Tin Complexes Containing an Atenolol Moiety as Photostabilizers for Poly(Vinyl Chloride)
Abstract
1. Introduction
2. Materials and Methods
2.1. General
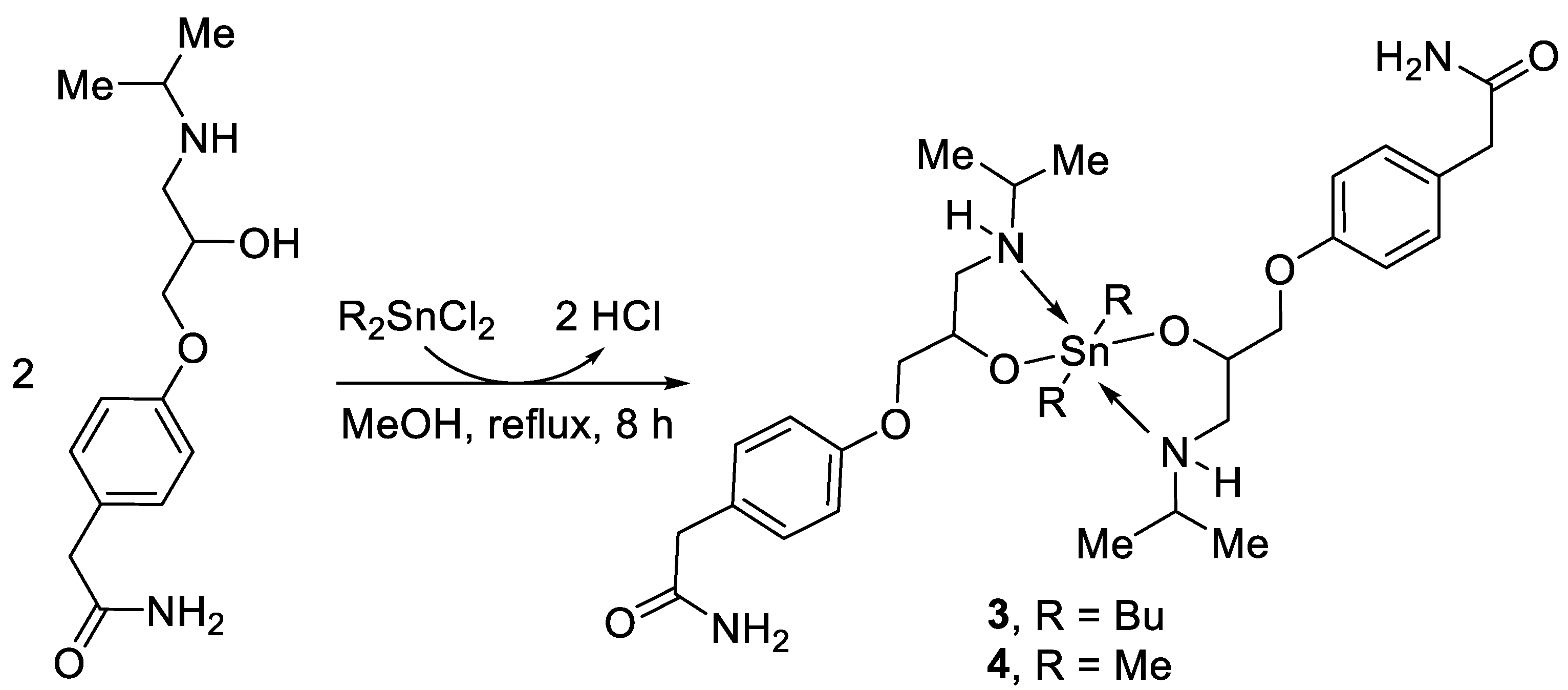
2.2. Synthesis of Complexes 1–4
2.3. Preparation of PVC Films
3. Results and Discussion
3.1. Synthesis of Complexes 1–4
3.2. FTIR Spectroscopy
3.3. Weight Loss
3.4. Surface Morphology
3.5. Photostabilization Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andrady, A.L.; Neal, M.A. Applications and societal benefits of plastics. Phil. Trans. R. Soc. B 2009, 364, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D. Polymer history. Des. Monomers Polym. 2008, 11, 1–15. [Google Scholar] [CrossRef]
- Crawford, C.B.; Quinn, B. Microplastic Pollutants, 1st ed.; Elsevier Science: Kidlington, UK, 2017. [Google Scholar]
- Titow, W.V. PVC Plastics Properties, Processing, and Applications; Elsevier Applied Science Publishers LTD: Amsterdam, The Netherlands, 1990. [Google Scholar]
- Burgess, R.H. Manufacture and Processing of PVC; Elsevier Applied Science Publishers LTD: London, UK, 2005. [Google Scholar]
- Folarin, O.M.; Sadiku, E.R. Thermal stabilizers for poly(vinyl chloride): A review. Int. J. Phys. Sci. 2011, 6, 4323–4330. [Google Scholar] [CrossRef]
- Cadogan, D.F.; Howick, C.J. Plasticizers. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar]
- Pimentel Real, L.E.; Ferraria, A.M.; Botelho do Rego, A.B. Comparison of different photo-oxidation conditions of poly(vinyl chloride) for outdoor applications. Polym. Test. 2008, 27, 743–751. [Google Scholar] [CrossRef]
- McNeill, I.C.; Memetea, L.; Cole, W.J. A study of the products of PVC thermal degradation. Polym. Degrad. Stab. 1995, 49, 181–191. [Google Scholar] [CrossRef]
- Starnes, W.H., Jr. Structural and mechanistic aspects of the thermal degradation of poly(vinyl chloride). Prog. Polym. Sci. 2002, 27, 2133–2170. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Yao, H. Thermal degradation of PVC: A review. Waste Manage. 2016, 48, 300–314. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Oraby, E.H.; Naby, A.S.A.; Mohamed, R.R. N-Phenyl-3-substituted 5-pyrazolone derivatives as organic stabilizers for rigid poly(vinyl chloride) against photodegradation. J. Appl. Polym. Sci. 2006, 101, 1543–1555. [Google Scholar] [CrossRef]
- Lemos, M.F.; Bohn, M.A. DMA of polyester-based polyurethane elastomers for composite rocket propellants containing different energetic plasticizers. J. Therm. Anal. Calorim. 2018, 131, 595–600. [Google Scholar] [CrossRef]
- Porta, M.; Zumeta, E. Implementing the Stockholm treaty on persistent organic pollutants. Occup. Environ. Med. 2002, 59, 651–652. [Google Scholar] [CrossRef]
- Fu, M.; Li, D.; Liu, H.; Ai, H.; Zhang, Y.; Zhang, L. Synergistic effects of zinc-mannitol alkoxide with calcium/zinc stearates and with β-diketone on thermal stability of rigid poly(vinyl chloride). J. Polym. Res. 2016, 23, 13. [Google Scholar] [CrossRef]
- Yousif, E.; El-Hiti, G.A.; Hussain, Z.; Altaie, A. Viscoelastic, spectroscopic and microscopic study of the photo irradiation effect on the stability of PVC in the presence of sulfamethoxazole Schiff’s bases. Polymers 2015, 7, 2190–2204. [Google Scholar] [CrossRef]
- Yousif, E.; Al-Amiery, A.A.; Kadihum, A.; Kadhum, A.H.; Mohamad, A. Photostabilizing efficiency of PVC in the presence of Schiff bases as photostabilizers. Molecules 2015, 20, 19886–19899. [Google Scholar] [CrossRef] [PubMed]
- Yousif, E.; Hasan, A.; El-Hiti, G.A. Spectroscopic, physical and topography of photochemical process of PVC films in the presence of Schiff base metal complexes. Polymers 2016, 8, 204. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.S.; El-Hiti, G.A.; Hameed, A.S.; Yousif, E.; Ahmed, A. New tetra-Schiff bases as efficient photostabilizers for poly(vinyl chloride). Molecules 2017, 22, 1506. [Google Scholar] [CrossRef] [PubMed]
- Shaalan, N.; Laftah, N.; El-Hiti, G.A.; Alotaibi, M.H.; Muslih, R.; Ahmed, D.S.; Yousif, E. Poly(vinyl chloride) photostabilization in the presence of Schiff bases containing a thiadiazole moiety. Molecules 2018, 23, 913. [Google Scholar] [CrossRef]
- Hashim, H.; El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, D.S.; Yousif, E. Fabrication of ordered honeycomb porous poly(vinyl chloride) thin film doped with a Schiff base and nickel(II) chloride. Heliyon 2018, 4, e00743. [Google Scholar] [CrossRef]
- El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, A.A.; Hamad, B.A.; Ahmed, D.S.; Ahmed, A.; Hashim, H.; Yousif, E. The morphology and performance of poly(vinyl chloride) containing melamine Schiff bases against ultraviolet light. Molecules 2019, 24, 803. [Google Scholar] [CrossRef]
- Balakit, A.A.; Ahmed, A.; El-Hiti, G.A.; Smith, K.; Yousif, E. Synthesis of new thiophene derivatives and their use as photostabilizers for rigid poly(vinyl chloride). Int. J. Polym. Sci. 2015, 2015, 510390. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Oraby, E.H.; Abdel Naby, A.S.; Mohammed, R.R. Anthraquinone derivatives as organic stabilizers for rigid poly(vinyl chloride) against photo-degradation. Eur. Polym. J. 2005, 41, 2530–2543. [Google Scholar] [CrossRef]
- Zhao, Y.; Dan, Y. Preparation and characterization of a high molecular weight UV-stabilizer based on a derivative of 2,4-dihydroxybenzophenone and its application in polymer materials. J. Appl. Polym. Sci. 2006, 102, 2203–2211. [Google Scholar] [CrossRef]
- Tomi, I.H.R.; Ali, G.Q.; Jawad, A.H.; Yousef, E. Synthesis and characterization of gallic acid derivatives and their utilized as organic photo-stabilizers for poly(vinyl chloride). J. Polym. Res. 2017, 24, 119. [Google Scholar] [CrossRef]
- El-Hiti, G.A.; Ahmed, D.S.; Yousif, E.; Alotaibi, M.H.; Satar, H.A.; Ahmed, A.A. Influence of polyphosphates on the physicochemical properties of poly(vinyl chloride) after irradiation with ultraviolet light. Polymers 2020, 12, 193. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.S.; El-Hiti, G.A.; Yousif, E.; Hameed, A.S. Polyphosphates as inhibitors for poly(vinyl chloride) photodegradation. Molecules 2017, 22, 1849. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, M.H.; El-Hiti, G.A.; Hashim, H.; Hameed, A.S.; Ahmed, D.S.; Yousif, E. SEM analysis of the tunable honeycomb structure of irradiated poly(vinyl chloride) films doped with polyphosphate. Heliyon 2018, 4, e01013. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, M.H.; El-Hiti, G.A.; Yousif, E.; Ahmed, D.S.; Hashim, H.; Hameed, A.S.; Ahmed, A. Evaluation of the use of polyphosphates as photostabilizers and in the formation of ball-like polystyrene materials. J. Polym. Res. 2019, 26, 161. [Google Scholar] [CrossRef]
- Mohammed, A.; El-Hiti, G.A.; Yousif, E.; Ahmed, A.A.; Ahmed, D.S.; Alotaibi, M.H. Protection of poly(vinyl chloride) films against photodegradation using various valsartan tin complexes. Polymers 2020, 12, 969. [Google Scholar] [CrossRef]
- Ali, M.M.; El-Hiti, G.A.; Yousif, E. Photostabilizing efficiency of poly(vinyl chloride) in the presence of organotin(IV) complexes as photostabilizers. Molecules 2016, 21, 1151. [Google Scholar] [CrossRef]
- Ghazi, D.; El-Hiti, G.A.; Yousif, E.; Ahmed, D.S.; Alotaibi, M.H. The effect of ultraviolet irradiation on the physicochemical properties of poly(vinyl chloride) films containing organotin(IV) complexes as photostabilizers. Molecules 2018, 23, 254. [Google Scholar] [CrossRef]
- Hadi, A.G.; Yousif, E.; El-Hiti, G.A.; Ahmed, D.S.; Jawad, K.; Alotaibi, M.H.; Hashim, H. Long-term effect of ultraviolet irradiation on poly(vinyl chloride) films containing naproxen diorganotin(IV) complexes. Molecules 2019, 24, 2396. [Google Scholar] [CrossRef]
- Hadi, A.G.; Jawad, K.; El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, A.A.; Ahmed, D.S.; Yousif, E. Photostabilization of poly(vinyl chloride) by organotin(IV) compounds against photodegradation. Molecules 2019, 24, 3557. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Li, C.; Pavlinek, V.; Saha, P.; Wang, H. Surface-modified antibacterial TiO2/Ag+ nanoparticles: Preparation and properties. Appl. Surf. Sci. 2006, 252, 4154–4160. [Google Scholar] [CrossRef]
- Birmingham, J.N. The effect of surface oxidation and titanium dioxide on exterior PVC color retention. J. Vinyl Addit. Technol. 1995, 1, 84–87. [Google Scholar] [CrossRef]
- Mallakpour, S.; Sadeghzadeh, R. A Benign and simple strategy for surface modification of Al2O3 nanoparticles with citric acid and L(+)-ascorbic acid and its application for the preparation of novel poly(vinyl chloride) nanocomposite films. Adv. Polym. Technol. 2017, 36, 409–417. [Google Scholar] [CrossRef]
- Sterzyński, T.; Tomaszewska, J.; Piszczek, K.; Skórczewska, K. The influence of carbon nanotubes on the PVC glass transition temperature. Compos. Sci. Technol. 2010, 70, 966–969. [Google Scholar] [CrossRef]
- Deanin, R.D.; Reynolds, H.H.; Ozcayir, Y. Thermal stabilization of polyvinyl chloride by group II metal laurates. J. Appl. Polym. Sci. 1969, 13, 1247–1252. [Google Scholar] [CrossRef]
- Mohammed, R.; El-Hiti, G.A.; Ahmed, A.; Yousif, E. Poly(vinyl chloride) doped by 2-(4-isobutylphenyl)propanoate metal complexes: Enhanced resistance to UV irradiation. Arab. J. Sci. Eng. 2017, 42, 4307–4315. [Google Scholar] [CrossRef]
- Nath, M.; Singh, H.; Kumar, P.; Kumar, A.; Song, X.; Eng, G. Organotin(IV) tryptophanylglycinates: Potential non-steroidal anti-inflammatory agents; crystal structure of dibutyltin(IV) tryptophanylglycinate. Applied Organometallic Chemistry 2009, 23, 347–358. [Google Scholar] [CrossRef]
- Masood, H.; Ali, S.; Mazhar, M. 1H, 13C, 119Sn NMR, mass, Mössbauer and biological studies of tri-, di- and chlorodiorganotin(IV) carboxylates. Turk. J. Chem. 2004, 28, 75–85. [Google Scholar]
- Alcock, N.W.; Culver, J.; Roe, S.M. Secondary bonding. Part 15. Influence of lone pairs on coordination: Comparison of diphenyl-tin(IV) and –tellurium(IV) carboxylates and dithiocarbamates. J. Chem. Soc. Dalton Trans 1992, 1477–1484. [Google Scholar] [CrossRef]
- Abd Mutalib, M.; Rahman, M.A.; Othman, M.H.D.; Ismail, A.F.; Jaafar, J. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-Ray (EDX) Spectroscopy. In Membrane Characterization; Hilal, N., Ismail, A.F., Matsuura, T., Oatley-Radcliffe, D., Eds.; Elsevier: Oxford, UK, 2017; Chapter 9; pp. 161–179. [Google Scholar] [CrossRef]
- Newbury, D.E. The new X-ray mapping: X-ray spectrum imaging above 100 kHz output count rate with the silicon drift detector. Microsc. Microanal. 2006, 12, 26–35. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gardette, J.L.; Gaumet, S.; Lemaire, J. Photooxidation of poly(viny1 chloride). 1. A reexamination of the mechanism. Macromolecules 1989, 22, 2576–2581. [Google Scholar] [CrossRef]
- Andrady, A.L.; Searle, N.D. Photodegradation of rigid PVC formulations. II. Spectral sensitivity to light-induced yellowing by polychromatic light. J. Appl. Polym. Sci. 1989, 37, 2789–2802. [Google Scholar] [CrossRef]
- Awaja, F.; Zhang, S.; Tripathi, M.; Nikiforov, A.; Pugno, N. Cracks, microcracks and fracture in polymer structures: Formation, detection, autonomic repair. Prog. Mater. Sci. 2016, 83, 536–573. [Google Scholar] [CrossRef]
- Valko, L.; Klein, E.; Kovařík, P.; Bleha, T.; Šimon, P. Kinetic study of thermal dehydrochlorination of poly(vinyl chloride) in the presence of oxygen: III. Statistical thermodynamic interpretation of the oxygen catalytic activity. Eur. Polym. J. 2001, 37, 1123–1132. [Google Scholar] [CrossRef]
- Mehmood, N.; Andreasson, E.; Kao-Walter, S. SEM observations of a metal foil laminated with a polymer film. Procedia Mater. Sci. 2014, 3, 1435–1440. [Google Scholar] [CrossRef]
- Nikafshar, S.; Zabihi, O.; Ahmadi, M.; Mirmohseni, A.; Taseidifar, M.; Naebe, M. The effects of UV light on the chemical and mechanical properties of a transparent epoxy-diamine system in the presence of an organic UV absorber. Materials 2017, 10, 180. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, J.; Shi, X.-M.; Jiang, G.-D. Different photodegradation processes of PVC with different average degrees of polymerization. J. Appl. Polym. Sci. 2008, 107, 528–540. [Google Scholar] [CrossRef]
- Zheng, X.-G.; Tang, L.-H.; Zhang, N.; Gao, Q.-H.; Zhang, C.-F.; Zhu, Z.-B. Dehydrochlorination of PVC materials at high temperature. Energy Fuels 2003, 17, 896–900. [Google Scholar] [CrossRef]










| Complex | Melting Point (°C) | Yield (%) | Measured % (Calculated; %) | ||
|---|---|---|---|---|---|
| C | H | N | |||
| 1 | 174–176 | 84 | 62.52(62.46) | 5.97 (5.90) | 4.50 (4.55) |
| 2 | 223–225 | 70 | 51.36 (51.28) | 7.94 (7.82) | 5.35 (5.44) |
| 3 | 208–211 | 69 | 56.69 (56.62) | 7.99 (7.92) | 7.25 (7.34) |
| 4 | 198–200 | 76 | 53.15 (53.03) | 7.20 (7.12) | 8.18 (8.25) |
| Complex | Wavenumber (ν; cm−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| OH | NH2 | C=O | Sn–C | Sn–O | Sn–N | |||
| asym | sym | ∆v (asym − sym) | ||||||
| 1 | 3375 | 3178 | 1639 | 1512 | 127 | 563 | 536 | 447 |
| 2 | 3356 | 3167 | 1674 | 1512 | 162 | 567 | 543 | 478 |
| 3 | 3332 | 3178 | 1670 | 1512 | 158 | 570 | 528 | 447 |
| 4 | 3332 | 3174 | 1639 | 1516 | 123 | 570 | 501 | 482 |
| Complex | 1H NMR (δ, ppm, J in Hz) |
|---|---|
| 1 | 7.89–7.82 (m, 6H, Ar), 7.49–7.41 (m, 9H, Ar), 7.19 (d, J = 7.9 Hz, 2H, Ar), 6.89 (d, J = 7.9 Hz, 2H, Ar), 6.85 (s, exch., 2H, NH2), 3.94 (br, 1H, CH–O), 3.87 (br, 2H, CH2–O), 3.31 (s, 2H, CH2C=O), 2.79–2.72 (m, 2H, CH and NH), 2.61 (m, 2H, CH2), 1.02 (d, J = 7.5 Hz, 6H, 2Me) |
| 2 | 6.72 (d, J = 7.8 Hz, 2H, Ar), 6.52 (d, J = 7.8 Hz, 2H, Ar), 6.36 (s, exch., 2H, NH2), 4.49 (s, exch., 1H, NH), 3.49 (br, 1H, CH–O), 3.41 (br, 2H, CH2–O), 2.85 (s, 2H, CH2C=O), 2.29–2.11 (m, 3H, CH2 and CH), 1.30–1.22 (m, 12H, 6 CH2), 1.06 (d, J = 7.5 Hz, 6H, 2 Me), 0.54 (t, J = 7.5 Hz, 6H, 2 Me) |
| 3 | 7.05 (s, exch., 4H, 2 NH2), 6.75 (d, J = 7.8 Hz, 4H, Ar), 6.45 (d, J = 7.8 Hz, 4H, Ar), 3.72 (br, 2H, 2 CH–O), 3.51 (br, 4H, 2 CH2–O), 2.87 (s, 4H, 2 CH2C=O), 2.75–2.59 (m, 4H, 2 CH and 2 NH), 2.43 (m, 4H, 2 CH2), 1.27–1.21 (m, 12H, 6 CH2), 0.99 (d, J = 7.6 Hz, 12H, 4 Me), 0.44 (t, J = 7.6 Hz, 6H, 2 Me) |
| 4 | 7.46 (s, exch., 4H, 2 NH2), 7.18 (d, J = 8.0 Hz, 4H, Ar), 6.87 (d, J = 8.0 Hz, 4H, Ar), 4.15 (br, 2H, 2 CH–O), 3.94 (br, 4H, 2 CH2–O), 3.21 (s, 4H, 2 CH2C=O), 3.05–3.01 (m, 4H, 2 CH and 2 NH), 2.87 (m, 4H, 2 CH2), 1.21 (d, J = 7.7 Hz, 12H, 4 Me), 0.72 (s, 6H, 2 Me) |
| Complex | 13C NMR (δ, ppm) |
|---|---|
| 1 | 172.5, 157.3, 136.0, 130.0, 128.9, 128.6, 128.4, 128.0, 114.2, 70.7, 68.1, 49.7, 48.3, 41.3, 22.5 |
| 2 | 172.1, 156.9, 129.5, 128.0, 113.8, 70.4, 68.0, 49.6, 47.7, 40.9, 40.1, 32.5, 27.8, 22.5, 13.7 |
| 3 | 172.1, 156.6, 129.6, 128.3, 113.8, 69.6, 65.5, 49.0, 47.2, 40.9, 27.4, 26.8, 25.4, 19.2, 13.2 |
| 4 | 172.6, 157.0, 130.0, 128.8, 114.3, 70.0, 65.8, 49.5, 47.4, 41.3, 19.4, 5.7 |
| PVC Film | Rq | Reduction in Rq (fold) |
|---|---|---|
| PVC | 322 | — |
| PVC + 1 | 43 | 7.5 |
| PVC + 2 | 75 | 4.3 |
| PVC + 3 | 70 | 4.6 |
| PVC + 4 | 81 | 4.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salam, B.; El-Hiti, G.A.; Bufaroosha, M.; Ahmed, D.S.; Ahmed, A.; Alotaibi, M.H.; Yousif, E. Tin Complexes Containing an Atenolol Moiety as Photostabilizers for Poly(Vinyl Chloride). Polymers 2020, 12, 2923. https://doi.org/10.3390/polym12122923
Salam B, El-Hiti GA, Bufaroosha M, Ahmed DS, Ahmed A, Alotaibi MH, Yousif E. Tin Complexes Containing an Atenolol Moiety as Photostabilizers for Poly(Vinyl Chloride). Polymers. 2020; 12(12):2923. https://doi.org/10.3390/polym12122923
Chicago/Turabian StyleSalam, Baneen, Gamal A. El-Hiti, Muna Bufaroosha, Dina S. Ahmed, Ahmed Ahmed, Mohammad Hayal Alotaibi, and Emad Yousif. 2020. "Tin Complexes Containing an Atenolol Moiety as Photostabilizers for Poly(Vinyl Chloride)" Polymers 12, no. 12: 2923. https://doi.org/10.3390/polym12122923
APA StyleSalam, B., El-Hiti, G. A., Bufaroosha, M., Ahmed, D. S., Ahmed, A., Alotaibi, M. H., & Yousif, E. (2020). Tin Complexes Containing an Atenolol Moiety as Photostabilizers for Poly(Vinyl Chloride). Polymers, 12(12), 2923. https://doi.org/10.3390/polym12122923