Physicochemical Characteristics of Arginine Enriched NaF Varnish: An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Varnish Preparation and Test Groups
- L-arginine + 5% NaF varnish groups:
- Group 1: 2% L-arginine + 5% NaF varnish (2% Arg-NaF);
- Group 2: 4% L-arginine + 5% NaF varnish (4% Arg-NaF) and;
- Group 3: 8% L-arginine + 5% NaF varnish (8% Arg-NaF).
- L-arginine monohydrochloride + 5% NaF varnish:
- Group 4: 2% L-arginine monohydrochloride + 5% NaF varnish (2% Arg.HCl-NaF);
- Group 5: 4% L-arginine monohydrochloride + 5% NaF varnish (4% Arg.HCl-NaF) and;
- Group 6: 8% L-arginine monohydrochloride + 5% NaF varnish (8% Arg.HCl-NaF).
- Controls:
- Group 7: 5% NaF varnish (NaF) and;
- Group 8: No varnish.
2.2. Physical Characterization
2.2.1. Varnish Adhesion
2.2.2. Varnish Viscosity
2.3. Chemical Characterization
2.3.1. Inorganic NaF and Organic F Extraction
2.3.2. Molecular Interaction of Arginine with Sodium Fluoride
2.3.3. Fluoride and Arginine Release Profiles
2.3.4. Fluoride Analysis
2.3.5. Arginine Detection
2.4. Statistical Analysis
3. Results
3.1. Physical Characterization
3.2. Chemical Characterization
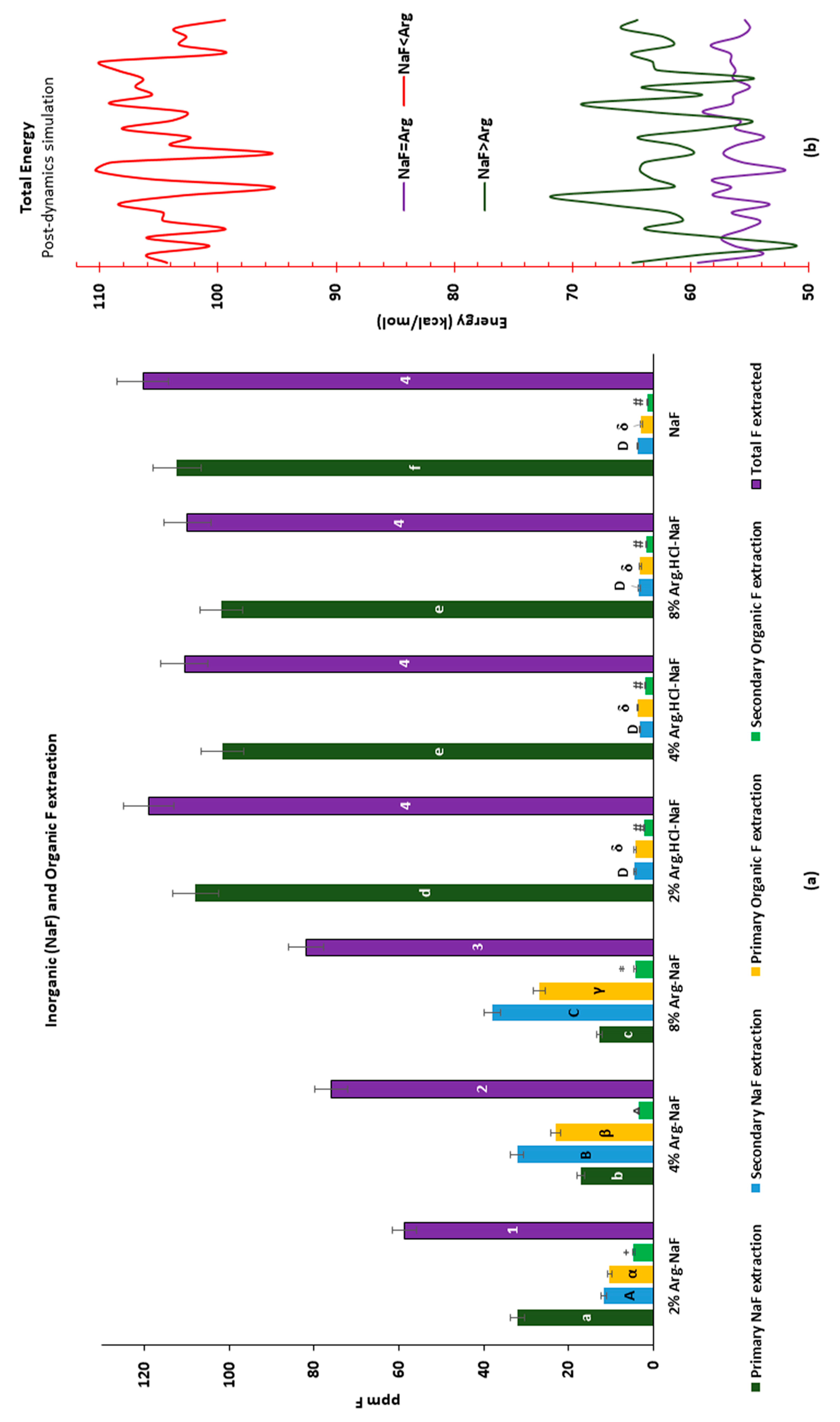
3.2.1. Inorganic NaF and Organic F Extraction
3.2.2. Molecular Interaction of Arginine with Sodium Fluoride
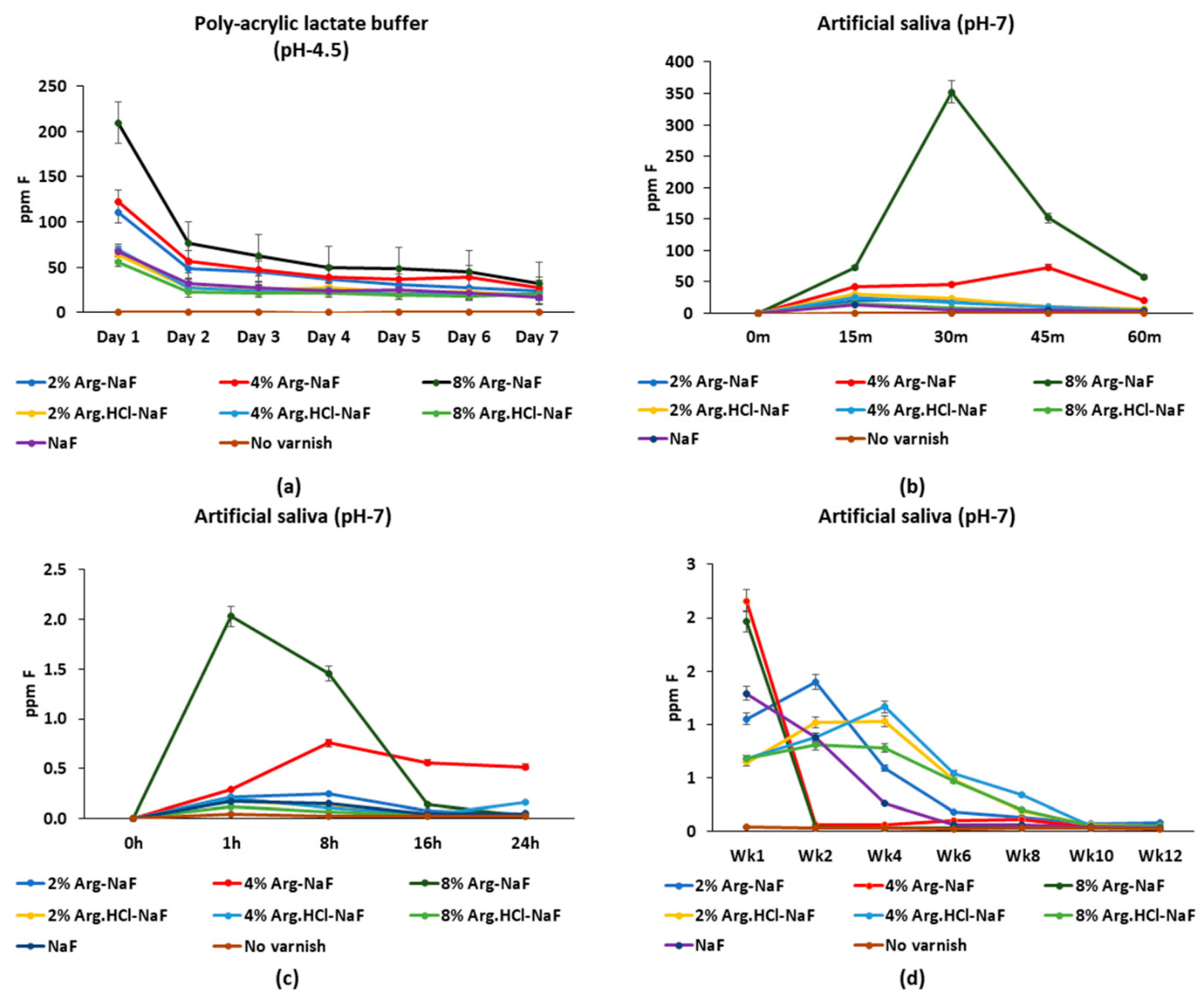
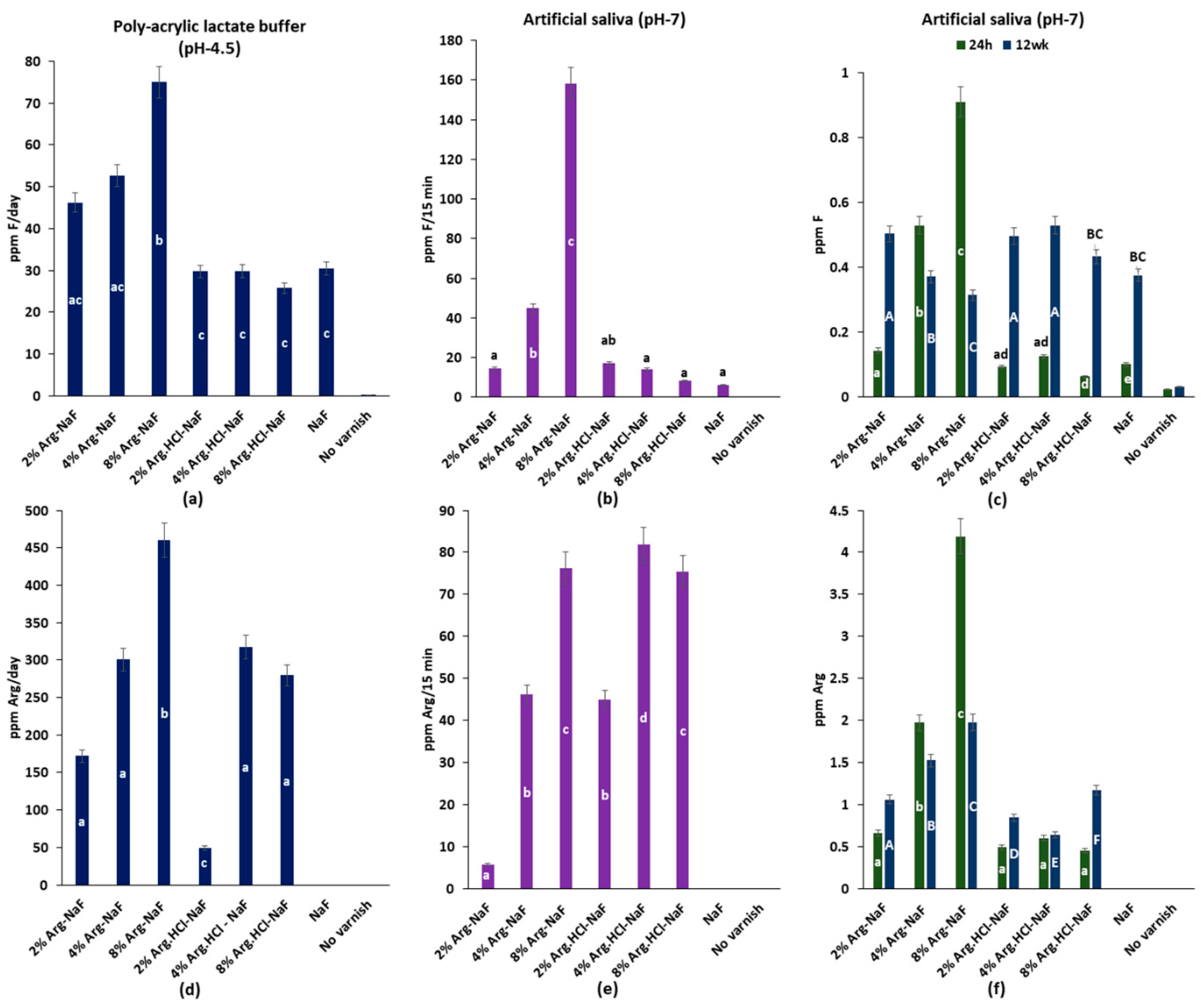
3.2.3. Fluoride and Arg Release Profile
Fluoride Release in Polyacrylic Lactate Buffer and Artificial Saliva
Arginine Release in Polyacrylic Lactate Buffer and Artificial Saliva
Integrated Fluoride/Arginine Release
4. Discussion
5. Conclusions
- L-arginine in 5% NaF varnish affected varnish physical and chemical properties; while L-arginine monohydrochloride in 5% NaF varnish had a non-contributory effect on varnish properties.
- Incorporating 2% L-arginine in 5% NaF varnish improved its physical properties and renders a stable matrix with enduring higher F/Arg release than control.
Author Contributions
Funding
Conflicts of Interest
References
- Kassebaum, N.J.; Smith, A.G.C.; Bernabé, E.; Fleming, T.D.; Reynolds, A.E.; Vos, T.; Murray, C.J.L.; Marcenes, W.; Abyu, G.Y.; Alsharif, U.; et al. Global, regional, and national prevalence, incidence, and disability-adjusted life years for oral conditions for 195 countries, 1990–2015: A systematic analysis for the global burden of diseases, injuries, and risk factors. J. Dent. Res. 2017, 96, 380–387. [Google Scholar] [CrossRef]
- Collaborators, G.B.D. Disease and Injury Incidence and Prevalence Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef] [Green Version]
- Philip, N.; Suneja, B.; Walsh, L. Beyond streptococcus mutans: Clinical implications of the evolving dental caries aetiological paradigms and its associated microbiome. Br. Dent. J. 2018, 224, 219–225. [Google Scholar] [CrossRef]
- MacHiulskiene, V.; Campus, G.; Carvalho, J.C.; Dige, I.; Ekstrand, K.R.; Jablonski-Momeni, A.; Maltz, M.; Manton, D.J.; Martignon, S.; Martinez-Mier, E.A.; et al. Terminology of Dental Caries and Dental Caries Management: Consensus Report of a Workshop Organized by ORCA and Cariology Research Group of IADR. Caries Res. 2020, 54, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.; Glenny, A.-M.; Worthington, H.V.; Marinho, V.C.; Appelbe, P. Fluoride toothpastes of different concentrations for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2019, 3, CD007868. [Google Scholar] [CrossRef] [PubMed]
- Marinho, V.C.C.; Worthington, H.V.; Walsh, T.; Clarkson, J.E. Fluoride varnishes for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Dang, M.H.; Jung, J.E.; Lee, D.W.; Song, K.Y.; Jeon, J.G. Recovery of acid production in Streptococcus mutans biofilms after short-term fluoride treatment. Caries Res. 2016, 50, 363–371. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, K.; Deng, M.; Exterkate, R.A.M.; Liu, C.; Zhou, X.; Cheng, L.; ten Cate, J.M. Effect of arginine on the growth and biofilm formation of oral bacteria. Arch. Oral Biol. 2017, 82, 256–262. [Google Scholar] [CrossRef]
- Agnello, M.; Cen, L.; Tran, N.C.; Shi, W.; McLean, J.S.; He, X. Arginine improves pH homeostasis via metabolism and microbiome modulation. J. Dent. Res. 2017, 96, 924–930. [Google Scholar] [CrossRef]
- Wijeyeweera, R.; Kleinberg, I. Arginolytic and ureolytic activities of pure cultures of human oral bacteria and their effects on the pH response of salivary sediment and dental plaque in vitro. Arch. Oral Biol. 1989, 34, 43–53. [Google Scholar] [CrossRef]
- Chakraborty, B.; Burne, R.A. Effects of arginine on Streptococcus mutans growth, virulence gene expression, and stress tolerance. Appl. Environ. Microbiol. 2017, 83, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.; Cheng, X.; Wang, L.; Qiu, W.; Wang, S.; Zhou, Y.; Li, M.; Li, Y.; Cheng, L.; Li, J.; et al. Combinatorial effects of arginine and fluoride on oral bacteria. J. Dent. Res. 2015, 94, 344–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, W.; Hu, D.Y.; Li, X.; Fan, X.; Zhang, Y.P.; Pretty, I.A.; Mateo, L.R.; Cummins, D.; Ellwood, R.P. The anti-caries efficacy of a dentifrice containing 1.5% arginine and 1450 ppm fluoride as sodium monofluorophosphate assessed using Quantitaive Light-induced Fluorescence (QLF). J. Dent. 2013, 41, S22–S28. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Hu, D.Y.; Li, X.; Fan, X.; Zhang, Y.P.; Pretty, I.A.; Mateo, L.R.; Cummins, D.; Ellwood, R.P. A clinical investigation using quantitative light-induced fluorescence (QLF) of the anticaries efficacy of a dentifrice containing 1.5% arginine and 1450 ppm fluoride as sodium monofluorophosphate. J. Clin. Dent. 2013, 24, A15–A22. [Google Scholar]
- Cummins, D. The development and validation of a new technology, based upon 1.5% arginine, an insoluble calcium compound and fluoride, for everyday use in the prevention and treatment of dental caries. J. Dent. 2013, 41, S1–S11. [Google Scholar] [CrossRef] [Green Version]
- Bijle, M.N.A.; Ekambaram, M.; Lo, E.C.; Yiu, C.K.Y. The combined enamel remineralization potential of arginine and fluoride toothpaste. J. Dent. 2018, 76, 75–82. [Google Scholar] [CrossRef]
- Lacombe, R. Pull Test. In Adhesion Measurement Methods; Taylor & Francis: Florida, NY, USA, 2005; pp. 14–17. ISBN 9781420028829. [Google Scholar]
- Shen, C.; Autio-Gold, J. Assessing fluoride concentration uniformity and fluoride release from three varnishes. J. Am. Dent. Assoc. 2002, 133, 176–182. [Google Scholar] [CrossRef]
- Shen, P.; Bagheri, R.; Walker, G.D.; Yuan, Y.; Stanton, D.P.; Reynolds, C.; Reynolds, E.C. Effect of calcium phosphate addition to fluoride containing dental varnishes on enamel demineralization. Aust. Dent. J. 2016, 61, 357–365. [Google Scholar] [CrossRef] [Green Version]
- Bolis, C.; Haertli, G.P.; Lendenmann, U. Fluoride varnishes—Is there a correlation between fluoride release and deposition on enamel? Oral Health Prev. Dent. 2015, 13, 545–556. [Google Scholar] [CrossRef]
- Jablonowski, B.; Bartoloni, J.; Hensley, D.; Vandewalle, K. Fluoride release from newly marketed fluoride varnishes. Quintessence Int. 2012, 43, 221–228. [Google Scholar]
- Ritwik, P.; Aubel, J.D.; Xu, X.; Fan, Y.; Hagan, J. Evaluation of short term fluoride release from fluoride varnishes. J. Clin. Pediatr. Dent. 2012, 36, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Srisilapanan, P.; Korwanich, N.; Yin, W.; Chuensuwonkul, C.; Mateo, L.R.; Zhang, Y.P.; Cummins, D.; Ellwood, R.P. Comparison of the efficacy of a dentifrice containing 1.5% arginine and 1450 ppm fluoride to a dentifrice containing 1450 ppm fluoride alone in the management of early coronal caries as assessed using Quantitative Light-induced Fluorescence. J. Dent. 2013, 41, S29–S34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abou Neel, E.A.; Aljabo, A.; Strange, A.; Ibrahim, S.; Coathup, M.; Young, A.M.; Bozec, L.; Mudera, V. Demineralization–remineralization dynamics in teeth and bone. Int. J. Nanomed. 2016, 11, 4743–4763. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Exterkate, R.A.M.; ten Cate, J.M. Factors Associated with Alkali Production from Arginine in Dental Biofilms. J. Dent. Res. 2012, 91, 1130–1134. [Google Scholar] [CrossRef] [Green Version]
- Fernández, C.E.; Tenuta, L.M.A.; Zárate, P.; Cury, J.A. Insoluble NaF in Duraphat® may prolong fluoride reactivity of varnish retained on dental surfaces. Braz. Dent. J. 2014, 25, 160–164. [Google Scholar] [CrossRef] [Green Version]
- Cruz, R.; Rölla, G. The effect of time of exposure on fluoride uptake by human enamel from acidulated fluoride solutions in vitro. Acta Odontol. Scand. 1992, 50, 51–56. [Google Scholar] [CrossRef]
- Cheng, X.; Xu, P.; Zhou, X.; Deng, M.; Cheng, L.; Li, M.; Li, Y.; Xu, X. Arginine promotes fluoride uptake into artificial carious lesions in vitro. Aust. Dent. J. 2015, 60, 104–111. [Google Scholar] [CrossRef]
- Milburn, J.L.; Henrichs, L.E. Substantive Fluoride Release from a New Fluoride Varnish Containing CXP. Dentistry 2015, 5. [Google Scholar] [CrossRef] [Green Version]
- Comar, L.; Souza, B.; Grizzo, L.; Buzalaf, M.; Magalhães, A. Evaluation of fluoride release from experimental TiF4 and NaF varnishes in vitro. J. Appl. Oral Sci. 2014, 22, 138–143. [Google Scholar] [CrossRef] [Green Version]
- Bonecker, M.J.S.; Rios, D.; Magalhaes, A.C.; Buzalaf, M.A.R.; Carvalho, T.S.; Peters, B.G.; Sampaio, F.C. Fluoride varnishes with calcium glycerophosphate: Fluoride release and effect on in vitro enamel demineralization. Braz. Oral Res. 2015, 29, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Manarelli, M.M.; Delbem, A.C.B.; Percinoto, C.; Pessan, J.P. Fluoride and sodium trimetaphosphate (TMP) release from fluoride varnishes supplemented with TMP. Braz. Oral Res. 2016, 30, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Varnish Weight on Substrates (g) | ||||
|---|---|---|---|---|
| Groups | 7-Day Demineralization Buffer | 1 h Artificial Saliva | 24 h Artificial Saliva | 12 w Artificial Saliva |
| 2% Arg -NaF | 0.67 ± 0.08 a | 0.20 ± 0.00 | 0.003 ± 0.001 | 0.003 ± 0.001 |
| 4% Arg -NaF | 0.77 ± 0.20 a | 0.20 ± 0.00 | 0.003 ± 0.001 | 0.003 ± 0.001 |
| 8% Arg -NaF | 0.72 ± 0.14 a | 0.20 ± 0.00 | 0.003 ± 0.001 | 0.003 ± 0.001 |
| 2% Arg.HCl -NaF | 0.69 ± 0.17 a | 0.20 ± 0.00 | 0.003 ± 0.001 | 0.003 ± 0.001 |
| 4% Arg.HCl -NaF | 0.79 ± 0.10 a | 0.20 ± 0.00 | 0.003 ± 0.001 | 0.003 ± 0.001 |
| 8% Arg.HCl -NaF | 0.59 ± 0.08 a | 0.20 ± 0.00 | 0.003 ± 0.001 | 0.003 ± 0.001 |
| NaF | 0.70 ± 0.13 a | 0.20 ± 0.00 | 0.003 ± 0.001 | 0.003 ± 0.001 |
| No varnish | 0.05 ± 0.00 b | - | - | - |
| One-way ANOVA with Tukey’s HSD post-hoc test; p < 0.05 is significant. Different superscripts identify significant differences between test groups | ||||
| p-value | <0.001 | 0.174 | 0.654 | 0.490 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bijle, M.N.; Ekambaram, M.; Lo, E.; Yiu, C. Physicochemical Characteristics of Arginine Enriched NaF Varnish: An In Vitro Study. Polymers 2020, 12, 2998. https://doi.org/10.3390/polym12122998
Bijle MN, Ekambaram M, Lo E, Yiu C. Physicochemical Characteristics of Arginine Enriched NaF Varnish: An In Vitro Study. Polymers. 2020; 12(12):2998. https://doi.org/10.3390/polym12122998
Chicago/Turabian StyleBijle, Mohammed Nadeem, Manikandan Ekambaram, Edward Lo, and Cynthia Yiu. 2020. "Physicochemical Characteristics of Arginine Enriched NaF Varnish: An In Vitro Study" Polymers 12, no. 12: 2998. https://doi.org/10.3390/polym12122998
APA StyleBijle, M. N., Ekambaram, M., Lo, E., & Yiu, C. (2020). Physicochemical Characteristics of Arginine Enriched NaF Varnish: An In Vitro Study. Polymers, 12(12), 2998. https://doi.org/10.3390/polym12122998







