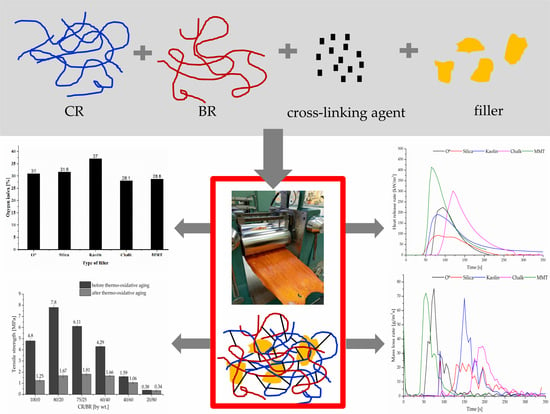
3.3. Mechanical, Dynamical and Thermal Properties of Filled CR/BR/Zn Vulcanizates
Different types of additives are used in the processing of rubbers into products include vulcanization accelerators, activators, plasticizers, dyes, pigments and fillers. Fillers are solid substances capable of changing the physical and chemical properties of rubber products by surface interaction or its lack thereof and by their own physical characteristics. Using a filler into the elastomers matrix allows to obtain the rubber composites of useful features. The fillers modify the physical and, to some extent, the chemical properties of the vulcanizates [
21]. In this study, silica, kaolin, chalk (in the amount of 30 phr) and montmorillonite (MMT, in the amount of 5 phr) were used as fillers into CR/BR/Zn (80/20/2.5 by wt %) compounds. Based on the obtained results, it was found that the incorporation of the filler into the studied blend significantly affects the cross-linking course and on the selected properties of cured products (
Table 6).
The cross-linking degree for the sample filled with kaolin or chalk was slightly lower compared to the unfilled CR/BR/Zn blend. The torque increment after 40 min of heating for the unfilled composite was 30.3 dNm, whereas ∆M
40 for the CR/BR/kaolin or CR/BR/chalk blend was 24.2 dNm or 29.4 dNm, respectively. More than twice the lower value of this parameter was observed in the case of the blend filled with silica (∆M
40 = 13.3 dNm). The decrease in this parameter is due to the fact that the precipitated silica adsorbs curative molecules on their surfaces, rendering the deactivation of vulcanization, and thus lower the cross-linking density of the prepared compounds is observed [
35,
36]. The viscosity of the CR/BR/Zn blend clearly increased in the sample containing silica, as evidenced by the highest value of the minimal moment (M
min = 21.2 dNm). This marked increase in viscosity is due to the flow restriction effect caused by solid particles of silica [
35]. A similar value of minimal torque (M
min~11.4 dNm) for the blend filled with kaolin, chalk or MMT was observed. In the case of the CR/BR/Zn blends filled with silica, kaolin or chalk the speed of vulcanization was similar (CRI~2.74 min
−1). However, for the MMT-filled blend the CRI value equal 3.25 min
−1, which may be due to the small filler’s amount in the total composition (only 5 phr). The lowest equilibrium swelling value (Q
VT = 6.52 mL/mL) in toluene was achieved in the case of chalk-filled vulcanizates, which is probably related to the strong adhesion of this filler to the polymer, which cannot be removed by the solvent molecules [
36]. However, the highest equilibrium swelling value in toluene (Q
VT = 11.56 mL/mL) was obtained for vulcanized filled with MMT, which indicates the lowest resistance to organic solvents of this compound.
A significant improvement of the tensile strength (
Table 6) is evident in the case of vulcanizate with the silica, kaolin or chalk relative to unfilled and MMT-filled CR/BR composites. The tensile strength for the filled (with silica, kaolin, chalk) CR/BR products was in the range from 9.62 to 10.08 MPa. The silica contains large amount of silanol groups (Si–OH) on the surface, it is considered as a highly polar and reactive filler. Therefore, silica is more compatible with chloroprene rubber. Hydrogen bonds are likely formed between CR and silica. It is possible that the positive hydrogen cation present in the silanol group of silica interacts with the negative chlorine atom in CR [
35]. The creation of additional bonds between silica and CR contributes to the improvement of mechanical properties. Kaolin and chalk reveal some chemical affinity to different elastomer into which they are incorporated to improve the physico-mechanical parameters. On the unmodified chalk surface, the entanglement of polymer chains is observed. Additionally, the chalk surface may react also with stearic acid present in the system, which leads to decreases anisotropy of chalk particles and at the same time increases its specific surface area. Therefore, the incorporation of the chalk improves the strength parameters of CR/BR/Zn vulcanizates [
37]. In the case of the CR/BR/Zn vulcanizates filled with kaolin, good mechanical properties can be caused by the particle’s shape of this filler that form thin lamellas, so that the tensile strength along the lamella direction is good [
38]. Conventional fillers, optionally with a coupling agent, are usually processed by direct incorporation into the rubber mixes. The incorporation of nanofillers into an elastomer makes it more difficult to obtain the good dispersion. Therefore, the successful application of nanofillers depends mainly on a good dispersion of these substances [
39]. The worst mechanical properties obtained for the vulcanizate filled with MMT may be related to incorrect dispersion of the filler in the elastomer matrix or the lowest cross-linking degree of this vulcanizate (∆M
40 = 17.8 dNm, Q
VT = 11.56 mL/mL). Additionally, the most attractive feature of nanocomposite is achieving excellent reinforcement effect only by very small filler loadings [
40]. The type of filler did not affect the value of elongation at break. For all samples, E
b was equal to about 660%. Nevertheless, the presence of silica and chalk in the vulcanizates produced increased their resistance to thermo-oxidative aging. In the case of vulcanizates containing silica, the tensile strength decreased from 10.08 MPa to 4.42 MPa, whereas TS’
b for the material filled with kaolin or montmorillonite was approximately 2.39 MPa. The low elongation at break that was examined after thermo-oxidative aging is also the reason for the very low aging coefficient (K ≤ 0.04).
These conclusions are consistent with the results of the dynamic analysis of tested materials. The calculated Payne effect allows to determine the interactions between the particles of fillers in the filled compounds. In this test, the dynamic properties were studied with strain sweep from a very small deformation to high deformation [
41]. In the filled vulcanizates, the reduction of storage modulus (G′) and the increment of loss modulus (G″) with the increasing amplitude deformation were observed (
Table 7,
Figure 3). For comparison, the dynamic properties of the unfilled sample were also presented. The changes in the minimum and maximum modules in filled composites were observed. The biggest decrease in the storage modulus was noticed in the case of the CR/BR/Zn/silica, which may result from the creation of the structure by the fillers interactions. Additionally, the decrease in the modulus was due to the increase in the surface area. Other fillers as kaolin, chalk and montmorillonite were less formable to create the ‘structure’, which was manifested by a much lesser change modulus.
Negative features of rubber materials include low thermal stability under exploitation conditions and too high flammability. Thermal properties of elastomers depend on the chemical structure of rubber macromolecules, method and degree of cross-linking and composition of rubber compounds. The conventionally cured butadiene rubber is a combustible material, determined by the oxygen index (OI) method. The OI value of conventional BR vulcanizates is equal to 17%. Chloroprene rubber cross-linked in a standard way, i.e., with zinc oxide and magnesium oxide, has a higher oxygen index (26%), which classifies it as a flame-retardant material. Based on this data thermal properties of the unfilled and filled CR/BR/Zn vulcanizates were investigated in this work. It was found that the combination of both elastomers and their cross-linking in the presence of zinc led to the production of incombustible materials. In the case of the CR/BR/Zn/kaolin, the highest value of the oxygen index (37%) was observed. Whereas the unfilled product or filled with silica, chalk or montmorillonite had the oxygen index above 28% (
Figure 4).
During the pyrolysis process, the fillers (kaolin, chalk) are decomposed with the release of large amounts of inert gases, e.g., carbon dioxide, water vapor. These gases dilute the gaseous, flammable, thermal decomposition products pass to the flame as well as they limit diffusion of oxygen into the combustion zone, which reduces the rate of thermal decomposition of the burned materials and increases their fire resistance.
The possibility of producing strong polymer-filler interactions (in the case of silica) helps to reduce the mobility of the segmental macromolecules, which results in an increase in the thermal stability of the vulcanizate and a reduction in its flammability [
42]. Very good fire properties of nanocomposites, such as the CR/BR/Zn/MMT compound, result from the structure of these materials. High temperatures and flames create a boundary layer on the surface of the material without cracks, isolating the polymer from fire and restricting access of combustible polymer decay products to the combustion zone [
43,
44]. Results obtained demonstrate the strong flame resistance of the tested CR/BR/Zn compositions, which does not require the incorporation of flame retardants.
The time of burning in air (tc) for four tested vulcanizates (unfilled and filled with silica, kaolin or MMT) did not exceed 5 s, which classifies these materials as self-extinguishing. The ts value of the CR/BR/Zn/chalk vulcanizate was 12 s. Burned samples behave differently, depending on the blend composition. All vulcanizates burn with a green glow, more or less intense, which is the result of the burning of chlorine present in CR. The cured CR/BR/Zn compounds containing silica were burned with a flame, which covered the sample in layers. Additionally, during the combustion, the sparking occurred and the sample delaminated and turn to ash. Due to the presence of kaolin in the CR/BR/Zn vulcanizate, the sample retained its shape after the burning, and the surface was covered with a layer of ash. The chalk-filled vulcanizate retained its shape after the burning, and the surface was covered with a black coating. The CR/BR/Zn vulcanizate containing montmorillonite burned with a smoky flame and after testing a frayed skeleton of the sample remained.
Although the method of oxygen index is commonly used for testing the flammability of polymers, the OI values obtained can only be used for a comparative assessment of flammability. It should be taken into account that the OI method is strict laboratory test. More detailed information is provided by the cone calorimeter (real fire conditions). It is assumed that the basic fire hazard properties of the material include: ignitability measured as the time to sustained ignition (TTI), total heat release (THR), heat release rate (HRR), effective heat of combustion (EHC) and average specific mass lost rate (MLR). The analysis of data presented in
Table 8 leads to the conclusion that the tested vulcanizates are materials with high resistance to burning. The average maximum heat release (HRR
max) of the unfilled CR/BR/Zn vulcanizates equal only 224.23 kW/m
2. For the CR/BR/Zn sample filled with silica, the HRR
max value decreased to 92.20 kW/m
2. The presence of nanofillers (MMT) in the tested compounds resulted in a slightly higher this parameter (414.01 kW/m
2) (
Figure 5). It should be stressed that during the combustion of the tested materials, regardless of their composition, the heat release rate is very low as compared to the commonly used rubber products such as cured acrylonitrile–butadiene rubbers, NBR18 and NBR39, for which this parameter amounts to 3569.23 and 3115.28 kW/m
2, respectively [
45].
The mass loss rate (MLR) of the studied composites, which proves the dynamics of material combustion dynamics, reached the highest value (49.99 g/m
2·s) in the case of the CR/BR/Zn vulcanizate containing montmorillonite. During the combustion of this compound, the greatest amount of heat is released, and the sample was burned in 97.7% (
Figure 6).
Based on the results obtained with the cone calorimeter the fire hazard was calculated, which is connected with the fire propagation rate (1/t
flashover), i.e., the inverse time to reach the flashover effect [
46]. The lowest fire hazard was observed for the CR/BR/Zn vulcanizates filled with silica or chalk (1/t
flashover equal 3.29 and 3.97 kW/m
2·s, respectively). Again, among the tested compounds, the sample containing nanofiller was characterized by the highest 1/t
flashover value (9.63 kW/m
2·s). It is worth noting that 1/t
flashover assessed on the basis of the ignition time and HRR
max of all the vulcanizates produced is much smaller (
Table 8) as compared with cured acrylonitrile-butadiene rubbers, NBR18 and NBR39, whose corresponding values amount to 65.87 and 65.88 kW/m
2·s, respectively [
45]. The obtained test results clearly indicate that the manufactured CR/BR/Zn products, both unfilled and filled, are non-flammable and pose a low fire hazard.