Natural Cellulose Fibers for Surgical Suture Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fiber Preparations
2.2. Fiber Characterization
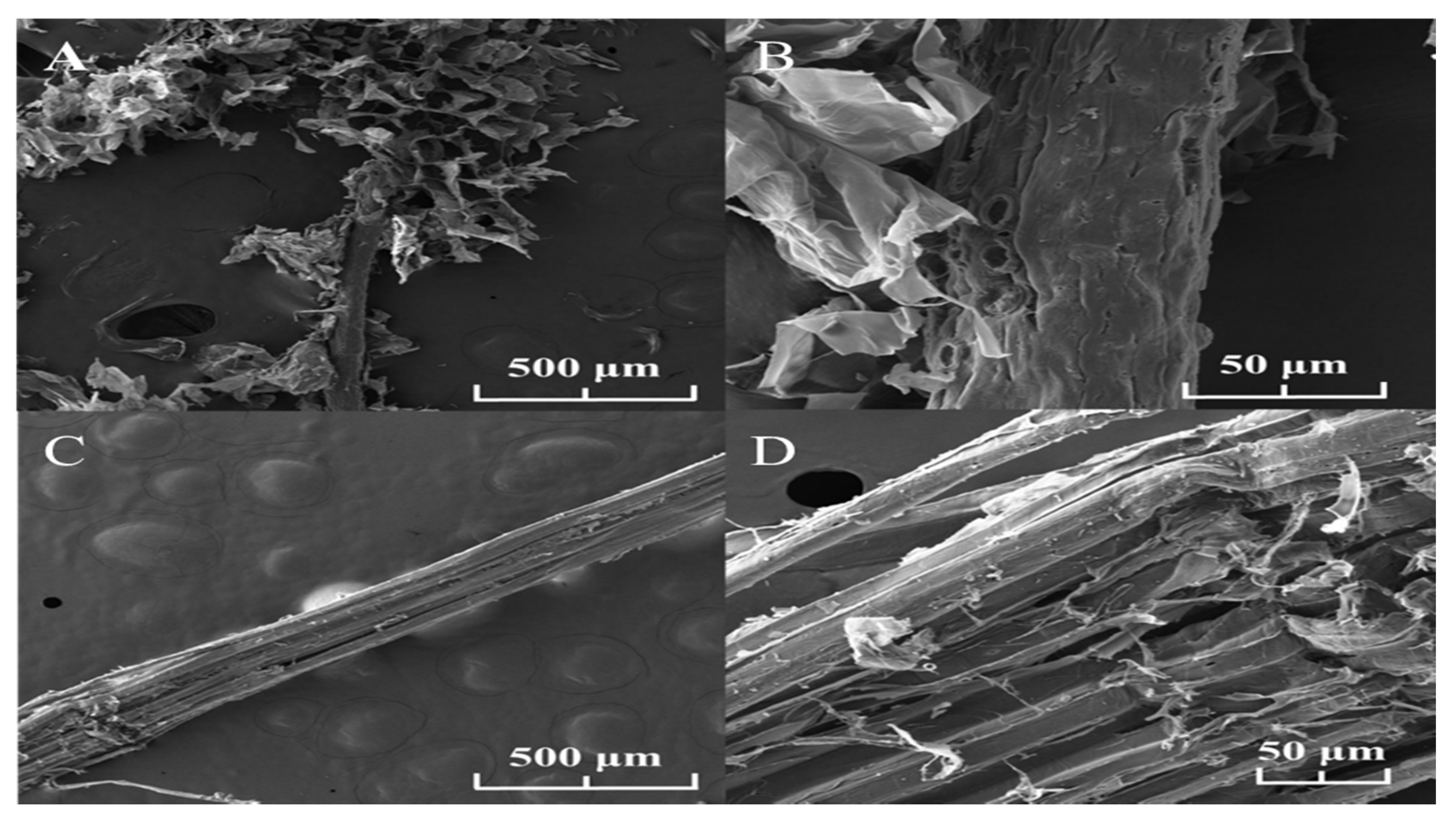
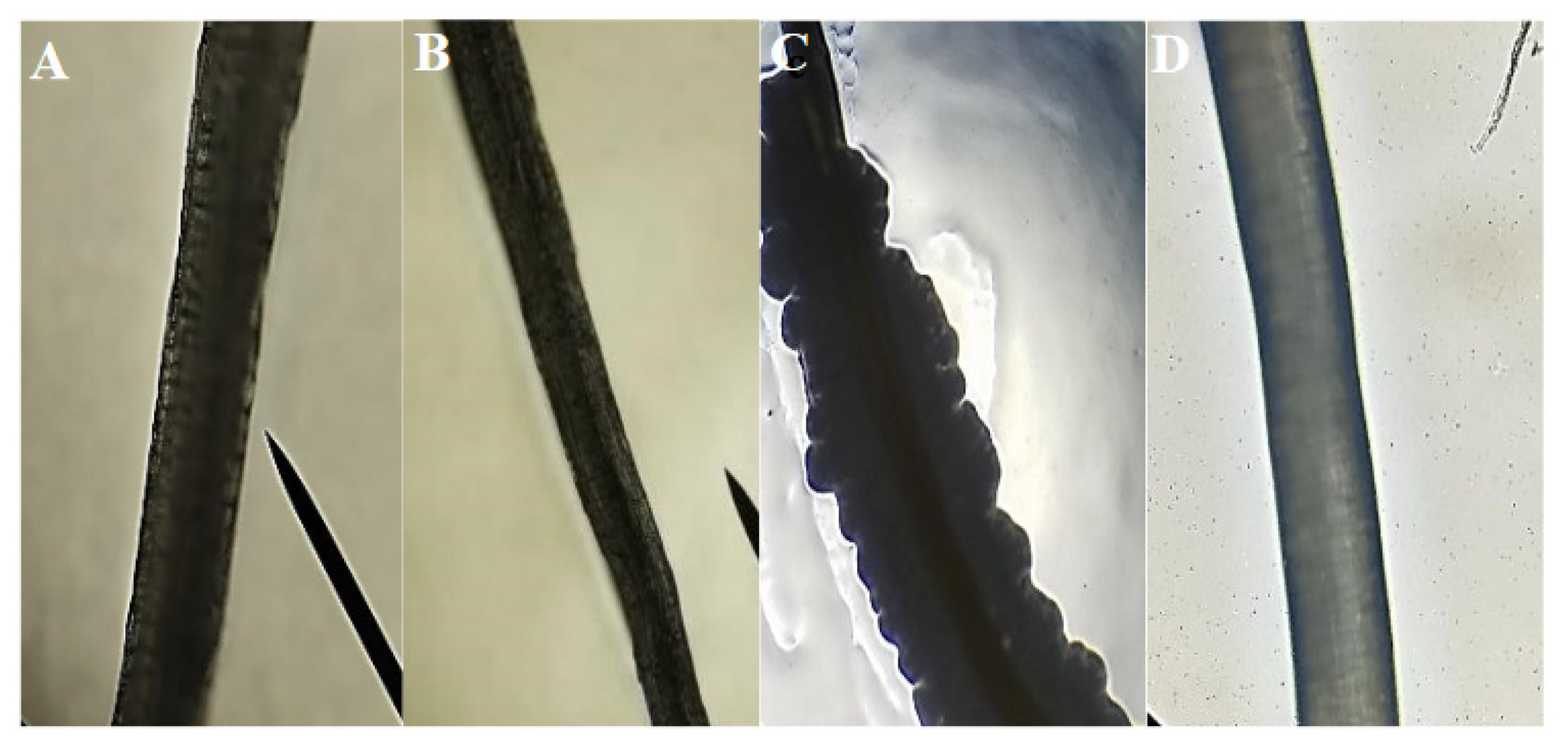
2.2.1. Scanning Electron Microscope (SEM)
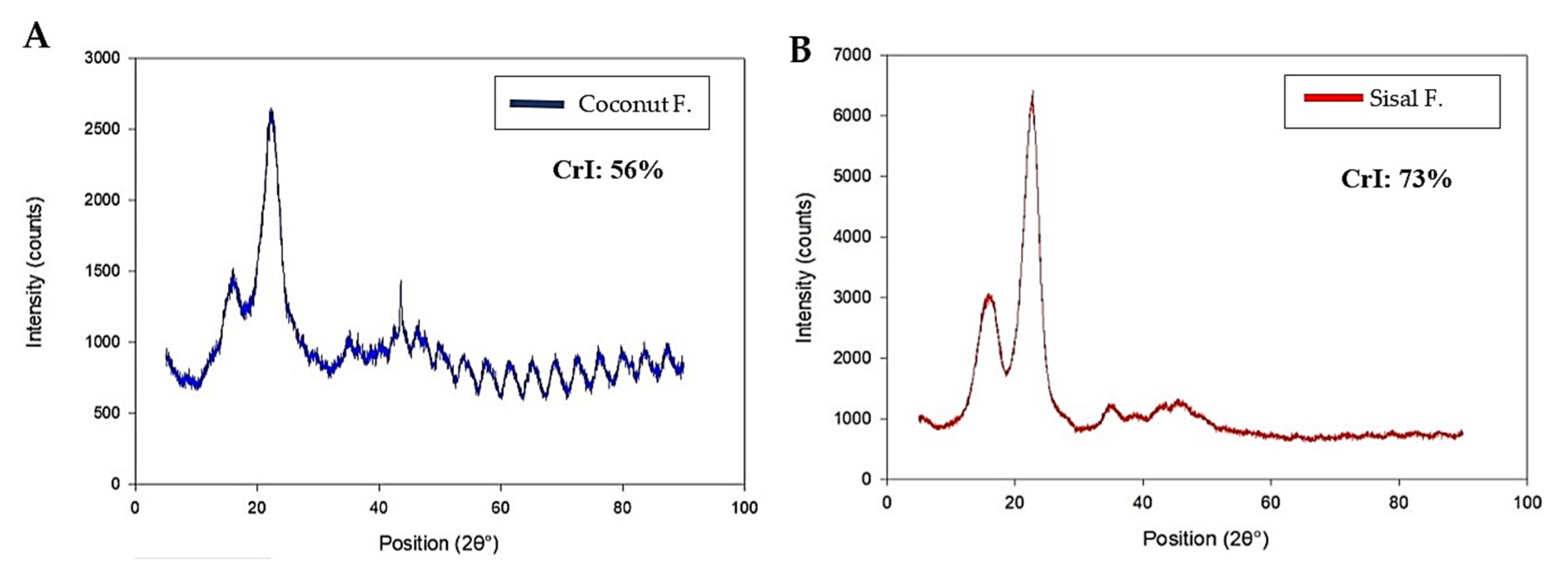
2.2.2. X-ray Diffraction (XRD)
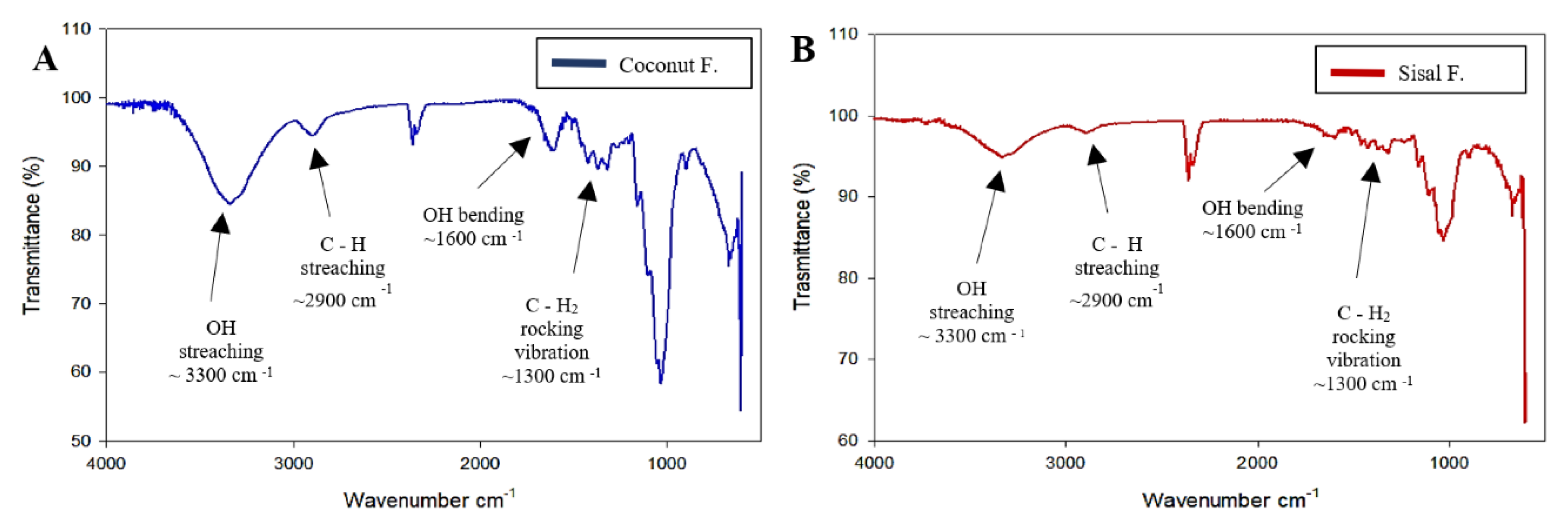
2.2.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.3. Mechanical Test of Fibers
2.4. Biodegradability Test of Fibers
2.5. In Vitro Antifouling Test of Fibers
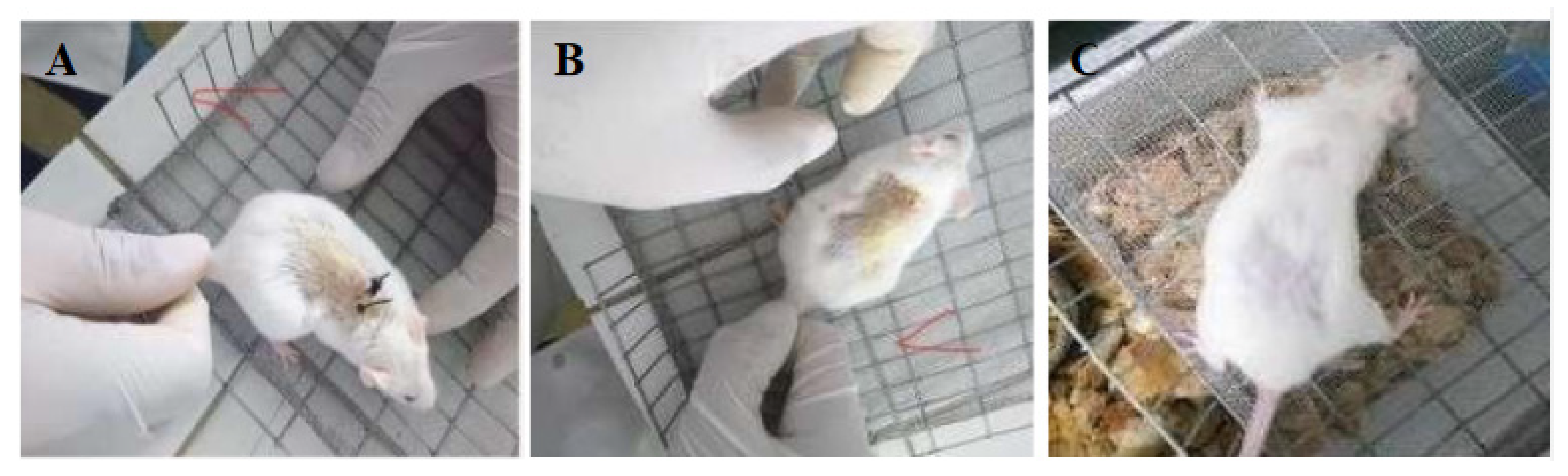
2.6. In Vivo Suture Testing
2.7. Statistical Analyses
3. Results
3.1. Characterization of Fibers
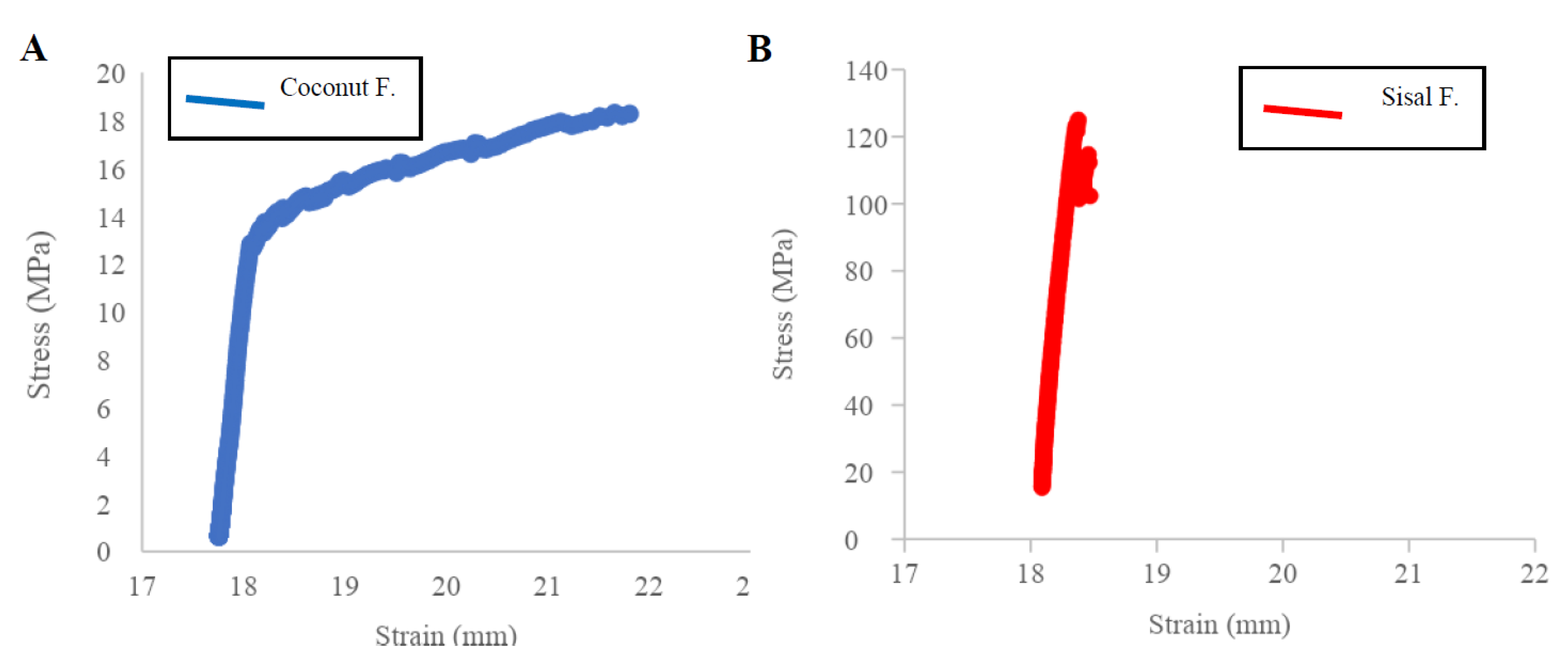
3.2. Mechanical Tests
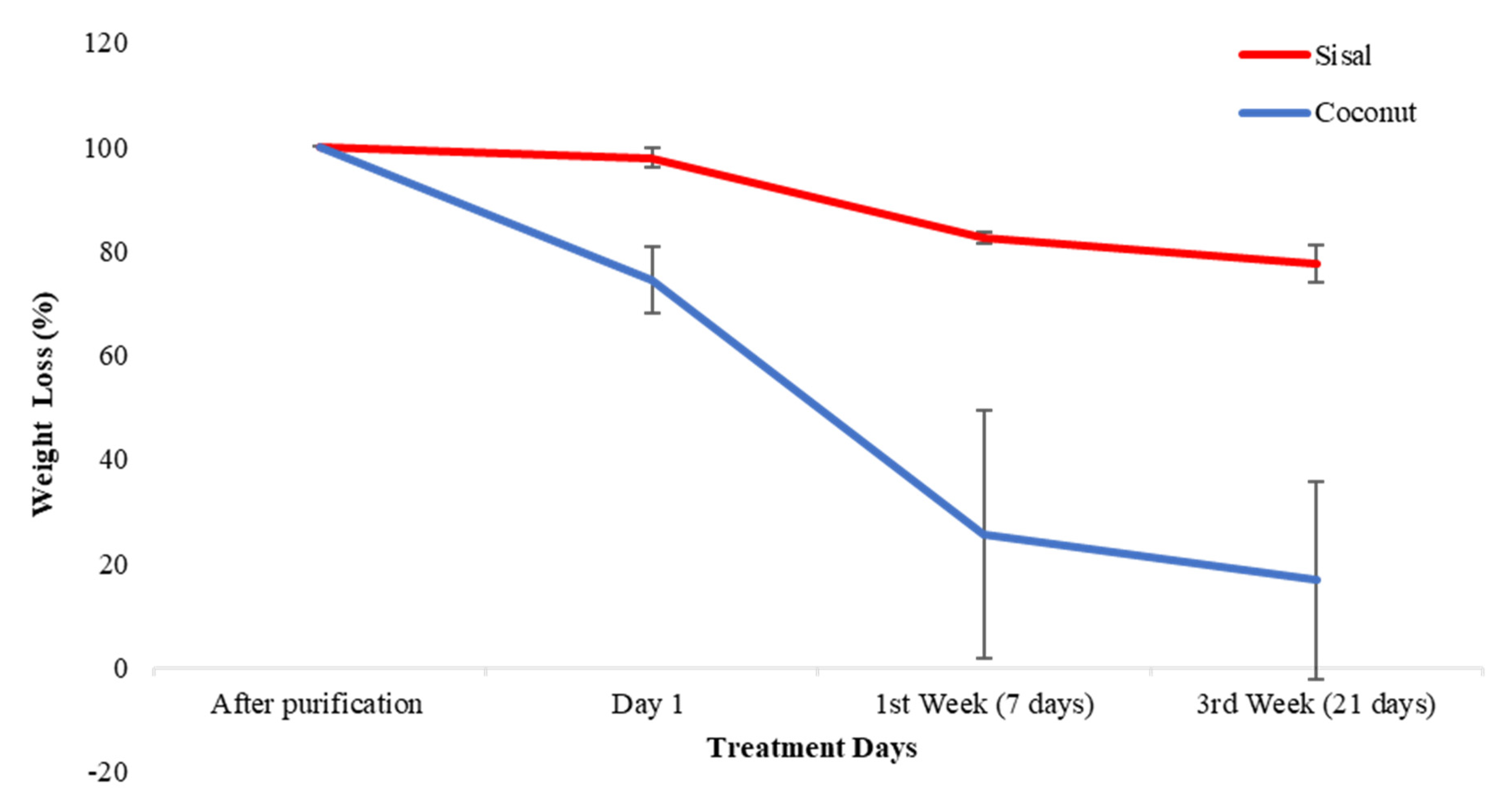
3.3. Biodegradability Test
3.4. In Vitro Antifouling Testing
- -
- No biofilm producer (0): The Of of the fiber is below the established cut-off point (ODf ≤ ODc).
- -
- Weak biofilm producer (+ or 1): The ODf of the fiber is between the cut-off point and the f value corresponding to it (ODc < ODf ≤ 2ODc).
- -
- Moderate biofilm producer (++ or 2): The ODf of the fiber is between twice the value of the cut-off point and the ODc value corresponding to the quadruple of it (2ODc < ODf ≤ 4ODc).
- -
3.5. In Vivo Suture Testing
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Raw Data of Mechanical Properties and Biodegradability Test of Coconut and Sisal Fibers
| Samples | UTS | Young’s Modulus | Fracture Strength | Yield Strength |
|---|---|---|---|---|
| (MPa) | (GPa) | (MPa) | (MPa) | |
| Sisal Fibers | ||||
| Sample 1 | 194.42 | 0.34 | 109.28 | 174.92 |
| Sample 2 | 110.79 | 2.27 | 79.21 | 98.87 |
| Sample 3 | 49.32 | 2.65 | 16.8 | 22.97 |
| Sample 4 | 200.84 | 5.8 | 180.28 | 161.27 |
| Coconut Fibers | ||||
| Sample 1 | 16.73 | 0.08 | 15.54 | 14.88 |
| Sample 2 | 17.21 | 0.03 | 17.21 | 10.37 |
| Sample 3 | 10.86 | 0.02 | 10.44 | 6.27 |
| Sample 4 | 30.08 | 0.04 | 30.08 | 18.28 |
| Weight Initial/mg (after Purification) | Weight/mg Day 1 | Weight/mg 1st Week—7 Days | Weight/mg 3rd Week—21 Days | |
|---|---|---|---|---|
| Coconut Fibers | ||||
| Sample 1 | 24.7 | 20.2 | 9.7 | 8.9 |
| Sample 2 | 24.2 | 17.4 | 4.9 | 4.7 |
| Sample 3 | 24.8 | 17.3 | 13.4 | 11.5 |
| Sisal Fibers | ||||
| Sample 1 | 25.4 | 25.1 | 21.1 | 19.3 |
| Sample 2 | 25.2 | 25 | 20.5 | 19.6 |
| Sample 3 | 25.9 | 24.8 | 21.7 | 21.3 |
Appendix B. Raw Data of the t-Student Test of Coconut, Sisal and Silk Fibers
| Fiber | N | Mean | Std. Deviation | |
|---|---|---|---|---|
| UTS | Coconut | 4 | 18.72 | 8.10492 |
| Sisal | 4 | 138.8425 | 72.41953 | |
| Silk | 4 | 217.5475 | 39.57996 | |
| Young Mod. | Coconut | 4 | 0.0425 | 0.0263 |
| Sisal | 4 | 2.765 | 2.26201 | |
| Silk | 4 | 5.0075 | 1.10885 |
| T | df | Sig. (2—Tailed) | Mean Difference | Std. Error Difference | 95% Confidence Interval of Difference | |||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| UTS | Equal variances assumed | −9.843 | 6 | 0 | −198.8275 | 20.2 | −248.256 | −149.398 |
| Equal variances not assumed | −9.843 | 3.251 | 0.002 | −198.8275 | 20.2 | −260.394 | −137.26 | |
| Young Mod. | Equal variances assumed | −8.953 | 6 | 0 | −4.965 | 0.55458 | −6.322 | −3.6079 |
| Equal variances not assumed | −8.953 | 3.003 | 0.003 | −4.965 | 0.55458 | −6.728 | −3.2011 | |
| t | df | Sig. Prob (2—Tailed) | Mean Difference | Std. Error Difference | 95% Confidence Interval of Difference | |||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| UTS | Equal variances assumed | −1.66 | 6 | 0.148 | −78.705 | 47.40419 | −194.698 | 37.28888 |
| Equal variances not assumed | −1.66 | 4.212 | 0.169 | −78.705 | 47.40419 | −207.743 | 50.33367 | |
| Young Mod. | Equal variances assumed | −1.254 | 6 | 0.257 | −2.2425 | 1.788883 | −6.6196 | 2.1346 |
| Equal variances not assumed | −1.254 | 3.631 | 0.285 | −2.2425 | 1.788883 | −7.41492 | 2.92992 | |
Appendix C. Raw Results of the Antibacterial and Antifouling Test
| Samples | OD 600 nm |
|---|---|
| Coconut + Bacteria | |
| Replica 1 | 0.702 |
| Replica 2 | 1.25 |
| Replica 3 | 0.845 |
| Sisal + Bacteria | |
| Replica 1 | 0.139 |
| Replica 2 | 0.295 |
| Replica 3 | 0.363 |
| Coconut + Bacteria + Antibiotic | |
| Replica 1 | 0.174 |
| Replica 2 | 0.225 |
| Replica 3 | 0.309 |
| Sisal + Bacteria + Antibiotic | |
| Replica 1 | 0.116 |
| Replica 2 | 0.095 |
| Replica 3 | 0.127 |
| Silk commercial + Bacteria | |
| Replica 1 | 1.2 |
| Replica 2 | 1.132 |
| Replica 3 | 1.422 |
| Silk commercial + Bacteria + Antibiotic | |
| Replica 1 | 0.248 |
| Replica 2 | 0.317 |
| Replica 3 | 0.219 |
References
- Dennis, C.; Sethu, S.; Nayak, S.; Mohan, L.; Morsi, Y.; Manivasagam, G. Suture materials—Current and emerging trends. J. Biomed. Mater. Res. Part A 2016, 104, 1544–1559. [Google Scholar] [CrossRef] [PubMed]
- Muffly, T.M.; Boyce, J.; Kieweg, S.L.; Bonham, A.J. Tensile Strength of a Surgeon’s or a Square Knot. J. Surg. Educ. 2010, 67, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Naleway, S.E.; Lear, W.; Kruzic, J.J.; Maughan, C. Mechanical properties of suture materials in general and cutaneous surgery. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 103, 735–742. [Google Scholar] [CrossRef]
- Byrne, M.; Aly, A. The Surgical Suture. Aesthet. Surg. J. 2019, 39, S67–S72. [Google Scholar] [CrossRef] [Green Version]
- Kandimalla, R.; Kalita, S.; Choudhury, B.; Devi, D.; Kalita, D.; Kalita, K.; Dash, S.; Kotoky, J. Fiber from ramie plant (Boehmeria nivea): A novel suture biomaterial. Mater. Sci. Eng. C 2016, 62, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Masini, B.D.; Stinner, D.J.; Waterman, S.M.; Wenke, J.C. Bacterial Adherence to Suture Materials. J. Surg. Educ. 2011, 68, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Shani, A.; Poliansky, V.; Mulla, H.; Rahamimov, N. Nylon Skin Sutures Carry a Lower Risk of Post-Operative Infection than Metal Staples in Open Posterior Spine Surgery: A Retrospective Case-Control Study of 270 Patients. Surg. Infect. 2020, 21, 440–444. [Google Scholar] [CrossRef]
- Figueroa, D.; Jauk, V.C.; Szychowski, J.M.; Garner, R.; Biggio, J.R.; Andrews, W.W.; Hauth, J.; Tita, A.T.N. Surgical Staples Compared with Subcuticular Suture for Skin Closure After Cesarean Delivery. Obstet. Gynecol. 2013, 121, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Lopez, C.A.; Navas, J.L.O. Determinación de la Frecuencia de Infecciones en el Sitio Operatorio y Factores de Riesgo Asociados en Pacientes Intervenidos Quirúrgicamente de Cirugía Abdominal de Emergencia en el Hospital Provincial Docente Ambato de Noviembre 2012 Hasta Abril del 2013. Bachelor’s Thesis, Pontificia Universidad Católica del Ecuador, Ambato, Ecuador, 2013. [Google Scholar]
- Leaper, D.; Fry, D.; Assadian, O. Perspectives in prevention and treatment of surgical site infection—A narrative review of the literature. Wounds 2013, 25, 313–323. [Google Scholar]
- Leaper, D.; Wilson, P.; Assadian, O.; Edmiston, C.; Kiernan, M.; Miller, A.; Bond-Smith, G.; Yap, J. The role of antimicrobial sutures in preventing surgical site infection. Ann. R. Coll. Surg. Engl. 2017, 99, 439–443. [Google Scholar] [CrossRef]
- Onesti, M.G.; Carella, S.; Scuderi, N. Effectiveness of antimicrobial-coated sutures for the prevention of surgical site infection: A review of the literature. Eur. Rev. Med Pharmacol. Sci. 2018, 22, 5729–5739. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Kubilay, N.Z.; Ren, J.; Allegranzi, B.; Bischoff, P.; Zayed, B.; Pittet, D.; Li, J. Antimicrobial-coated sutures to decrease surgical site infections: A systematic review and meta-analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 36, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Matz, D.; Teuteberg, S.; Wiencierz, A.; Soysal, S.D.; Heizmann, O. Do antibacterial skin sutures reduce surgical site infections after elective open abdominal surgery?—Study protocol of a prospective, randomized controlled single center trial. Trials 2019, 20, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Justinger, C.; Moussavian, M.R.; Schlueter, C.; Kopp, B.; Kollmar, O.; Schilling, M.K. Antibiotic coating of abdominal closure sutures and wound infection. Surgery 2009, 145, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, C.; Pandey, I.; Himanshu, P.; Ramteke, P.W.; Pandey, A.C.; Mishra, S.B.; Patil, S. Electrospun Nanofibrous Scaffold as a Potential Carrier of Antimicrobial Therapeutics for Diabetic Wound Healing and Tissue Regeneration; Elsevier Inc.: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Hauck, O.; Allen, N.S.; Lees, G.; Rowe, H.; Verran, J. Preliminary Studies into Wash-Fast Antimicrobial Treatments of Polyester; Woodhead Publishing: New Delhi, India, 2010; pp. 122–125. [Google Scholar] [CrossRef]
- Guadarrama-Reyes, S.C.; Scougall-Vilchis, R.J.; Luckie, R.A.M.; Mendieta, V.S.; López-Castañares, R. Antimicrobial Effect of Silk and Catgut Suture Threads Coated with Biogenic Silver Nanoparticles Silver. In Nanoparticles: Fabrication, Characterization and Applications; IntechOpen Limited: London, UK, 2018; p. 249. [Google Scholar] [CrossRef]
- Vehvilainen, M. Wet-Spinning of Cellulosic Fibres from Water-Based Solution Prepared from Enzyme- Treated Pulp. Ph.D. Thesis, Tampere University of Technology, Tampere, Finland, 2015. [Google Scholar]
- Zhang, S.; Liu, X.; Wang, H.; Peng, J.; Wong, K.K.Y. Silver nanoparticle-coated suture effectively reduces inflammation and improves mechanical strength at intestinal anastomosis in mice. J. Pediatr. Surg. 2014, 49, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yu, J.; Li, H.; Wang, K.; Wu, G.; Wang, B.; Liu, M.; Zhang, Y.; Wang, P.; Zhang, J.; et al. Controllable Drug Release Behavior of Polylactic Acid (PLA) Surgical Suture Coating with Ciprofloxacin (CPFX)—Polycaprolactone (PCL)/Polyglycolide (PGA). Polymers 2020, 12, 288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, I.; Boulton, A.J.; Rizvi, S.; Carlos, W.; Dickenson, E.; A Smith, N.; Reed, M. The use of triclosan-coated sutures to prevent surgical site infections: A systematic review and meta-analysis of the literature. BMJ Open 2019, 9, e029727. [Google Scholar] [CrossRef] [Green Version]
- Laas, E.; Poilroux, C.; Bézu, C.; Coutant, C.; Uzan, S.; Rouzier, R.; Chéreau, E. Antibacterial-Coated Suture in Reducing Surgical Site Infection in Breast Surgery: A Prospective Study. Int. J. Breast Cancer 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Cheung, H.-Y.; Lau, K.-T.; Ho, M.-P.; Mosallam, A. Study on the Mechanical Properties of Different Silkworm Silk Fibers. J. Compos. Mater. 2009, 43, 2521–2531. [Google Scholar] [CrossRef]
- Koh, L.-D.; Cheng, Y.; Teng, C.-P.; Khin, Y.-W.; Loh, X.-J.; Tee, S.-Y.; Low, M.; Ye, E.; Yu, H.-D.; Zhang, Y.W.; et al. Structures, mechanical properties and applications of silk fibroin materials. Prog. Polym. Sci. 2015, 46, 86–110. [Google Scholar] [CrossRef]
- Teshome, A.; Onyari, J.M.; Raina, S.; Kabaru, J.M.; Vollrath, F. Mechanical and thermal degradation properties of silk from African wild silkmoths. J. Appl. Polym. Sci. 2012, 127, 289–297. [Google Scholar] [CrossRef]
- Chen, X.; Hou, D.; Tang, X.; Wang, L. Quantitative physical and handling characteristics of novel antibacterial braided silk suture materials. J. Mech. Behav. Biomed. Mater. 2015, 50, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Hockenberger, A.; Karaca, E. Investigating Fracture Mechanisms of Some Non-Absorbable Sutures In Vivo; Woodhead Publishing Limited: London, UK, 2010; pp. 430–436. [Google Scholar] [CrossRef]
- Thilagavathi, G.; Viju, S. Silk as a Suture Material; Woodhead Publishing: London, UK, 2015; pp. 219–232. [Google Scholar] [CrossRef]
- Manavalan, R.A.; Mukhopadhyay, A. Surgical Sutures: Performance, Development and Use. J. Biomimetics, Biomater. Tissue Eng. 2008, 1, 1–36. [Google Scholar] [CrossRef]
- Ercan, U.K.; Ibis, F.; Dikyol, C.; Horzum, N.; Karaman, O.; Yıldırım, Ç.; Çukur, E.; Demirci, E.A. Prevention of bacterial colonization on non-thermal atmospheric plasma treated surgical sutures for control and prevention of surgical site infections. PLoS ONE 2018, 13, e0202703. [Google Scholar] [CrossRef] [Green Version]
- Yu, F.; Li, L.; Teng, H.; Shi, D.; Jiang, Q. Robots in orthopedic surgery. Ann. Jt. 2018, 3, 15. [Google Scholar] [CrossRef]
- Chen, X.; Hou, D.; Wang, L.; Zhang, Q.; Zou, J.; Sun, G. Antibacterial Surgical Silk Sutures Using a High-Performance Slow-Release Carrier Coating System. ACS Appl. Mater. Interfaces 2015, 7, 22394–22403. [Google Scholar] [CrossRef]
- Rathinamoorthy, R.; Sasikala, L.; Thilagavathi, G. Textiles-As biomedical implants, types and functional requirements. J. Inst. Eng. Part TX Text. Eng. Div. 2009, 90, 37–44. [Google Scholar]
- Cao, Y.; Wang, B.-C. Biodegradation of Silk Biomaterials. Int. J. Mol. Sci. 2009, 10, 1514–1524. [Google Scholar] [CrossRef] [Green Version]
- Chu, C. Materials for Absorbable and Nonabsorbable Surgical Sutures. In Biotextiles as Medical Implants; Woodhead Publishing: London, UK, 2013; pp. 275–334. [Google Scholar] [CrossRef]
- Bekele, T.; Bhokre, A.P.; Tesfaye, A. Tissue reactivity and suture handling characteristics of “jimat” against silk and chromic gut in cat thigh muscle: A comparative study. Vet. World 2015, 8, 958–969. [Google Scholar] [CrossRef]
- Ibujes, M.; Plaza, J. Propuesta de Revestimiento Basado en las Propiedades Acusticas—Termicas de la Hoja de la Palma de Coco. Bachelor’s Thesis, Universidad Laica Vicente Rocafuerte de Guayaquil, Guayaquil, Ecuador, 2013. [Google Scholar]
- Rubio, M.; Soto, A. Estudio de la Factibilidad para la Implementacion de una Micro-Empresa Productora de Fibra de dos Variedades de Agave Cabuya Negra (Agave americana L.) y Agave Sisal (Agave Sisalana Perrine) para la Elaboracion de Artesanias en la Provincia de Cotopaxi. Bachelor’s Thesis, Universidad Tecnica de Cotopaxi, Latacunga, Ecuador, 2015. [Google Scholar]
- Nair, V.; Khosla, P.; Ramachandran, M. Review on mechanical properties of various natural fibers reinforced composites. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 2001–2004. [Google Scholar]
- Guerrero, V. Obtencion y Caracterizacion de Materiales Compuestos de Matriz Poliester Reforzados con Fibra de Cabuya Mediante Estratificacion. Bachelor’s Thesis, National Polytechnic School, Quito, Ecuador, 2012. [Google Scholar]
- Sample Preparation Techniques for Soil, Plant, and Animal Samples. In Immunity in Insects; Humana Press: New York, NY, USA, 2016; pp. 117–123.
- Hu, Y.; Hamed, O.; Salghi, R.; Abidi, N.; Jodeh, S.; Hattb, R. Extraction and Characterization of Cellulose from Agricultural Waste Argan Press Cake. Cellul. Chem. Technol. 2017, 51, 263–272. [Google Scholar]
- Costa, L.A.D.S.; Assis, D.D.J.; Gomes, G.V.; Da Silva, J.B.; Fonsêca, A.F.; Druzian, J.I. Extraction and Characterization of Nanocellulose from Corn Stover. Mater. Today Proc. 2015, 2, 287–294. [Google Scholar] [CrossRef]
- Siddiquee, M.; Helali, M. Effects of Fiber Length and Fiber Ratio on the Biodegradability of Jute Polymer Composites. Int. J. Sci. Eng. Res. 2014, 2, 64–69. [Google Scholar]
- Edmiston, C.E.; Krepel, C.J.; Marks, R.M.; Rossi, P.J.; Sanger, J.; Goldblatt, M.; Graham, M.B.; Rothenburger, S.; Collier, J.; Seabrook, G.R. Microbiology of Explanted Suture Segments from Infected and Noninfected Surgical Patients. J. Clin. Microbiol. 2013, 51, 417–421. [Google Scholar] [CrossRef] [Green Version]
- Merritt, J.H.; Kadouri, D.E.; O’Toole, G.A. Growing and Analyzing Static Biofilms. Curr. Protoc. Microbiol. 2011, 22, 1B.1.1–1B.1.17. [Google Scholar] [CrossRef] [Green Version]
- Wilson, C.; Lukowicz, R.; Merchant, S.; Valquier-Flynn, H.; Caballero, J.; Sandoval, J.; Okuom, M.; Huber, C.; Brooks, T.D.; Wilson, E.; et al. Quantitative and Qualitative Assessment Methods for Biofilm Growth: A Mini-review. Res. Rev. J. Eng. Technol. 2017, 6. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30214915%0Ahttp://www.bmedcentral.nih.gov/articlerender.fcgi?artid=PMC6133255 (accessed on 14 December 2020).
- Byrne, B.A. Laboratory Diagnosis of Bacterial Infections, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2007; pp. 236–244. [Google Scholar]
- Gooch, J.W. Disk Diffusion Method. In Laboratory Manual of Standardized Methods for Antimicrobial Sensitivity Tests for Bacteria Isolated from Aquatic Animals and Environment; Southeast Asian Fisheries Development Center: Bangkok, Thailand, 2004; pp. 13–29. [Google Scholar] [CrossRef]
- Simmonds, R.C. Bioethics and Animal Use in Programs of Research, Teaching, and Testing. In Management of Animal Care and Use Programs in Research, Education, and Testing; CRC Press: Boca Raton, FL, USA; Taylor&Francis: Boca Raton, FL, USA, 2017; pp. 35–62. [Google Scholar]
- Popa, V.; Lascar, I.; Valcu, M.; Sebe, I.T.; Caraban, B.; Margina, A.C. Bioethics in animal experimentation. ARS Med. Tomitana 2015, 21, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Parirokh, M.; Asgary, S.; Eghbal, M.J. The Effect of Different Suture Removal Time Intervals on Surgical Wound Healing. Iran. Endod. J. 2006, 1, 81–86. [Google Scholar]
- Trott, A.T. Suture Removal and Wound Aftercare. In Wounds and Lacerations, 4th ed.; Elsevier BV: Amsterdam, The Netherlands, 2012; pp. 288–293. [Google Scholar] [CrossRef]
- Hospodarova, V.; Singovszka, E.; Stevulova, N. Characterization of Cellulosic Fibers by FTIR Spectroscopy for Their Further Implementation to Building Materials. Am. J. Anal. Chem. 2018, 9, 303–310. [Google Scholar] [CrossRef] [Green Version]
- The Official Manual of GeoGebra. 2016, pp. 40–48. Available online: https://wiki.geogebra.org/GeoGebra-en-Manual.pdf (accessed on 12 March 2018).
- Stepanović, S.; Vuković, D.; Hola, V.; di Bonaventura, G.; Djukić, S.; Ćircović, I.; Ruzicka, F. Quantification of biofilm in microtiter plates. Apmis 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Gómez, J.; Gómez-Lus, M.L.; Bas, P.; Ramos, C.; Cafini, F.; Maestre, J.R.; Prieto, J. Es la cuantificación del biofilm un elemento diferenciador en la patogenia de bacilos gramnegativos? Rev. Esp. Quimioter. 2013, 26, 97–102. [Google Scholar]
- Balani, K.; Verma, V.; Agarwal, A.; Narayan, R. Physical, Thermal, and Mechanical Properties of Polymers. Biosurfaces 2015, 329–344. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Sharma, C.P. Review Paper: Absorbable Polymeric Surgical Sutures: Chemistry, Production, Properties, Biodegradability, and Performance. J. Biomater. Appl. 2010, 25, 291–366. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.E.; Um, I.C. Effect of molecular weight and concentration on crystallinity and post drawing of wet spun silk fibroin fiber. Fibers Polym. 2014, 15, 153–160. [Google Scholar] [CrossRef]
- Bhat, N.V.; Nadiger, G.S. Crystallinity in silk fibers: Partial acid hydrolysis and related studies. J. Appl. Polym. Sci. 1980, 25, 921–932. [Google Scholar] [CrossRef]
- Benčina, M.; Mavrič, T.; Junkar, I.; Bajt, A.; Krajnović, A.; Lakota, K.; Žigon, P.; Sodin-Semrl, S.; Kralj-Iglič, V.; Iglič, A. The Importance of Antibacterial Surfaces in Biomedical Applications. Advances in Biomembranes and Lipid Self-Assembly 2018, 28, 115–165. [Google Scholar] [CrossRef]
- Donlan, R. Biofilms: Microbial Life on Surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Song, J.; Birbach, N.L.; Hinestroza, J.P. Deposition of silver nanoparticles on cellulosic fibers via stabilization of carboxymethyl groups. Cellul. 2012, 19, 411–424. [Google Scholar] [CrossRef]
- Al-Maharma, A.Y.; Al-Huniti, N.S. Critical Review of the Parameters Affecting the Effectiveness of Moisture Absorption Treatments Used for Natural Composites. J. Compos. Sci. 2019, 3, 27. [Google Scholar] [CrossRef] [Green Version]
- Funabashi, M.; Ninomiya, F.; Kunioka, M. Biodegradability Evaluation of Polymers by ISO 14855-2. Int. J. Mol. Sci. 2009, 10, 3635–3654. [Google Scholar] [CrossRef] [Green Version]
- Zuo, B.; Dai, L.; Wu, Z. Analysis of structure and properties of biodegradable regenerated silk fibroin fibers. J. Mater. Sci. 2006, 41, 3357–3361. [Google Scholar] [CrossRef]
- Kathju, S.; Nistico, L.; Tower, I.; Lasko, L.-A.; Stoodley, P. Bacterial Biofilms on Implanted Suture Material Are a Cause of Surgical Site Infection. Surg. Infect. 2014, 15, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Dhom, J.; Bloes, D.A.; Peschel, A.; Hofmann, U.K. Bacterial adhesion to suture material in a contaminated wound model: Comparison of monofilament, braided, and barbed sutures. J. Orthop. Res. 2016, 35, 925–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzetti, M.; Dogša, I.; Stošicki, T.; Stopar, D.; Kalin, M.; Kobe, S.; Novak, S. The Influence of Surface Modification on Bacterial Adhesion to Titanium-Based Substrates. ACS Appl. Mater. Interfaces 2015, 7, 1644–1651. [Google Scholar] [CrossRef]
- Hammuel, C.; Yebpella, G.G.; A Shallangwa, G.; Magomya, A.M.; Agbajp, A.S. Phytochemical and antimicrobial screening of methanol and aqueous extracts of Agave sisalana. Acta Pol. Pharm. Drug Res. 2011, 68, 535–539. [Google Scholar]
- Kohli, D.; Hugar, S.M.; Bhat, K.G.; Shah, P.P.; Mundada, M.V.; Badakar, C.M. Comparative evaluation of the antimicrobial susceptibility and cytotoxicity of husk extract of Cocos nucifera and chlorhexidine as irrigating solutions against Enterococcus Faecalis, Prevotella Intermedia and Porphyromonas Gingivalis—An in-vitro study. J. Indian Soc. Pedod. Prev. Dent. 2018, 36, 142–150. [Google Scholar] [CrossRef]
- Chen, X.; Wei, L. Preparation of Antibacterial Silk and Analysis of Interface Formation Mechanism. J. Eng. Fibers Fabr. 2014, 9, 120–125. [Google Scholar] [CrossRef]
- Tan, N.S.; Wahli, W. Studying Wound Repair in the Mouse. Curr. Protoc. Mouse Biol. 2013, 3, 171–185. [Google Scholar] [CrossRef]
- Gazivoda, D.; Pelemis, D.; Vujaskovic, G. A clinical study on the influence of suturing material on oral wound healing. Vojn. Pregl. 2015, 72, 765–769. [Google Scholar] [CrossRef]


| Fibers | ODf Level |
|---|---|
| Commercial silk (3-0) | Moderate biofilm producer (++) |
| Coconut | Moderate biofilm producer (++) |
| Sisal | Weak biofilm producer (+) |
| Parameters | Crystallinity Index (%) | Average Mechanical Properties | Average Biodegradability (% Total Weight Loss) | Bacterial Plaque Formation | |
|---|---|---|---|---|---|
| UTS (MPa) | Young’s Modulus (GPa) | ||||
| Silk | 58–64 [61,62] | 217.55 ± 39.58 | 5.00 ± 1.11 | 0.60 | ++ |
| Coconut | 56.04 | 18.72 ± 8.10 | 0.04 ± 0.02 | 83.15% ± 18.96 | ++ |
| Sisal | 73.65 | 138.84 ± 72.41 | 2.76 ± 2.26 | 22.48% ± 3.58 | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guambo, M.P.R.; Spencer, L.; Vispo, N.S.; Vizuete, K.; Debut, A.; Whitehead, D.C.; Santos-Oliveira, R.; Alexis, F. Natural Cellulose Fibers for Surgical Suture Applications. Polymers 2020, 12, 3042. https://doi.org/10.3390/polym12123042
Guambo MPR, Spencer L, Vispo NS, Vizuete K, Debut A, Whitehead DC, Santos-Oliveira R, Alexis F. Natural Cellulose Fibers for Surgical Suture Applications. Polymers. 2020; 12(12):3042. https://doi.org/10.3390/polym12123042
Chicago/Turabian StyleGuambo, María Paula Romero, Lilian Spencer, Nelson Santiago Vispo, Karla Vizuete, Alexis Debut, Daniel C. Whitehead, Ralph Santos-Oliveira, and Frank Alexis. 2020. "Natural Cellulose Fibers for Surgical Suture Applications" Polymers 12, no. 12: 3042. https://doi.org/10.3390/polym12123042
APA StyleGuambo, M. P. R., Spencer, L., Vispo, N. S., Vizuete, K., Debut, A., Whitehead, D. C., Santos-Oliveira, R., & Alexis, F. (2020). Natural Cellulose Fibers for Surgical Suture Applications. Polymers, 12(12), 3042. https://doi.org/10.3390/polym12123042










