Design of Thienothiophene-Based Copolymers with Various Side Chain-End Groups for Efficient Polymer Solar Cells
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.1.1. 4-Bromothiophene-3-carbaldehyde (1)
2.1.2. Ethyl Thieno[3,4-b]thiophene-2-carboxylate (2)
2.1.3. Thieno[3,4-b]thiophene-2ylmethanol (3)
2.1.4. Thieno[3,4-b]thiophene-2carbaldehyde (4)
2.1.5. 4,6-Dibromothieno[3,2-c]thiophene-2-carbaldehyde (5)
2.1.6. 2-((4,6-Dibromothieno[3,4-b]thiophen-2-yl)methylene)malononitrile (6)
2.1.7. (E)-2-(benzo[d]thiazol-2-yl)-3-(4,6-dibromothieno[3,4-b]thiophen-2-yl)acrylonitrile (7)
2.1.8. (Z)-5-((4,6-dibromothieno[3,4-b]thiophen-2-yl)methylene)-3-ethyl-2-thioxothiazolidin-4-one (8)
2.1.9. Synthesis of P1
2.1.10. Synthesis of P2
2.1.11. Synthesis of P3
2.2. Characterization of Copolymers
2.3. Fabrication and Characterization of PSCs
3. Results and Discussion
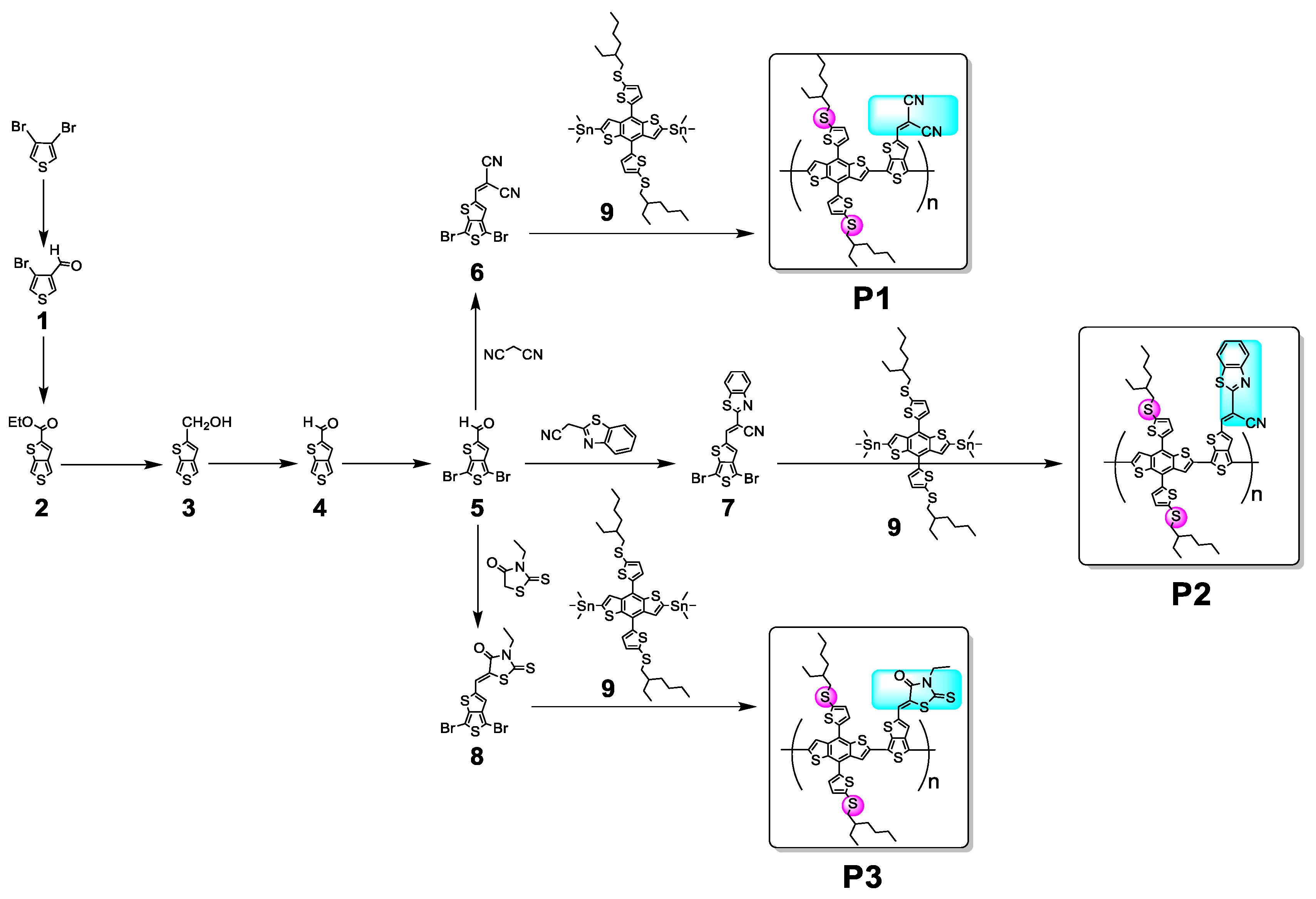
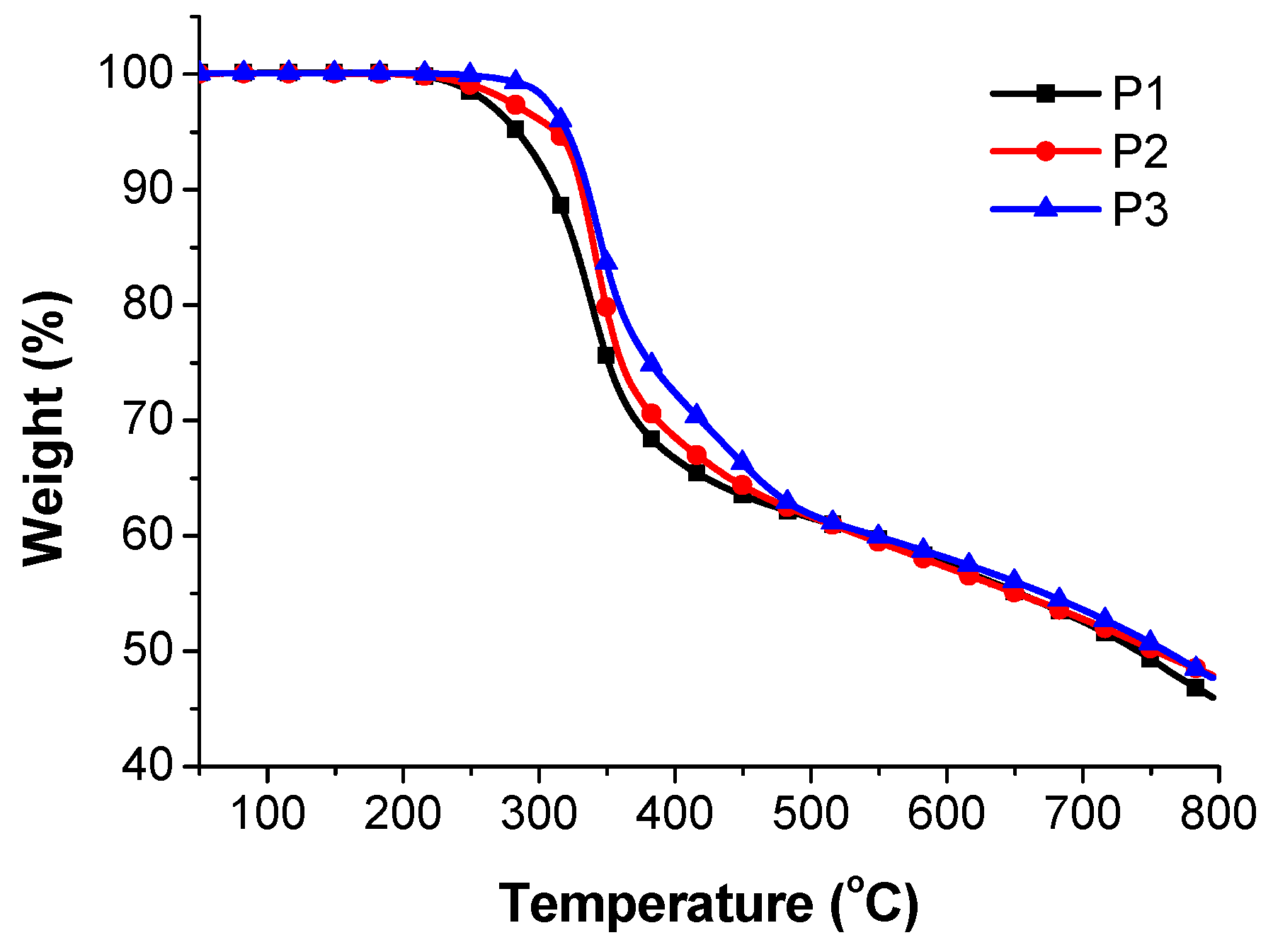
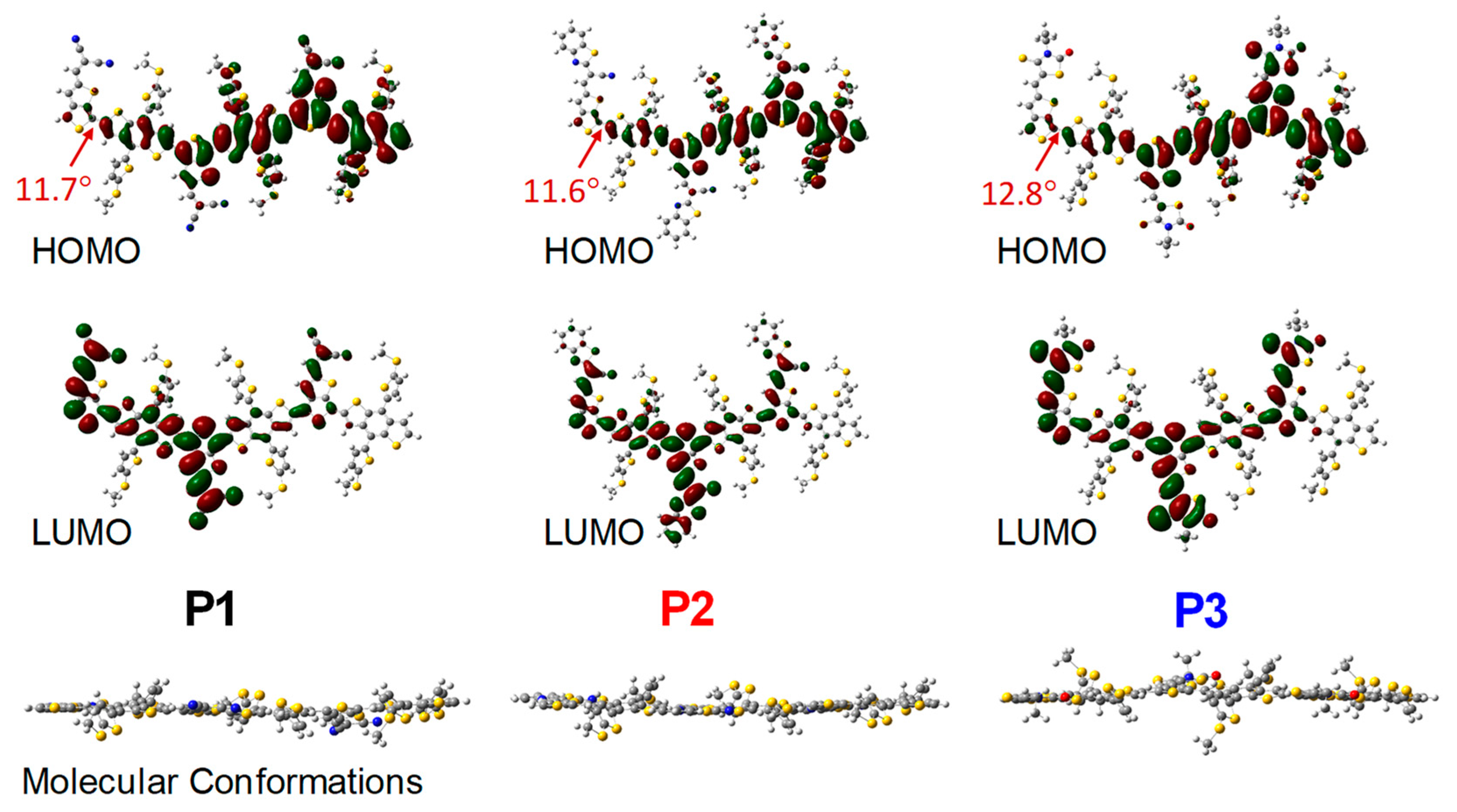
3.1. Characterization of Copolymers
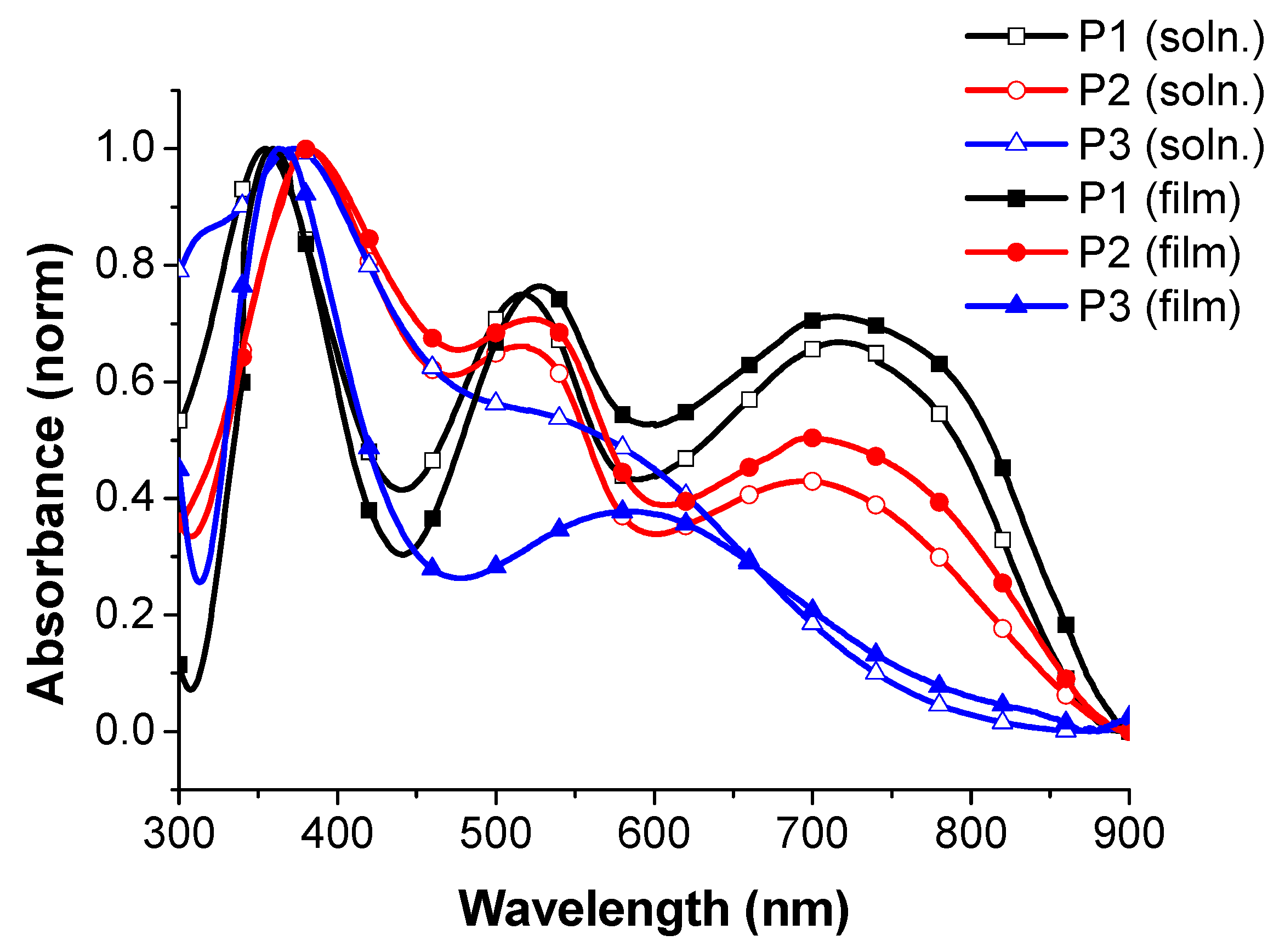
3.2. Photophysical Properties
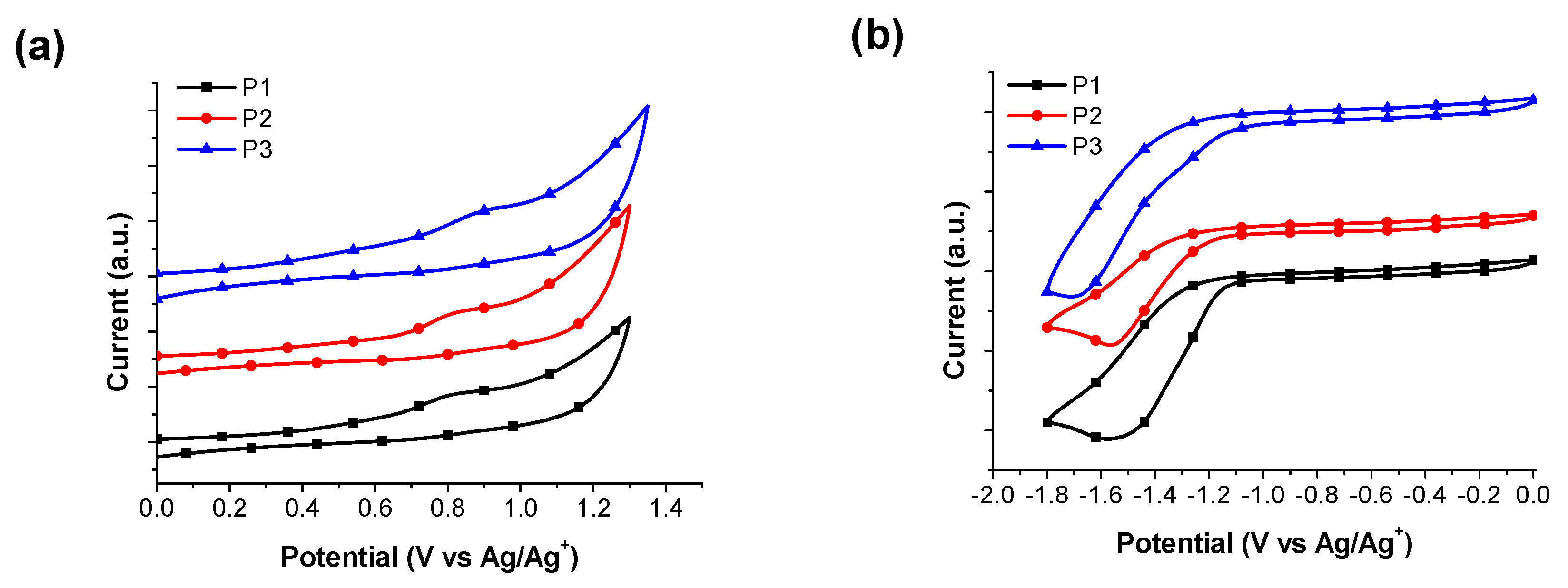
3.3. Energy Levels and Energy Gaps
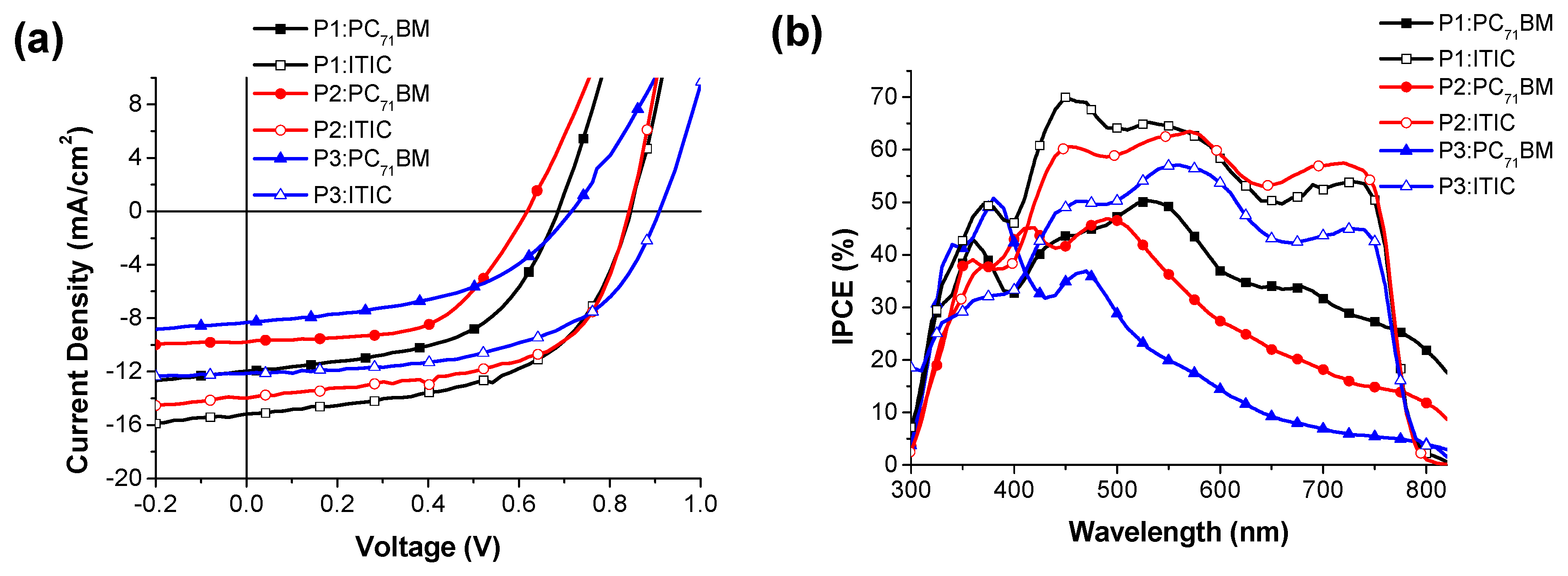
3.4. Photovoltaic Properties of PSCs
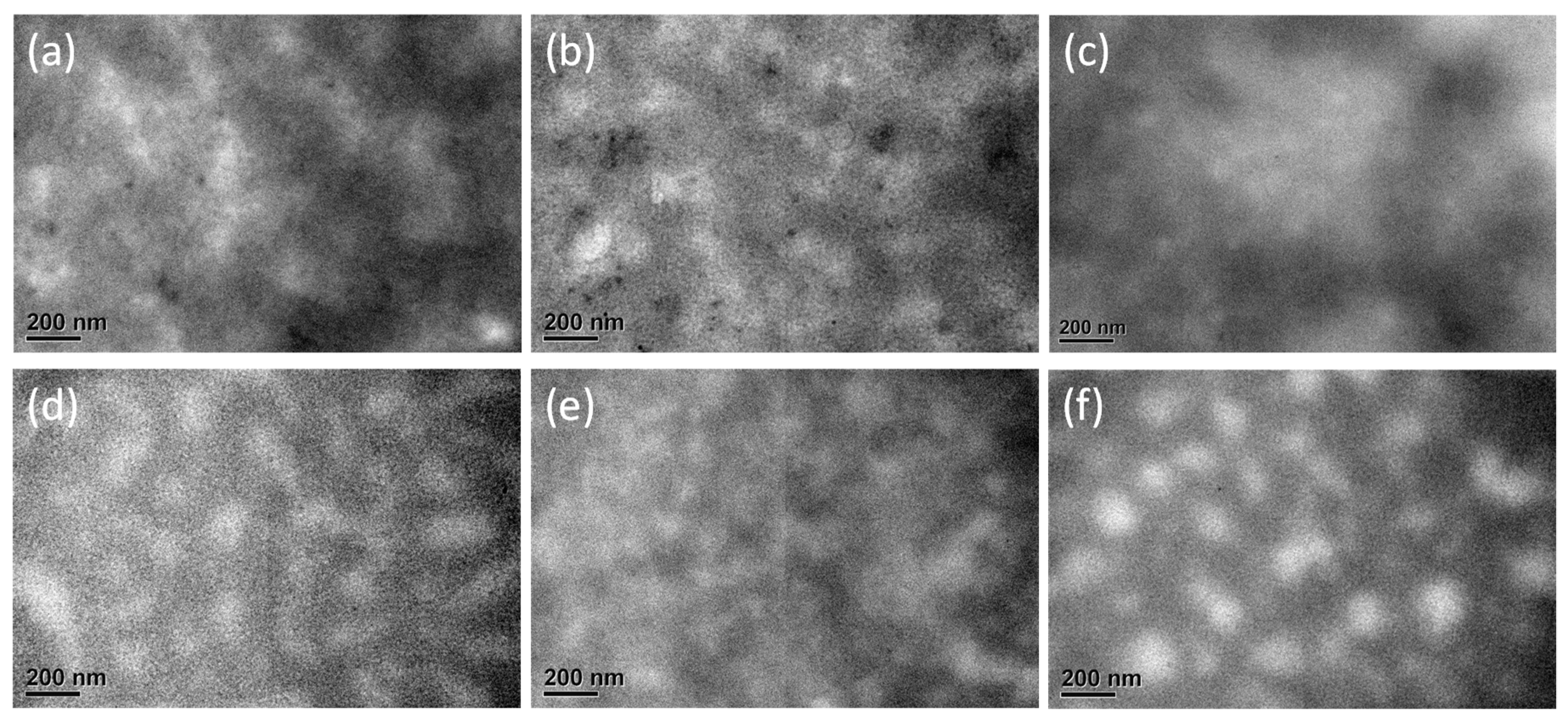
3.5. Analysis of Surface Morphologies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lu, L.; Zheng, T.; Wu, Q.; Schneider, A.M.; Zhao, D.; Yu, L. Recent advances in bulk heterojunction polymer solar cells. Chem. Rev. 2015, 115, 12666–12731. [Google Scholar] [CrossRef]
- Low, F.W.; Lai, C.W.; Hamid, S.B.A. Easy preparation of ultrathin reduced graphene oxide sheets at a high stirring speed. Ceram. Int. 2015, 41, 5798–5806. [Google Scholar] [CrossRef]
- Kashif, M.; Ngaini, Z.; Harry, A.V.; Vekariya, R.L.; Ahmad, A.; Zuo, Z.; Sahari, S.K.; Hussain, S.; Khan, Z.A.; Alarifi, A. An experimental and DFT study on novel dyes incorporated with natural dyes on titanium dioxide (TiO2) towards solar cell application. Appl. Phys. A Mater. 2020, 126, 716. [Google Scholar] [CrossRef]
- Zhao, W.; Li, S.; Yao, H.; Zhang, S.; Yang, B.; Hou, J. Molecular optimization enables over 13% efficiency in organic solar cells. J. Am. Chem. Soc. 2017, 139, 7148–7151. [Google Scholar] [CrossRef]
- Zhang, Y.; Kan, B.; Sun, Y.; Wang, Y.; Xia, R.; Ke, X.; Yi, Y.-Q.-Q.; Li, C.; Yip, H.-L.; Wan, X.; et al. Nonfullerene tandem organic solar cells with high performance of 14.11%. Adv. Mater. 2018, 30, 1707508. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, B.; Zhao, H.; Wang, L.; Wang, W.; Cong, Z.; Liu, J.; Ma, W.; Gao, C. Effects of Monofluorinated positions at the end-capping groups on the performances of twisted non-fullerene acceptor-based polymer solar cells. ACS Appl. Mater. Interfaces 2020, 12, 789–797. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, Y.; Wang, W.; Yan, C.; Rech, J.; Zhang, M.; You, W.; Lu, X.; Zhan, X. Pairing 1D/2D-conjugation donors/acceptors towards high-performance organic solar cells. Mater. Chem. Front. 2019, 3, 276–283. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, Y.; Jin, K.; Qin, J.; Xu, J.; Li, W.; Xiong, J.; Liu, J.; Xiao, Z.; Sun, K.; et al. 18% Efficiency organic solar cells. Sci. Bull. 2020, 65, 272–275. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.F.; Zou, Y.P. Conjugated polymer photovoltaic materials with broad absorption band and high Charge carrier mobility. Adv. Mater. 2008, 20, 2952–2958. [Google Scholar] [CrossRef]
- Chen, J.W.; Cao, Y. Development of novel conjugated donor polymers for high-efficiency bulk-heterojunction photovoltaic devices. Acc. Chem. Res. 2009, 42, 1709–1718. [Google Scholar] [CrossRef]
- Laquindanum, J.G.; Katz, H.E.; Lovinger, A.J.; Dodabalapur, A. Benzodithiophene Rings as Semiconductor Building Blocks. Adv. Mater. 1997, 9, 36–39. [Google Scholar] [CrossRef]
- Pan, H.; Li, Y.; Wu, Y.; Liu, P.; Ong, B.S.; Zhu, S.; Xu, G. Low-temperature, solution-processed, high-mobility polymer semiconductors for thin-film transistors. J. Am. Chem. Soc. 2007, 129, 4112–4113. [Google Scholar] [CrossRef]
- Piliego, C.; Holcombe, T.W.; Douglas, J.D.; Woo, C.H.; Beaujuge, P.M.; Fréchet, J.M. Synthetic control of structural order in N-alkylthieno[3,4-c]pyrrole-4,6-dione-based polymers for efficient solar cells. J. Am. Chem. Soc. 2010, 132, 7595–7597. [Google Scholar] [CrossRef]
- Cabanetos, C.; El Labban, A.; Bartelt, J.A.; Douglas, J.D.; Mateker, W.R.; Fréchet, J.M.; McGehee, M.D.; Beaujuge, P.M. Linear side chains in benzo[1,2-b:4,5-b′]dithiophene–thieno[3,4-c]pyrrole-4,6-dione polymers direct self-assembly and solar cell performance. J. Am. Chem. Soc. 2013, 135, 4656–4659. [Google Scholar] [CrossRef]
- Warnan, J.; Cbanetos, C.; Bude, R.; El Labban, A.; Li, L.; Beaujuge, P.M. Electron-deficient N-alkyloyl derivatives of thieno [3,4-c] pyrrole-4,6-dione yield efficient polymer solar cells with open-circuit voltages. Chem. Mater. 2014, 26, 2829–2835. [Google Scholar] [CrossRef]
- Wang, E.; Hou, L.; Wang, Z.; Hellström, S.; Zhang, F.; Inganäs, O.; Andersson, M.R. An easily synthesized blue polymer for high-performance polymer solar cells. Adv. Mater. 2010, 22, 5240–5244. [Google Scholar] [CrossRef]
- Zhou, E.; Cong, J.; Tajima, K.; Hashimoto, K. Synthesis and photovoltaic properties of donor–acceptor copolymers based on 5,8-dithien-2-yl-2,3-diphenylquinoxaline. Chem. Mater. 2010, 22, 4890–4895. [Google Scholar] [CrossRef]
- Gedefaw, D.; Tessarolo, M.; Zhuang, W.; Kroon, R.; Wang, E.; Bolognesi, M.; Seri, M.; Muccini, M.; Andersson, M.R. Conjugated polymers based on benzodithiophene and fluorinated quinoxaline for bulk heterojunction solar cells: Thiophene versus thieno[3,2-b]thiophene as π-conjugated spacers. Polym. Chem. 2014, 5, 2083–2093. [Google Scholar] [CrossRef]
- Park, S.H.; Roy, A.; Beaupre, S.; Cho, S.; Coates, N.; Moon, J.S.; Moses, D.; Leclerc, M.; Lee, K.; Heeger, A.J. Bulk heterojunction solar cells with internal quantum efficiency approaching 100%. Nat. Photonics 2009, 3, 297–302. [Google Scholar] [CrossRef]
- Subbiah, J.; Purushothaman, B.; Chen, M.; Qin, T.; Gao, M.; Vak, D.; Scholes, F.H.; Chen, X.; Watkins, S.E.; Wilson, G.J.; et al. Organic solar cells using a high-molecular-weight benzodithiophene-benzothiadiazole copolymer with an efficiency of 9.4%. Adv. Mater. 2015, 27, 702–705. [Google Scholar] [CrossRef]
- Qin, R.; Li, W.; Li, C.; Du, C.; Veit, C.; Schleiermacher, H.-F.; Andersson, M.; Bo, Z.; Liu, Z.; Inganäs, O.; et al. A planar copolymer for high efficiency polymer solar cells. J. Am. Chem. Soc. 2009, 131, 14612–14613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ye, L.; Wang, Q.; Li, Z.; Guo, X.; Huo, L.; Fan, H.; Hou, J. Enhanced photovoltaic performance of diketopyrrolopyrrole (DPP)-based polymers with extended π conjugation. J. Phys. Chem. C 2013, 117, 9550–9557. [Google Scholar] [CrossRef]
- Dou, L.; Gao, J.; Richard, E.; You, J.; Chen, C.-C.; Cha, K.C.; He, Y.; Li, G.; Yang, Y. Systematic investigation of benzodithiophene- and diketopyrrolopyrrole-based low-bandgap polymers designed for single junction and tandem polymer solar cells. J. Am. Chem. Soc. 2012, 134, 10071–10079. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.T.; You, J.B.; Yang, J.; Chen, C.C.; He, Y.J.; Murase, S.; Moriarty, T.; Emery, K.; Li, G.; Yang, Y. Tandem polymer solar cells featuring a spectrally matched low-bandgap polymer. Nat. Photonics 2012, 6, 180–185. [Google Scholar] [CrossRef]
- Liang, Y.; Feng, D.; Wu, Y.; Tsai, S.-T.; Li, G.; Ray, C.; Yu, L. Highly efficient solar cell polymers developed via fine-tuning of structural and electronic properties. J. Am. Chem. Soc. 2009, 131, 7792–7799. [Google Scholar] [CrossRef]
- Huo, L.; Zhang, S.; Guo, X.; Xu, F.; Li, Y.; Hou, J. Replacing alkoxy groups with alkylthienyl groups: A feasible approach to improve the properties of photovoltaic polymers. Angew. Chem. Int. Ed. 2011, 50, 9697–9702. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, S.; Zhao, W.; Yao, H.; Hou, J. Highly efficient 2D-conjugated benzodithiophene-based photovoltaic polymer with linear alkylthio side chain. Chem. Mater. 2014, 26, 3603–3605. [Google Scholar] [CrossRef]
- Yao, H.; Ye, L.; Zhang, H.; Li, S.; Zhang, S.; Hou, J. Molecular design of benzodithiophene-based organic photovoltaic materials. Chem. Rev. 2016, 116, 7397–7457. [Google Scholar] [CrossRef]
- Feng, K.; Xu, X.; Li, Z.; Li, Y.; Li, K.; Yu, T.; Peng, Q. Low band gap benzothiophene–thienothiophene copolymers with conjugated alkylthiothieyl and alkoxycarbonyl cyanovinyl side chains for photovoltaic applications. Chem. Commun. 2015, 51, 6290–6292. [Google Scholar] [CrossRef]
- Li, Y.; Chang, C.Y.; Chen, Y.; Song, Y.; Li, C.Z.; Yip, H.L.; Jen, A.K.-Y.; Li, C. The effect of thieno[3,2-b]thiophene on the absorption, charge mobility and photovoltaic performance of diketopyrrolopyrrole-based low bandgap conjugated polymers. J. Mater. Chem. C 2013, 1, 7526–7533. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, Z.; Xia, J.; Tsai, S.-T.; Wu, Y.; Li, G.; Ray, C.; Yu, L. For the bright future-bulk heterojunction polymer solar cells with power conversion efficiency of 7.4%. Adv. Mater. 2010, 22, E135–E138. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.-H.; Jhuo, H.-J.; Cheng, Y.-S.; Chen, S.-A. Fullerene derivative-doped zinc oxide nanofilm as the cathode of inverted polymer solar cells with low-bandgap polymer (PTB7-Th) for high performance. Adv. Mater. 2013, 25, 4766–4771. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-D.; Cui, C.; Li, Y.-Q.; Zhou, L.; Ou, Q.-D.; Li, C.; Li, Y.; Tang, J.-X. Single-junction polymer solar cells exceeding 10% power conversion efficiency. Adv. Mater. 2015, 27, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Wong, W.-Y.; Li, Y. Improvement of open-circuit voltage and photovoltaic properties of 2D-conjugated polymers by alkylthio substitution. Energy Environ. Sci. 2014, 7, 2276–2284. [Google Scholar] [CrossRef]
- Huang, Y.; Hou, L.; Zhang, S.; Guo, X.; Han, C.C.; Li, Y.; Hou, J. Sulfonyl: A new application of electron-withdrawing substituent in highly efficient photovoltaic polymer. Chem. Commun. 2011, 47, 8904–8906. [Google Scholar] [CrossRef]
- Kim, J.-H.; Park, J.B.; Yoon, S.C.; Jung, I.H.; Hwang, D.-H. Enhanced and controllable open-circuit voltage using 2D-conjugated benzodithiophene (BDT) homopolymers by alkylthio substitution. J. Mater. Chem. C 2016, 4, 2170–2177. [Google Scholar] [CrossRef]
- Sun, Y.; Seo, J.H.; Takacs, C.J.; Seifter, J.; Heeger, A.J. Inverted polymer solar cells integrated with a low-temperature-annealed sol-gel-derived ZnO film as an electron transport layer. Adv. Mater. 2011, 23, 1679–1683. [Google Scholar] [CrossRef]
- Park, J.H.; Seo, Y.G.; Yoon, D.H.; Lee, Y.-S.; Lee, S.-H.; Pyo, M.; Zong, K. A concise synthesis and electrochemical behavior of functionalized poly(thieno[3,4-b]thiophenes): New conjugated polymers with low bandgap. Eur. Polym. J. 2010, 46, 1790–1795. [Google Scholar] [CrossRef]
- Chen, L.; Shen, P.; Zhang, Z.-G.; Li, Y. Side-chain engineering of benzodithiophene–thiophene copolymers with conjugated side chains containing the electron-withdrawing ethylrhodanine group. J. Mater. Chem. A 2015, 3, 12005–12015. [Google Scholar] [CrossRef]
- Wen, S.; Chen, W.; Fan, M.; Duan, L.; Qiu, M.; Sun, M.; Han, L.; Yang, R. A diketopyrrolopyrrole-based low bandgap polymer with enhanced photovoltaic performances through backbone twisting. J. Mater. Chem. A 2016, 4, 18174–18180. [Google Scholar] [CrossRef]
- Chao, P.; Wang, H.; Mo, D.; Meng, H.; Chen, W.; He, F. Synergistic effects of chlorination and a fully two-dimensional side-chain design on molecular energy level modulation toward non-fullerene photovoltaics. J. Mater. Chem. A 2018, 6, 2942–2951. [Google Scholar] [CrossRef]
- Li, Z.; Liu, C.; Gan, G.; Sun, N.; Li, X.; Tong, Y.; Wang, H.; Hao, Y. Regio-asymmetric polymers based on fluorinated benzothiadiazole–benzodithiophene for polymer solar cells with a high open-circuit voltage. New J. Chem. 2019, 43, 3801–3809. [Google Scholar] [CrossRef]
- Zhu, T.; Liu, D.; Zhang, K.; Li, Y.; Liu, Z.; Gao, X.; Bao, X.; Sun, M.; Yang, R. Rational design of asymmetric benzodithiophene based photovoltaic polymers for efficient solar cells. J. Mater. Chem. A 2018, 6, 948–956. [Google Scholar] [CrossRef]
- Gu, C.; Liu, D.; Wang, J.; Niu, Q.; Gu, C.; Shahid, B.; Yu, B.; Cong, H.; Yang, R. Alkylthienyl substituted asymmetric 2D BDT and DTBT-based polymer solar cells with a power conversion efficiency of 9.2%. J. Mater. Chem. A 2018, 6, 2371–2378. [Google Scholar] [CrossRef]
- Wang, X.; Tong, J.; Guo, P.; Li, Y.; Li, H.; Xia, Y.; Wang, F. Low-bandgap conjugated polymers based on alkylthiothienyl-substituted benzodithiophene for efficient bulk heterojunction polymer solar cells. Polymer 2017, 122, 96–104. [Google Scholar] [CrossRef]

| Copolymers | Mn a (g/mol) | Mw a (g/mol) | PDI (Mw/Mn) | Tdb (°C) |
|---|---|---|---|---|
| P1 | 16,250 | 36,800 | 1.65 | 285 |
| P2 | 21,000 | 25,900 | 1.23 | 314 |
| P3 | 12,820 | 18,200 | 1.42 | 321 |
| Copolymers | In Solution a (nm) | In Film b (nm) | HOMO/LUMO c (eV) | d (V) |
|---|---|---|---|---|
| P1 | 355, 518, 717 | 359, 528, 718 | −5.50/−3.69 | 1.81 |
| P2 | 382, 518, 704 | 381, 524, 707 | −5.54/−3.65 | 1.89 |
| P3 | 373, 539 | 363, 587 | −5.60/−3.72 | 1.88 |
| Active Layer | Voc (V) | Jsc (mA/cm2) | FF (%) | PCE (%) | Thickness (nm) |
|---|---|---|---|---|---|
| P1:PC71BM | 0.682 ± 0.009 | 11.97 ± 0.15 | 54.09 ± 0.17 | 4.41 ± 0.24 | 120 |
| P1:ITIC | 0.842 ± 0.008 | 15.17 ± 0.17 | 56.76 ± 0.15 | 7.25 ± 0.31 | 115 |
| P2:PC71BM | 0.622 ± 0.009 | 9.78 ± 0.11 | 56.37 ± 0.12 | 3.43 ± 0.18 | 110 |
| P2:ITIC | 0.841 ± 0.009 | 13.98 ± 0.22 | 59.09 ± 0.19 | 6.95 ± 0.40 | 120 |
| P3:PC71BM | 0.702 ± 0.009 | 8.00 ± 0.10 | 50.11 ± 0.11 | 2.82 ± 0.15 | 125 |
| P3:ITIC | 0.902 ± 0.010 | 12.13 ± 0.19 | 55.69 ± 0.21 | 6.09 ± 0.37 | 108 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chao, Y.-C.; Chen, J.-H.; Chiou, Y.-J.; Kao, P.-l.; Wu, J.-L.; Chen, C.-T.; Chan, L.-H.; Jeng, R.-J. Design of Thienothiophene-Based Copolymers with Various Side Chain-End Groups for Efficient Polymer Solar Cells. Polymers 2020, 12, 2964. https://doi.org/10.3390/polym12122964
Chao Y-C, Chen J-H, Chiou Y-J, Kao P-l, Wu J-L, Chen C-T, Chan L-H, Jeng R-J. Design of Thienothiophene-Based Copolymers with Various Side Chain-End Groups for Efficient Polymer Solar Cells. Polymers. 2020; 12(12):2964. https://doi.org/10.3390/polym12122964
Chicago/Turabian StyleChao, Ying-Chieh, Jhe-Han Chen, Yi-Jie Chiou, Po-lin Kao, Jhao-Lin Wu, Chin-Ti Chen, Li-Hsin Chan, and Ru-Jong Jeng. 2020. "Design of Thienothiophene-Based Copolymers with Various Side Chain-End Groups for Efficient Polymer Solar Cells" Polymers 12, no. 12: 2964. https://doi.org/10.3390/polym12122964
APA StyleChao, Y.-C., Chen, J.-H., Chiou, Y.-J., Kao, P.-l., Wu, J.-L., Chen, C.-T., Chan, L.-H., & Jeng, R.-J. (2020). Design of Thienothiophene-Based Copolymers with Various Side Chain-End Groups for Efficient Polymer Solar Cells. Polymers, 12(12), 2964. https://doi.org/10.3390/polym12122964






