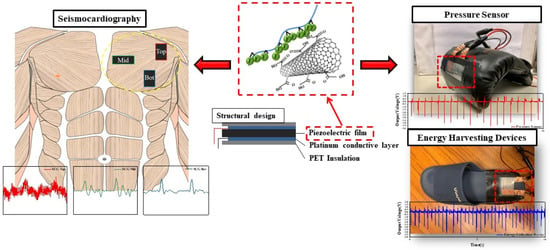
Enhanced Piezoelectric Properties of Poly(Vinylidenefluoride-Co-Trifluoroethylene)/Carbon-Based Nanomaterial Composite Films for Pressure Sensing Applications
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Carbon Nanomaterial/Poly(Vinylidenefluoride-Co-Trifluoroethylene) Composite Piezoelectric Film
2.3. Synthesis of Styrene Maleic Anhydride (SMA)-Amide
2.4. Preparation of Composite Piezoelectric Film with Modified Carbon Nanotube Dispersion
2.5. Characterization and Instruments
3. Results and Discussion
3.1. Poly(vinylidenefluoride-co-trifluoroethylene (PVDF-TrFE) Annealing Process and Polarization Treatment
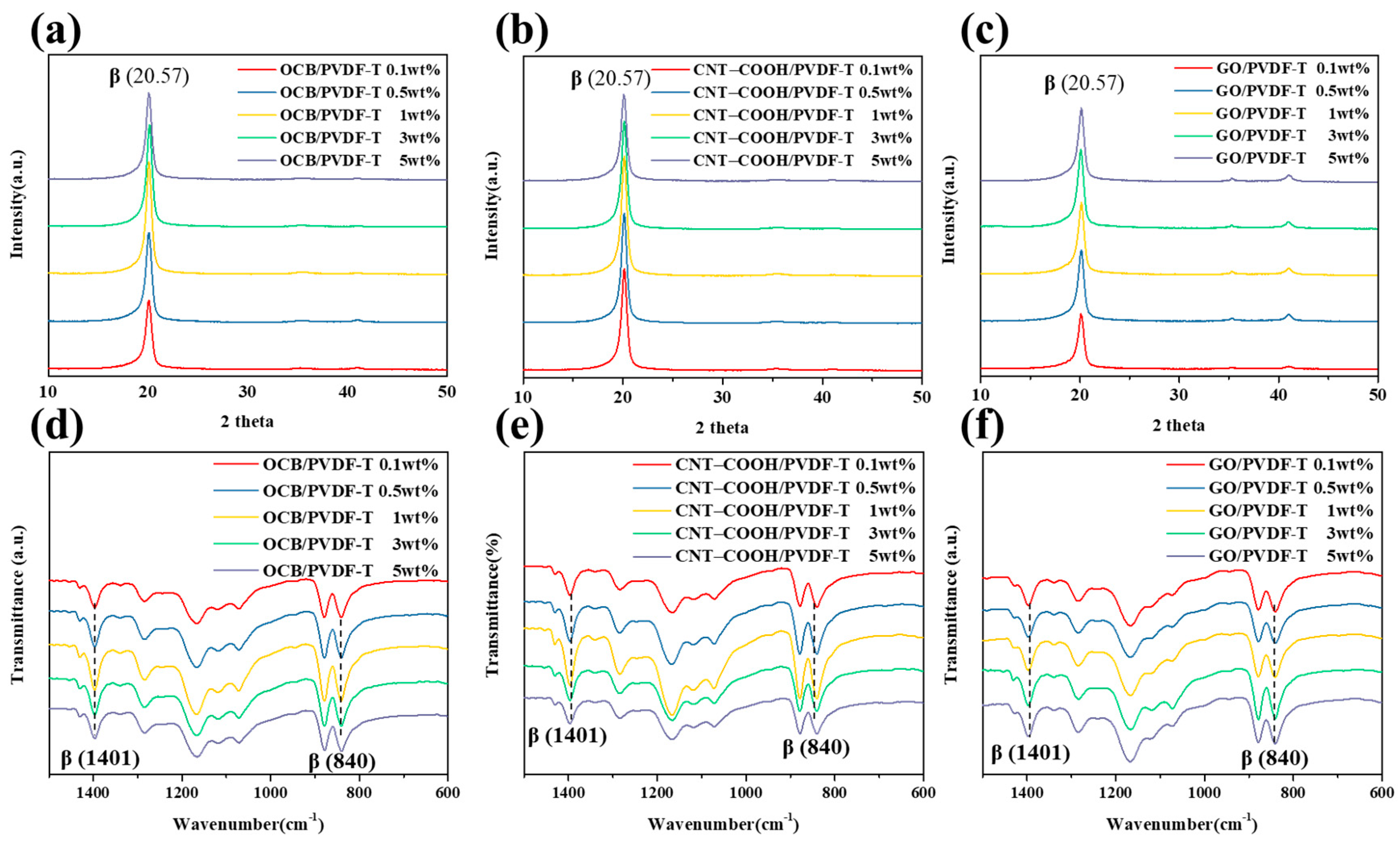
3.2. Carbon Nanomaterials/PVDF-TrFE Piezoelectric Composite Film
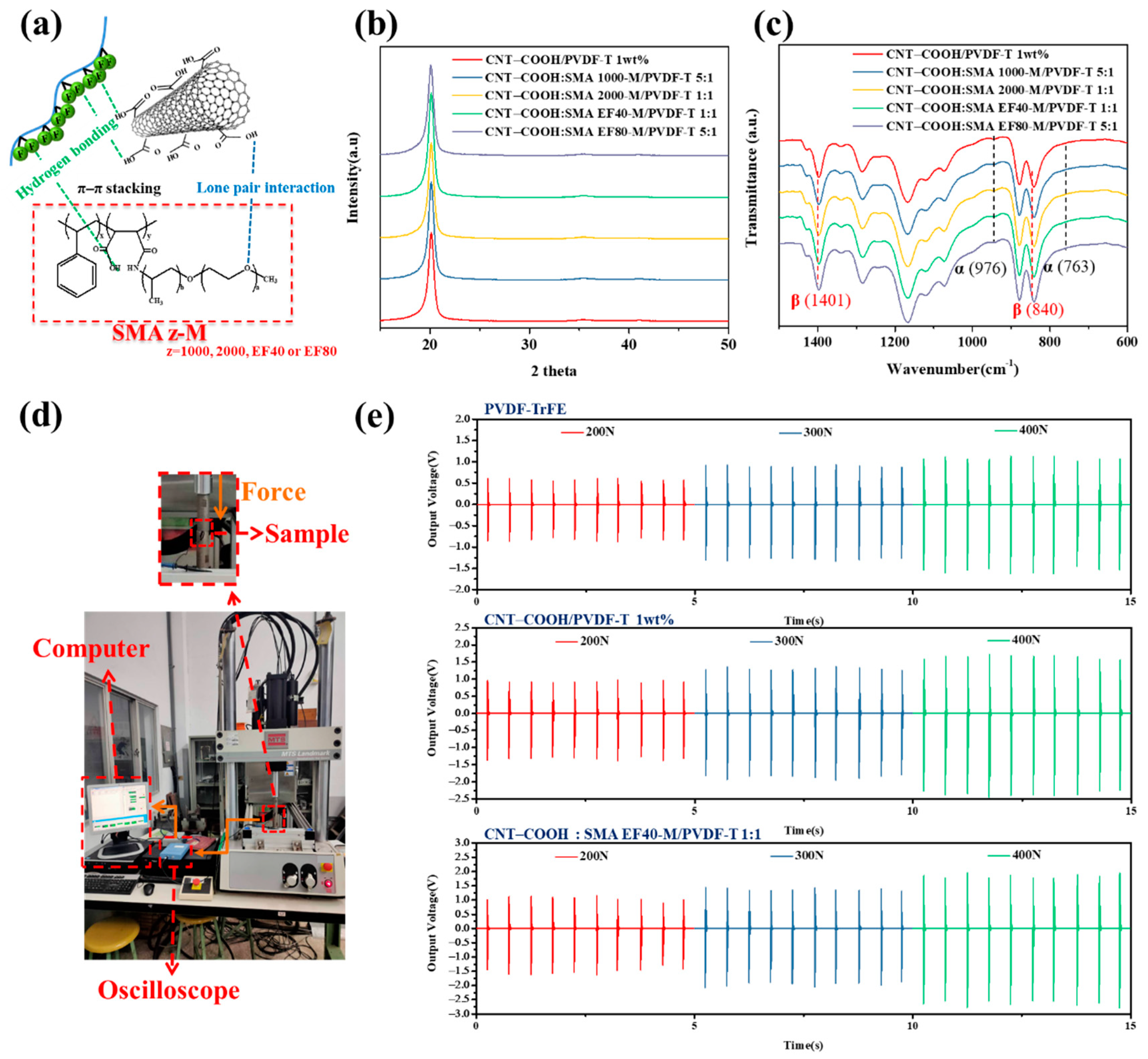
3.3. Dispersion Stability of CNT–COOH Using Polymeric Dispersant
3.4. CNT–COOH:SMAz-M/PVDF-T Piezoelectric Composite Film
3.5. Application of the CNT–COOH:SMAEF40-M/PVDF-T Piezoelectric Composite Film
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, X.; Yang, S.; Sun, Z.; Cui, N.; Zhao, P.; Tang, Q.; Tong, Y.; Liu, Y. Enhancing the intrinsic stretchability of micropatterned gold film by covalent linkage of carbon nanotubes for wearable electronics. ACS Appl. Electron. Mater. 2019, 1, 1295–1303. [Google Scholar] [CrossRef]
- Al-Milaji, K.N.; Huang, Q.; Li, Z.; Ng, T.N.; Zhao, H. Direct embedment and alignment of silver nanowires by inkjet printing for stretchable conductors. ACS Appl. Electron. Mater. 2020, 2, 3289–3298. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, X.; Ma, Y.; Feng, X. Stretchable self-powered generator for multiple functional detection. ACS Appl. Electron. Mater. 2020, 2, 3577–3584. [Google Scholar] [CrossRef]
- Gielen, D.; Boshell, F.; Saygin, D.; Bazilian, M.D.; Wagner, N.; Gorini, R. The role of renewable energy in the global energy transformation. Energy Strat. Rev. 2019, 24, 38–50. [Google Scholar] [CrossRef]
- Bardineh, Y.H.; Mohamadian, F.; Ahmadi, M.H.; Akbarianrad, N. Medical and dental applications of renewable energy systems. Int. J. Low-Carbon Technol. 2018, 13, 320–326. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, H.; Qi, L.; Zhang, Z.; Yuan, Y.; Liu, Y. A renewable energy harvesting system using a mechanical vibration rectifier (MVR) for railroads. Appl. Energy 2017, 204, 1535–1543. [Google Scholar] [CrossRef]
- Khan, F.U.; Qadir, M.U. State-of-the-art in vibration-based electrostatic energy harvesting. J. Micromech. Microeng. 2016, 26, 103001. [Google Scholar] [CrossRef]
- Sharma, H.B.; Sarma, H.N.K.; Mansingh, A. Ferroelectric and dielectric properties of sol-gel processed barium titanate ceramics and thin films. J. Mater. Sci. 1999, 34, 1385–1390. [Google Scholar] [CrossRef]
- Ponnamma, D.; Chamakh, M.M.; Alahzm, A.M.; Salim, N.V.; Hameed, N.; Al-Maadeed, M. Core-shell nanofibers of polyvinylidene fluoride-based nanocomposites as piezoelectric nanogenerators. Polymers 2020, 12, 2344. [Google Scholar] [CrossRef]
- Benz, M.; Euler, W.B. Determination of the crystalline phases of poly(vinylidene fluoride) under different preparation conditions using differential scanning calorimetry and infrared spectroscopy. J. Appl. Polym. Sci. 2003, 89, 1093–1100. [Google Scholar] [CrossRef]
- Zhang, Q.M.; Bharti, V.; Zhao, X. Giant Electrostriction and relaxor ferroelectric behavior in electron-irradiated poly(vinylidene fluoride-trifluoroethylene) copolymer. Science 1998, 280, 2101–2104. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, H.; Królikowski, B.; Chylińska, M.; Klimiec, E.; Bajer, D. Piezoelectric films based on polyethylene modified by aluminosilicate filler. Polymers 2019, 11, 1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortunato, M.; Cavallini, D.; De Bellis, G.; Marra, F.; Tamburrano, A.; Sarto, M.S. Phase inversion in PVDF films with enhanced piezoresponse through spin-coating and quenching. Polymers 2019, 11, 1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Wang, J.; Zhang, M.; Zhou, W. Rheology of carbon nanotubes–filled poly(vinylidene fluoride) composites. Ind. Eng. Chem. Res. 2012, 51, 6705–6713. [Google Scholar] [CrossRef]
- Zhang, L.; Zha, D.-A.; Du, T.; Mei, S.; Shi, Z.; Jin, Z. Formation of superhydrophobic microspheres of poly(vinylidene fluoride– hexafluoropropylene)/graphene composite via gelation. Langmuir 2011, 27, 8943–8949. [Google Scholar] [CrossRef]
- Abdolmaleki, H.; Agarwala, S. PVDF-BaTiO3 nanocomposite inkjet inks with enhanced β-phase crystallinity for printed electronics. Polymers 2020, 12, 2430. [Google Scholar] [CrossRef]
- Singh, D.; Choudhary, A.; Garg, A. Flexible and robust piezoelectric polymer nanocomposites based energy harvesters. ACS Appl. Mater. Interfaces 2018, 10, 2793–2800. [Google Scholar] [CrossRef]
- Xu, P.; Fu, W.; Cui, Z.; Ding, Y. Synergistic promotion of polar phase crystallization of PVDF by ionic liquid with PEG segment. Appl. Surf. Sci. 2018, 444, 480–484. [Google Scholar] [CrossRef]
- Karan, S.K.; Mandal, D.; Khatua, B.B. Self-powered flexible Fe-doped RGO/PVDF nanocomposite: An excellent material for a piezoelectric energy harvester. Nanoscale 2015, 7, 10655–10666. [Google Scholar] [CrossRef]
- Shuai, C.; Zeng, Z.; Yang, Y.-W.; Qi, F.; Peng, S.; Yang, W.; He, C.; Wang, G.; Qian, G. Graphene oxide assists polyvinylidene fluoride scaffold to reconstruct electrical microenvironment of bone tissue. Mater. Des. 2020, 190, 108564. [Google Scholar] [CrossRef]
- Bhavanasi, V.; Kumar, V.; Parida, K.; Wang, J.; Lee, P.S. Enhanced piezoelectric energy harvesting performance of flexible PVDF-TrFE bilayer films with graphene oxide. ACS Appl. Mater. Interfaces 2016, 8, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Levi, N.; Czerw, R.; Xing, S.; Iyer, P.; Carroll, D.L. Properties of polyvinylidene difluoride−carbon nanotube blends. Nano Lett. 2004, 4, 1267–1271. [Google Scholar] [CrossRef]
- Pariy, I.O.; Ivanova, A.A.; Shvartsman, V.V.; Lupascu, D.C.; Sukhorukov, G.B.; Ludwig, T.; Bartasyte, A.; Mathur, S.; Surmenev, R.A.; Surmenev, R.A. Piezoelectric response in hybrid micropillar arrays of poly(vinylidene fluoride) and reduced graphene oxide. Polymers 2019, 11, 1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, C.-W.; Ou, G.-B. Facile preparation of highly electrically conductive films of silver nanoparticles finely dispersed in polyisobutylene-b-poly(oxyethylene)-b-polyisobutylene triblock copolymers and graphene oxide hybrid surfactants. RSC Adv. 2015, 5, 102462–102468. [Google Scholar] [CrossRef]
- Chiu, C.-W.; Lin, C.-A.; Hong, P.-D. Melt-spinning and thermal stability behavior of TiO2 nanoparticle/polypropylene nanocomposite fibers. J. Polym. Res. 2010, 18, 367–372. [Google Scholar] [CrossRef]
- Huang, P.-Y.; Chiu, C.-W.; Huang, C.-Y.; Shen, S.-Y.; Lee, Y.-C.; Cheng, C.-C.; Jeng, R.-J.; Lin, J.-J. Facile fabrication of flexible electrodes and immobilization of silver nanoparticles on nanoscale silicate platelets to form highly conductive nanohybrid films for wearable electronic devices. Nanomaterials 2019, 10, 65. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-C.; Chiu, C.-W. Immobilization and 3D hot-junction formation of gold nanoparticles on two-dimensional silicate nanoplatelets as substrates for high-efficiency surface-enhanced Raman scattering detection. Nanomaterials 2019, 9, 324. [Google Scholar] [CrossRef] [Green Version]
- Barretta, R.; De Sciarra, F.M. Variational nonlocal gradient elasticity for nano-beams. Int. J. Eng. Sci. 2019, 143, 73–91. [Google Scholar] [CrossRef]
- Barretta, R.; Caporale, A.; Faghidian, S.A.; Luciano, R.; De Sciarra, F.M.; Medaglia, C.M. A stress-driven local-nonlocal mixture model for Timoshenko nano-beams. Compos. Part B Eng. 2019, 164, 590–598. [Google Scholar] [CrossRef]
- Chiu, C.-W.; Li, J.-W.; Huang, C.-Y.; Yang, S.-S.; Soong, Y.-C.; Lin, C.-L.; Lee, J.C.-M.; Sanchez, W.A.L.; Cheng, C.-C.; Suen, M.-C. Controlling the structures, flexibility, conductivity stability of three-dimensional conductive networks of silver nanoparticles/carbon-based nanomaterials with nanodispersion and their application in wearable electronic sensors. Nanomaterials 2020, 10, 1009. [Google Scholar] [CrossRef]
- Arat, R.; Uyanık, N. Study of the morphological and thermal properties of polystyrene nanocomposites based on modified halloysite nanotubes with styrene-maleic anhydride copolymers. Mater. Today Commun. 2017, 13, 255–262. [Google Scholar] [CrossRef]
- Li, J.-W.; Tsen, W.-C.; Tsou, C.-H.; Suen, M.-C.; Chiu, C.-W. Synthetic environmentally friendly castor oil based-polyurethane with carbon black as a microphase separation promoter. Polymers 2019, 11, 1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.-T.; Li, J.-W.; Tsou, C.-H.; Pang, J.-C.; Chung, R.-J.; Chiu, C.-W. Polyurethane/nanosilver-doped halloysite nanocomposites: Thermal, mechanical properties, and antibacterial properties. Polymers 2020, 12, 2729. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Ma, L.; Wen, S.; Luo, J. Fracture of the intermolecular hydrogen bond network structure of glycerol modified by carbon nanotubes. J. Phys. Chem. C 2018, 122, 19931–19936. [Google Scholar] [CrossRef]

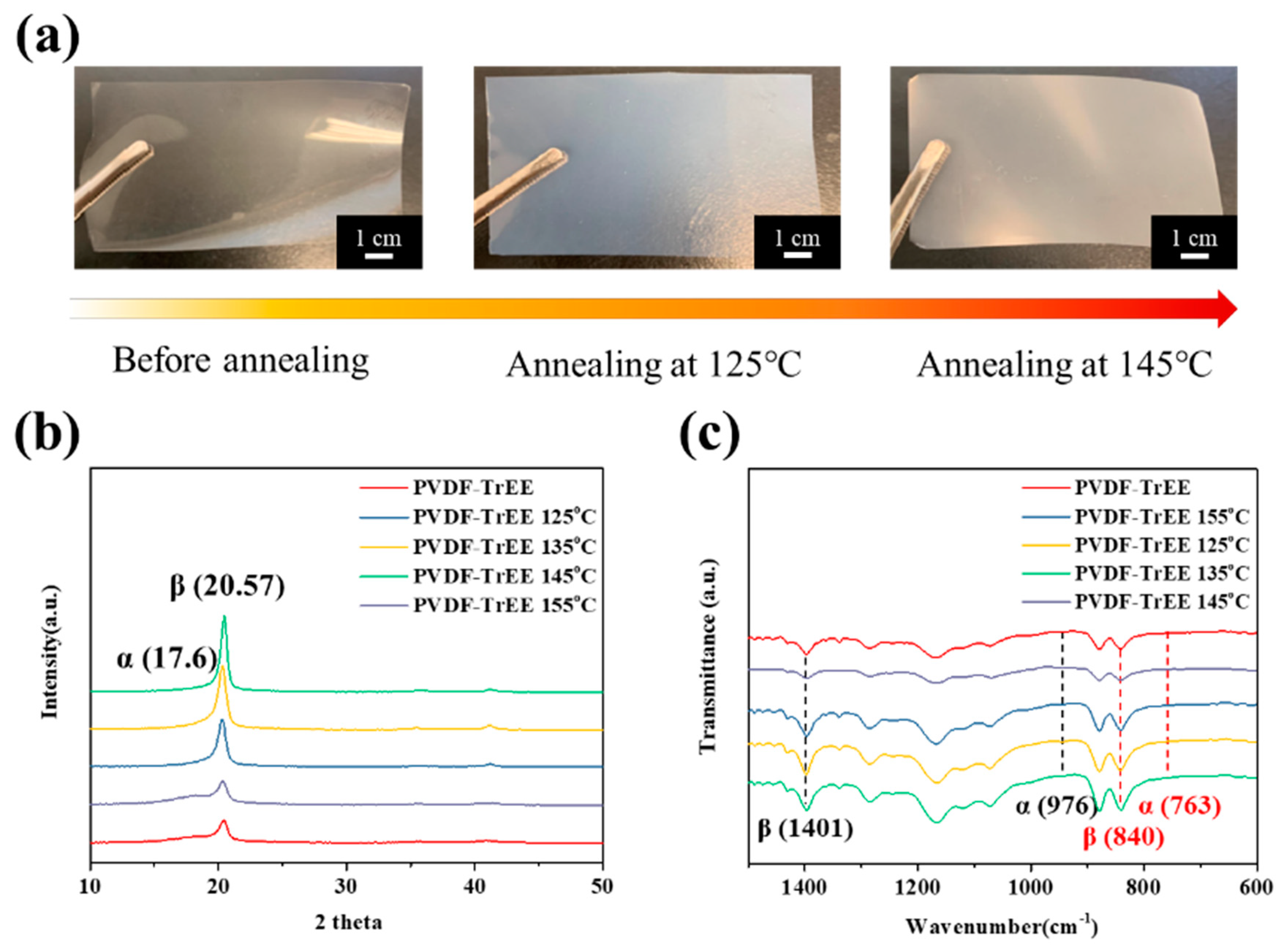



| Sample Name | PVDF-TrFE | PVDF-TrFE 135 °C | PVDF-TrFE 145 °C | PVDF-TrFE 155 °C | |||||
|---|---|---|---|---|---|---|---|---|---|
| Applied voltage (V) | 0 | 0 | 0 | 1000 | 2000 | 3000 | 4000 | 5000 | 0 |
| β/β0 | — | 91.1% | 131.7% | — | — | — | — | — | 23.9% |
| Crystallinity | 56.8% | 70.4% | 71.9% | — | — | — | — | — | 61.8% |
| F(β) | 62.1% | 66.8% | 69.5% | 70.4% | 70.8% | 71.3% | 73.6% | 73.6% | 55.6% |
| d33 (pC/N) | 1.8 ± 0.6 | — | 2.3 ± 0.8 | — | — | — | 19.8 ± 1.0 | — | — |
| Sample | Dispersant | Content (wt%) | Crystallinity (%) | F(β) (%) | d33 (pC/N) |
|---|---|---|---|---|---|
| OCB/PVDF-TrFE | — | 0.1 | 78.9 | 74.4 | — |
| — | 0.5 | 80.6 | 79.6 | — | |
| — | 1.0 | 83.1 | 80.9 | 23.2 ± 1.4 | |
| — | 3.0 | 82.4 | 78.3 | — | |
| — | 5.0 | 81.2 | 74.3 | — | |
| CNT–COOH/PVDF-TrFE | — | 0.1 | 82.1 | 75.2 | — |
| — | 0.5 | 82.9 | 77.9 | — | |
| — | 1.0 | 83.4 | 82.8 | 24.3 ± 1.3 | |
| SMA 1000 | 1.0 | 83.5 | 84.2 | — | |
| SMA 2000 | 1.0 | 83.6 | 85.1 | — | |
| SMA EF40 | 1.0 | 83.8 | 86.4 | 26.4 ± 1.3 | |
| SMA EF80 | 1.0 | 83.4 | 84.5 | — | |
| — | 3.0 | 81.8 | 76.6 | — | |
| — | 5.0 | 79.7 | 74.7 | — | |
| GO/PVDF-TrFE | — | 0.1 | 73.0 | 74.8 | — |
| — | 0.5 | 75.2 | 75.7 | — | |
| — | 1.0 | 75.4 | 76.5 | — | |
| — | 3.0 | 76.6 | 77.3 | 20.6 ± 1.3 | |
| — | 5.0 | 75.5 | 76.8 | — |
| Sample Name | 200 N | 300 N | 400 N | |||
|---|---|---|---|---|---|---|
| Max Voltage (V) | Avg. Voltage (V) | Max Voltage (V) | Avg. Voltage (V) | Max Voltage (V) | Avg. Voltage (V) | |
| PVDF-TrFE | 0.96 | 0.71 | 1.32 | 1.04 | 1.66 | 1.31 |
| CNT–COOH/PVDF-T 1 wt% | 1.46 | 1.22 | 1.98 | 1.61 | 2.50 | 2.16 |
| CNT–COOH:SMA EF40-M/PVDF-T 1:1 | 1.57 | 1.34 | 2.06 | 1.75 | 2.72 | 2.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.-W.; Huang, C.-Y.; Chen, K.-Y.; Chen, J.-X.; Hsu, X.-Y.; Chen, Y.-F.; Kuo, C.-F.J.; Cheng, C.-C.; Suen, M.-C.; Chiu, C.-W. Enhanced Piezoelectric Properties of Poly(Vinylidenefluoride-Co-Trifluoroethylene)/Carbon-Based Nanomaterial Composite Films for Pressure Sensing Applications. Polymers 2020, 12, 2999. https://doi.org/10.3390/polym12122999
Li J-W, Huang C-Y, Chen K-Y, Chen J-X, Hsu X-Y, Chen Y-F, Kuo C-FJ, Cheng C-C, Suen M-C, Chiu C-W. Enhanced Piezoelectric Properties of Poly(Vinylidenefluoride-Co-Trifluoroethylene)/Carbon-Based Nanomaterial Composite Films for Pressure Sensing Applications. Polymers. 2020; 12(12):2999. https://doi.org/10.3390/polym12122999
Chicago/Turabian StyleLi, Jia-Wun, Chen-Yang Huang, Kuan-Yu Chen, Jian-Xun Chen, Xiao-Yong Hsu, Yan-Feng Chen, Chung-Feng Jeffrey Kuo, Chih-Chia Cheng, Maw-Cherng Suen, and Chih-Wei Chiu. 2020. "Enhanced Piezoelectric Properties of Poly(Vinylidenefluoride-Co-Trifluoroethylene)/Carbon-Based Nanomaterial Composite Films for Pressure Sensing Applications" Polymers 12, no. 12: 2999. https://doi.org/10.3390/polym12122999
APA StyleLi, J.-W., Huang, C.-Y., Chen, K.-Y., Chen, J.-X., Hsu, X.-Y., Chen, Y.-F., Kuo, C.-F. J., Cheng, C.-C., Suen, M.-C., & Chiu, C.-W. (2020). Enhanced Piezoelectric Properties of Poly(Vinylidenefluoride-Co-Trifluoroethylene)/Carbon-Based Nanomaterial Composite Films for Pressure Sensing Applications. Polymers, 12(12), 2999. https://doi.org/10.3390/polym12122999