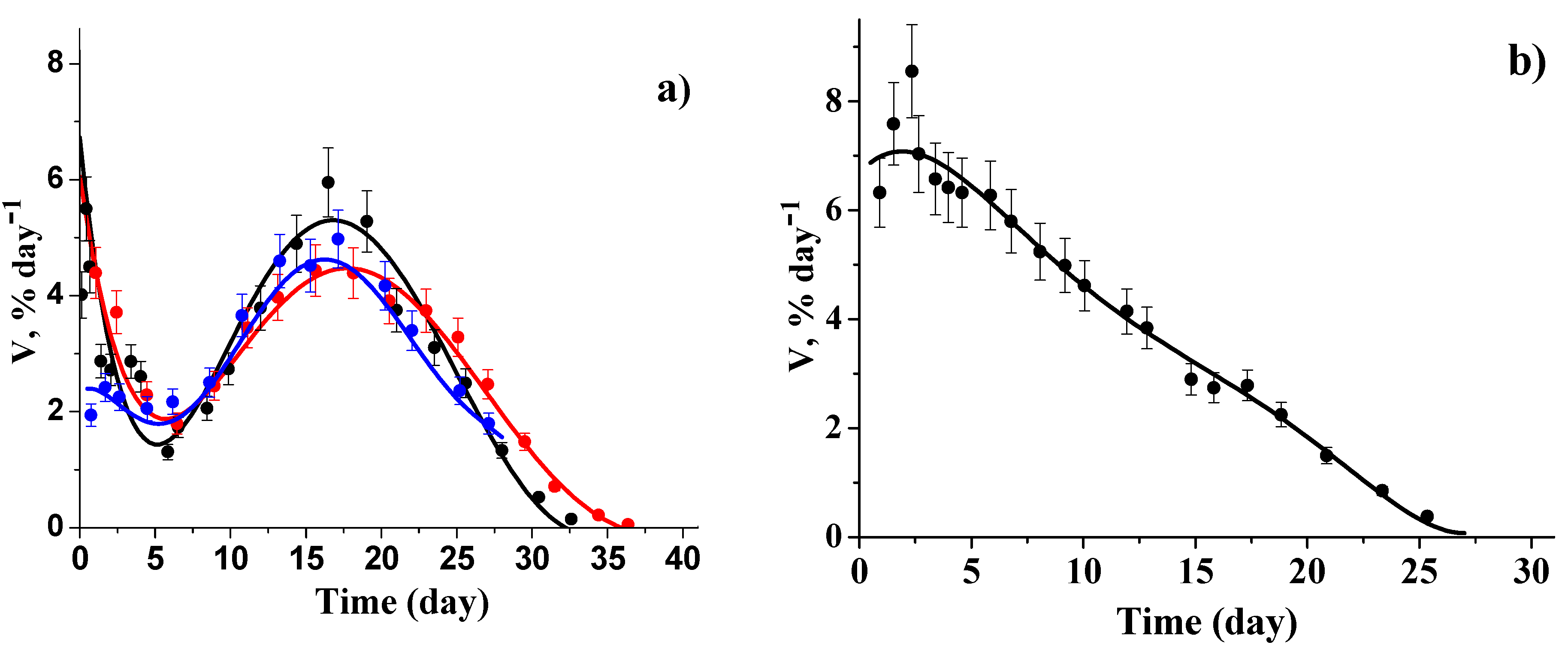
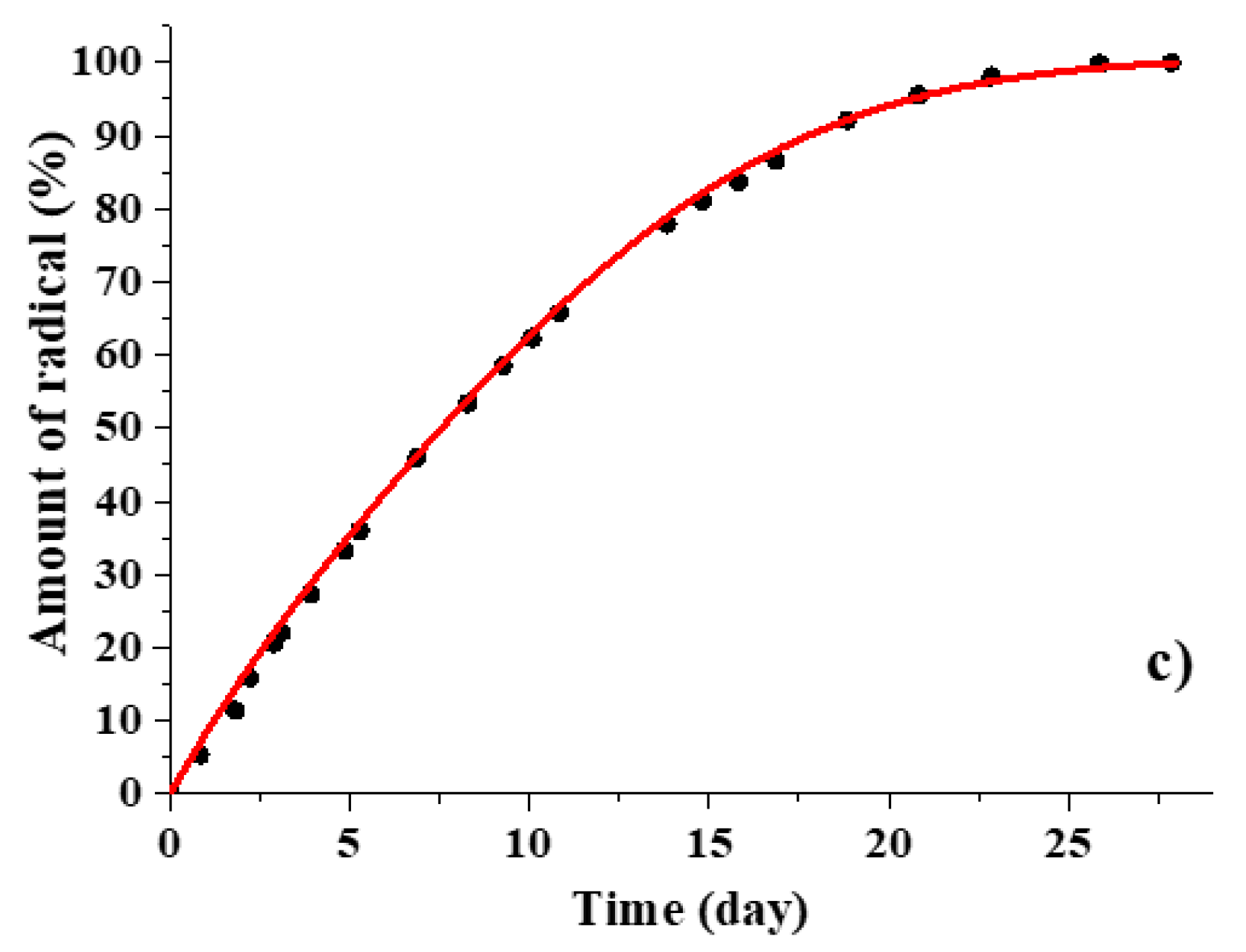
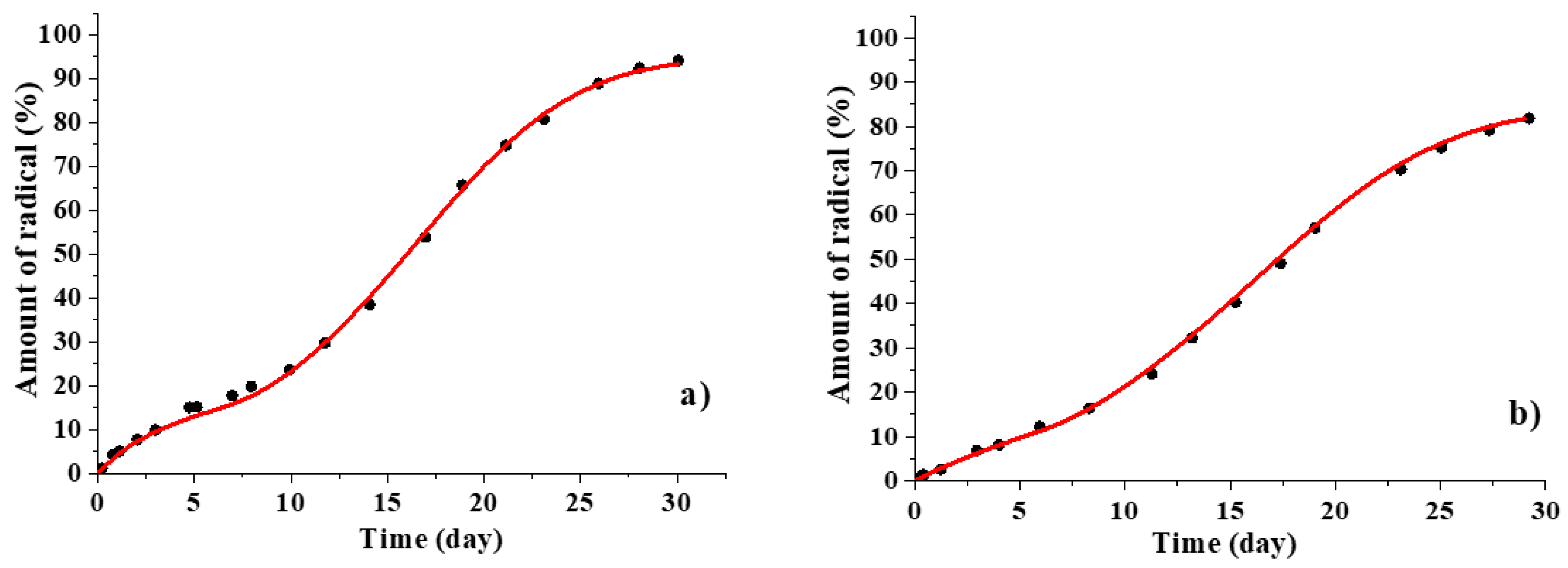
3.2. Kinetic Regularities of the Dopants Release from the Poly(d,l-lactide) Films
3.3. Mathematical Modeling of the Release Process
- (1)
- The dry polymer film is structurally uniform.
- (2)
- The dopant is distributed uniformly in a dry sample.
- (3)
- Diffusion through the side surface of the film is negligible.
- (4)
- Perfect-sink conditions are used.
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hench, L.L.; Jones, J.R. Biomaterials, Artificial Organs and Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2005; ISBN 9781855737372. [Google Scholar]
- Giandalia, G.; De Caro, V.; Cordone, L.; Giannola, L.I. Trehalose–hydroxyethylcellulose microspheres containing vancomycin for topical drug delivery. Eur. J. Pharm. Biopharm. 2001, 52, 83–89. [Google Scholar] [CrossRef]
- Chrzanowska, O.; Struszczyk, M.H.; Krucinska, I.; Puchalski, M.; Herczyńska, L.; Chrzanowski, M. Elaboration of small-diameter vascular prostheses—Selection of appropriate sterilisation method. J. Appl. Polym. Sci. 2014, 131, 9611–9620. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P. An Overview of Poly(lactic-co-glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Domínguez-Robles, J.; Donnelly, R.; Larrañeta, E. Implantable Polymeric Drug Delivery Devices: Classification, Manufacture, Materials, and Clinical Applications. Polymers 2018, 10, 1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, L.; Liu, J.; Zhang, J.; Deng, L.; Dong, A. pH-sensitive nanoparticles prepared from amphiphilic and biodegradable methoxy poly(ethylene glycol)-block-(polycaprolactone-graft-poly(methacrylic acid)) for oral drug delivery. Polym. Chem. 2013, 4, 1430–1438. [Google Scholar] [CrossRef]
- Montes, A.; Gordillo, M.D.; Pereyra, C.; De los Santos, D.M.; Martínez de la Ossa, E.J. Ibuprofen–polymer precipitation using supercritical CO2 at low temperature. J. Supercrit. Fluids 2014, 94, 91–101. [Google Scholar] [CrossRef]
- Elvira, C.; Fanovich, A.; Fernández, M.; Fraile, J.; San Román, J.; Domingo, C. Evaluation of drug delivery characteristics of microspheres of PMMA–PCL–cholesterol obtained by supercritical-CO2 impregnation and by dissolution–evaporation techniques. J. Control. Release 2004, 99, 231–240. [Google Scholar] [CrossRef]
- Mashak, A.; Mobedi, H.; Mahdavi, H. A Comparative Study of Progesterone and Lidocaine Hydrochloride Release from Poly(L-lactide) Films. Pharm. Sci. 2015, 21, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Ramchandani, M.; Robinson, D. In vitro and in vivo release of ciprofloxacin from PLGA 50:50 implants. J. Control. Release 1998, 54, 167–175. [Google Scholar] [CrossRef]
- Cabezas, L.I.; Fernández, V.; Mazarro, R.; Gracia, I.; De Lucas, A.; Rodríguez, J.F. Production of biodegradable porous scaffolds impregnated with indomethacin in supercritical CO2. J. Supercrit. Fluids 2012, 63, 155–160. [Google Scholar] [CrossRef]
- Guney, O.; Akgerman, A. Synthesis of controlled-release products in supercritical medium. AIChE J. 2002, 48, 856–866. [Google Scholar] [CrossRef]
- Park, M.W.; Bae, H.K. Dye distribution in supercritical dyeing with carbon dioxide. J. Supercrit. Fluids 2002, 22, 65–73. [Google Scholar] [CrossRef]
- Nazem, N.; Taylor, L.T.; Rubira, A.F. Metallized poly(etherether ketone) films achieved by supercritical fluid impregnation of a silver precursor followed by thermal curing. J. Supercrit. Fluids 2002, 23, 43–57. [Google Scholar] [CrossRef]
- Said-Galiyev, E.; Nikitin, L.; Vinokur, R.; Gallyamov, M.; Kurykin, M.; Petrova, O.; Lokshin, B.; Volkov, I.; Khokhlov, A.; Schaumburg, K. New chelate complexes of copper and iron: Sythesis and impregnation into a polymer matrix from solution in supercritical carbon dioxide. Ind. Eng. Chem. Res. 2000, 39, 4891–4896. [Google Scholar] [CrossRef]
- Li, D.; Liu, Z.; Han, B.; Song, L.; Yang, G.; Jiang, T. Preparation of nanometer dispersed polypropylene/polystyrene interpenetrating network using supercritical CO2 as a swelling agent. Polymer 2002, 43, 5363–5367. [Google Scholar] [CrossRef]
- Tang, M.; Wen, T.-Y.; Du, T.-B.; Chen, Y.-P. Synthesis of electrically conductive polypyrrole–polystyrene composites using supercritical carbon dioxide. Eur. Polym. J. 2003, 39, 143–149. [Google Scholar] [CrossRef]
- Fu, Y.; Kao, W.J. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin. Drug Deliv. 2010, 7, 429–444. [Google Scholar] [CrossRef]
- Strobel, C.; Bormann, N.; Kadow-Romacker, A.; Schmidmaier, G.; Wildemann, B. Sequential release kinetics of two (gentamicin and BMP-2) or three (gentamicin, IGF-I and BMP-2) substances from a one-component polymeric coating on implants. J. Control. Release 2011, 156, 37–45. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, Z.; Feng, S.-S. d-α-Tocopheryl polyethylene glycol 1000 succinate (TPGS) modified poly(l-lactide) (PLLA) films for localized delivery of paclitaxel. Int. J. Pharm. 2008, 350, 166–171. [Google Scholar] [CrossRef]
- Shen, E.; Kipper, M.J.; Dziadul, B.; Lim, M.K.; Narasimhan, B. Mechanistic relationships between polymer microstructure and drug release kinetics in bioerodible polyanhydrides. J. Control. Release 2002, 82, 115–125. [Google Scholar] [CrossRef]
- Molavi, F.; Barzegar-Jalali, M.; Hamishehkar, H. Polyester based polymeric nano and microparticles for pharmaceutical purposes: A review on formulation approaches. J. Control. Release 2020, 320, 265–282. [Google Scholar] [CrossRef] [PubMed]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Ford, J.L.; Mitchell, K.; Rowe, P.; Armstrong, D.J.; Elliott, P.N.C.; Rostron, C.; Hogan, J.E. Mathematical modelling of drug release from hydroxypropylmethylcellulose matrices: Effect of temperature. Int. J. Pharm. 1991, 71, 95–104. [Google Scholar] [CrossRef]
- Kao, C.-C.; Chen, S.-C.; Sheu, M.-T. Lag time method to delay drug release to various sites in the gastrointestinal tract. J. Control. Release 1997, 44, 263–270. [Google Scholar] [CrossRef]
- Raman, C.; Berkland, C.; Kim, K.; Pack, D.W. Modeling small-molecule release from PLG microspheres: Effects of polymer degradation and nonuniform drug distribution. J. Control. Release 2005, 103, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Lindner, W.D.; Lippold, B.C. Drug Release From Hydrocolloid Embeddings with High or Low Susceptibility to Hydrodynamic Stress. Pharm. Res. Off. J. Am. Assoc. Pharm. Sci. 1995, 12, 1781–1785. [Google Scholar]
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J. Control. Release 2001, 73, 121–136. [Google Scholar] [CrossRef]
- Wada, R.; Hyon, S.; Ikada, Y. Kinetics of diffusion-mediated drug release enhanced by matrix degradation. J. Control. Release 1995, 37, 151–160. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Zhang, Y.; Zhu, C. Sustained release of isoniazid from polylactide microspheres prepared using solid/oil drug loading method for tuberculosis treatment. Sci. China Life Sci. 2016, 59, 724–731. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Braatz, R.D. A mechanistic model for drug release in PLGA biodegradable stent coatings coupled with polymer degradation and erosion. J. Biomed. Mater. Res. Part A 2015, 103, 2269–2279. [Google Scholar] [CrossRef] [Green Version]
- Mader, K.; Bittner, B.; Li, Y.; Wohlauf, W.; Kissel, T. Monitoring microviscosity and microacidity of the albumin microenvironment inside degrading microparticles from poly(lactide-co-glycolide) (PLG) or ABA-triblock polymers containing hydrophobic poly(lactide-co-glycolide) A blocks and hydrophilic poly(ethyleneoxide) B Blocks. Pharm. Res. 1998, 15, 787–793. [Google Scholar] [PubMed]
- Mäder, K.; Crémmilleux, Y.; Domb, A.J.; Dunn, J.R.; Swartz, H.M. In Vitro/In Vivo Comparison of Drug Release and Polymer Erosion from Biodegradable P(FAD-SA) Polyanhydrides—A Noninvasive Approach by the Combined Use of Electron Paramagnetic Resonance Spectroscopy and Nuclear Magnetic Resonance Imaging. Pharm. Res. 1997, 14, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Mäder, K.; Bacic, G.; Domb, A.; Elmalak, O.; Langer, R.; Swartz, H.M. Noninvasive in Vivo Monitoring of Drug Release and Polymer Erosion from Biodegradable Polymers by EPR Spectroscopy and NMR Imaging. J. Pharm. Sci. 1997, 86, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Golubeva, E.N.; Chumakova, N.A.; Kuzin, S.V.; Grigoriev, I.A.; Kalai, T.; Korotkevich, A.A.; Bogorodsky, S.E.; Krotova, L.I.; Popov, V.K.; Lunin, V.V. Paramagnetic bioactives encapsulated in poly(d,l-lactide) microparticules: Spatial distribution and in vitro release kinetics. J. Supercrit. Fluids 2020, 158, 104748. [Google Scholar] [CrossRef]
- Akovantseva, A.A.; Bagratashvili, V.N.; Chumakova, N.A.; Golubeva, E.N.; Gromov, O.I.; Kuzin, S.V.; Melnikov, M.Y.; Timashev, P.S. Impregnation of Polycarbonate by Paramagnetic Probe 2,2,6,6-Tetramethyl-4-Hydroxy-Piperidine-1-Oxyl (TEMPOL) in Supercritical CO2. Appl. Magn. Reson. 2018. [Google Scholar] [CrossRef]
- Uddin, M.A.; Yu, H.; Wang, L.; Naveed, K.U.R.; Haq, F.; Amin, B.U.; Mehmood, S.; Nazir, A.; Xing, Y.; Shen, D. Recent progress in EPR study of spin labeled polymers and spin probed polymer systems. J. Polym. Sci. 2020, 58, 1924–1948. [Google Scholar] [CrossRef]
- Han, X.; Ding, L.; Wang, Y.; Pan, J.; Sinka, C. A phenomenological model for the degradation of biodegradable polymers. Biomaterials 2008, 29, 3393–3401. [Google Scholar] [CrossRef]
- Proikakis, C.S.; Mamouzelos, N.J.; Tarantili, P.A.; Andreopoulos, A.G. Swelling and hydrolytic degradation of poly(d,l-lactic acid) in aqueous solutions. Polym. Degrad. Stab. 2006, 91, 614–619. [Google Scholar] [CrossRef]
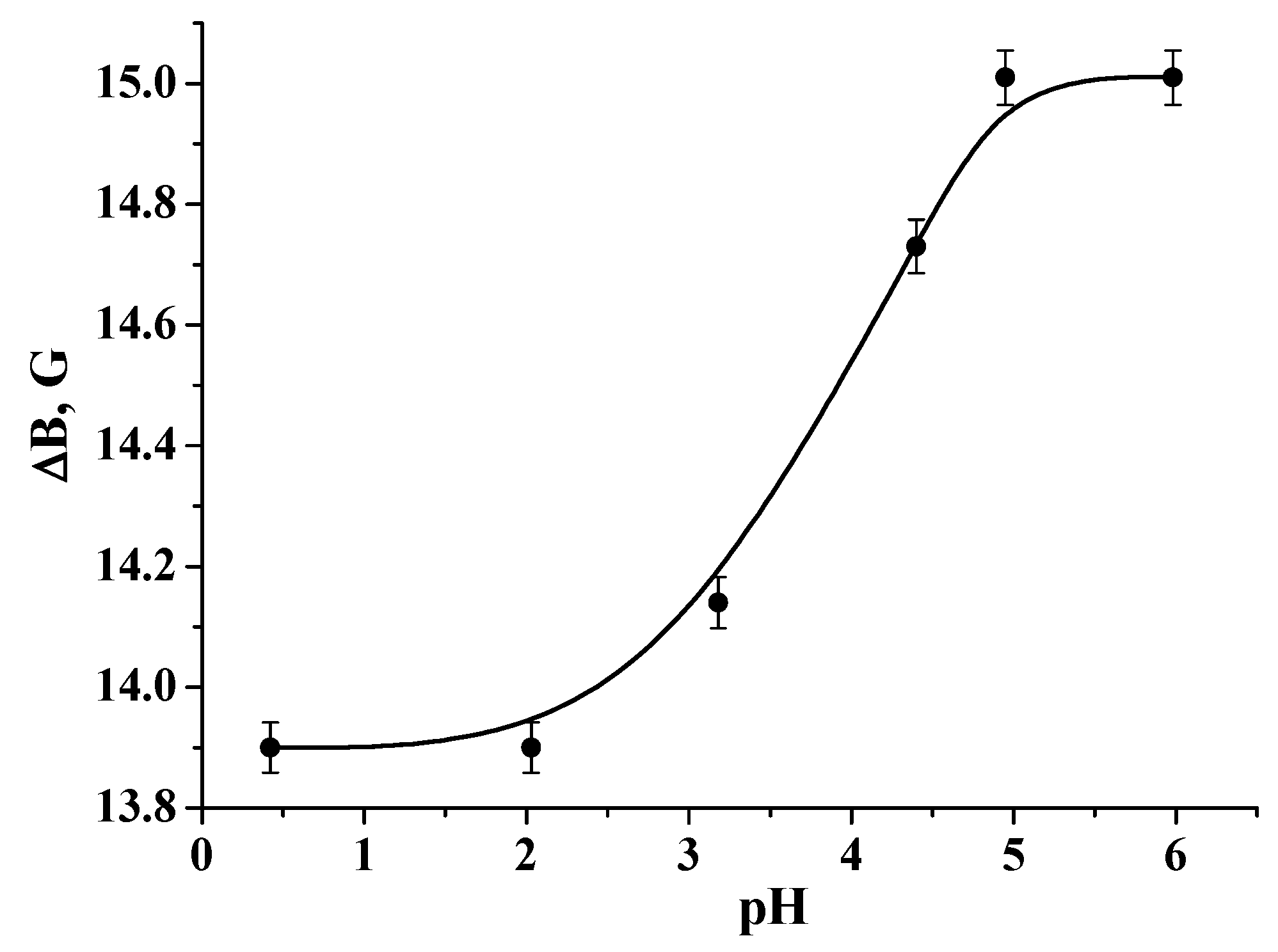
- Khramtsov, V.V.; Weiner, L.M.; Grigoriev, I.A.; Volodarsky, L.B. Proton exchange in stable nitroxyl radicals. EPR study of the pH of aqueous solutions. Chem. Phys. Lett. 1982, 91, 69–72. [Google Scholar] [CrossRef]
- Kirilyuk, I.A.; Bobko, A.A.; Khramtsov, V.V.; Grigor’ev, I.A. Nitroxides with two pK values—Useful spin probes for pH monitoring within a broad range. Org. Biomol. Chem. 2005, 3, 1269–1274. [Google Scholar] [CrossRef]
- Minaev, N.V.; Yusupov, V.I.; Bagratashvili, B.N. Laboratory Setup for the Study and Carrying Out Physical and Chemical Processes. U.S. Patent No. 147199, 20 October 2014. [Google Scholar]
- Chumakova, N.A.; Golubeva, E.N.; Ivanova, T.A.; Vorobieva, N.N.; Timashev, P.S.; Bagratashvili, V.N. EPR Diagnostics of d,l-Polylactide Porous Matrices Formed in Supercritical CO2. Russ. J. Phys. Chem. B 2018, 12, 1255–1260. [Google Scholar] [CrossRef]
- Eaton, G.R.; Eaton, S.S.; Barr, D.P.; Weber, R.T. Quantitative EPR; Springer: Vienna, Austria, 2010; Volume 369. [Google Scholar]
- Chumakova, N.A.; Kuzin, S.V.; Grechishnikov, A.I. Spectral Convolution for Quantitative Analysis in EPR Spectroscopy. Appl. Magn. Reson. 2019, 50, 1125–1147. [Google Scholar] [CrossRef]
- Duling, D.R. Simulation of Multiple Isotropic Spin-Trap EPR Spectra. J. Magn. Reson. Ser. B 1994, 104, 105–110. [Google Scholar] [CrossRef]
- Zaitsev, K.V.; Piskun, Y.A.; Oprunenko, Y.F.; Karlov, S.S.; Zaitseva, G.S.; Vasilenko, I.V.; Churakov, A.V.; Kostjuk, S.V. Controlled ring-opening homo- and copolymerization of ε-caprolactone and d,l-lactide by iminophenolate aluminum complexes: An efficient approach toward well-defined macromonomers. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 1237–1250. [Google Scholar] [CrossRef]
- Kokorin, A. (Ed.) Nitroxides—Theory, Experiment and Applications; InTech: London, UK, 2012; ISBN 978-953-51-0722-4. [Google Scholar]
- Garlotta, D. A literature review of poly(lactic acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Banerjee, D.; Bhat, S.N.; Bhat, S.V.; Leporini, D. ESR evidence for 2 coexisting liquid phases in deeply supercooled bulk water. Proc. Natl. Acad. Sci. USA 2009, 106, 11448–11453. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, B.M.; Schwendeman, S.P. Characterization of the initial burst release of a model peptide from poly(d,l-lactide-co-glycolide) microspheres. J. Control. Release 2002, 82, 289–307. [Google Scholar] [CrossRef]
- Messaritaki, A.; Black, S.J.; Van Der Walle, C.F.; Rigby, S.P. NMR and confocal microscopy studies of the mechanisms of burst drug release from PLGA microspheres. J. Control. Release 2005, 108, 271–281. [Google Scholar] [CrossRef]
- Mäder, K.; Gallez, B.; Liu, K.J.; Swartz, H.M. Non-invasive in vivo characterization of release processes in biodegradable polymers by low-frequency electron paramagnetic resonance spectroscopy. Biomaterials 1996, 17, 457–461. [Google Scholar] [CrossRef]
- Kishioka, S.Y.; Ohsaka, T.; Tokuda, K. Electrochemical studies of acid-promoted disproportionation of nitroxyl radical. Electrochim. Acta 2003, 48, 1589–1594. [Google Scholar] [CrossRef]
- Bobko, A.A.; Kirilyuk, I.A.; Grigor’ev, I.A.; Zweier, J.L.; Khramtsov, V. V Reversible reduction of nitroxides to hydroxylamines: Roles for ascorbate and glutathione. Free Radic. Biol. Med. 2007, 42, 404–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagtap, A.P.; Krstic, I.; Kunjir, N.C.; Hänsel, R.; Prisner, T.F.; Sigurdsson, S.T. Sterically shielded spin labels for in-cell EPR spectroscopy: Analysis of stability in reducing environment. Free Radic. Res. 2015, 49, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Nagura, K.; Bogdanov, A.; Chumakova, N.; Vorobiev, A.K.; Moronaga, S.; Imai, H.; Matsuda, T.; Noda, Y.; Maeda, T.; Koizumi, S.; et al. Size-tunable MRI-visible nitroxide-based magnetic mixed micelles: Preparation, stability, and theranostic application. Nanotechnology 2019, 30. [Google Scholar] [CrossRef] [PubMed]
- Alexis, F.; Venkatraman, S.; Rath, S.K.; Gan, L.H. Some insight into hydrolytic scission mechanisms in bioerodible polyesters. J. Appl. Polym. Sci. 2006, 102, 3111–3117. [Google Scholar] [CrossRef]
- Antheunis, H.; van der Meer, J.-C.; de Geus, M.; Kingma, W.; Koning, C.E. Improved Mathematical Model for the Hydrolytic Degradation of Aliphatic Polyesters. Macromolecules 2009, 42, 2462–2471. [Google Scholar] [CrossRef]
- Xu, Y.; Kim, C.S.; Saylor, D.M.; Koo, D. Polymer degradation and drug delivery in PLGA-based drug–polymer applications: A review of experiments and theories. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1692–1716. [Google Scholar] [CrossRef] [PubMed]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef]
- Huang, J.; Mazzara, J.M.; Schwendeman, S.P.; Thouless, M.D. Self-healing of pores in PLGAs. J. Control. Release 2015, 206, 20–29. [Google Scholar] [CrossRef]
- Reinhold, S.E.; Desai, K.G.H.; Zhang, L.; Olsen, K.F.; Schwendeman, S.P. Self-healing microencapsulation of biomacromolecules without organic solvents. Angew. Chem. Int. Ed. 2012, 51, 10800–10803. [Google Scholar] [CrossRef]
- Antheunis, H.; van der Meer, J.-C.; de Geus, M.; Heise, A.; Koning, C.E. Autocatalytic Equation Describing the Change in Molecular Weight during Hydrolytic Degradation of Aliphatic Polyesters. Biomacromolecules 2010, 11, 1118–1124. [Google Scholar] [CrossRef]
- Borovikov, P.I.; Sviridov, A.P.; Antonov, E.N.; Dunaev, A.G.; Krotova, L.I.; Fatkhudinov, T.K.; Popov, V.K. Model of aliphatic polyesters hydrolysis comprising water and oligomers diffusion. Polym. Degrad. Stab. 2019, 159, 70–78. [Google Scholar] [CrossRef]
- Siparsky, G.L.; Voorhees, K.J.; Dorgan, J.R.; Schilling, K. Water Transport in Polylactic Acid (PLA), PLA/Polycaprolactone Copolymers, and PLA/Polyethylene Glycol Blends. J. Environ. Polym. Degrad. 1997, 5, 125–136. [Google Scholar]
- Sevim, K.; Pan, J. A model for hydrolytic degradation and erosion of biodegradable polymers. Acta Biomater. 2018, 66, 192–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.M.; Garreau, H.; Vert, M. Structure-property relationships in the case of the degradation of massive aliphatic poly-(α-hydroxy acids) in aqueous media—Part 1: Poly(dl-lactic acid). J. Mater. Sci. Mater. Med. 1990, 1, 123–130. [Google Scholar] [CrossRef]
- Jonnalagadda, S.; Robinson, D.H. Effect of thickness and PEG addition on the hydrolytic degradation of PLLA. J. Biomater. Sci. Polym. Ed. 2004, 15, 1317–1326. [Google Scholar] [CrossRef]
- Grizzi, I.; Garreau, H.; Li, S.; Vert, M. Hydrolytic degradation of devices based on poly(dl-lactic acid) size-dependence. Biomaterials 1995, 16, 305–311. [Google Scholar] [CrossRef]




| Mn | Mw | Đ | |
|---|---|---|---|
| PDL 04 | 14,800 | 36,100 | 2.4 |
| PDL 04, after SCF processing | 15,200 | 35,800 | 2.4 |
| PDL 02 | 7200 | 15,600 | 2.2 |
| Time | Average Number of Pores per 100 μm2 |
|---|---|
| 2 h | 16 |
| 2 days | 7 |
| 4 days | 8 |
| 6 days | 4 |
| 9 days | 8 |
| 11 days | 10 |
| System | D0, m2·Day−1 | , m−1 | , Day−1 | , Day | A, m2·Day−5/2 | ||
|---|---|---|---|---|---|---|---|
| 1 | TEMPOL/200 μm | 7.5 × 10−11 | 5.0 × 104 | 1.4 | 0.5 | 6.1 | 1.35 × 10−11 |
| 1 | TEMPOL/200 μm | 7.5 × 10−11 | 2.5 × 104 | 1.0 | 0.3 | 5.9 | 0.94 × 10−11 |
| 2 | TEMPOL/50 μm | 2.4 × 10−11 | 0.9 × 104 | 0.33 | 1.0 | 2.6 | 4.77 × 10−13 |
| 3 | ATI/200 μm | 6.9 × 10−11 | 1.4 × 104 | 0.17 | 0.9 | 5.8 | 1.11 × 10−11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chumakova, N.A.; Golubeva, E.N.; Kuzin, S.V.; Ivanova, T.A.; Grigoriev, I.A.; Kostjuk, S.V.; Melnikov, M.Y. New Insight into the Mechanism of Drug Release from Poly(d,l-lactide) Film by Electron Paramagnetic Resonance. Polymers 2020, 12, 3046. https://doi.org/10.3390/polym12123046
Chumakova NA, Golubeva EN, Kuzin SV, Ivanova TA, Grigoriev IA, Kostjuk SV, Melnikov MY. New Insight into the Mechanism of Drug Release from Poly(d,l-lactide) Film by Electron Paramagnetic Resonance. Polymers. 2020; 12(12):3046. https://doi.org/10.3390/polym12123046
Chicago/Turabian StyleChumakova, Natalia A., Elena N. Golubeva, Sergei V. Kuzin, Tatiana A. Ivanova, Igor A. Grigoriev, Sergey V. Kostjuk, and Mikhail Ya. Melnikov. 2020. "New Insight into the Mechanism of Drug Release from Poly(d,l-lactide) Film by Electron Paramagnetic Resonance" Polymers 12, no. 12: 3046. https://doi.org/10.3390/polym12123046






