Self-Assembly of Molecular Brushes with Polyimide Backbone and Amphiphilic Block Copolymer Side Chains in Selective Solvents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Structure Characterization of Molecular Brushes
2.2. Determination of Molar Mass and Hydrodynamic Characteristics and Investigation of Self-Assembly of Molecular Brushes in Dilute Solutions
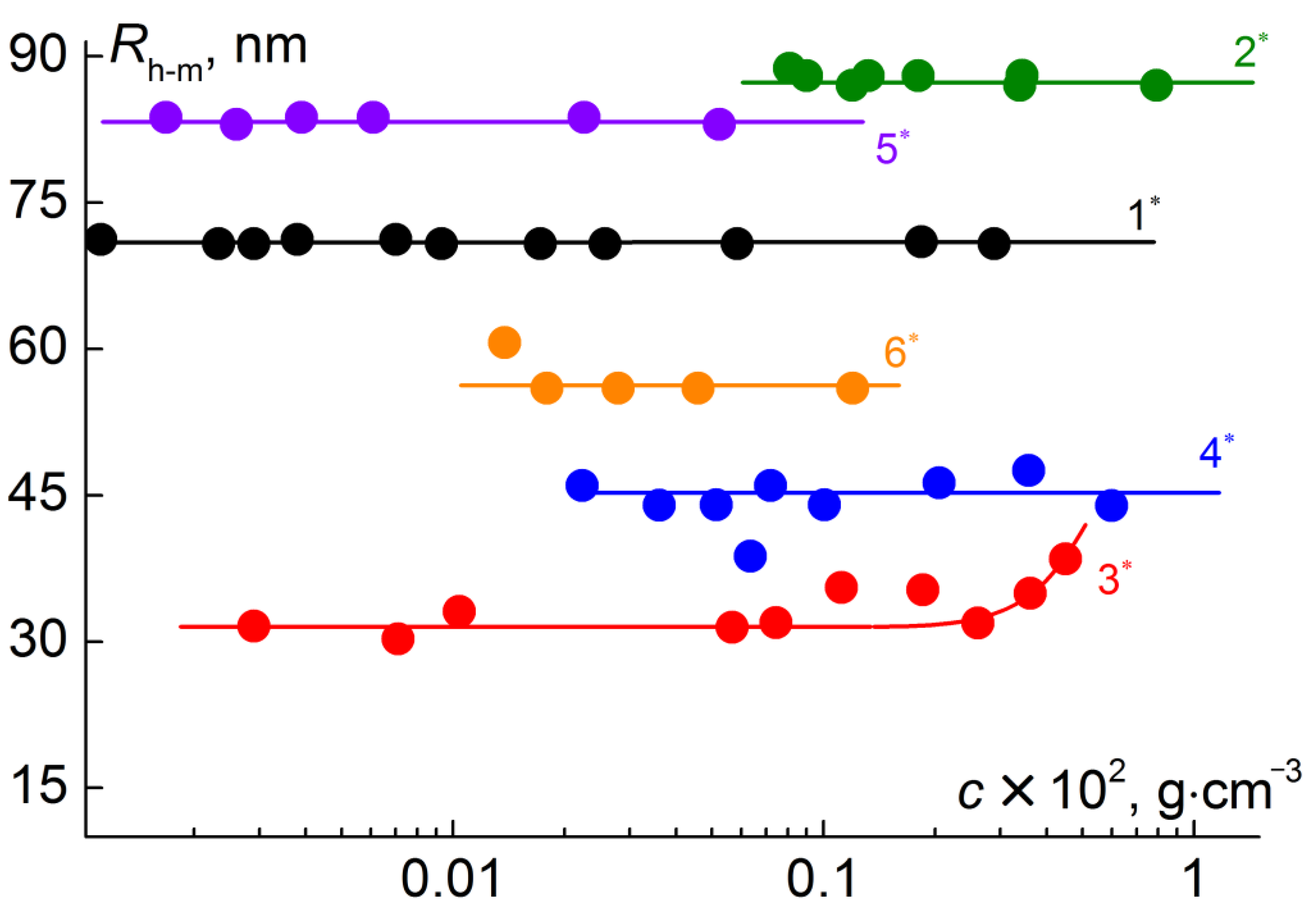
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, M.; Muller, A.H.E. Cylindrical Polymer Brushes. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 3461–3469. [Google Scholar] [CrossRef]
- Wurm, F.; Frey, H. Linear-dendritic block-copolymers: The state of the art and exciting perspectives. Prog. Polym. Sci. 2011, 36, 1–52. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Pitsikalis, M.; Pispas, S.; Iatrou, H. Polymers with complex architecture by living anionic polymerization. Chem. Rev. 2001, 101, 3747–3792. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, S.; Weng, Z.; Gao, C. Hyperbranched polymers: Advances from synthesis to applications. Chem. Soc. Rev. 2015, 44, 4091–4130. [Google Scholar] [CrossRef]
- Voit, B.I.; Lederer, A. Hyperbranched and highly branched polymer architectures—Synthetic strategies and major characterization aspects. Chem. Rev. 2009, 109, 5924–5973. [Google Scholar] [CrossRef]
- Li, M.; Pester, C.W. Mixed Polymer Brushes for “Smart” Surfaces. Polymers 2020, 12, 1553. [Google Scholar] [CrossRef]
- Passeno, L.M.; Mackay, E.M.; Baker, L.G. Conformational changes of linear-dendrimer diblock copolymers in dilute solution. Macromolecules 2006, 39, 740–746. [Google Scholar] [CrossRef]
- Schulze, U.; Fónagy, T.; Komber, H.; Pompe, G.; Pionteck, J.; Iván, B. Synthesis of Poly(propene-g-styrene) Graft Copolymers by Metallocene Catalyzed Copolymerization of Propene with Allyl-Terminated Polystyrene Macromonomer Obtained via Quasiliving Atom Transfer Radical Polymerization and the Effect of the Grafts on Blending Polypropene with Polystyrene. Macromolecules 2003, 36, 4719–4726. [Google Scholar]
- Zhang, D.; Ortiz, C. Synthesis and Single Molecule Force Spectroscopy of Graft Copolymers of Poly(2-hydroxyethyl methacrylate-g-ethylene glycol). Macromolecules 2004, 37, 4271–4282. [Google Scholar] [CrossRef] [Green Version]
- Gu, L.; Shen, Z.; Zhang, S.; Lu, G.; Zhang, X.; Huang, X. Novel Amphiphilic Centipede-Like Copolymer Bearing Polyacrylate Backbone and Poly(ethylene glycol) and Polystyrene Side Chains. Macromolecules 2007, 40, 4486–4493. [Google Scholar] [CrossRef]
- Mu, D.; Li, J.-Q.; Cong, X.-S.; Zhang, H. Mesoscopic Detection of the Influence of a Third Component on the Self-Assembly Structure of A2B Star Copolymer in Thin Films. Polymers 2019, 11, 1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodchenko, S.; Amirova, A.; Milenin, S.; Ryzhkov, A.; Talalaevа, E.; Kalinina, A.; Kurlykin, M.; Tenkovtsev, A.; Filippov, A. Amphiphilic molecular brushes with regular polydimethylsiloxane backbone and poly-2-isopropyl-2-oxazoline side chains. Synthesis, characterization and conformation in solution. Eur. Polym. J. 2020, 140, 110035. [Google Scholar] [CrossRef]
- Ayres, N. Polymer brushes: Applications in biomaterials and nanotechnology. Polym. Chem. 2010, 1, 769–777. [Google Scholar] [CrossRef]
- Hao, J. Self-Assembled Structures: Properties and Applications in Solution and on Surfaces, 3rd ed.; Taylor and Francis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2011; pp. 1–202. [Google Scholar]
- Sheiko, S.S.; Sumerlin, B.S.; Matyjaszewski, K. Cylindrical molecular brushes: Synthesis, characterization, and properties. Prog. Polym. Sci. 2008, 33, 759–785. [Google Scholar] [CrossRef]
- Xie, G.; Martinez, M.R.; Olszewski, M.; Sheiko, S.S.; Matyjaszewski, K. Molecular bottlebrushes as novel materials. Biomacromolecules 2019, 20, 27–54. [Google Scholar] [CrossRef] [PubMed]
- Müllner, M.; Müller, A.H.E. Cylindrical polymer brushes—Anisotropic building blocks, unimolecular templates and particulate nanocarriers. Polymer 2016, 98, 389–401. [Google Scholar] [CrossRef]
- Pelras, T.; Mahon, C.S.; Müllner, M. Synthesis and applications of compartmentalised molecular polymer brushes. Angew. Chem. 2018, 57, 6982–6994. [Google Scholar] [CrossRef]
- Ivanov, I.V.; Meleshko, T.K.; Kashina, A.V.; Yakimansky, A.V. Amphiphilic multicomponent molecular brushes. Russ. Chem. Rev. 2019, 88, 1248–1290. [Google Scholar] [CrossRef]
- Verduzco, R.; Li, X.; Pesek, S.L.; Stein, G.E. Structure, function, self-assembly, and applications of bottlebrush copolymers. Chem. Soc. Rev. 2015, 44, 2405–2420. [Google Scholar] [CrossRef] [Green Version]
- Qiu, L.Y.; Bae, Y.H. Polymer Architecture and Drug Delivery. Pharm. Res. 2006, 23, 1–30. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Tsarevsky, N.V. Nanostructured functional materials prepared by atom transfer radical polymerization. Nat. Chem. 2009, 1, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Tezuka, Y.; Oike, H. Topological polymer chemistry. Prog. Polym. Sci. 2002, 27, 1069–1122. [Google Scholar] [CrossRef]
- Palacios-Hernandez, T.; Luo, H.; Garcia, E.A.; Pacheco, L.A.; Herrera-Alonso, M. Nanoparticles from amphiphilic heterografted macromolecular brushes with short backbones. Macromolecules 2018, 51, 2831–2837. [Google Scholar] [CrossRef]
- Onbulak, S.; Rzayev, J. Synthesis and one-dimensional assembly of cylindrical polymer nanoparticles prepared from tricomponent bottlebrush copolymers. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3868–3874. [Google Scholar] [CrossRef]
- Luo, H.; Szymusiak, M.; Garcia, E.A.; Lock, L.L.; Cui, H.; Liu, Y.; Herrera-Alonso, M. Solute-triggered morphological transitions of an amphiphilic heterografted brush copolymer as a single-molecule drug carrier. Macromolecules 2017, 50, 2201–2206. [Google Scholar] [CrossRef]
- Nam, J.; Kim, Y.; Kim, J.G.; Seo, M. Self-assembly of monolayer vesicles via backbone-shiftable synthesis of janus core–shell bottlebrush polymer. Macromolecules 2019, 52, 9484–9494. [Google Scholar] [CrossRef]
- Li, H.; Liu, H.; Nie, T.; Chen, Y.; Wang, Z.; Huang, H.; Liu, L.; Chen, Y. Molecular bottlebrush as a unimolecular vehicle with tunable shape for photothermal cancer therapy. Biomaterials 2018, 178, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Müllner, M.; Dodds, S.J.; Nguyen, T.-H.; Senyschyn, D.; Porter, C.J.H.; Boyd, B.J.; Caruso, F. Size and rigidity of cylindrical polymer brushes dictate long circulating properties In Vivo. ACS Nano 2015, 9, 1294–1304. [Google Scholar] [CrossRef]
- Müllner, M. Molecular polymer brushes in nanomedicine. Macromol. Chem. Phys. 2016, 217, 2209–2222. [Google Scholar] [CrossRef]
- Huang, K.; Canterbury, D.P.; Rzayev, J. Organosoluble polypyrrole nanotubes from core–shell bottlebrush copolymers. Chem. Commun. 2010, 46, 6326–6328. [Google Scholar] [CrossRef]
- Huang, K.; Rzayev, J. Well-defined organic nanotubes from multicomponent bottlebrush copolymers. J. Am. Chem. Soc. 2009, 131, 6880–6885. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Canterbury, D.P.; Rzayev, J. Synthesis of segmented polylactide molecular brushes and their transformation to open-end nanotubes. Macromolecules 2010, 43, 6632–6638. [Google Scholar] [CrossRef]
- Müllner, M.; Yuan, J.; Weiss, S.; Walther, A.; Förtsch, M.; Drechsler, M.; Müller, A.H.E. Water-soluble organo−silica hybrid nanotubes templated by cylindrical polymer brushes. J. Am. Chem. Soc. 2010, 132, 16587–16592. [Google Scholar] [CrossRef] [PubMed]
- Müllner, M.; Lunkenbein, T.; Schieder, M.; Gröschel, A.H.; Miyajima, N.; Förtsch, M.; Breu, J.; Caruso, F.; Müller, A.H.E. Template-directed mild synthesis of anatase hybrid nanotubes within cylindrical core–shell–corona polymer brushes. Macromolecules 2012, 45, 6981–6988. [Google Scholar]
- Yuan, J.; Lu, Y.; Schacher, F.; Lunkenbein, T.; Weiss, S.; Schmalz, H.; Müller, A.H.E. Template-Directed synthesis of hybrid titania nanowires within core−shell bishydrophilic cylindrical polymer brushes. Chem. Mater. 2009, 21, 4146–4154. [Google Scholar] [CrossRef]
- Yuan, J.; Drechsler, M.; Xu, Y.; Zhang, M.; Müller, A.H.E. Cadmium selenide nanowires within core–shell cylindrical polymer brushes: Synthesis, characterization and the double-loading process. Polymer 2008, 49, 1547–1554. [Google Scholar] [CrossRef]
- Yuan, J.; Schacher, F.; Drechsler, M.; Hanisch, A.; Lu, Y.; Ballauff, M.; Müller, A.H.E. Stimuli-responsive organosilica hybrid nanowires decorated with metal nanoparticles. Chem. Mater. 2010, 22, 2626–2634. [Google Scholar] [CrossRef]
- Yuan, J.; Müller, A.H.E. One-dimensional organic–inorganic hybrid nanomaterials. Polymer 2010, 51, 4015–4036. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Yuan, J.; Fang, B.; Drechsler, M.; Müllner, M.; Bolisetty, S.; Ballauff, M.; Müller, A.H.E. Hybrids of magnetic nanoparticles with double hydrophilic core/shell cylindrical polymer brushes and their alignment in a magnetic field. Adv. Funct. Mater. 2010, 20, 4182–4189. [Google Scholar] [CrossRef]
- Bai, J.; Wang, X.; Fu, P.; Cui, Z.; Zhao, Q.; Pang, X.; Liu, M. Highly water-dispersed superparamagnetic magnetite colloidal nanocrystal clusters from multifunctional polymeric nanoreactors: Synthesis and properties. RSC Adv. 2016, 6, 9429–9435. [Google Scholar] [CrossRef]
- Zhang, M.; Estournès, C.; Bietsch, W.; Müller, A.H.E. Superparamagnetic hybrid nanocylinders. Adv. Funct. Mater. 2004, 14, 871–882. [Google Scholar] [CrossRef]
- Meleshko, T.K.; Ivanov, I.V.; Kashina, A.V.; Bogorad, N.N.; Simonova, M.A.; Zakharova, N.V.; Filippov, A.P.; Yakimansky, A.V. Diphilic macromolecular brushes with a polyimide backbone and poly(methacrylic acid) blocks in side chains. Polym. Sci. Ser. B 2018, 60, 35–50. [Google Scholar] [CrossRef]
- Yakimansky, A.V.; Meleshko, T.K.; Ilgach, D.M.; Bauman, M.A.; Anan’eva, T.D.; Klapshina, L.G.; Lermontova, S.A.; Balalaeva, I.V.; Douglas, W.E. Novel regular polyimide-graft-poly(methacrylic acid) brushes: Synthesis and possible applications as nanocontainers of cyanoporphyrazine agents for photodynamic therapy. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 4267–4281. [Google Scholar] [CrossRef]
- Tarabukina, E.; Amirova, A.; Belyaeva, E.; Krasova, A.; Simonova, M.; Filippov, A.; Meleshko, T.; Ilgach, D.; Bogorad, N.; Yakimansky, A. Conformational characteristics of polyimide initiator for the synthesis of poly(methylmethacrylate) grafted block- copolymers. J. Macromol. Sci. Part B Phys. 2013, 52, 1545–1557. [Google Scholar] [CrossRef]
- Filippov, A.P.; Belyaeva, E.V.; Meleshko, T.K.; Yakimansky, A.V. Solution behavior of polyimide-graft-polystyrene copolymers in selective solvents. J. Polym. Sci. Part B 2014, 52, 1539–1546. [Google Scholar] [CrossRef]
- Filippov, A.P.; Krasova, A.S.; Tarabukina, E.B.; Meleshko, T.K.; Yakimansky, A.V.; Sheiko, S.S. The behavior of amphiphilic molecular brushes with polyimide backbone and poly(methyl methacrylate) and polystyrene side chains in the vicinity of θ point. Polym. Sci. Ser. C 2018, 60, 219–227. [Google Scholar] [CrossRef]
- Burchard, W. Solution properties of branched macromolecules. Adv. Polym. Sci. 1999, 143, 113–194. [Google Scholar]
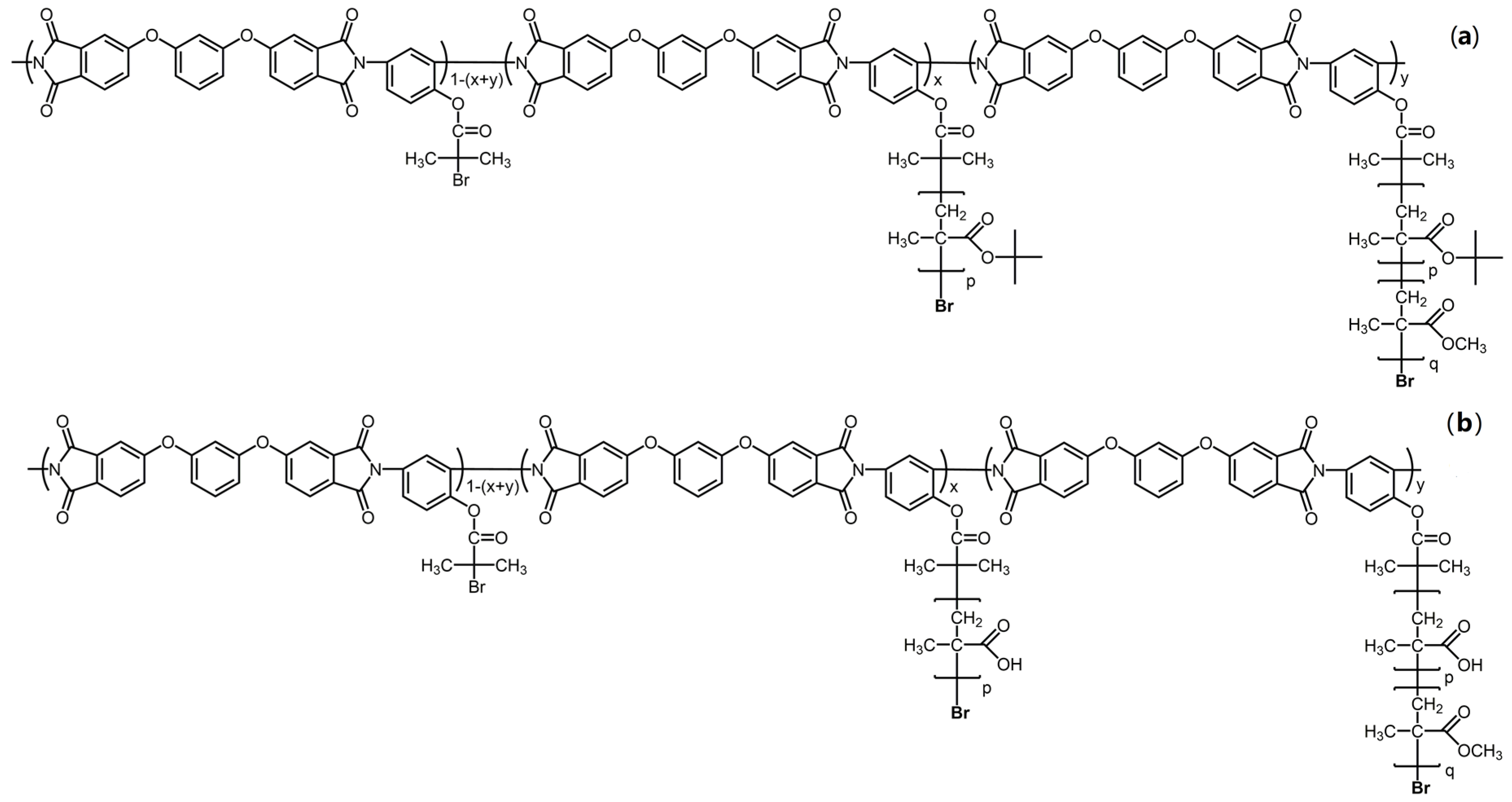
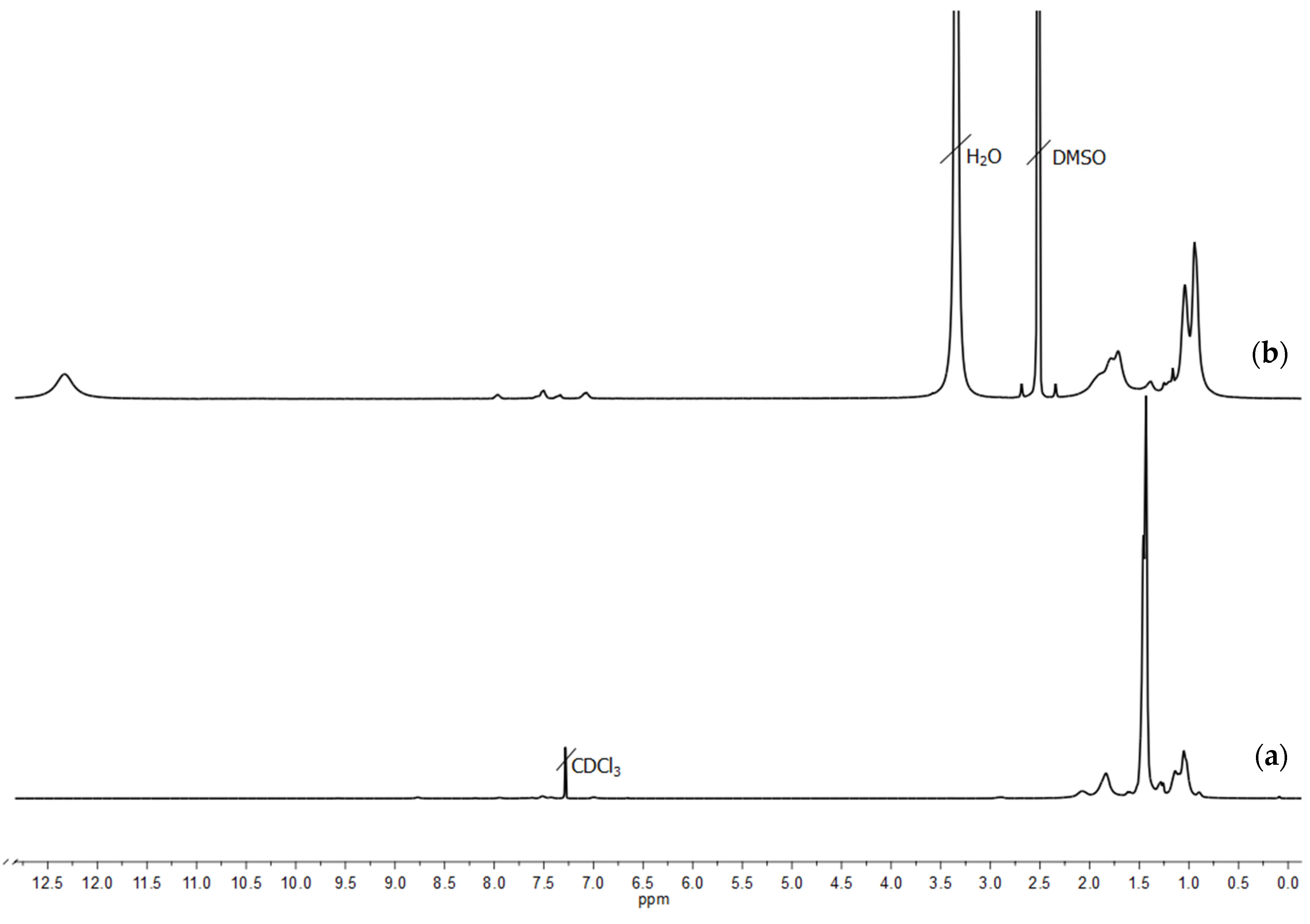
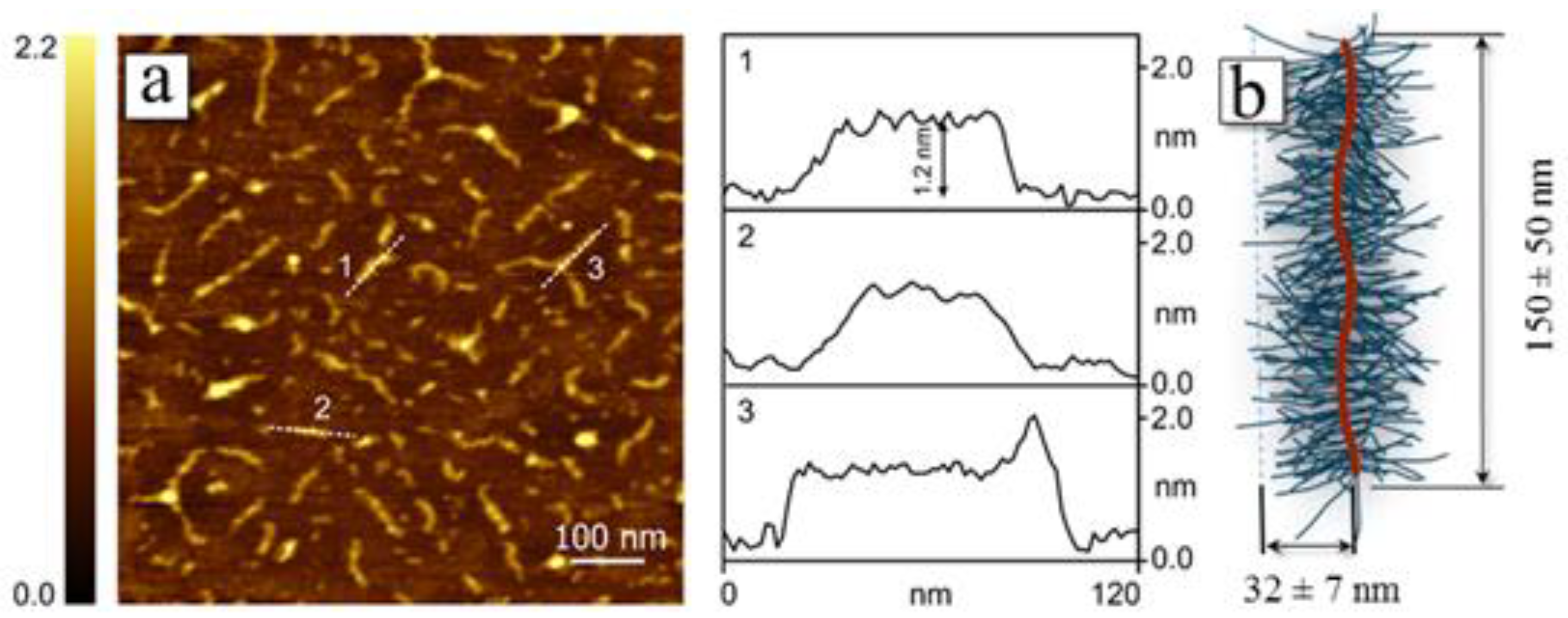
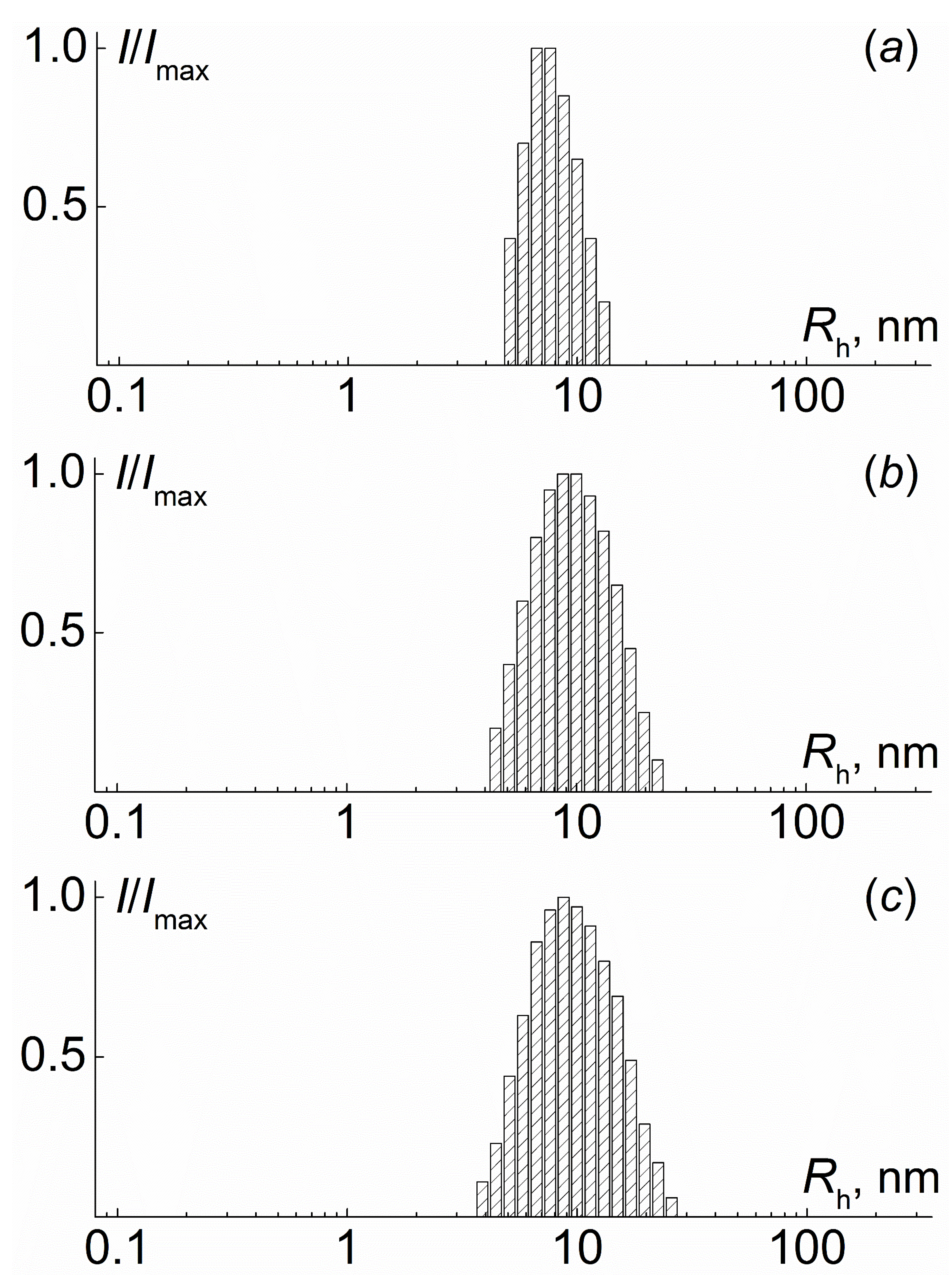
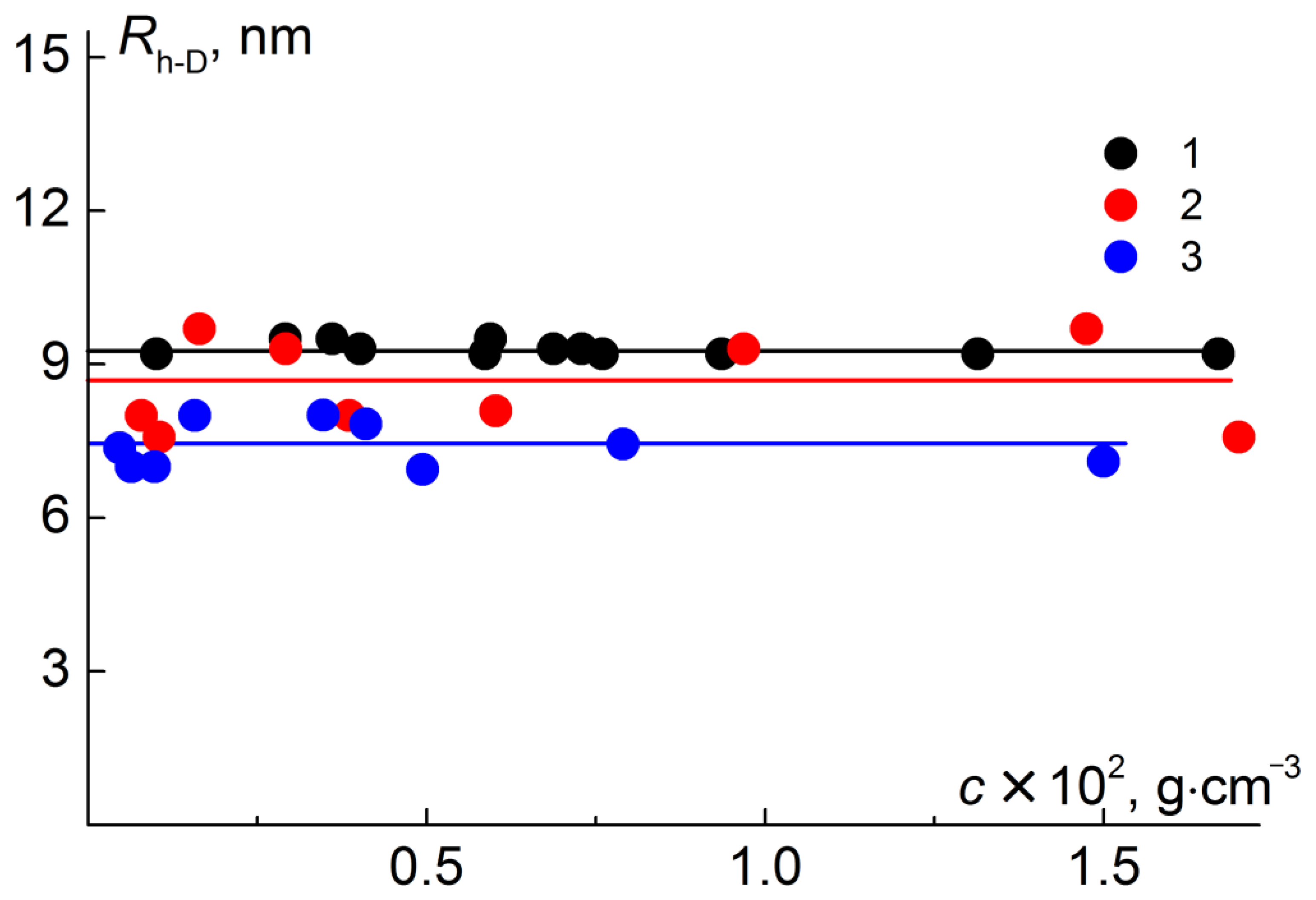
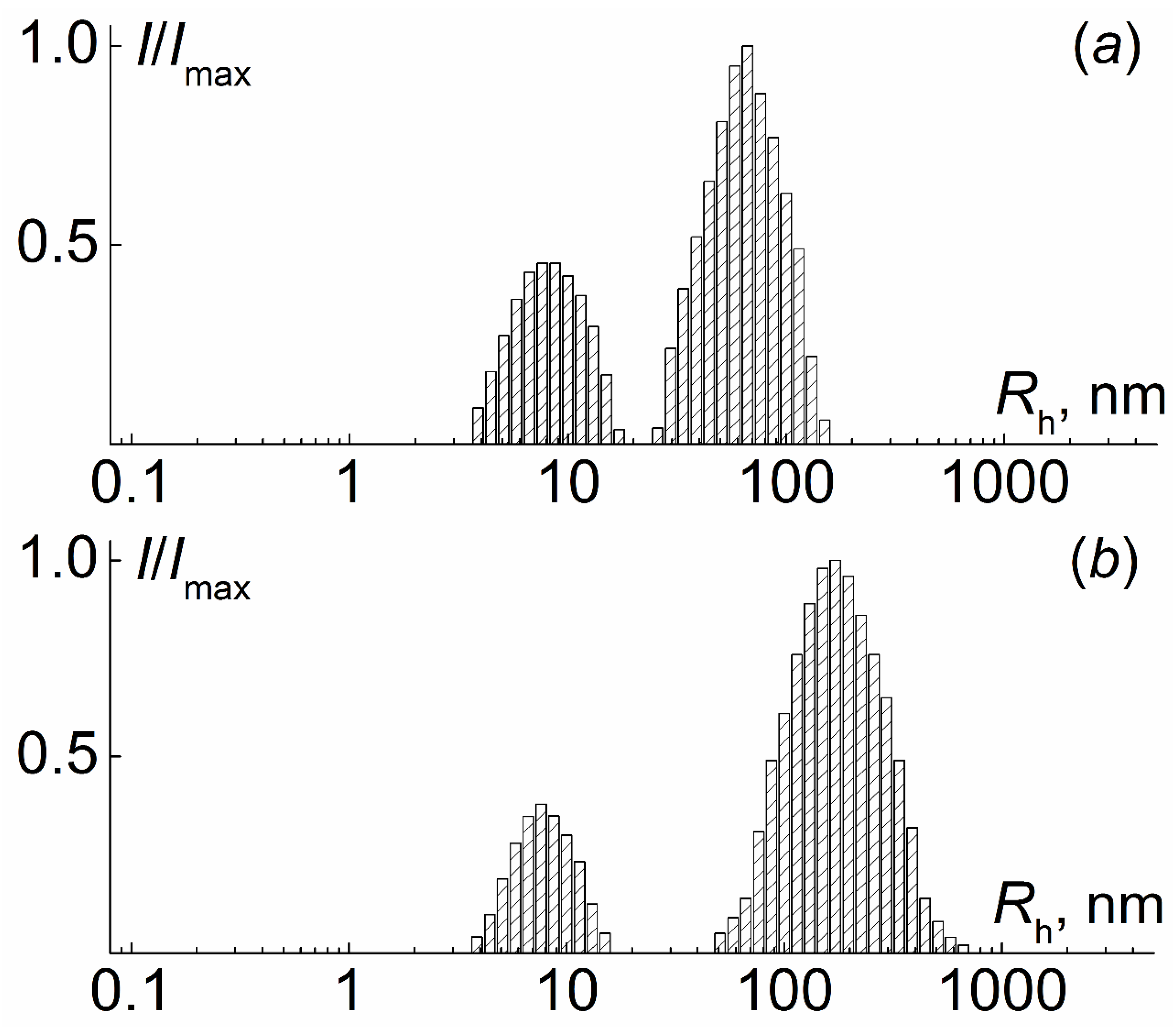
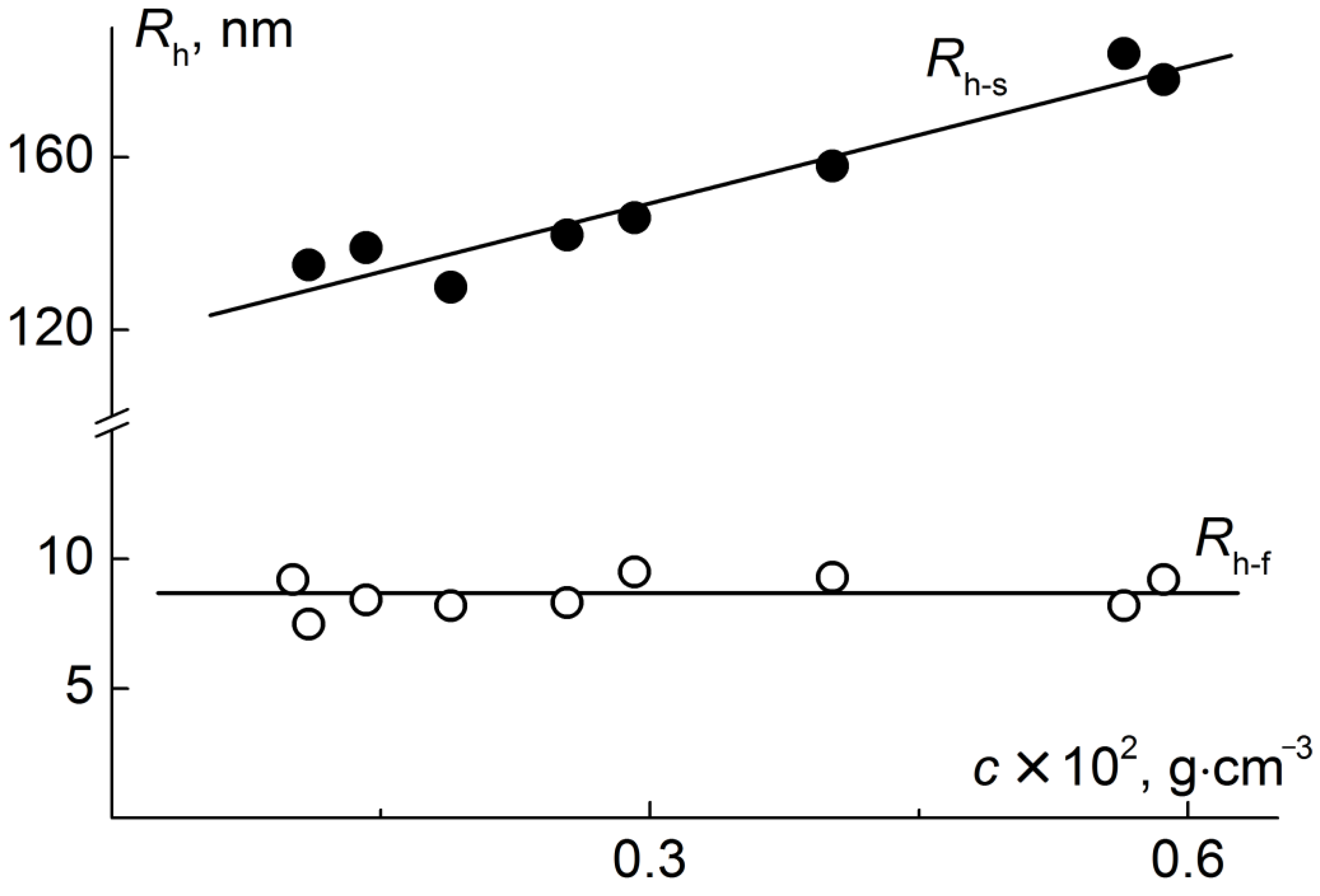
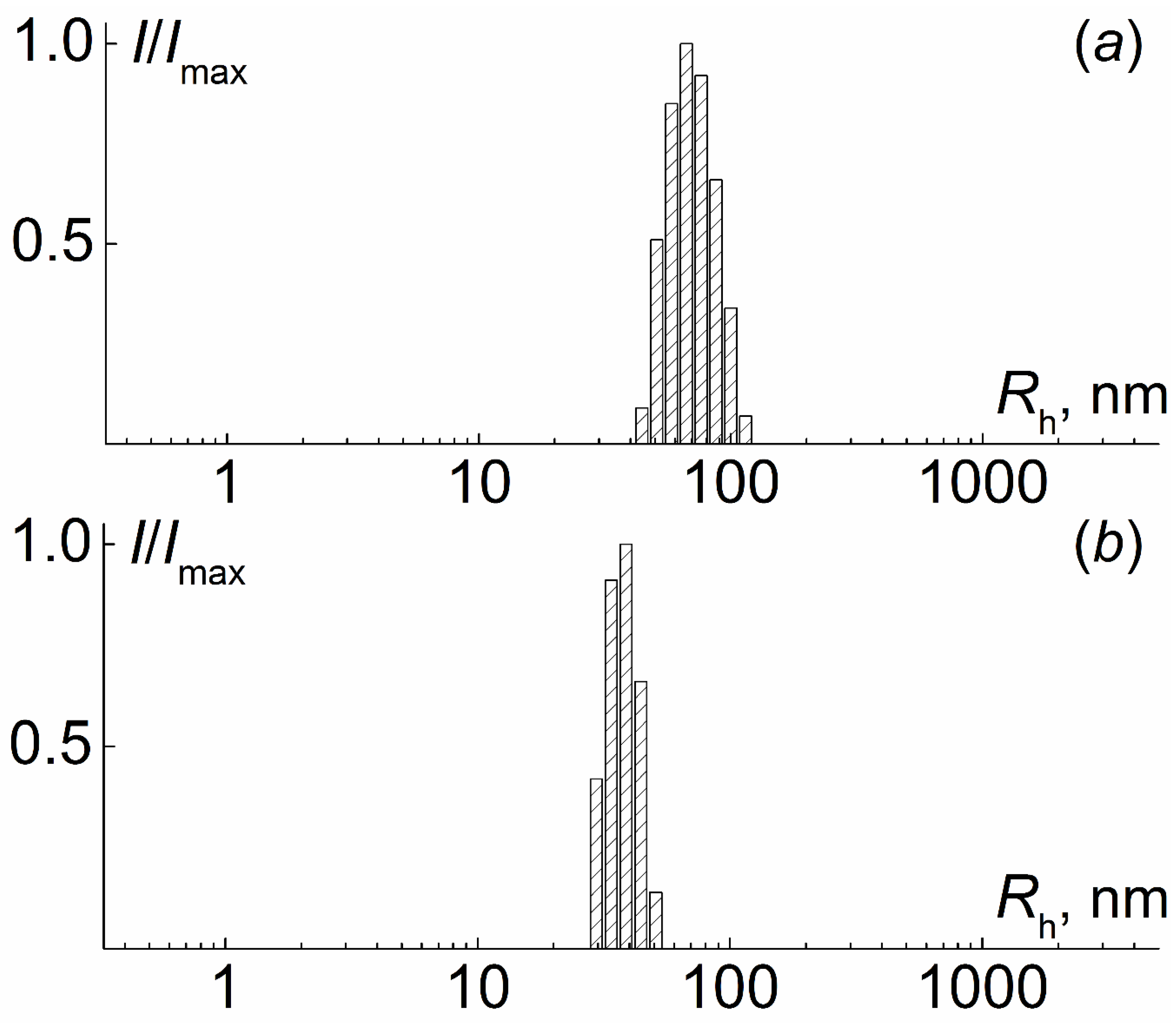
| N | Characteristics of Polyimide Backbone | Characteristics of Side Chains | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mn × 10−3 | NPI | Ð | PtBMA (x) | PtBMA-b-PMMA (y) | Molar Fraction of PI Units with Homo-PtBMA (x) and PtBMA-b-PMMA (y) Side Chains | Average Polymerization Degree of PtBMA (p) and PMMA (q) Blocks | |||||
| Mn × 10−3 | Ð | Mn × 10−3 | Ð | x | y | p | q | ||||
| 1 | 24 | 37 | 2.8 | 7.0 | 1.3 | 37 | 1.4 | 0.5 | 0.1 | 50 | 300 |
| 2 | 31 | 49 | 2.5 | 9.8 | 1.6 | 11 | 1.6 | 0.4 | 0.6 | 70 | 10 |
| 3 | 31 | 49 | 2.5 | 8.6 | 1.5 | 25 | 1.3 | 0.2 | 0.8 | 60 | 170 |
| 4 | 31 | 49 | 2.5 | 8.6 | 1.5 | 10.5 | 1.5 | 0.6 | 0.4 | 60 | 20 |
| 5 | 31 | 49 | 2.5 | 8.6 | 1.5 | 45 | 1.3 | 0.3 | 0.7 | 60 | 360 |
| 6 | 31 | 49 | 2.5 | 8.6 | 1.5 | 20 | 1.7 | 0.4 | 0.6 | 60 | 110 |
| Solvent | Solvent Characteristics | Solubility of the Structural Elements of Molecular Brushes | |||||
|---|---|---|---|---|---|---|---|
| ρ, g × cm−3 | η0, cP | n0 | PI | PtBMA | PMAA | PMMA | |
| DMF | 0.94 | 0.80 | 1.428 | + | + | − | + |
| Chloroform | 1.49 | 0.57 | 1.446 | ± | + | − | + |
| THF | 0.89 | 0.46 | 1.405 | − | + | − | + |
| Ethanol | 0.79 | 1.08 | 1.359 | − | − | + | − |
| Polymers | Solvents | Mw × 10−3, g·mol−1 | Rh-D, nm |
|---|---|---|---|
| PI | DMF | 39 | 7.3 |
| PI-g-PtBMA | Chloroform | 500 | 7.4 |
| PI-g-(PtBMA-b-PMMA) | Chloroform | 870 | 9.3 |
| PI-g-(PMAA-b-PMMA) | Chloroform | 690 | 8.6 |
| Samples * | x | y | p | q | Rh-m, nm | Rg, nm | Rg/Rh-m |
|---|---|---|---|---|---|---|---|
| 1 | 0.5 | 0.1 | 50 | 300 | 70 | 64 | 0.9 |
| 2 | 0.4 | 0.6 | 70 | 10 | 87 | 81 | 0.9 |
| 3 | 0.2 | 0.8 | 60 | 170 | 34 | 16 | 0.5 |
| 4 | 0.6 | 0.4 | 60 | 20 | 45 | 49 | 1.1 |
| 5 | 0.3 | 0.7 | 60 | 360 | 84 | 56 | 0.7 |
| 6 | 0.4 | 0.6 | 60 | 110 | 57 | 42 | 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonova, M.; Ivanov, I.; Meleshko, T.; Kopyshev, A.; Santer, S.; Yakimansky, A.; Filippov, A. Self-Assembly of Molecular Brushes with Polyimide Backbone and Amphiphilic Block Copolymer Side Chains in Selective Solvents. Polymers 2020, 12, 2922. https://doi.org/10.3390/polym12122922
Simonova M, Ivanov I, Meleshko T, Kopyshev A, Santer S, Yakimansky A, Filippov A. Self-Assembly of Molecular Brushes with Polyimide Backbone and Amphiphilic Block Copolymer Side Chains in Selective Solvents. Polymers. 2020; 12(12):2922. https://doi.org/10.3390/polym12122922
Chicago/Turabian StyleSimonova, Maria, Ivan Ivanov, Tamara Meleshko, Alexey Kopyshev, Svetlana Santer, Alexander Yakimansky, and Alexander Filippov. 2020. "Self-Assembly of Molecular Brushes with Polyimide Backbone and Amphiphilic Block Copolymer Side Chains in Selective Solvents" Polymers 12, no. 12: 2922. https://doi.org/10.3390/polym12122922
APA StyleSimonova, M., Ivanov, I., Meleshko, T., Kopyshev, A., Santer, S., Yakimansky, A., & Filippov, A. (2020). Self-Assembly of Molecular Brushes with Polyimide Backbone and Amphiphilic Block Copolymer Side Chains in Selective Solvents. Polymers, 12(12), 2922. https://doi.org/10.3390/polym12122922






