Progress and Prospects of Polymer-Based Drug Delivery Systems for Bone Tissue Regeneration
Abstract
:1. Introduction
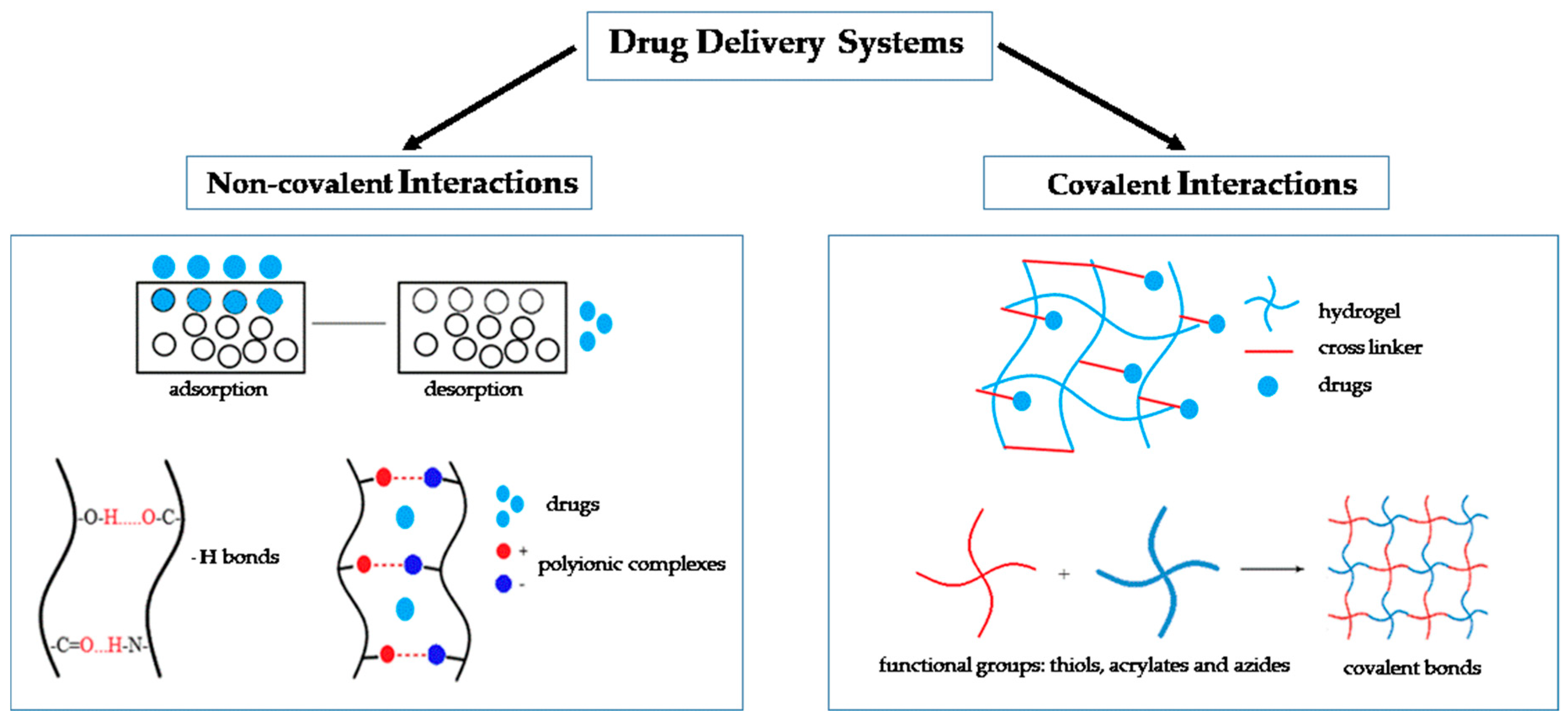
2. Types of Drug Delivery Systems
2.1. Non-Covalent Interactions
2.2. Covalent Interactions
3. Biodegradable Polymers for Drug Delivery
3.1. Natural Polymers for Drug Delivery
3.1.1. Collagen
3.1.2. Chitosan
3.1.3. Hyaluronic Acid
3.1.4. Alginate
3.1.5. Fibrin
3.2. Synthetic Polymers for Drug Delivery
3.2.1. Poly (ε-caprolactone)
3.2.2. Poly(lactic-co-glycolic acid)
3.2.3. Poly(lactic acid)
3.2.4. Polyethylene Glycol
4. Polymer Nanoparticle- and Microparticle-Based Drug Delivery Systems for Bone Regeneration
5. Clinical Trials of Polymer-Based Drug Delivery Systems for Bone Tissue Regeneration
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ghiasi, M.S.; Chen, J.; Vaziri, A.; Rodriguez, E.K.; Nazarian, A. Bone fracture healing in mechanobiological modeling: A review of principles and methods. Bone Rep. 2017, 6, 87–100. [Google Scholar]
- Marongiu, G.; Dolci, A.; Verona, M.; Capone, A. The biology and treatment of acute long-bones diaphyseal fractures: Overview of the current options for bone healing enhancement. Bone Rep. 2020, 12, 100249. [Google Scholar]
- Sen, M.K.; Miclau, T. Autologous iliac crest bone graft: Should it still be the gold standard for treating nonunions? Injury 2007, 38 (Suppl. 1), S75–S80. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Calcei, J.G.; Rodeo, S.A. Orthobiologics for Bone Healing. Clin. Sports Med. 2019, 38, 79–95. [Google Scholar] [CrossRef]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, Y.; Kawai, T.; Goto, K.; Matsuda, S. Clinical application of injectable growth factor for bone regeneration: A systematic review. Inflamm. Regen. 2019, 39, 20. [Google Scholar] [CrossRef]
- El Bialy, I.; Jiskoot, W.; Reza Nejadnik, M. Formulation, Delivery and Stability of Bone Morphogenetic Proteins for Effective Bone Regeneration. Pharm. Res. 2017, 34, 1152–1170. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Guo, J.; Zhou, Y.; Wu, G. The roles of bone morphogenetic proteins and their signaling in the osteogenesis of adipose-derived stem cells. Tissue Eng. Part B Rev. 2014, 20, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Ramly, E.P.; Alfonso, A.R.; Kantar, R.S.; Wang, M.M.; Siso, J.R.D.; Ibrahim, A.; Coelho, P.G.; Flores, R.L. Safety and efficacy of recombinant human bone morphogenetic protein-2 (rhBMP-2) in craniofacial surgery. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2347. [Google Scholar] [CrossRef]
- Carreira, A.C.; Lojudice, F.H.; Halcsik, E.; Navarro, R.D.; Sogayar, M.C.; Granjeiro, J.M. Bone morphogenetic proteins: Facts, challenges, and future perspectives. J. Dent. Res. 2014, 93, 335–345. [Google Scholar] [CrossRef]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef]
- Zeng, Y.; Hoque, J.; Varghese, S. Biomaterial-assisted local and systemic delivery of bioactive agents for bone repair. Acta Biomater. 2019, 93, 152–168. [Google Scholar] [CrossRef]
- Aoki, K.; Saito, N. Biodegradable polymers as drug delivery systems for bone regeneration. Pharmaceutics 2020, 12, 95. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A unique polymer for drug delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef]
- Yan, Q.; Xiao, L.Q.; Tan, L.; Sun, W.; Wu, T.; Chen, L.W.; Mei, Y.; Shi, B. Controlled release of simvastatin-loaded thermo-sensitive PLGA-PEG-PLGA hydrogel for bone tissue regeneration: In vitro and in vivo characteristics. J. Biomed. Mater. Res. Part A 2015, 103, 3580–3589. [Google Scholar] [CrossRef]
- Olthof, M.G.L.; Kempen, D.H.R.; Herrick, J.L.; Yaszemski, M.J.; Dhert, W.J.A.; Lu, L. Effect of different sustained bone morphogenetic protein-2 release kinetics on bone formation in poly(propylene fumarate) scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 477–487. [Google Scholar] [CrossRef]
- Filippi, M.; Born, G.; Chaaban, M.; Scherberich, A. Natural polymeric scaffolds in bone regeneration. Front Bioeng. Biotechnol. 2020, 8, 474. [Google Scholar] [CrossRef]
- Chen, Y.X.; Zhu, R.; Xu, Z.L.; Ke, Q.F.; Zhang, C.Q.; Guo, Y.P. Self-assembly of pifithrin-α-loaded layered double hydroxide/chitosan nanohybrid composites as a drug delivery system for bone repair materials. J. Mater. Chem. B 2017, 5, 2245–2253. [Google Scholar] [CrossRef]
- Sionkowska, A. Current research on the blends of natural and synthetic polymers as new biomaterials. Prog. Polym. Sci. 2011, 36, 1254–1276. [Google Scholar] [CrossRef]
- Brudno, Y.; Mooney, D.J. On-demand drug delivery from local depots. J. Control. Release 2015, 219, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chen, Y.; Ho, E.A.; Liu, S. Reversibly pH-responsive polyurethane membranes for on-demand intravaginal drug delivery. Acta Biomater. 2017, 47, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Chen, J.; Zhao, S.; Ding, J.; Chen, X. Thermo-sensitive polypeptide hydrogel for locally sequential delivery of two-pronged antitumor drugs. Acta Biomater. 2017, 58, 44–53. [Google Scholar] [CrossRef]
- Sultankulov, B.; Berillo, D.; Sultankulova, K.; Tokay, T.; Saparov, A. Progress in the development of chitosan-based biomaterials for tissue engineering and regenerative medicine. Biomolecules 2019, 9, 470. [Google Scholar] [CrossRef] [Green Version]
- Mansurov, N.; Chen, W.C.; Awada, H.; Huard, J.; Wang, Y.; Saparov, A. A controlled release system for simultaneous delivery of three human perivascular stem cell-derived factors for tissue repair and regeneration. J. Tissue Eng. Regen. Med. 2018, 12, e1164–e1172. [Google Scholar] [CrossRef]
- Mitchell, A.C.; Briquez, P.S.; Hubbell, J.A.; Cochran, J.R. Engineering growth factors for regenerative medicine applications. Acta Biomater. 2016, 30, 1–12. [Google Scholar] [CrossRef]
- Atienza-Roca, P.; Cui, X.; Hooper, G.J.; Woodfield, T.B.; Lim, K.S. Growth factor delivery systems for tissue engineering and regenerative medicine. In Cutting-Edge Enabling Technologies for Regenerative Medicine; Springer: New York, NY, USA, 2018; pp. 245–269. [Google Scholar]
- Draenert, F.G.; Nonnenmacher, A.L.; Kämmerer, P.W.; Goldschmitt, J.; Wagner, W. BMP-2 and bFGF release and in vitro effect on human osteoblasts after adsorption to bone grafts and biomaterials. Clin. Oral Implant. Res. 2013, 24, 750–757. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Lu, W.W.; Zhen, W.; Yang, D.; Peng, S. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Mater. 2017, 9, e435. [Google Scholar] [CrossRef]
- Nurkesh, A.; Jaguparov, A.; Jimi, S.; Saparov, A. Recent advances in the controlled release of growth factors and cytokines for improving cutaneous wound healing. Front. Cell Dev. Biol. 2020, 8, 638. [Google Scholar] [CrossRef]
- Jimi, S.; Jaguparov, A.; Nurkesh, A.; Sultankulov, B.; Saparov, A. Sequential delivery of cryogel released growth factors and cytokines accelerates wound healing and improves tissue regeneration. Front. Bioeng. Biotechnol. 2020, 8, 345. [Google Scholar] [CrossRef]
- Bouyer, M.; Guillot, R.; Lavaud, J.; Plettinx, C.; Olivier, C.; Curry, V.; Boutonnat, J.; Coll, J.-L.; Peyrin, F.; Josserand, V. Surface delivery of tunable doses of BMP-2 from an adaptable polymeric scaffold induces volumetric bone regeneration. Biomaterials 2016, 104, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; La, W.-G.; Bhang, S.H.; Jeon, J.-Y.; Lee, J.H.; Kim, B.-S. Heparin-conjugated fibrin as an injectable system for sustained delivery of bone morphogenetic protein-2. Tissue Eng. Part A 2010, 16, 1225–1233. [Google Scholar] [CrossRef]
- De Witte, T.M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lienemann, P.S.; Lutolf, M.P.; Ehrbar, M. Biomimetic hydrogels for controlled biomolecule delivery to augment bone regeneration. Adv. Drug Deliv. Rev. 2012, 64, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Silva, E.A.; Mooney, D.J. Growth factor delivery-based tissue engineering: General approaches and a review of recent developments. J. R. Soc. Interface 2011, 8, 153–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Censi, R.; Di Martino, P.; Vermonden, T.; Hennink, W.E. Hydrogels for protein delivery in tissue engineering. J. Control. Release Off. J. Control. Release Soc. 2012, 161, 680–692. [Google Scholar] [CrossRef]
- Busra, M.F.M.; Lokanathan, Y. Recent development in the fabrication of collagen scaffolds for tissue engineering applications: A review. Curr. Pharm. Biotechnol. 2019, 20, 992–1003. [Google Scholar] [CrossRef]
- Sheikh, Z.; Javaid, M.A.; Hamdan, N.; Hashmi, R. Bone regeneration using bone morphogenetic proteins and various biomaterial carriers. Materials 2015, 8, 1778–1816. [Google Scholar] [CrossRef]
- Hyun, S.J.; Han, D.K.; Choi, S.H.; Chai, J.K.; Cho, K.S.; Kim, C.K.; Kim, C.S. Effect of recombinant human bone morphogenetic protein-2, -4, and -7 on bone formation in rat calvarial defects. J. Periodontol. 2005, 76, 1667–1674. [Google Scholar] [CrossRef]
- Nakamura, T.; Shirakata, Y.; Shinohara, Y.; Miron, R.J.; Furue, K.; Noguchi, K. Osteogenic potential of recombinant human bone morphogenetic protein-9/absorbable collagen sponge (rhBMP-9/ACS) in rat critical size calvarial defects. Clin. Oral Investig. 2017, 21, 1659–1665. [Google Scholar] [CrossRef]
- Lo, K.W.-H.; Ulery, B.D.; Ashe, K.M.; Laurencin, C.T. Studies of bone morphogenetic protein-based surgical repair. Adv. Drug Deliv. Rev. 2012, 64, 1277–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zara, J.N.; Siu, R.K.; Zhang, X.; Shen, J.; Ngo, R.; Lee, M.; Li, W.; Chiang, M.; Chung, J.; Kwak, J. High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue Eng. Part A 2011, 17, 1389–1399. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, V.; Sinha, M. A review on carrier systems for bone morphogenetic protein-2. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 904–925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; He, Q.-F.; Zhang, T.-H.; Yu, X.-L.; Liu, Q.; Deng, F.-I. Improvement in the delivery system of bone morphogenetic protein-2: A new approach to promote bone formation. Biomed. Mater. 2012, 7, 045002. [Google Scholar] [CrossRef] [PubMed]
- Osidak, E.; Osidak, M.; Sivogrivov, D.; Portnaya, T.; Grunina, T.; Soboleva, L.; Lunin, V.; Karyagina, A.; Domogatskii, S. Regulation of the binding of the BMP-2 growth factor with collagen by blood plasma fibronectin. Appl. Biochem. Microbiol. 2014, 50, 200–205. [Google Scholar] [CrossRef]
- Liu, K.; Meng, C.X.; Lv, Z.Y.; Zhang, Y.J.; Li, J.; Li, K.Y.; Liu, F.Z.; Zhang, B.; Cui, F.Z. Enhancement of BMP-2 and VEGF carried by mineralized collagen for mandibular bone regeneration. Regen. Biomater. 2020, 7, 435–440. [Google Scholar] [CrossRef]
- Fujioka-Kobayashi, M.; Schaller, B.; Saulacic, N.; Pippenger, B.E.; Zhang, Y.; Miron, R.J. Absorbable collagen sponges loaded with recombinant bone morphogenetic protein 9 induces greater osteoblast differentiation when compared to bone morphogenetic protein 2. Clin. Exp. Dent. Res. 2017, 3, 32–40. [Google Scholar] [CrossRef]
- Levengood, S.K.L.; Zhang, M. Chitosan-based scaffolds for bone tissue engineering. J. Mater. Chem. B 2014, 2, 3161–3184. [Google Scholar] [CrossRef]
- Saravanan, S.; Leena, R.S.; Selvamurugan, N. Chitosan based biocomposite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1354–1365. [Google Scholar] [CrossRef]
- Venkatesan, J.; Anil, S.; Kim, S.K.; Shim, M.S. Chitosan as a vehicle for growth factor delivery: Various preparations and their applications in bone tissue regeneration. Int. J. Biol. Macromol. 2017, 104, 1383–1397. [Google Scholar] [CrossRef]
- Sultankulov, B.; Berillo, D.; Kauanova, S.; Mikhalovsky, S.; Mikhalovska, L.; Saparov, A. Composite cryogel with polyelectrolyte complexes for growth factor delivery. Pharmaceutics 2019, 11, 650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef]
- Aguilar, A.; Zein, N.; Harmouch, E.; Hafdi, B.; Bornert, F.; Offner, D.; Clauss, F.; Fioretti, F.; Huck, O.; Benkirane-Jessel, N.; et al. Application of chitosan in bone and dental engineering. Molecules 2019, 24, 3009. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Kang, Y.; Mercado-Pagán, Á.E.; Maloney, W.J.; Yang, Y. In vitro evaluation of photo-crosslinkable chitosan-lactide hydrogels for bone tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1393–1406. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Bedigrew, K.; Guda, T.; Maloney, W.J.; Park, S.; Wenke, J.C.; Yang, Y.P. Novel osteoinductive photo-cross-linkable chitosan-lactide-fibrinogen hydrogels enhance bone regeneration in critical size segmental bone defects. Acta Biomater. 2014, 10, 5021–5033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, I.-H.; Jeong, B.-C.; Kook, M.-S.; Kim, S.-H.; Koh, J.-T. Evaluation of a thiolated chitosan scaffold for local delivery of BMP-2 for osteogenic differentiation and ectopic bone formation. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Yu, B.; Huang, Q.; Liu, R.; Feng, Q.; Cai, Q.; Mi, S. In vitro BMP-2 peptide release from thiolated chitosan based hydrogel. Int. J. Biol. Macromol. 2016, 93, 314–321. [Google Scholar] [CrossRef]
- Yun, Y.-P.; Kim, S.E.; Kang, E.Y.; Kim, H.-J.; Park, K.; Song, H.-R. The effect of bone morphogenic protein-2 (BMP-2)-immobilizing heparinized-chitosan scaffolds for enhanced osteoblast activity. Tissue Eng. Regen. Med. 2013, 10, 122–130. [Google Scholar] [CrossRef]
- Kim, S.; Fan, J.; Lee, C.-S.; Chen, C.; Bubukina, K.; Lee, M. Heparinized chitosan stabilizes the bioactivity of BMP-2 and potentiates the osteogenic efficacy of demineralized bone matrix. J. Biol. Eng. 2020, 14, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Hemshekhar, M.; Thushara, R.M.; Chandranayaka, S.; Sherman, L.S.; Kemparaju, K.; Girish, K.S. Emerging roles of hyaluronic acid bioscaffolds in tissue engineering and regenerative medicine. Int. J. Biol. Macromol. 2016, 86, 917–928. [Google Scholar] [CrossRef]
- Zhao, N.; Wang, X.; Qin, L.; Zhai, M.; Yuan, J.; Chen, J.; Li, D. Effect of hyaluronic acid in bone formation and its applications in dentistry. J. Biomed. Mater. Res. Part A 2016, 104, 1560–1569. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.K.; Choi, J.H.; Shin, M.E.; Kim, J.W.; Kim, P.Y.; Kim, N.; Song, J.E.; Khang, G. Evaluation of cartilage regeneration of chondrocyte encapsulated gellan gum-based hyaluronic acid blended hydrogel. Int. J. Biol. Macromol. 2019, 141, 51–59. [Google Scholar] [CrossRef]
- Bhakta, G.; Lim, Z.X.; Rai, B.; Lin, T.; Hui, J.H.; Prestwich, G.D.; van Wijnen, A.J.; Nurcombe, V.; Cool, S.M. The influence of collagen and hyaluronan matrices on the delivery and bioactivity of bone morphogenetic protein-2 and ectopic bone formation. Acta Biomater. 2013, 9, 9098–9106. [Google Scholar] [CrossRef] [PubMed]
- Kisiel, M.; Martino, M.M.; Ventura, M.; Hubbell, J.A.; Hilborn, J.; Ossipov, D.A. Improving the osteogenic potential of BMP-2 with hyaluronic acid hydrogel modified with integrin-specific fibronectin fragment. Biomaterials 2013, 34, 704–712. [Google Scholar] [CrossRef]
- Bae, M.S.; Ohe, J.Y.; Lee, J.B.; Heo, D.N.; Byun, W.; Bae, H.; Kwon, Y.D.; Kwon, I.K. Photo-cured hyaluronic acid-based hydrogels containing growth and differentiation factor 5 (GDF-5) for bone tissue regeneration. Bone 2014, 59, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.S.; Yang, D.H.; Lee, J.B.; Heo, D.N.; Kwon, Y.D.; Youn, I.C.; Choi, K.; Hong, J.H.; Kim, G.T.; Choi, Y.S.; et al. Photo-cured hyaluronic acid-based hydrogels containing simvastatin as a bone tissue regeneration scaffold. Biomaterials 2011, 32, 8161–8171. [Google Scholar] [CrossRef]
- Ray, P.; Maity, M.; Barik, H.; Sahoo, G.S.; Hasnain, M.S.; Hoda, M.N.; Nayak, A.K. Alginate-based hydrogels for drug delivery applications. In Alginates in Drug Delivery; Elsevier: New York, NY, USA, 2020; pp. 41–70. [Google Scholar]
- Pawar, S.N.; Edgar, K.J. Chemical modification of alginates in organic solvent systems. Biomacromolecules 2011, 12, 4095–4103. [Google Scholar] [CrossRef]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef]
- Pawar, S.N.; Edgar, K.J. Alginate esters via chemoselective carboxyl group modification. Carbohydr. Polym. 2013, 98, 1288–1296. [Google Scholar] [CrossRef]
- Su, J.; Xu, H.; Sun, J.; Gong, X.; Zhao, H. Dual delivery of BMP-2 and bFGF from a new nano-composite scaffold, loaded with vascular stents for large-size mandibular defect regeneration. Int. J. Mol. Sci. 2013, 14, 12714–12728. [Google Scholar] [CrossRef] [Green Version]
- Choi, D.H.; Park, C.H.; Kim, I.H.; Chun, H.J.; Park, K.; Han, D.K. Fabrication of core-shell microcapsules using PLGA and alginate for dual growth factor delivery system. J. Control. Release Off. J. Control. Release Soc. 2010, 147, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Kanczler, J.M.; Ginty, P.J.; White, L.; Clarke, N.M.; Howdle, S.M.; Shakesheff, K.M.; Oreffo, R.O. The effect of the delivery of vascular endothelial growth factor and bone morphogenic protein-2 to osteoprogenitor cell populations on bone formation. Biomaterials 2010, 31, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.; Wang, C.; Yuan, X.; Liu, Z.; Dziak, R.; Yang, S. Combination of controlled release platelet-rich plasma alginate beads and bone morphogenetic protein-2 genetically modified mesenchymal stem cells for bone regeneration. J. Periodontol. 2016, 87, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Bujoli, B.; Scimeca, J.C.; Verron, E. Fibrin as a multipurpose physiological platform for bone tissue engineering and targeted delivery of bioactive compounds. Pharmaceutics 2019, 11, 556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noori, A.; Ashrafi, S.J.; Vaez-Ghaemi, R.; Hatamian-Zaremi, A.; Webster, T.J. A review of fibrin and fibrin composites for bone tissue engineering. Int. J. Nanomed. 2017, 12, 4937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khodakaram-Tafti, A.; Mehrabani, D.; Shaterzadeh-Yazdi, H. An overview on autologous fibrin glue in bone tissue engineering of maxillofacial surgery. Dent. Res. J. 2017, 14, 79. [Google Scholar]
- Martino, M.M.; Hubbell, J.A. The 12th-14th type III repeats of fibronectin function as a highly promiscuous growth factor-binding domain. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2010, 24, 4711–4721. [Google Scholar] [CrossRef]
- Yang, H.S.; La, W.G.; Cho, Y.M.; Shin, W.; Yeo, G.D.; Kim, B.S. Comparison between heparin-conjugated fibrin and collagen sponge as bone morphogenetic protein-2 carriers for bone regeneration. Exp. Mol. Med. 2012, 44, 350–355. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Casanova, S.; Martin-Saavedra, F.M.; Escudero-Duch, C.; Falguera Uceda, M.I.; Prieto, M.; Arruebo, M.; Acebo, P.; Fabiilli, M.L.; Franceschi, R.T.; Vilaboa, N. Local delivery of bone morphogenetic protein-2 from near infrared-responsive hydrogels for bone tissue regeneration. Biomaterials 2020, 241, 119909. [Google Scholar] [CrossRef]
- Rao, S.H.; Harini, B.; Shadamarshan, R.P.K.; Balagangadharan, K.; Selvamurugan, N. Natural and synthetic polymers/bioceramics/bioactive compounds-mediated cell signalling in bone tissue engineering. Int. J. Biol. Macromol. 2018, 110, 88–96. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, A.S.; Asif, A.; Yar, M.; Haycock, J.W.; Rehman, I.U. Recent concepts in biodegradable polymers for tissue engineering paradigms: A critical review. Int. Mater. Rev. 2019, 64, 91–126. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, G.; Vernekar, V.N.; Kuyinu, E.L.; Laurencin, C.T. Poly (lactic acid)-based biomaterials for orthopaedic regenerative engineering. Adv. Drug Deliv. Rev. 2016, 107, 247–276. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current Concepts in Scaffolding for Bone Tissue Engineering. Arch. Bone Jt. Surg. 2018, 6, 90–99. [Google Scholar] [PubMed]
- Miroshnichenko, S.; Timofeeva, V.; Permykova, E.; Ershov, S.; Kiryukhantsev-Korneev, P.; Dvořaková, E.; Shtansky, D.V.; Zajíčková, L.; Solovieva, A.; Manakhov, A. Plasma-coated polycaprolactone nanofibers with covalently bonded platelet-rich plasma enhance adhesion and growth of human fibroblasts. Nanomaterials 2019, 9, 637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldemir Dikici, B.; Dikici, S.; Reilly, G.C.; MacNeil, S.; Claeyssens, F. A novel bilayer polycaprolactone membrane for guided bone regeneration: Combining electrospinning and emulsion templating. Materials 2019, 12, 2643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pazarçeviren, A.E.; Dikmen, T.; Altunbaş, K.; Yaprakçı, V.; Erdemli, Ö.; Keskin, D.; Tezcaner, A. Composite clinoptilolite/PCL-PEG-PCL scaffolds for bone regeneration: In vitro and in vivo evaluation. J. Tissue Eng. Regen. Med. 2020, 14, 3–15. [Google Scholar] [CrossRef]
- Wang, H.; Tong, D.; Wang, L.; Chen, L.; Yu, N.; Li, Z. A facile strategy for fabricating PCL/PEG block copolymer with excellent enzymatic degradation. Polym. Degrad. Stab. 2017, 140, 64–73. [Google Scholar] [CrossRef]
- Huang, B.; Caetano, G.; Vyas, C.; Blaker, J.J.; Diver, C.; Bártolo, P. Polymer-ceramic composite scaffolds: The effect of hydroxyapatite and β-tri-calcium phosphate. Materials 2018, 11, 129. [Google Scholar] [CrossRef] [Green Version]
- Bittner, S.M.; Smith, B.T.; Diaz-Gomez, L.; Hudgins, C.D.; Melchiorri, A.J.; Scott, D.W.; Fisher, J.P.; Mikos, A.G. Fabrication and mechanical characterization of 3D printed vertical uniform and gradient scaffolds for bone and osteochondral tissue engineering. Acta Biomater. 2019, 90, 37–48. [Google Scholar] [CrossRef]
- Park, S.H.; Park, S.A.; Kang, Y.G.; Shin, J.W.; Park, Y.S.; Gu, S.R.; Wu, Y.R.; Wei, J.; Shin, J.W. PCL/β-TCP Composite scaffolds exhibit positive osteogenic differentiation with mechanical stimulation. Tissue Eng. Regen. Med. 2017, 14, 349–358. [Google Scholar] [CrossRef]
- Bao, X.; Zhu, L.; Huang, X.; Tang, D.; He, D.; Shi, J.; Xu, G. 3D biomimetic artificial bone scaffolds with dual-cytokines spatiotemporal delivery for large weight-bearing bone defect repair. Sci. Rep. 2017, 7, 7814. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Liu, Y.; Pan, Y.; Miszuk, J.M.; Sun, H. One-pot porogen free method fabricated porous microsphere-aggregated 3D PCL scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2699–2710. [Google Scholar] [CrossRef] [PubMed]
- Junka, R.; Yu, X. Polymeric nanofibrous scaffolds laden with cell-derived extracellular matrix for bone regeneration. Mater. Sci. Eng. C 2020, 113, 110981. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Cosme, J.G.; Xu, T.; Miszuk, J.M.; Picciani, P.H.; Fong, H.; Sun, H. Three dimensional electrospun PCL/PLA blend nanofibrous scaffolds with significantly improved stem cells osteogenic differentiation and cranial bone formation. Biomaterials 2017, 115, 115–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [Green Version]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110698. [Google Scholar] [CrossRef]
- Generali, M.; Kehl, D.; Capulli, A.K.; Parker, K.K.; Hoerstrup, S.P.; Weber, B. Comparative analysis of poly-glycolic acid-based hybrid polymer starter matrices for in vitro tissue engineering. Colloids Surf. B Biointerfaces 2017, 158, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.; Han, J.; Sharma, N.; Msallem, B.; Jeong, W.; Son, J.; Kunz, C.; Kang, H.W.; Thieringer, F.M. In vitro mechanical and biological properties of 3D printed polymer composite and β-tricalcium phosphate scaffold on human dental pulp stem cells. Materials 2020, 13, 3057. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Fu, L.; Ye, S.; Wang, M.; Zhou, Y. Fabrication and application of novel porous scaffold in situ-loaded graphene oxide and osteogenic peptide by cryogenic 3d printing for repairing critical-sized bone defect. Molecules 2019, 24, 1669. [Google Scholar] [CrossRef] [Green Version]
- Deng, N.; Sun, J.; Li, Y.; Chen, L.; Chen, C.; Wu, Y.; Wang, Z.; Li, L. Experimental study of rhBMP-2 chitosan nano-sustained release carrier-loaded PLGA/nHA scaffolds to construct mandibular tissue-engineered bone. Arch. Oral Biol. 2019, 102, 16–25. [Google Scholar] [CrossRef]
- Das, A.; Fishero, B.A.; Christophel, J.J.; Li, C.J.; Kohli, N.; Lin, Y.; Dighe, A.S.; Cui, Q. Poly(lactic-co-glycolide) polymer constructs cross-linked with human BMP-6 and VEGF protein significantly enhance rat mandible defect repair. Cell Tissue Res. 2016, 364, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Han, M.A.; Shin, J.Y.; Jeon, J.H.; Lee, S.J.; Yoon, M.Y.; Kim, H.J.; Choi, E.J.; Do, S.H.; Yang, V.C.; et al. Intra-articular delivery of synovium-resident mesenchymal stem cells via BMP-7-loaded fibrous PLGA scaffolds for cartilage repair. J. Control. Release Off. J. Control. Release Soc. 2019, 302, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Jakus, A.E.; Rutz, A.L.; Jordan, S.W.; Kannan, A.; Mitchell, S.M.; Yun, C.; Koube, K.D.; Yoo, S.C.; Whiteley, H.E.; Richter, C.P.; et al. Hyperelastic “bone”: A highly versatile, growth factor-free, osteoregenerative, scalable, and surgically friendly biomaterial. Sci. Transl. Med. 2016, 8, 358ra127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajebi, S.; Mohammadi Nasr, S.; Rabiee, N.; Bagherzadeh, M.; Ahmadi, S.; Rabiee, M.; Tahriri, M.; Tayebi, L.; Hamblin, M.R. Bioresorbable composite polymeric materials for tissue engineering applications. Int. J. Polym. Mater. Polym. Biomater. 2020, 1–15. [Google Scholar] [CrossRef]
- Botlhoko, O.J.; Ramontja, J.; Ray, S.S. A new insight into morphological, thermal, and mechanical properties of melt-processed polylactide/poly (ε-caprolactone) blends. Polym. Degrad. Stab. 2018, 154, 84–95. [Google Scholar] [CrossRef]
- Li, H.; Qiao, T.; Song, P.; Guo, H.; Song, X.; Zhang, B.; Chen, X. Star-shaped PCL/PLLA blended fiber membrane via electrospinning. J. Biomater. Sci. Polym. Ed. 2015, 26, 420–432. [Google Scholar] [CrossRef]
- Qiao, T.; Song, P.; Guo, H.; Song, X.; Zhang, B.; Chen, X. Reinforced electrospun PLLA fiber membrane via chemical crosslinking. Eur. Polym. J. 2016, 74, 101–108. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; Botta, L.; Rigogliuso, S.; Ghersi, G. Preparation of three-layered porous PLA/PEG scaffold: Relationship between morphology, mechanical behavior and cell permeability. J. Mech. Behav. Biomed. Mater. 2016, 54, 8–20. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, X.; Zhao, D.; Jiang, W.; Du, Z.; Li, Q.; Jiang, C.; Han, D. Three dimensional printed polylactic acid-hydroxyapatite composite scaffolds for prefabricating vascularized tissue engineered bone: An in vivo bioreactor model. Sci. Rep. 2017, 7, 15255. [Google Scholar] [CrossRef]
- Mohammadi, M.; Alibolandi, M.; Abnous, K.; Salmasi, Z.; Jaafari, M.R.; Ramezani, M. Fabrication of hybrid scaffold based on hydroxyapatite-biodegradable nanofibers incorporated with liposomal formulation of BMP-2 peptide for bone tissue engineering. Nanomedicine 2018, 14, 1987–1997. [Google Scholar] [CrossRef]
- Ma, D.; An, G.; Liang, M.; Liu, Y.; Zhang, B.; Wang, Y. A composited PEG-silk hydrogel combining with polymeric particles delivering rhBMP-2 for bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 65, 221–231. [Google Scholar] [CrossRef]
- Yang, T.; Hu, Y.; Wang, C.; Binks, B.P. Fabrication of hierarchical macroporous biocompatible scaffolds by combining pickering high internal phase emulsion templates with three-dimensional printing. ACS Appl. Mater. Interfaces 2017, 9, 22950–22958. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Ma, S.; Jin, L.; Liu, Z.; Liu, D.; Zhang, X.; Cai, Q.; Yang, X. Repairing a bone defect with a three-dimensional cellular construct composed of a multi-layered cell sheet on electrospun mesh. Biofabrication 2017, 9, 025036. [Google Scholar] [CrossRef] [PubMed]
- Saroia, J.; Yanen, W.; Wei, Q.; Zhang, K.; Lu, T.; Zhang, B. A review on biocompatibility nature of hydrogels with 3D printing techniques, tissue engineering application and its future prospective. Bio Des. Manuf. 2018, 1, 265–279. [Google Scholar] [CrossRef]
- Wang, J.Z.; You, M.L.; Ding, Z.Q.; Ye, W.B. A review of emerging bone tissue engineering via PEG conjugated biodegradable amphiphilic copolymers. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 1021–1035. [Google Scholar] [CrossRef] [PubMed]
- Kutikov, A.B.; Song, J. Biodegradable peg-based amphiphilic block copolymers for tissue engineering applications. ACS Biomater. Sci. Eng. 2015, 1, 463–480. [Google Scholar] [CrossRef] [Green Version]
- Eğri, S.; Eczacıoğlu, N. Sequential VEGF and BMP-2 releasing PLA-PEG-PLA scaffolds for bone tissue engineering: I. Design and in vitro tests. Artif. Cells Nanomed. Biotechnol. 2017, 45, 321–329. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.; Cai, B.; Wang, L.; Cai, L.; Wang, Z.; Xie, J.; Lv, Q.X.; Yuan, Y.; Liu, C.; Shen, S.G. A viscoelastic PEGylated poly(glycerol sebacate)-based bilayer scaffold for cartilage regeneration in full-thickness osteochondral defect. Biomaterials 2020, 253, 120095. [Google Scholar] [CrossRef]
- Yang, Y.; Aghazadeh-Habashi, A.; Panahifar, A.; Wu, Y.; Bhandari, K.H.; Doschak, M.R. Bone-targeting parathyroid hormone conjugates outperform unmodified PTH in the anabolic treatment of osteoporosis in rats. Drug Deliv. Transl. Res. 2017, 7, 482–496. [Google Scholar] [CrossRef]
- Venkanna, A.; Kwon, O.W.; Afzal, S.; Jang, C.; Cho, K.H.; Yadav, D.K.; Kim, K.; Park, H.G.; Chun, K.H.; Kim, S.Y.; et al. Pharmacological use of a novel scaffold, anomeric N,N-diarylamino tetrahydropyran: Molecular similarity search, chemocentric target profiling, and experimental evidence. Sci. Rep. 2017, 7, 12535. [Google Scholar] [CrossRef] [Green Version]
- Stevens, M.M. Biomaterials for bone tissue engineering. Mater. Today 2008, 11, 18–25. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, F.X.; Chan, J.M.; Wang, A.Z.; Langer, R.S.; Farokhzad, O.C. Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Jalali, N.; Moztarzadeh, F.; Mozafari, M.; Asgari, S.; Motevalian, M.; Alhosseini, S.N. Surface modification of poly (lactide-co-glycolide) nanoparticles by d-α-tocopheryl polyethylene glycol 1000 succinate as potential carrier for the delivery of drugs to the brain. Colloids Surf. A Physicochem. Eng. Asp. 2011, 392, 335–342. [Google Scholar] [CrossRef]
- Venkatpurwar, V.; Shiras, A.; Pokharkar, V. Porphyran capped gold nanoparticles as a novel carrier for delivery of anticancer drug: In vitro cytotoxicity study. Int. J. Pharm 2011, 409, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Canfarotta, F.; Whitcombe, M.J.; Piletsky, S.A. Polymeric nanoparticles for optical sensing. Biotechnol. Adv. 2013, 31, 1585–1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.; Morshed, R.A.; Auffinger, B.; Tobias, A.L.; Lesniak, M.S. Multifunctional nanoparticles for brain tumor imaging and therapy. Adv. Drug Deliv. Rev. 2014, 66, 42–57. [Google Scholar] [CrossRef] [Green Version]
- Jo, J.; Aoki, I.; Tabata, Y. Design of iron oxide nanoparticles with different sizes and surface charges for simple and efficient labeling of mesenchymal stem cells. J. Control. Release Off. J. Control. Release Soc. 2010, 142, 465–473. [Google Scholar] [CrossRef]
- Zhou, C.-J.; Wang, S.-H.; Yu, Z.; Rong, P.-F.; Chen, Z.-Z.; Liu, J.-Y.; Zhou, J.-D. Folate-conjugated Fe3O4 nanoparticles for in vivo tumor labeling. Trans. Nonferrous Met. Soc. China 2013, 23, 2079–2084. [Google Scholar] [CrossRef]
- Sur, S.; Rathore, A.; Dave, V.; Reddy, K.R.; Chouhan, R.S.; Sadhu, V. Recent developments in functionalized polymer nanoparticles for efficient drug delivery system. Nano Struct. Nano Objects 2019, 20, 100397. [Google Scholar] [CrossRef]
- Calori, I.R.; Braga, G.; de Jesus, P.D.C.C.; Bi, H.; Tedesco, A.C. Polymer scaffolds as drug delivery systems. Eur. Polym. J. 2020, 129, 109621. [Google Scholar] [CrossRef]
- George, A.; Shah, P.A.; Shrivastav, P.S. Natural biodegradable polymers based nano-formulations for drug delivery: A review. Int. J. Pharm. 2019, 561, 244–264. [Google Scholar] [CrossRef] [PubMed]
- Ikono, R.; Li, N.; Pratama, N.H.; Vibriani, A.; Yuniarni, D.R.; Luthfansyah, M.; Bachtiar, B.M.; Bachtiar, E.W.; Mulia, K.; Nasikin, M.; et al. Enhanced bone regeneration capability of chitosan sponge coated with TiO(2) nanoparticles. Biotechnol. Rep. 2019, 24, e00350. [Google Scholar] [CrossRef]
- Iannazzo, D.; Pistone, A.; Salamò, M.; Galvagno, S. Hybrid ceramic/polymer composites for bone tissue regeneration. In Hybrid Polymer Composite Materials; Elsevier: New York, NY, USA, 2017; pp. 125–155. [Google Scholar]
- Khan, S.; Garg, M.; Chockalingam, S.; Gopinath, P.; Kundu, P.P. TiO(2) doped chitosan/poly (vinyl alcohol) nanocomposite film with enhanced mechanical properties for application in bone tissue regeneration. Int. J. Biol. Macromol. 2020, 143, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, Y.; Ge, J.; Ma, P.X.; Lei, B. In situ silica nanoparticles-reinforced biodegradable poly (citrate-siloxane) hybrid elastomers with multifunctional properties for simultaneous bioimaging and bone tissue regeneration. Appl. Mater. Today 2018, 10, 153–163. [Google Scholar] [CrossRef]
- Tamburaci, S.; Tihminlioglu, F. Chitosan-hybrid poss nanocomposites for bone regeneration: The effect of poss nanocage on surface, morphology, structure and in vitro bioactivity. Int. J. Biol. Macromol. 2020, 142, 643–657. [Google Scholar] [CrossRef]
- Vukajlovic, D.; Parker, J.; Bretcanu, O.; Novakovic, K. Chitosan based polymer/bioglass composites for tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 955–967. [Google Scholar] [CrossRef]
- Ignjatović, N.; Wu, V.; Ajduković, Z.; Mihajilov-Krstev, T.; Uskoković, V.; Uskoković, D. Chitosan-PLGA polymer blends as coatings for hydroxyapatite nanoparticles and their effect on antimicrobial properties, osteoconductivity and regeneration of osseous tissues. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 60, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Balagangadharan, K.; Trivedi, R.; Vairamani, M.; Selvamurugan, N. Sinapic acid-loaded chitosan nanoparticles in polycaprolactone electrospun fibers for bone regeneration in vitro and in vivo. Carbohydr. Polym. 2019, 216, 1–16. [Google Scholar] [CrossRef]
- Makvandi, P.; Ali, G.W.; Della Sala, F.; Abdel-Fattah, W.I.; Borzacchiello, A. Hyaluronic acid/corn silk extract based injectable nanocomposite: A biomimetic antibacterial scaffold for bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110195. [Google Scholar] [CrossRef]
- Hashemi, S.F.; Mehrabi, M.; Ehterami, A.; Gharravi, A.M.; Bitaraf, F.S.; Salehi, M. In-vitro and in-vivo studies of PLA/PCL/gelatin composite scaffold containing ascorbic acid for bone regeneration. J. Drug Deliv. Sci. Technol. 2020, 102077. [Google Scholar] [CrossRef]
- Ou, Q.; Huang, K.; Fu, C.; Huang, C.; Fang, Y.; Gu, Z.; Wu, J.; Wang, Y. Nanosilver-incorporated halloysite nanotubes/gelatin methacrylate hybrid hydrogel with osteoimmunomodulatory and antibacterial activity for bone regeneration. Chem. Eng. J. 2020, 382, 123019. [Google Scholar] [CrossRef]
- Amirian, J.; Makkar, P.; Lee, G.H.; Paul, K.; Lee, B.T. Incorporation of alginate-hyaluronic acid microbeads in injectable calcium phosphate cement for improved bone regeneration. Mater. Lett. 2020, 272, 127830. [Google Scholar] [CrossRef]
- Patel, A.; Zaky, S.H.; Schoedel, K.; Li, H.; Sant, V.; Beniash, E.; Sfeir, C.; Stolz, D.B.; Sant, S. Design and evaluation of collagen-inspired mineral-hydrogel nanocomposites for bone regeneration. Acta Biomater. 2020, 112, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Purohit, S.D.; Singh, H.; Bhaskar, R.; Yadav, I.; Chou, C.F.; Gupta, M.K.; Mishra, N.C. Gelatin-alginate-cerium oxide nanocomposite scaffold for bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 116, 111111. [Google Scholar] [CrossRef]
- Dong, J.; Tao, L.; Abourehab, M.A.S.; Hussain, Z. Design and development of novel hyaluronate-modified nanoparticles for combo-delivery of curcumin and alendronate: Fabrication, characterization, and cellular and molecular evidences of enhanced bone regeneration. Int. J. Biol. Macromol. 2018, 116, 1268–1281. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Ahmad, S.A.; Chaudhary, N.; Hoda, M.N.; Nayak, A.K. Biodegradable polymer matrix nanocomposites for bone tissue engineering. In Applications of Nanocomposite Materials in Orthopedics; Elsevier: New York, NY, USA, 2019; pp. 1–37. [Google Scholar]
- Okamoto, M.; John, B. Synthetic biopolymer nanocomposites for tissue engineering scaffolds. Prog. Polym. Sci. 2013, 38, 1487–1503. [Google Scholar] [CrossRef]
- Neufurth, M.; Wang, X.; Wang, S.; Steffen, R.; Ackermann, M.; Haep, N.D.; Schröder, H.C.; Müller, W.E.G. 3D printing of hybrid biomaterials for bone tissue engineering: Calcium-polyphosphate microparticles encapsulated by polycaprolactone. Acta Biomater. 2017, 64, 377–388. [Google Scholar] [CrossRef]
- Shen, J.; Wang, W.; Zhai, X.; Chen, B.; Qiao, W.; Li, W.; Li, P.; Zhao, Y.; Meng, Y.; Qian, S. 3D-printed nanocomposite scaffolds with tunable magnesium ionic microenvironment induce in situ bone tissue regeneration. Appl. Mater. Today 2019, 16, 493–507. [Google Scholar] [CrossRef]
- Babilotte, J.; Martin, B.; Guduric, V.; Bareille, R.; Agniel, R.; Roques, S.; Héroguez, V.; Dussauze, M.; Gaudon, M.; Le Nihouannen, D. Development and characterization of a PLGA-HA composite material to fabricate 3D-printed scaffolds for bone tissue engineering. Mater. Sci. Eng. C 1920, 118, 111334. [Google Scholar] [CrossRef]
- Awasthi, G.P.; Kaliannagounder, V.K.; Maharjan, B.; Lee, J.Y.; Park, C.H.; Kim, C.S. Albumin-induced exfoliation of molybdenum disulfide nanosheets incorporated polycaprolactone/zein composite nanofibers for bone tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 116, 111162. [Google Scholar] [CrossRef]
- Wang, C.; Huang, W.; Zhou, Y.; He, L.; He, Z.; Chen, Z.; He, X.; Tian, S.; Liao, J.; Lu, B.; et al. 3D printing of bone tissue engineering scaffolds. Bioact. Mater. 2020, 5, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.X.; Ding, Z.Y.; Zhou, X.B.; Li, S.T.; Xie de, M.; Li, Z.Z.; Sun, G.D. In vitro and in vivo evaluation of the developed PLGA/HAp/Zein scaffolds for bone-cartilage interface regeneration. Biomed. Environ. Sci. BES 2015, 28, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Wang, W.; Uo, M.; Ohkawa, S.; Akasaka, T.; Tamura, K.; Cui, F.; Watari, F. A three-layered nano-carbonated hydroxyapatite/collagen/PLGA composite membrane for guided tissue regeneration. Biomaterials 2005, 26, 7564–7571. [Google Scholar] [CrossRef] [PubMed]
- Terukina, T.; Naito, Y.; Tagami, T.; Morikawa, Y.; Henmi, Y.; Prananingrum, W.; Ichikawa, T.; Ozeki, T. Research paper. J. Drug Deliv. Sci. Technol. 2016, 33, 136–142. [Google Scholar] [CrossRef]
- Nath, S.D.; Son, S.; Sadiasa, A.; Min, Y.K.; Lee, B.T. Preparation and characterization of PLGA microspheres by the electrospraying method for delivering simvastatin for bone regeneration. Int. J. Pharm. 2013, 443, 87–94. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, J.; Qiao, W.; Zhao, Y.; Wong, K.H.M.; Chu, P.K.; Bian, L.; Wu, S.; Zheng, Y.; Cheung, K.M.C.; et al. Precisely controlled delivery of magnesium ions thru sponge-like monodisperse PLGA/nano-MgO-alginate core-shell microsphere device to enable in-situ bone regeneration. Biomaterials 2018, 174, 1–16. [Google Scholar] [CrossRef]
- Yuan, Z.; Wei, P.; Huang, Y.; Zhang, W.; Chen, F.; Zhang, X.; Mao, J.; Chen, D.; Cai, Q.; Yang, X. Injectable PLGA microspheres with tunable magnesium ion release for promoting bone regeneration. Acta Biomater. 2019, 85, 294–309. [Google Scholar] [CrossRef]
- Govoni, M.; Lamparelli, E.P.; Ciardulli, M.C.; Santoro, A.; Oliviero, A.; Palazzo, I.; Reverchon, E.; Vivarelli, L.; Maso, A.; Storni, E.; et al. Demineralized bone matrix paste formulated with biomimetic PLGA microcarriers for the vancomycin hydrochloride controlled delivery: Release profile, citotoxicity and efficacy against S. aureus. Int. J. Pharm. 2020, 582, 119322. [Google Scholar] [CrossRef]
- Vaccaro, A.R.; Patel, T.; Fischgrund, J.; Anderson, D.G.; Truumees, E.; Herkowitz, H.; Phillips, F.; Hilibrand, A.; Albert, T.J. A pilot safety and efficacy study of OP-1 putty (rhBMP-7) as an adjunct to iliac crest autograft in posterolateral lumbar fusions. Eur. Spine J. 2003, 12, 495–500. [Google Scholar] [CrossRef] [Green Version]
- Vaccaro, A.R.; Patel, T.; Fischgrund, J.; Anderson, D.G.; Truumees, E.; Herkowitz, H.; Phillips, F.; Hilibrand, A.; Albert, T.J. A 2-year follow-up pilot study evaluating the safety and efficacy of op-1 putty (rhbmp-7) as an adjunct to iliac crest autograft in posterolateral lumbar fusions. Eur. Spine J. 2005, 14, 623–629. [Google Scholar] [CrossRef] [Green Version]
- White, A.P.; Vaccaro, A.R.; Hall, J.A.; Whang, P.G.; Friel, B.C.; McKee, M.D. Clinical applications of BMP-7/OP-1 in fractures, nonunions and spinal fusion. Int. Orthop. 2007, 31, 735–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ristiniemi, J.; Flinkkilä, T.; Hyvönen, P.; Lakovaara, M.; Pakarinen, H.; Jalovaara, P. RhBMP-7 accelerates the healing in distal tibial fractures treated by external fixation. J. Bone Jt. Surg. Br. Vol. 2007, 89, 265–272. [Google Scholar] [CrossRef]
- McKay, W.F.; Peckham, S.M.; Badura, J.M. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE Bone Graft). Int. Orthop. 2007, 31, 729–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Y.D.; Jiang, W.M.; Yang, H.L.; Shi, J.H. Exploratory meta-analysis on dose-related efficacy and complications of rhBMP-2 in anterior cervical discectomy and fusion: 1,539,021 cases from 2003 to 2017 studies. J. Orthop. Transl. 2020, 24, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Triplett, R.G.; Nevins, M.; Marx, R.E.; Spagnoli, D.B.; Oates, T.W.; Moy, P.K.; Boyne, P.J. Pivotal, randomized, parallel evaluation of recombinant human bone morphogenetic protein-2/absorbable collagen sponge and autogenous bone graft for maxillary sinus floor augmentation. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2009, 67, 1947–1960. [Google Scholar] [CrossRef] [PubMed]
- Fiorellini, J.P.; Howell, T.H.; Cochran, D.; Malmquist, J.; Lilly, L.C.; Spagnoli, D.; Toljanic, J.; Jones, A.; Nevins, M. Randomized study evaluating recombinant human bone morphogenetic protein-2 for extraction socket augmentation. J. Periodontol. 2005, 76, 605–613. [Google Scholar] [CrossRef] [Green Version]
- Aro, H.T.; Govender, S.; Patel, A.D.; Hernigou, P.; Perera de Gregorio, A.; Popescu, G.I.; Golden, J.D.; Christensen, J.; Valentin, A. Recombinant human bone morphogenetic protein-2: A randomized trial in open tibial fractures treated with reamed nail fixation. J. Bone Jt. Surg. Am. Vol. 2011, 93, 801–808. [Google Scholar] [CrossRef]
- Daniels, T.R.; Younger, A.S.; Penner, M.J.; Wing, K.J.; Le, I.L.; Russell, I.S.; Lalonde, K.A.; Evangelista, P.T.; Quiton, J.D.; Glazebrook, M.; et al. Prospective Randomized Controlled Trial of Hindfoot and Ankle Fusions Treated With rhPDGF-BB in Combination With a β-TCP-Collagen Matrix. Foot Ankle Int. 2015, 36, 739–748. [Google Scholar] [CrossRef]
- Daniels, T.R.; Anderson, J.; Swords, M.P.; Maislin, G.; Donahue, R.; Pinsker, E.; Quiton, J.D. Recombinant Human Platelet-Derived Growth Factor BB in Combination with a Beta-Tricalcium Phosphate (rhPDGF-BB/β-TCP)-Collagen Matrix as an Alternative to Autograft. Foot Ankle Int. 2019, 40, 1068–1078. [Google Scholar] [CrossRef]
- Hollinger, J.O.; Hart, C.E.; Hirsch, S.N.; Lynch, S.; Friedlaender, G.E. Recombinant human platelet-derived growth factor: Biology and clinical applications. J. Bone Jt. Surg. Am. Vol. 2008, 90 (Suppl. 1), 48–54. [Google Scholar] [CrossRef]
- Min, S.H.; Kang, N.E.; Song, S.I.; Lee, J.K. Regenerative effect of recombinant human bone morphogenetic protein-2/absorbable collagen sponge (rhBMP-2/ACS) after sequestrectomy of medication-related osteonecrosis of the jaw (MRONJ). J. Korean Assoc.Oral Maxillofac. Surg. 2020, 46, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, K.J.; Kim, B.J.; Kim, C.H.; Kim, J.H. Immediate reconstruction of mandibular defect after treatment of medication-related osteonecrosis of the jaw (MRONJ) with rhBMP-2/ACS and miniplate: Review of 3 cases. Int. J. Surg. Case Rep. 2020, 66, 25–29. [Google Scholar] [CrossRef] [PubMed]
| Source | Polymer | Advantages | Limitations | Reference |
|---|---|---|---|---|
| Natural | Collagen | The most abundant protein, capable of altering its mechanical properties by crosslinking, safety, biocompatibility | Strong burst release, causing inflammation, swelling, ectopic bone formation and osteolysis | Busra and Lokanathan, 2019 Sheikh et al., 2015 Zara et al., 2011 |
| Chitosan | Biocompatibility, biodegradability, antibacterial and wound healing activity, bioadhesion, presence of functional groups on the surface, enabling physical and chemical functionalization | Requirement for additional modifications for controlled and sustained release of osteopromotive drugs | Levengood and Zhang, 2014 Saravanan et al., 2016 Venkatesan et al., 2017 | |
| Hyaluronic acid | Good biocompatibility, biodegradability, high viscoelasticity, hydrophilicity, non-immunogenicity, capability to modification, low burst release, sustained drug release | Low mechanical and physical properties, fast degradation rate, complicated crosslinking method | Hemshekhar et al., 2016 Zhao et al., 2016, Kim et al., 2019 | |
| Alginate | Good biodegradability, biocompatibility, non-immunogenicity, capability of modification, significant promotion of the proliferation and osteogenic differentiation | Poor cell attachment ability, limited availability of cellular adhesion sites | Ray et al., 2020 Su et al., 2013, Purohit et al., 2020 | |
| Fibrin | Adhesion, hemostasis, sealant capability, presence of multiple binding sites | Requirement for additional modification | Bujoli et al., 2019 Noori et al., 2017 Khodakaram-Tafti et al., 2017 Martino and Hubbell, 2010 Censi et al., 2012 | |
| Synthetic | Poly (ε-caprolactone) | Elasticity, biocompatibility, bioabsorbtion and biodegradability | Limited polymer-cell interaction due to its hydrophobicity and mechanical properties, used as one of the scaffold components | Narayanan et al., 2016 Ghassemi et al., 2018 Miroshnichenko et al., 2019 Aldemir Dikici et al., 2019 Pazarçeviren et al., 2020 Wang et al., 2017 |
| Poly(lactic-co-glycolic acid) | Biocompatibility, biodegradability, increased adhesion and cell viability and proliferation, sustained release of GF, induced osteogenic differentiation, quick integration with surrounding tissues | Rapid biodegradation | Turnbull et al., 2018 Bharadwaz and Jayasuriya, 2020 Cao et al., 2020 Zhang et al., 2019 Deng et al., 2019 Das et al., 2016 Kim et al., 2019 Jakus et al., 2016 Generali et al., 2017 | |
| Polyethylene glycol | Hydrophilicity, biocompatibility, non-immunogenicity, biodegradability | Absence of functional groups on the surface, used as one of the composite components | Saroia et al., 2018 Wang et al., 2019 Kutikov and Song, 2015 Eğri and Eczacıoğlu, 2017 |
| Source | Polymer | Formulation | Function | Reference |
|---|---|---|---|---|
| Natural | Chitosan | Nanocomposites | Improved osteoblast adhesion and proliferation, osteocalcin secretion and biomineralization of cells | Tamburaci et al., 2020 |
| Sponge | Improved biomineralization and osteogenic induction | Ikono et al., 2019 | ||
| Nanocomposite film | Accelerated tissue ingrowth, enhanced capability to mimic human bone extracellular matrix, vascularization, antibacterial efficacy, compatibility with human erythrocytes, advanced cell attachment and high proliferation with human osteoblasts | Khan et al., 2019 | ||
| Nanoparticle-containing electrospun fibers | Increased bioavailability and osteogenic capability | Balagangadharan et al., 2019 | ||
| Coated nanoparticles | Improved antimicrobial activity | Ignjatović et al., 2016 | ||
| Hyaluronic acid | Nanocomposite | Improved antibacterial activity, high bone differentiation of mesenchymal stem cells | Makvandi et al., 2020 | |
| Nanoparticle | Sustained delivery of alendronate and curcumin, increased proliferation, differentiation and mineralization of MC3T3-E1 cells | Dong et al., 2018 | ||
| Gelatin | Composite scaffold | Improved cell proliferation and bone healing, cytocompatibility, osteoinductivity | Hashemi et al., 2020 | |
| Nanocomposite scaffold | Accelerated differentiation of mesenchymal stem cells to osteoblast and reduce free radicals | Purohit et al., 2020 | ||
| Nanotubes/hydrogel | Enhanced osteogenic differentiation, osteoimmunomodulatory and antibacterial activities | Ou et al., 2020 | ||
| Alginate | Nanocomposite scaffold | Improved bio-mineralization | Purohit et al., 2020 | |
| Microbeads | Enhanced bone formation, higher injectability and washout resistance | Amirian et al., 2020 | ||
| Collagen | Nanocomposite | Enhanced bone regeneration, improved healing and tissue remodeling | Patel et al., 2020 | |
| Three-layered composite membrane | Guided tissue regeneration | Liao et al., 2005 | ||
| Synthetic | Poly(-caprolactone) (PCL) | Composite nanofibers | Improved fiber morphology, cell attachment, proliferation, differentiation, biomineralization, calcium-phosphate deposition | Awasthi et al., 2020 |
| Nanocomposite 3D matrix | Enhanced osteogenic differentiation, early bone defect repair, tissue mineralization | Shen et al., 2019 | ||
| PLGA | Nanocomposite | Induced osteogenic effects and successful bone defect repair in vivo, BMP2 delivery | Deng et al., 2019 | |
| Microspheres | Controlled delivery of magnesium ions, enhanced cell attachment, proliferation, osteogenic differentiation, cell migration of bone marrow mesenchymal stromal cells, promotion of mineral depositions | Yuan et al., 2019 | ||
| Scaffolds | Improved cell attachment, proliferation, induction of cartilage formation | Lin et al., 2018 | ||
| Microspheres | Sustained Simvastatin delivery, induced proliferation of MC3T3-E1 cells, increased differentiation and bone mineralization | Terukina et al., 2016 | ||
| Microspheres | Sustained drug release, good biocompatibility | Nath et al., 2013 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogay, V.; Mun, E.A.; Kudaibergen, G.; Baidarbekov, M.; Kassymbek, K.; Zharkinbekov, Z.; Saparov, A. Progress and Prospects of Polymer-Based Drug Delivery Systems for Bone Tissue Regeneration. Polymers 2020, 12, 2881. https://doi.org/10.3390/polym12122881
Ogay V, Mun EA, Kudaibergen G, Baidarbekov M, Kassymbek K, Zharkinbekov Z, Saparov A. Progress and Prospects of Polymer-Based Drug Delivery Systems for Bone Tissue Regeneration. Polymers. 2020; 12(12):2881. https://doi.org/10.3390/polym12122881
Chicago/Turabian StyleOgay, Vyacheslav, Ellina A. Mun, Gulshakhar Kudaibergen, Murat Baidarbekov, Kuat Kassymbek, Zharylkasyn Zharkinbekov, and Arman Saparov. 2020. "Progress and Prospects of Polymer-Based Drug Delivery Systems for Bone Tissue Regeneration" Polymers 12, no. 12: 2881. https://doi.org/10.3390/polym12122881
APA StyleOgay, V., Mun, E. A., Kudaibergen, G., Baidarbekov, M., Kassymbek, K., Zharkinbekov, Z., & Saparov, A. (2020). Progress and Prospects of Polymer-Based Drug Delivery Systems for Bone Tissue Regeneration. Polymers, 12(12), 2881. https://doi.org/10.3390/polym12122881









