Resistivity-Temperature Behavior of Intrinsically Conducting Bis(3-methoxysalicylideniminato)nickel Polymer
Abstract
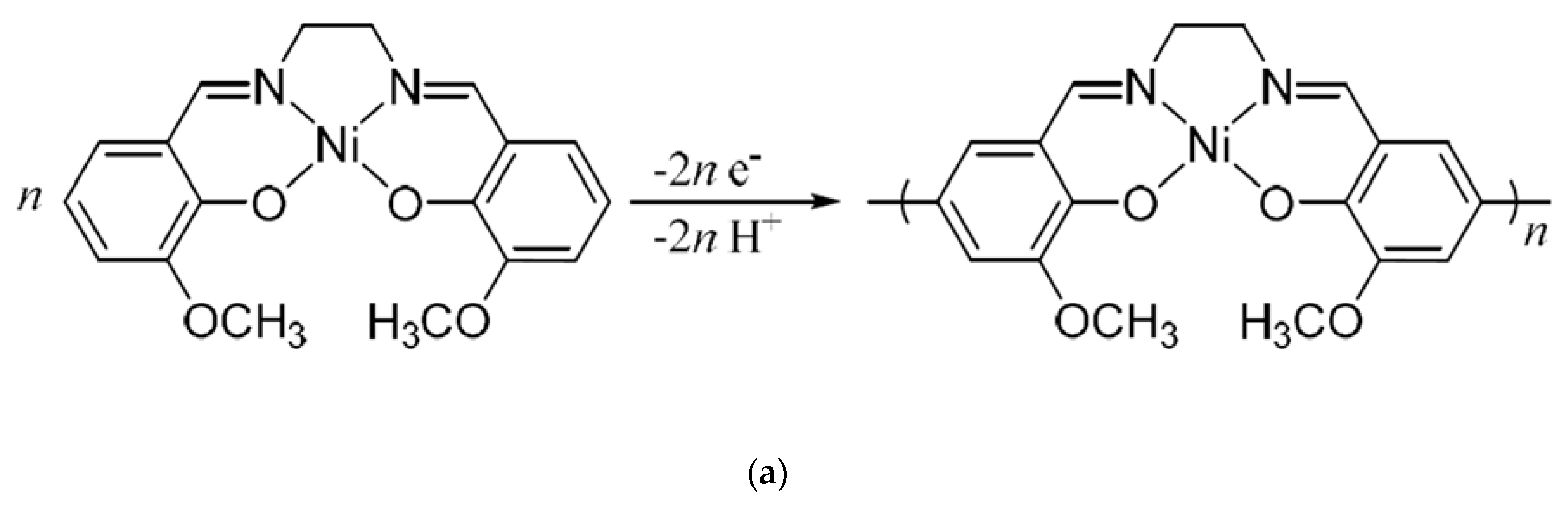
:1. Introduction
2. Materials and Methods
2.1. Materials
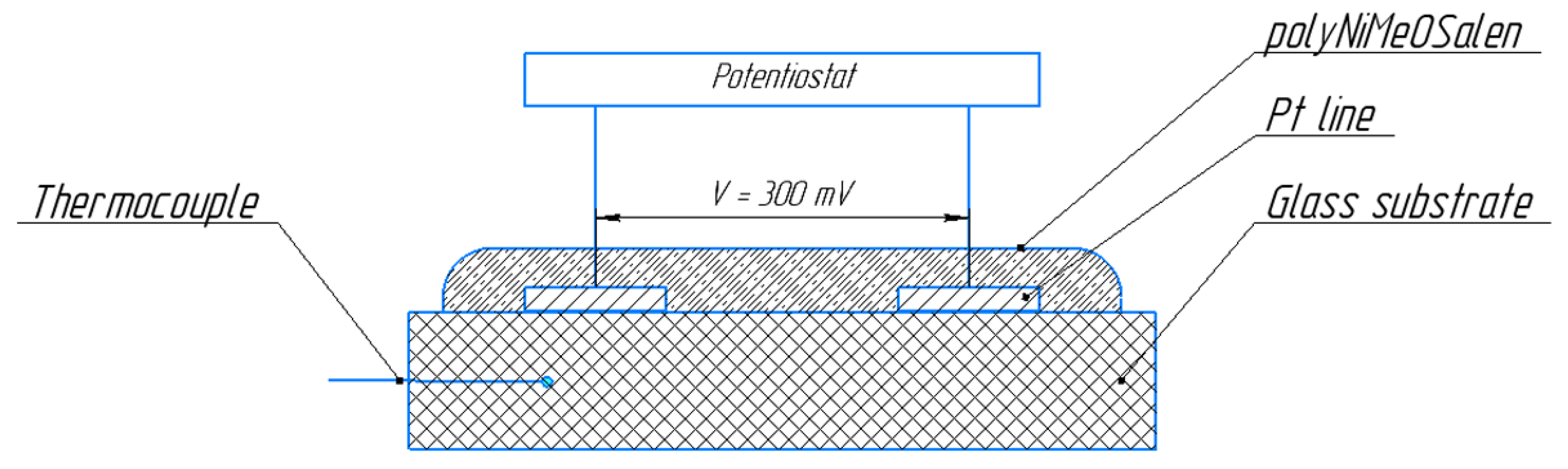
2.2. Temperature Tests
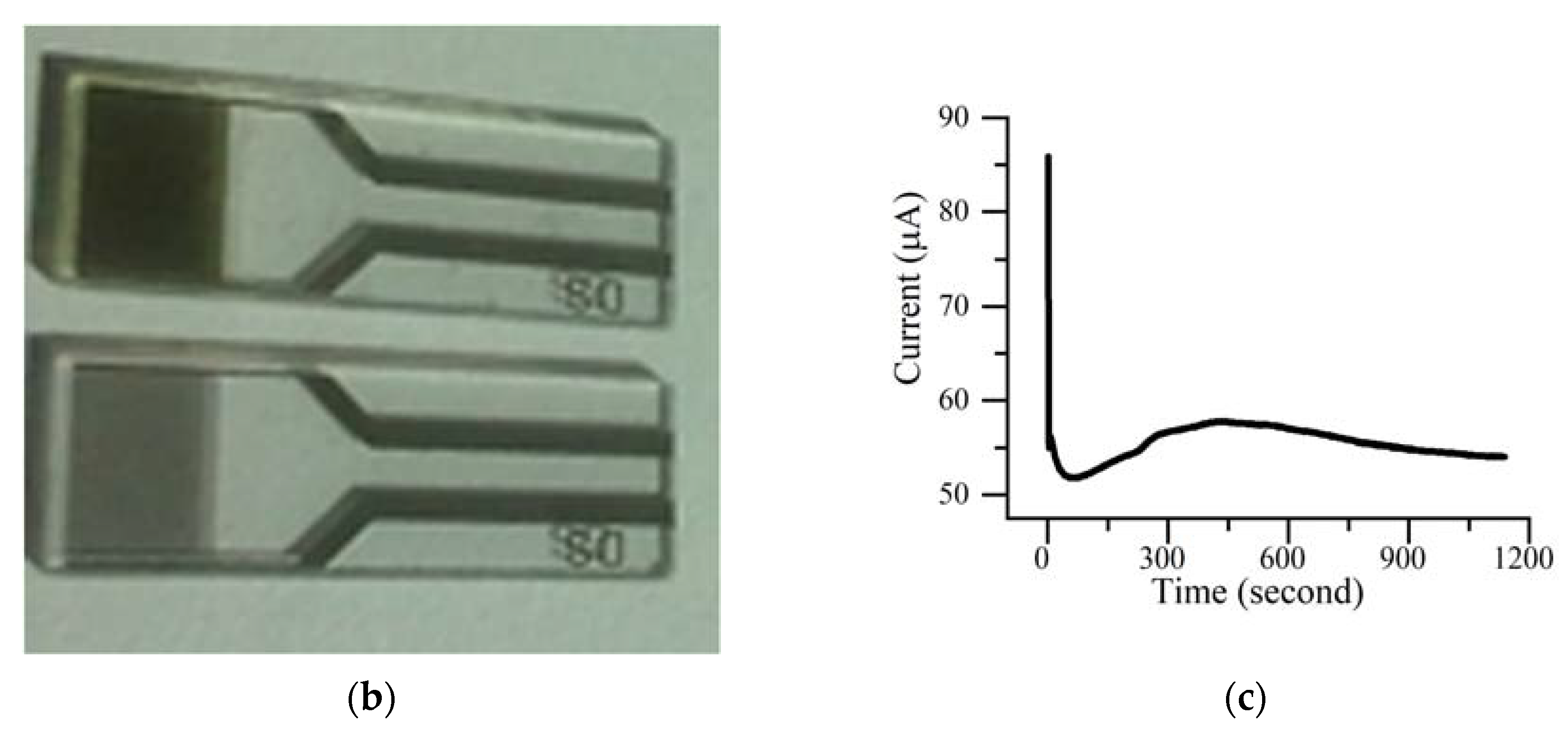
2.3. In Situ Conductivity Measurements in Solution
2.4. Characterization
3. Results and Discussion
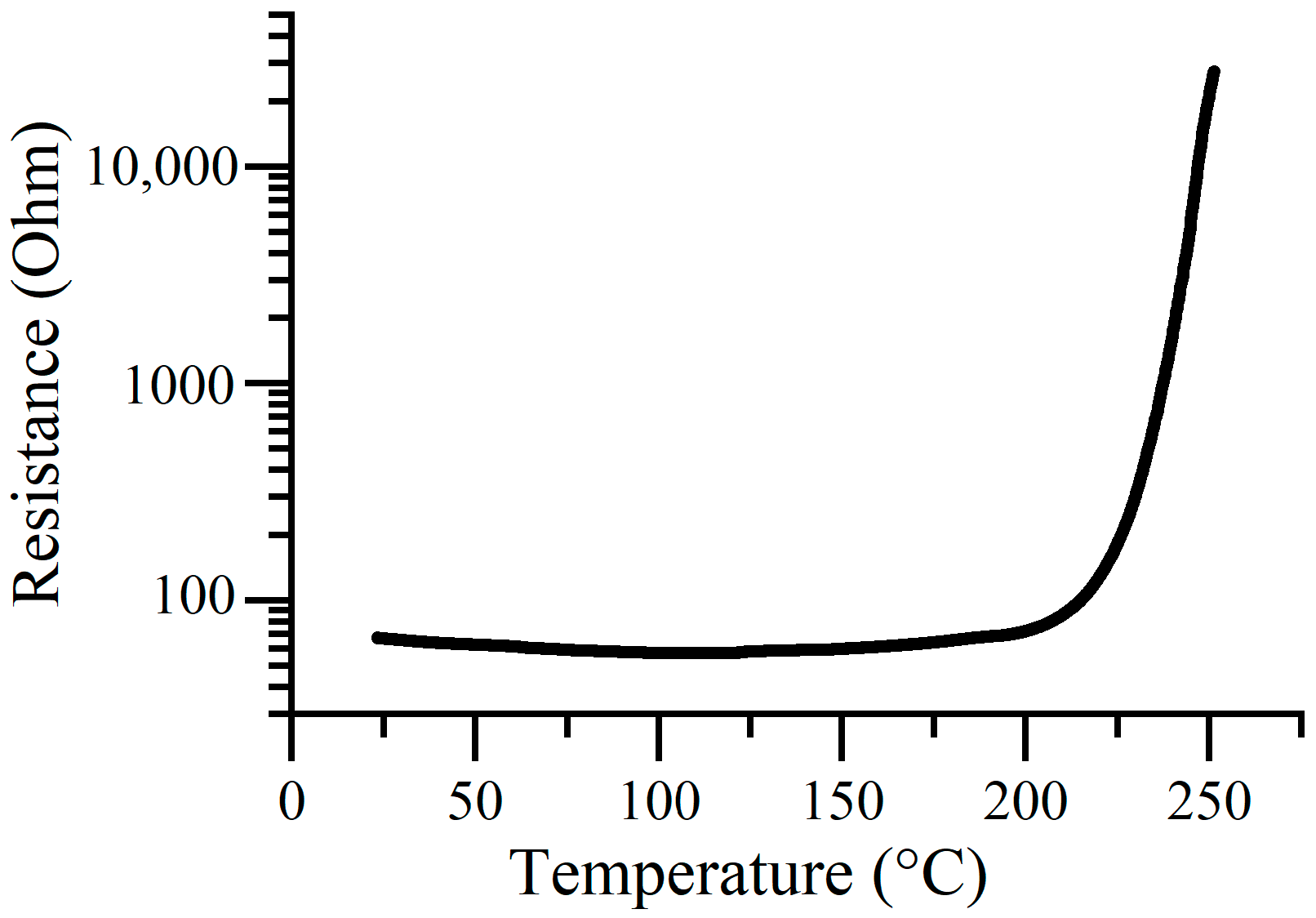
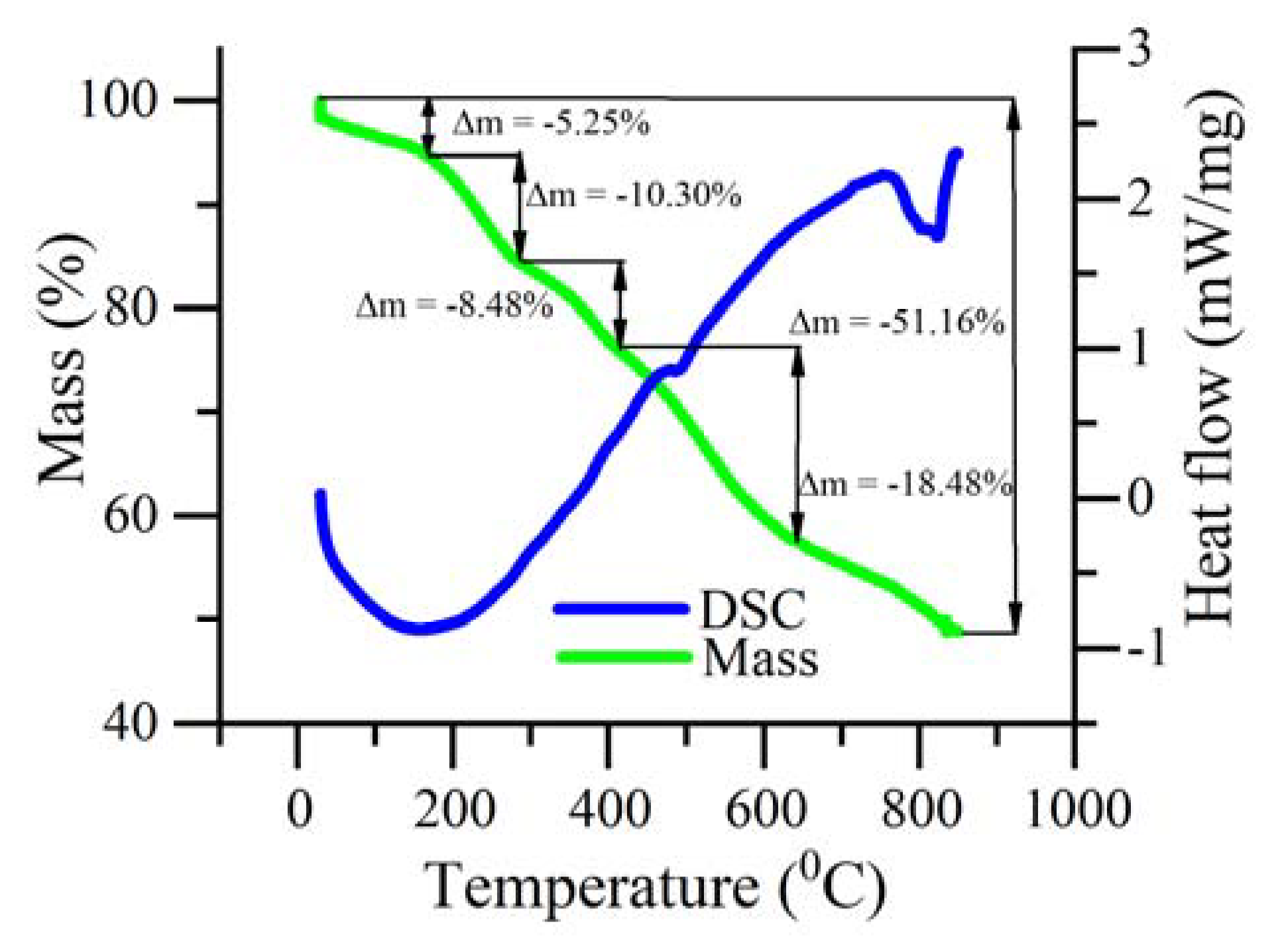
3.1. Temperature Dependence
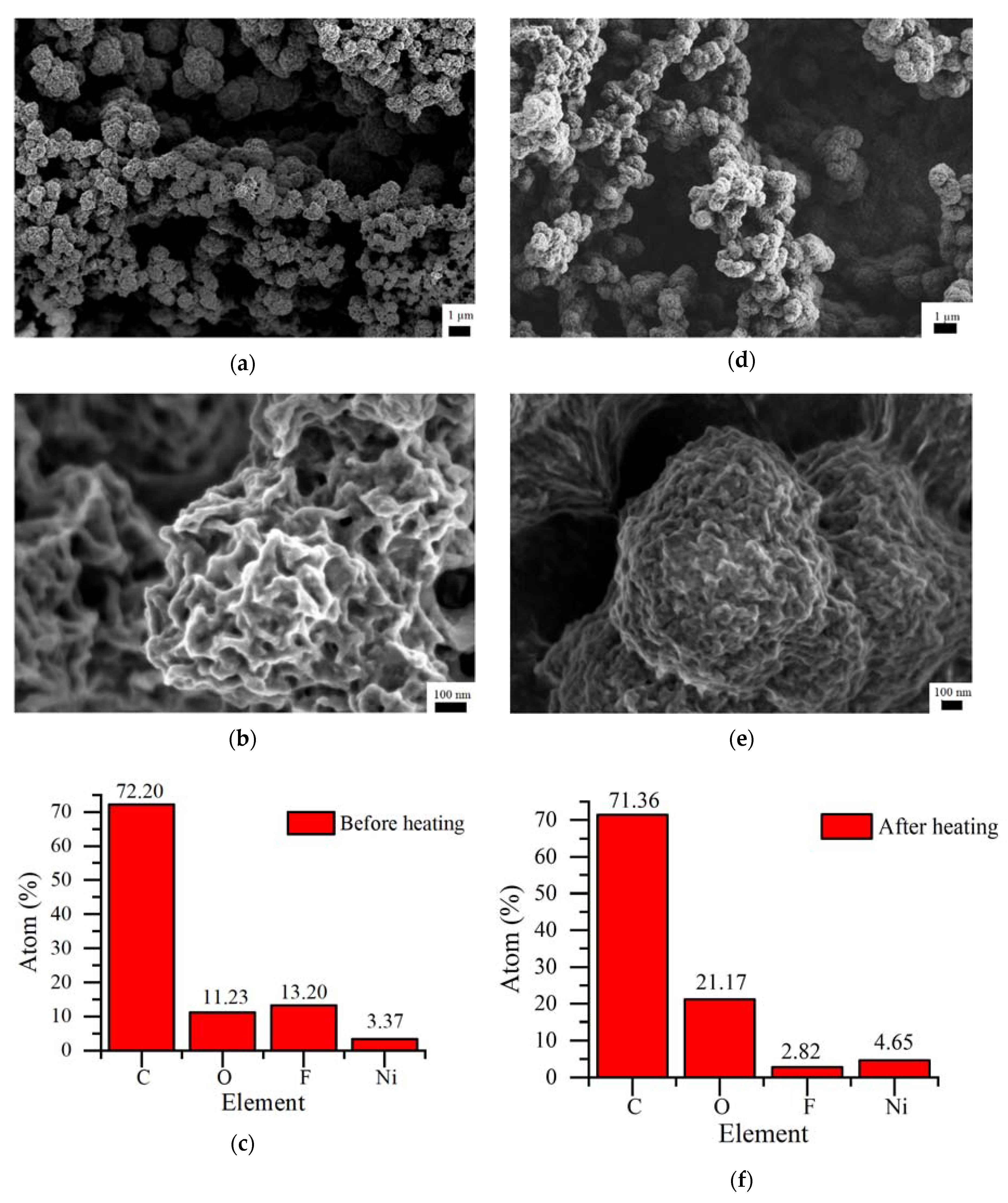
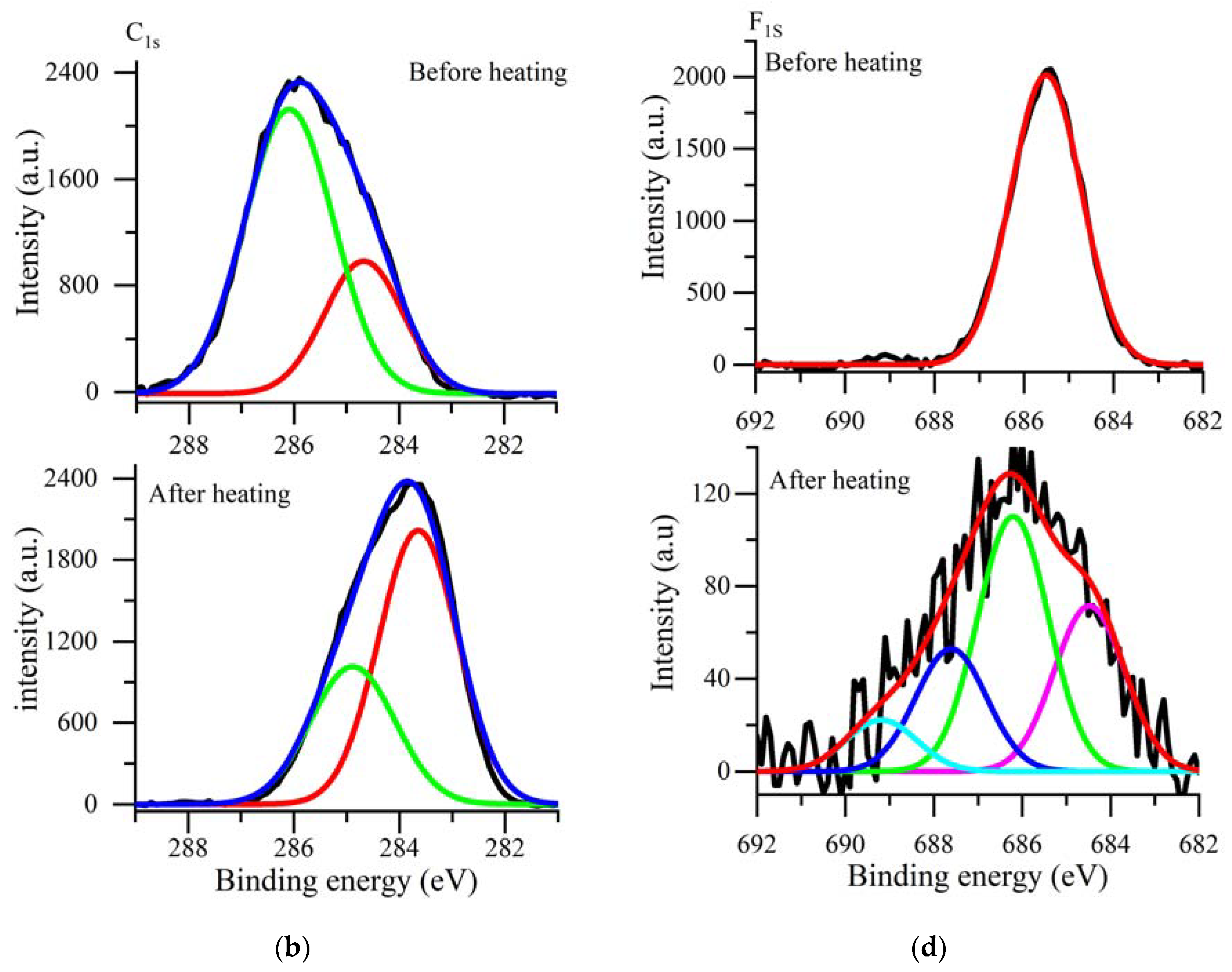
3.2. XPS Analysis
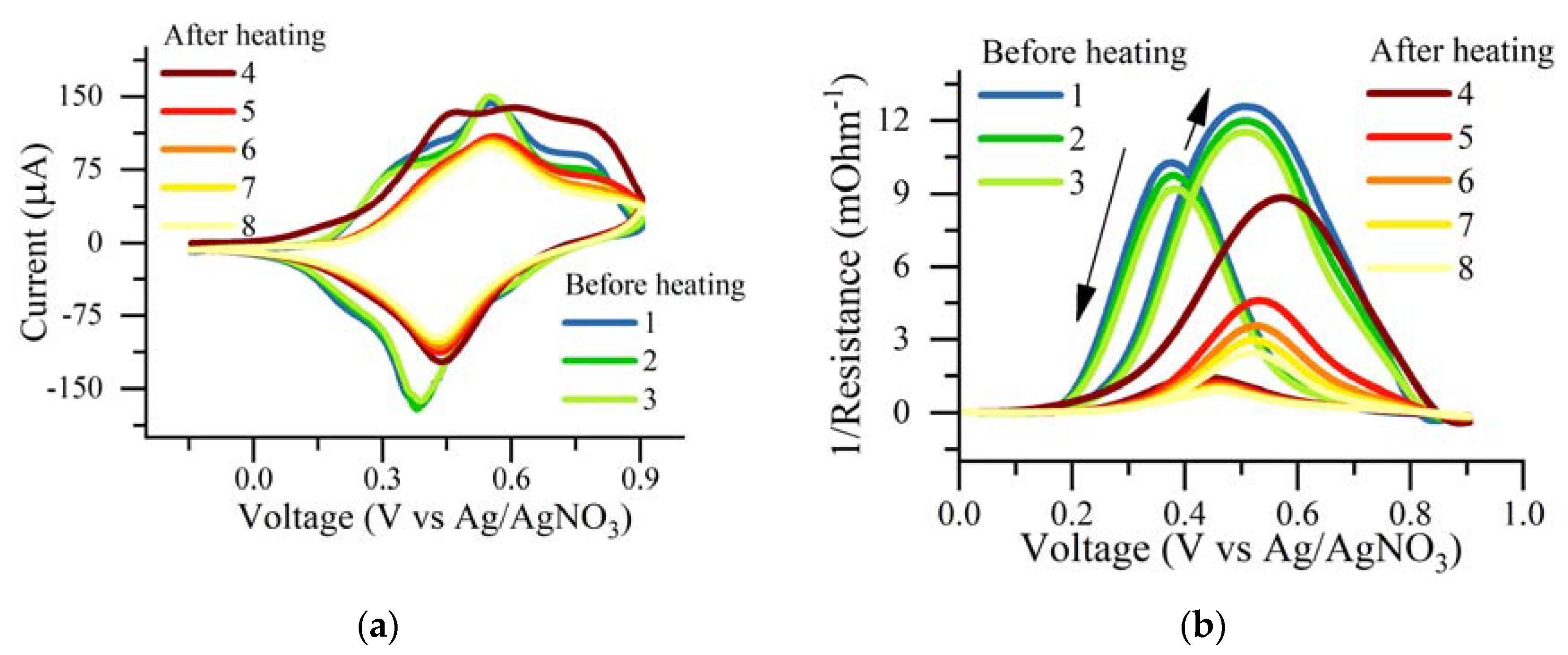
3.3. Cyclic Voltammetry and Conductivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Australian Trade and Investment Commission. The Lithium-Ion Battery Value Chain New Economy Opportunities for Australia. 2018, p. 56. Available online: https://apo.org.au/node/210341 (accessed on 5 December 2020).
- Ouyang, D.; Chen, M.; Huang, Q.; Weng, J.; Wang, Z.; Wang, J. A review on the thermal hazards of the lithium-ion battery and the corresponding countermeasures. Appl. Sci. 2019, 9, 2483. [Google Scholar] [CrossRef] [Green Version]
- Kulova, T.L. New electrode materials for lithium-ion batteries. Russ. J. Electrochem. 2013, 49, 1–25. [Google Scholar] [CrossRef]
- Kise, M.; Yoshioka, S.; Hamano, K.; Takemura, D.; Nishimura, T.; Urushibata, H.; Yoshiyasu, H. Development of new safe electrode for lithium rechargeable battery. J. Power Sources 2005, 146, 775–778. [Google Scholar] [CrossRef]
- Kise, M.; Yoshioka, S.; Kuriki, H. Relation between composition of the positive electrode and cell performance and safety of lithium-ion PTC batteries. J. Power Sources 2007, 174, 861–866. [Google Scholar] [CrossRef]
- Chen, Z.; Hsu, P.-C.; Lopez, J.; Li, Y.; To, J.W.; Liu, N.; Wang, C.; Andrew, S.C.; Liu, J.; Cui, Y.; et al. Fast and reversible thermoresponsive polymer switching materials for safer batteries. Nat. Energy 2016, 1, 1–2. [Google Scholar] [CrossRef]
- Feng, X.M.; Ai, X.P.; Yang, H.X. A positive-temperature-coefficient electrode with thermal cut-off mechanism for use in rechargeable lithium batteries. Electrochem. Commun. 2004, 6, 1021–1024. [Google Scholar] [CrossRef]
- Zhang, H.; Pang, J.; Ai, X.; Cao, Y.; Yang, H.; Lu, S. Poly(3-butylthiophene)-based positive-temperature-coefficient electrodes for safer lithium-ion batteries. Electrochim. Acta. 2016, 187, 173–178. [Google Scholar] [CrossRef]
- Xia, L.; Li, S.; Ai, X.; Yang, H.; Cao, Y. Temperature-sensitive cathode materials for safer lithium-ion batteries. Energy Environ. Sci. 2011, 4, 2845–2848. [Google Scholar] [CrossRef]
- Ji, W.; Wang, F.; Liu, D.; Qian, J.; Cao, Y.; Chen, Z.; Yang, H.; Ai, X. Building thermally stable Li-ion batteries using a temperature-responsive cathode. J. Mater. Chem. A 2016, 4, 11239–11246. [Google Scholar] [CrossRef]
- Eliseeva, S.N.; Alekseeva, E.V.; Vereshchagin, A.A.; Volkov, A.I.; Vlasov, P.S.; Konev, A.S.; Levin, O.V. Nickel-salen type polymers as cathode materials for rechargeable lithium batteries. Macromol. Chem. Phys. 2017, 218, 1700361. [Google Scholar] [CrossRef]
- Polozhentseva, Y.A.; Karushev, M.P.; Rumyantsev, A.M.; Chepurnaya, I.A.; Timonov, A.M. A lithium-ion supercapacitor with a positive electrode based on a carbon material modified by polymeric complexes of nickel with schiff bases. Tech. Phys. Lett. 2020, 46, 196–199. [Google Scholar] [CrossRef]
- Basistaya, A.O.; Karushev, M.P.; Chepurnaya, I.A.; Bykov, V.A.; Timonov, A.M. A new conducting polymer for lithium-ion batteries. Tech. Phys. Lett. 2020, 46, 77–79. [Google Scholar] [CrossRef]
- O’Meara, C.; Karushev, M.P.; Polozhentceva, I.A.; Dharmasena, S.; Cho, H.; Yurkovich, B.J.; Kogan, S.; Kim, J.H. Nickel-salen-type polymer as conducting agent and binder for carbon-free cathodes in lithium-ion batteries. ACS Appl. Mater. Interfaces 2018, 11, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Alekseeva, E.V.; Chepurnaya, I.A.; Malev, V.V.; Timonov, A.M.; Levin, O.V. Polymeric nickel complexes with salen-type ligands for modification of supercapacitor electrodes: Impedance studies of charge transfer and storage properties. Electrochim. Acta 2017, 225, 378–391. [Google Scholar] [CrossRef]
- Beletskii, E.V.; Volosatova, Y.A.; Eliseeva, S.N.; Levin, O.V. The effect of electrode potential on the conductivity of polymer complexes of nickel with salen ligands. Russ. J. Electrochem. 2019, 55, 339–345. [Google Scholar] [CrossRef]
- Karlsson, C.; Suga, T. Quantifying TEMPO Redox Polymer Charge Transport toward the Organic Radical Battery. ACS Appl. Mater. Interfaces 2017, 9. [Google Scholar] [CrossRef]
- Anderson, T.; Roth, S. Conducting polymers: Electrical transport and current applications. Braz. J. Phys. 1994, 24, 746. [Google Scholar]
- Kaneto, K.; Yoshino, K.; Inuishi, Y. Electrical and optical properties of polythiophene prepared by electrochemical polymerization. Solid State Commun. 1983, 46, 389–391. [Google Scholar] [CrossRef]
- Bharti, M.; Singh, A.; Samanta, S.; Aswal, D.K. Conductive polymers for thermoelectric power generation. Prog. Mater. Sci. 2018, 93, 270–310. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Kang, F. Enhanced electrochemical performance of salen-type transition metal polymer with electron-donating substituents. Ionics 2019, 25, 1045–1055. [Google Scholar] [CrossRef]
- Abee, M.W.; Cox, D.F. BF3 adsorption on α-Cr2O3 (1012): Probing the lewis basicity of surface oxygen anions. J. Phys. Chem. B 2001, 105, 8375–8380. [Google Scholar] [CrossRef]
- Toman, P.; Menšík, M.; Bartkowiak, W.; Pfleger, J. Modelling of the charge carrier mobility in disordered linear polymer materials. Phys. Chem. Chem. Phys. 2017, 19, 7760–7771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anishchenko, D.V.; Levin, O.V.; Malev, V.V. Quasi-equilibrium voltammetric curves of polaron-conducting polymer films. Electrochim. Acta 2016, 188, 480–489. [Google Scholar] [CrossRef]
- Ofer, D.; Crooks, R.M.; Wrighton, M.S. Potential dependence of the conductivity of highly oxidized polythiophenes, polypyrroles, and polyaniline: Finite windows of high conductivity. J. Am. Chem. Soc. 1990, 112, 7869–7879. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beletskii, E.; Ershov, V.; Danilov, S.; Lukyanov, D.; Alekseeva, E.; Levin, O. Resistivity-Temperature Behavior of Intrinsically Conducting Bis(3-methoxysalicylideniminato)nickel Polymer. Polymers 2020, 12, 2925. https://doi.org/10.3390/polym12122925
Beletskii E, Ershov V, Danilov S, Lukyanov D, Alekseeva E, Levin O. Resistivity-Temperature Behavior of Intrinsically Conducting Bis(3-methoxysalicylideniminato)nickel Polymer. Polymers. 2020; 12(12):2925. https://doi.org/10.3390/polym12122925
Chicago/Turabian StyleBeletskii, Evgenii, Valentin Ershov, Stepan Danilov, Daniil Lukyanov, Elena Alekseeva, and Oleg Levin. 2020. "Resistivity-Temperature Behavior of Intrinsically Conducting Bis(3-methoxysalicylideniminato)nickel Polymer" Polymers 12, no. 12: 2925. https://doi.org/10.3390/polym12122925
APA StyleBeletskii, E., Ershov, V., Danilov, S., Lukyanov, D., Alekseeva, E., & Levin, O. (2020). Resistivity-Temperature Behavior of Intrinsically Conducting Bis(3-methoxysalicylideniminato)nickel Polymer. Polymers, 12(12), 2925. https://doi.org/10.3390/polym12122925







