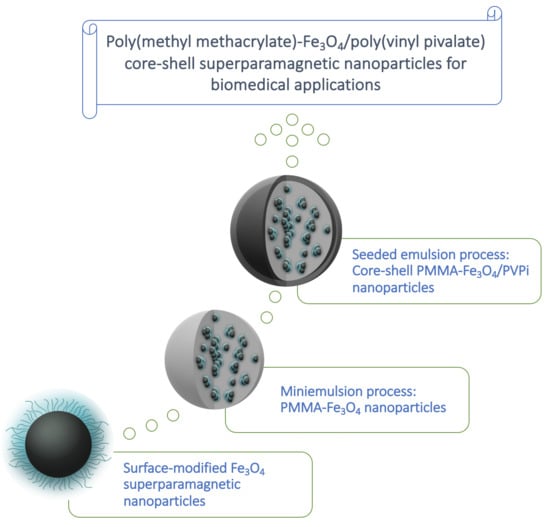
Well Defined Poly(Methyl Methacrylate)-Fe3O4/Poly(Vinyl Pivalate) Core–Shell Superparamagnetic Nanoparticles: Design and Evaluation of In Vitro Cytotoxicity Activity Against Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
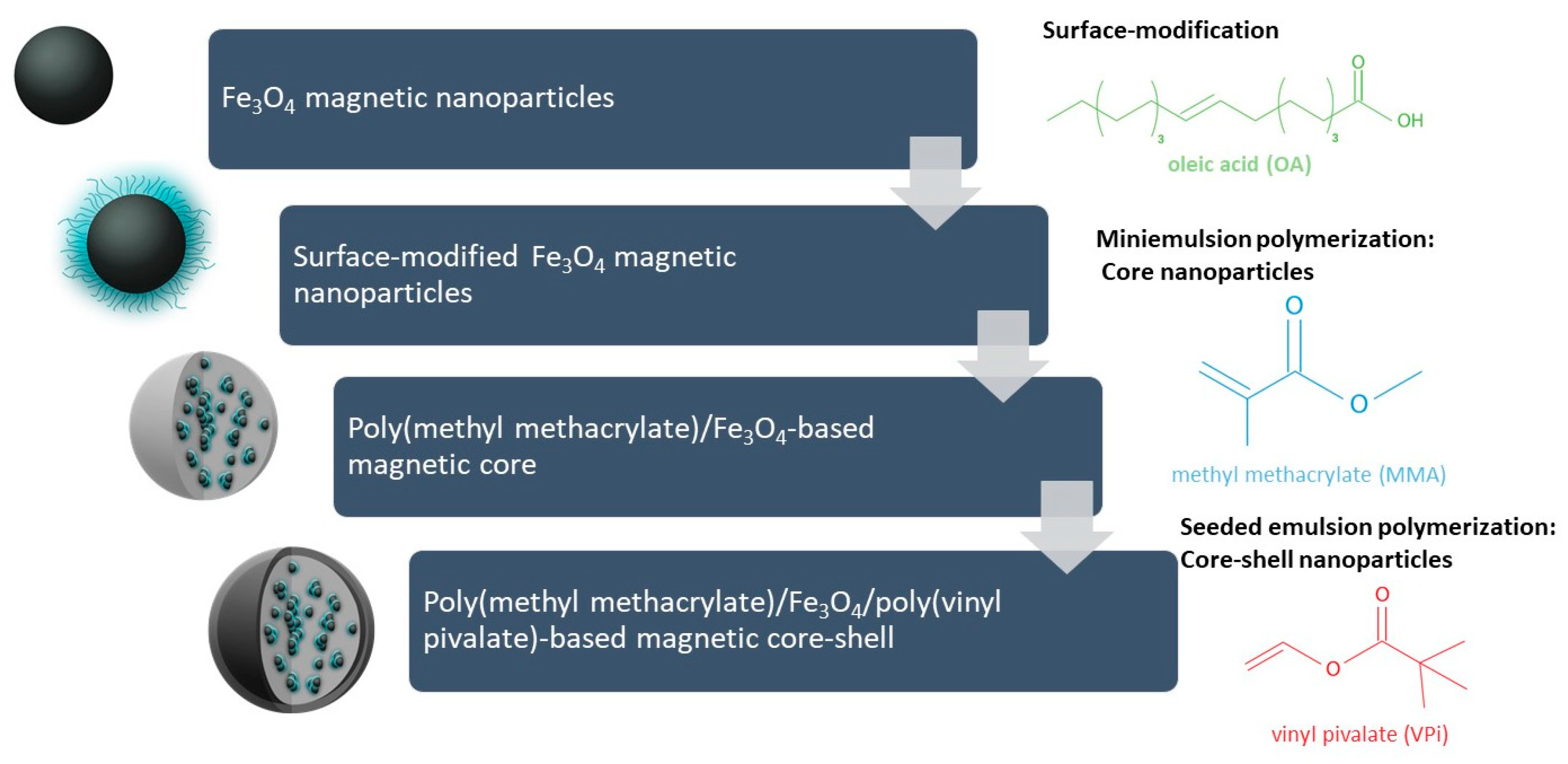
2.1. Synthesis of Magnetic Nanoparticles (OM)
2.2. Coating of Magnetic Nanoparticles with Oleic Acid (OM-OA)
2.3. Synthesis of PMMA/OM-OA Magnetic Nanocomposite
2.4. Production of Poly(Vinyl Pivalate) Shell via Seeded Emulsion Polymerization
2.5. Sample Characterization
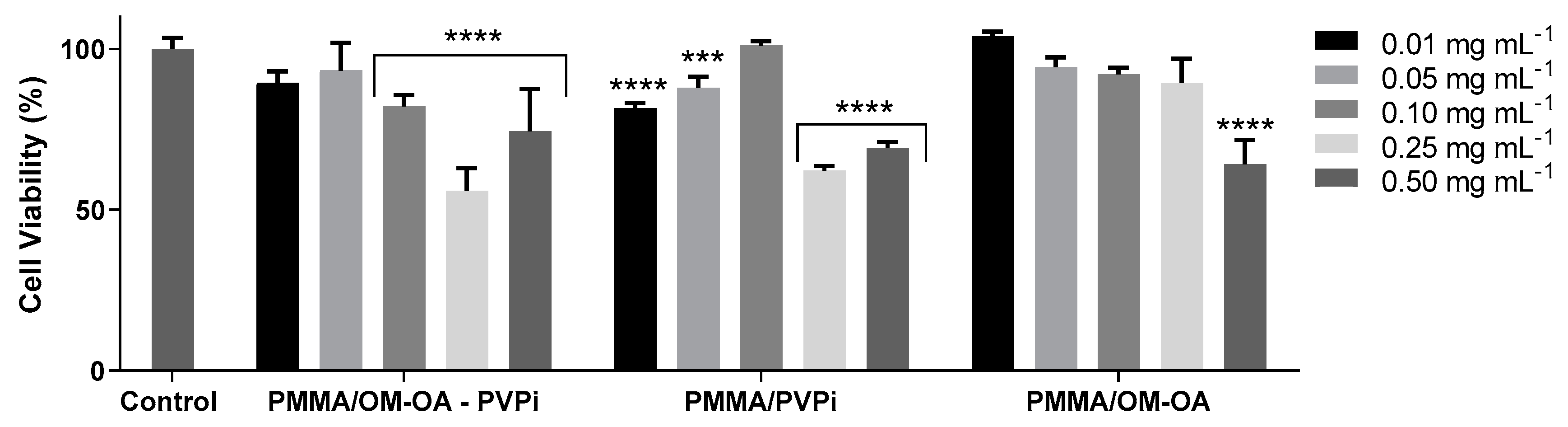
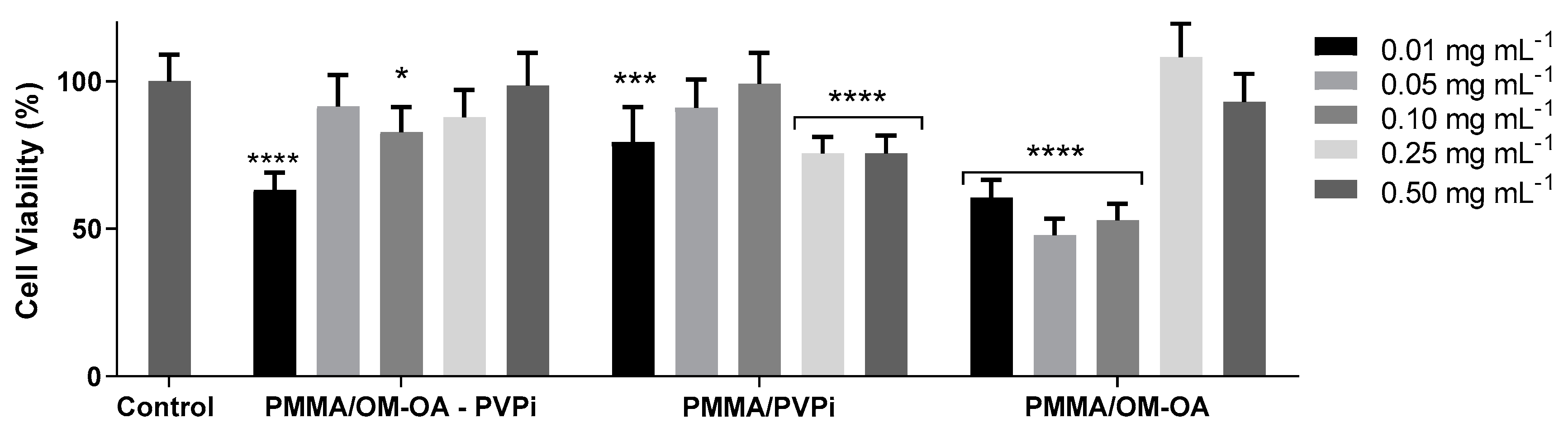
2.6. In Vitro Cytotoxicity Assays
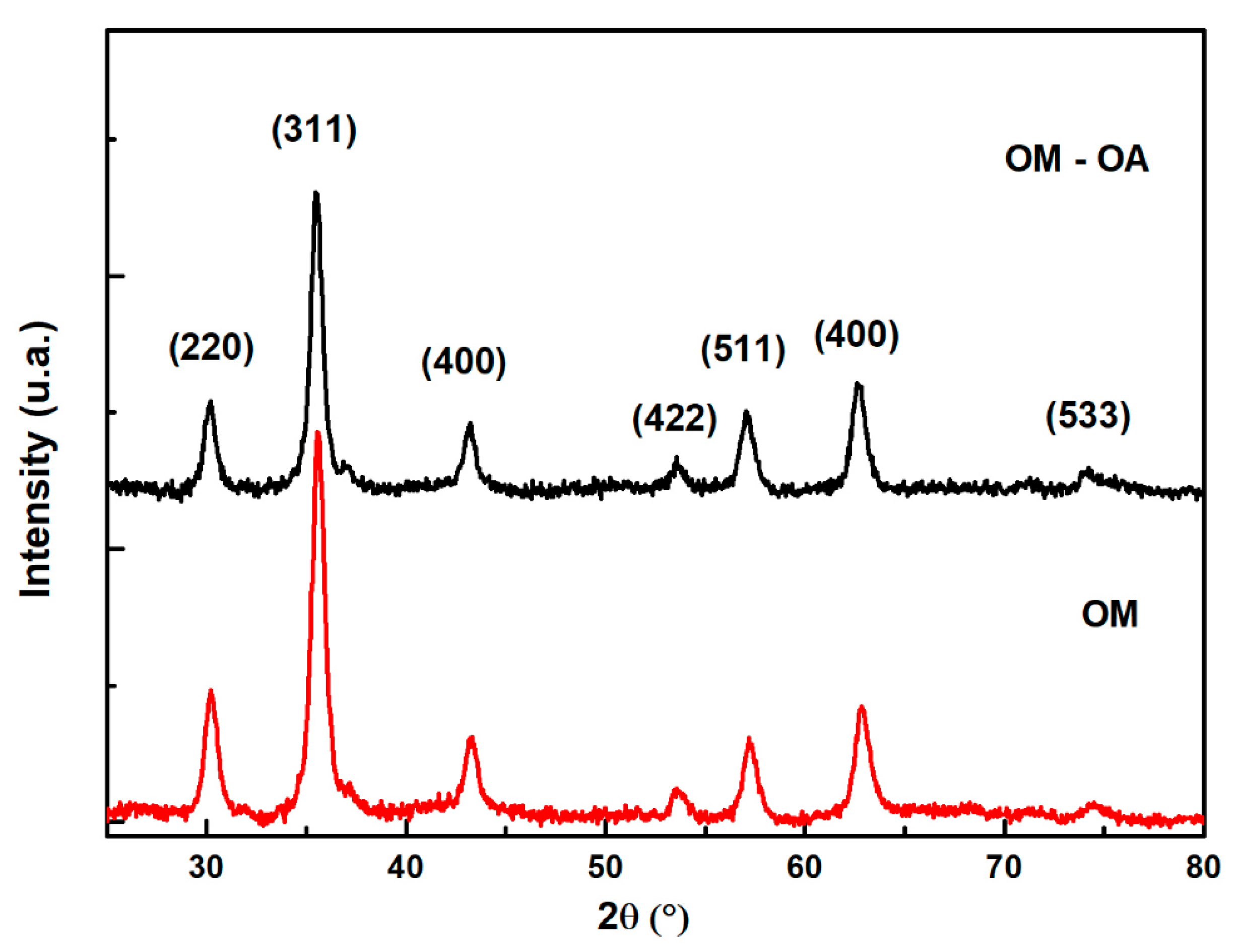
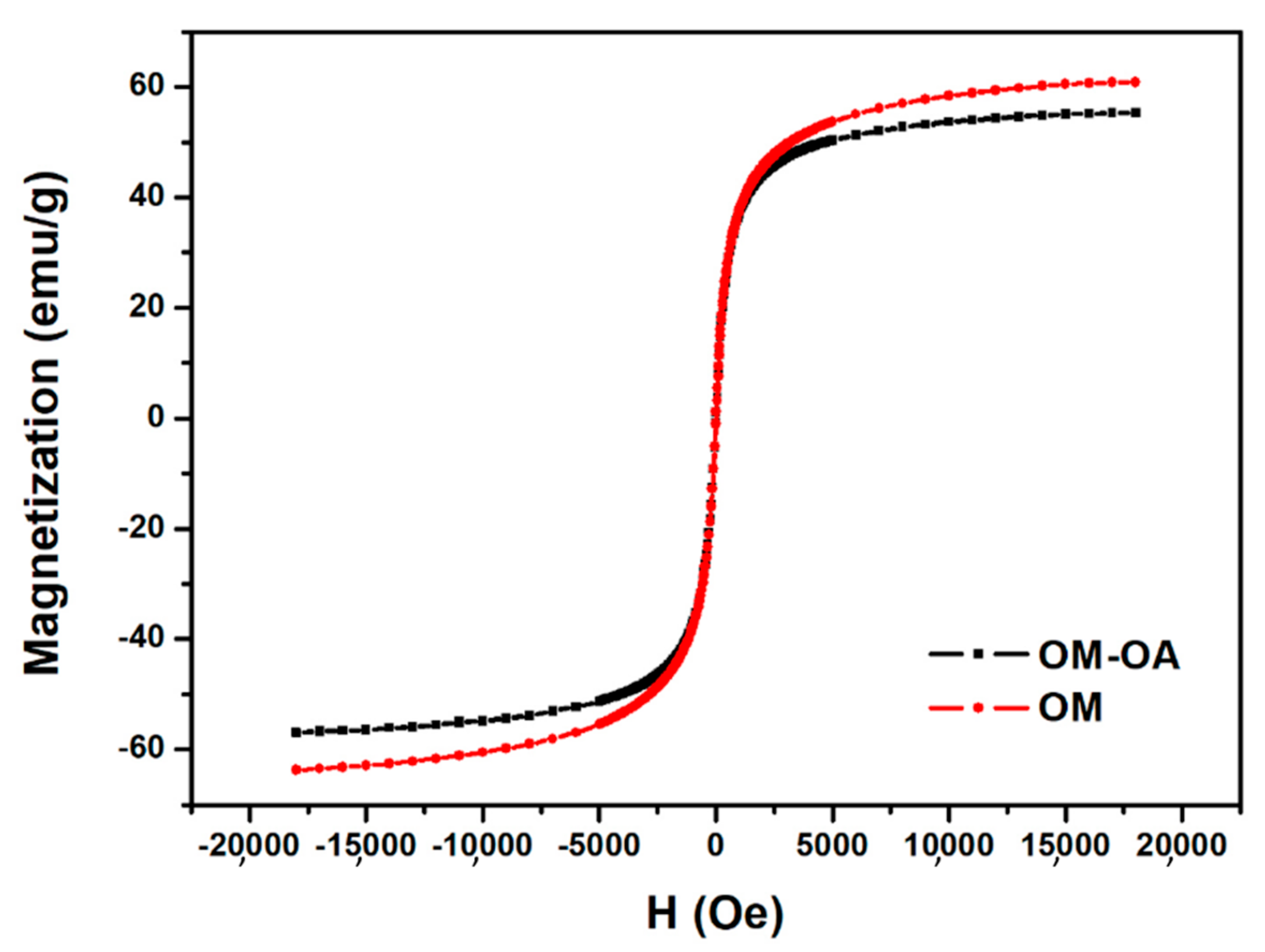
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dutra, G.V.S.; Neto, W.S.; Dutra, J.P.S.; Machado, F. Implantable medical devices and tissue engineering: An overview of manufacturing processes and the use of polymeric matrices for manufacturing and coating their surfaces. Curr. Med. Chem. 2020, 27, 1580–1599. [Google Scholar] [CrossRef] [PubMed]
- Teo, A.J.T.; Mishra, A.; Park, I.; Kim, Y.-J.; Park, W.-T.; Yoon, Y.-J. Polymeric Biomaterials for Medical Implants and Devices. ACS Biomater. Sci. Eng. 2016, 2, 454–472. [Google Scholar] [CrossRef]
- Chen, X.; Lv, H.; Ye, M.; Wang, S.; Ni, E.; Zeng, F.; Cao, C.; Luo, F.; Yan, J. Novel superparamagnetic iron oxide nanoparticles for tumor embolization application: Preparation, characterization and double targeting. Int. J. Pharm. 2012, 426, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Rittikulsittichai, S.; Singhana, B.; Bryan, W.W.; Sarangi, S.; Jamison, A.C.; Brazdeikis, A.; Lee, T.R. Preparation, characterization, and utilization of multi-functional magnetic-fluorescent composites for bio-imaging and magnetic hyperthermia therapy. RSC Adv. 2013, 3, 7838–7849. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Shen, S.; Wu, D. Progress of recyclable magnetic particles for biomedical applications. J. Mater. Chem. B 2018, 6, 366–380. [Google Scholar] [CrossRef]
- Chatterjee, K.; Sarkar, S.; Jagajjanani Rao, K.; Paria, S. Core/shell nanoparticles in biomedical applications. Adv. Colloid Interface Sci. 2014, 209, 8–39. [Google Scholar] [CrossRef]
- Jiang, Q.L.; Zheng, S.W.; Hong, R.Y.; Deng, S.M.; Guo, L.; Hu, R.L.; Gao, B.; Huang, M.; Cheng, L.F.; Liu, G.H.; et al. Folic acid-conjugated Fe3O4 magnetic nanoparticles for hyperthermia and MRI in vitro and in vivo. Appl. Surf. Sci. 2014, 307, 224–233. [Google Scholar] [CrossRef]
- Bakhtiary, Z.; Saei, A.A.; Hajipour, M.J.; Raoufi, M.; Vermesh, O.; Mahmoudi, M. Targeted superparamagnetic iron oxide nanoparticles for early detection of cancer: Possibilities and challenges. Nanomedicine 2016, 12, 287–307. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Jun, Y.-W.; Huh, Y.-M.; Choi, J.-S.; Lee, J.-H.; Song, H.-T.; Kim, S.; Yoon, S.; Kim, K.-S.; Shin, J.-S.; Suh, J.-S.; et al. Nanoscale Size Effect of Magnetic Nanocrystals and Their Utilization for Cancer Diagnosis via Magnetic Resonance Imaging. J. Am. Chem. Soc. 2005, 127, 5732–5733. [Google Scholar] [CrossRef]
- Singh, A.P.; Biswas, A.; Shukla, A.; Maiti, P. Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal Transduc. Target. Ther. 2019, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Lin, Z.Y.; Yildirimer, L.; Dhinakar, A.; Zhao, X.; Wu, J. Polymer-based nanoparticles for protein delivery: Design, strategies and applications. J. Mater. Chem. B 2016, 4, 4060–4071. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhu, A.M.; Liu, Q.L.; Zhang, Q.G. Fe3O4/poly(N-Isopropylacrylamide)/Chitosan Composite Microspheres with Multiresponsive Properties. Ind. Eng. Chem. Res. 2008, 47, 7700–7706. [Google Scholar] [CrossRef]
- Koo, H.Y.; Chang, S.T.; Choi, W.S.; Park, J.-H.; Kim, D.-Y.; Velev, O.D. Emulsion-Based Synthesis of Reversibly Swellable, Magnetic Nanoparticle-Embedded Polymer Microcapsules. Chem. Mater. 2006, 18, 3308–3313. [Google Scholar] [CrossRef]
- Rouhi, M.; Mansour Lakouraj, M.; Baghayeri, M.; Hasantabar, V. Novel conductive magnetic nanocomposite based on poly (indole-co-thiophene) as a hemoglobin diagnostic biosensor: Synthesis, characterization and physical properties. Int. J. Polymer. Mater. 2017, 66, 12–19. [Google Scholar] [CrossRef]
- Neves, J.S.; Souza Júnior, F.G.; Suarez, P.A.Z.; Umpierre, A.P.; Machado, F. In situ Production of Polystyrene Magnetic Nanocomposites through a Batch Suspension Polymerization Process. Macromol. Mater. Eng. 2011, 296, 1107–1118. [Google Scholar] [CrossRef]
- Araujo, R.T.; Ferreira, G.R.; Segura, T.; Souza, F.G.; Machado, F. An experimental study on the synthesis of poly(vinyl pivalate)-based magnetic nanocomposites through suspension polymerization process. Eur. Polym. J. 2015, 68, 441–459. [Google Scholar] [CrossRef]
- Ferreira, G.R.; Segura, T.; Souza, F.G.; Umpierre, A.P.; Machado, F. Synthesis of poly(vinyl acetate)-based magnetic polymer microparticles. Eur. Polym. J. 2012, 48, 2050–2069. [Google Scholar] [CrossRef]
- Piazza, R.D.; Nunes, E.S.; Viali, W.R.; Silva, S.W.; Aragón, F.H.; Coaquira, J.A.H.; Morais, P.C.; Marques, R.F.C.; Jafelicci, M. Magnetic nanohydrogel obtained by miniemulsion polymerization of poly(acrylic acid) grafted onto derivatized dextran. Carbohydr. Polym. 2017, 178, 378–385. [Google Scholar] [CrossRef]
- Gao, F.; Wu, X.; Wu, D.; Yu, J.; Yao, J.; Qi, Q.; Cao, Z.; Cui, Q.; Mi, Y. Preparation of degradable magnetic temperature- and redox-responsive polymeric/Fe3O4 nanocomposite nanogels in inverse miniemulsions for loading and release of 5-fluorouracil. Colloids Surf. A Physicochem. Eng. Asp. 2020, 587, 124363. [Google Scholar] [CrossRef]
- Chiaradia, V.; Valério, A.; Feuser, P.E.; Oliveira, D.; Araújo, P.H.H.; Sayer, C. Incorporation of superparamagnetic nanoparticles into poly(urea-urethane) nanoparticles by step growth interfacial polymerization in miniemulsion. Colloids Surf. A Physicochem. Eng. Asp. 2015, 482, 596–603. [Google Scholar] [CrossRef]
- Goode, J.A.; Matson, M.B. Embolisation of cancer: What is the evidence? Cancer Imaging 2004, 4, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Coldwell, D.M.; Stokes, K.R.; Yakes, W.F. Embolotherapy: Agents, clinical applications, and techniques. Radiographics 1994, 14, 623–643. [Google Scholar] [CrossRef] [PubMed]
- Schwaner, S.L.; Haug, S.B.; Matsumoto, A.H. Overview of embolotherapy: Agents, indications, applications, and nursing management. Periop. Nurs. Clin. 2010, 5, 137–176. [Google Scholar] [CrossRef]
- Asua, J.M. Challenges for industrialization of miniemulsion polymerization. Progr. Polym. Sci. 2014, 39, 1797–1826. [Google Scholar] [CrossRef]
- Asua, J.M. Miniemulsion polymerization. Progr. Polym. Sci. 2002, 27, 1283–1346. [Google Scholar] [CrossRef]
- Crespy, D.; Landfester, K. Miniemulsion polymerization as a versatile tool for the synthesis of functionalized polymers. Beilstein J. Org. Chem. 2010, 6, 1132–1148. [Google Scholar] [CrossRef]
- Gharieh, A.; Khoee, S.; Mahdavian, A.R. Emulsion and miniemulsion techniques in preparation of polymer nanoparticles with versatile characteristics. Adv. Colloid Interface Sci. 2019, 269, 152–186. [Google Scholar] [CrossRef]
- Ramli, R.A.; Laftah, W.A.; Hashim, S. Core–shell polymers: A review. RSC Adv. 2013, 3, 15543–15565. [Google Scholar] [CrossRef]
- Stubbs, J.; Tsavalas, J.; Carrier, R.; Sundberg, D. The Structural Evolution of Composite Latex Particles During Starve-Fed Emulsion Polymerization: Modeling and Experiments for Kinetically Frozen Morphologies. Macromol. React. Eng. 2010, 4, 424–431. [Google Scholar] [CrossRef]
- Pei, X.; Zhai, K.; Tan, Y.; Xu, K.; Lu, C.; Wang, P.; Wang, T.; Chen, C.; Tao, Y.; Dai, L.; et al. Synthesis of monodisperse starch-polystyrene core-shell nanoparticles via seeded emulsion polymerization without stabilizer. Polymer 2017, 108, 78–86. [Google Scholar] [CrossRef]
- Law, W.-C.; Yong, K.-T.; Roy, I.; Xu, G.; Ding, H.; Bergey, E.J.; Zeng, H.; Prasad, P.N. Optically and magnetically doped organically modified silica nanoparticles as efficient magnetically guided biomarkers for two-photon imaging of live cancer cells. J. Phys. Chem. C 2008, 112, 7972–7977. [Google Scholar] [CrossRef]
- Nagarajan, S.; Yong, Z. Use of core/shell structured nanoparticles for biomedical applications. Recent Pat. Biomed. Eng. 2008, 1, 34–42. [Google Scholar]
- Rahman, M.M.; Chehimi, M.M.; Fessi, H.; Elaissari, A. Highly temperature responsive core–shell magnetic particles: Synthesis, characterization and colloidal properties. J. Colloid Interface Sci. 2011, 360, 556–564. [Google Scholar] [CrossRef]
- Chen, L.; Hong, L.; Lin, J.-C.; Meyers, G.; Harris, J.; Radler, M. Epoxy-acrylic core-shell particles by seeded emulsion polymerization. J. Colloid Interface Sci. 2016, 473, 182–189. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.; Demirci, S.; Qin, S.; Xu, Z.; Olson, E.; Liu, F.; Palm, D.; Yong, X.; Jiang, S. Morphology evolution of Janus dumbbell nanoparticles in seeded emulsion polymerization. J. Colloid Interface Sci. 2019, 543, 34–42. [Google Scholar] [CrossRef]
- Li, W.S.J.; Ladmiral, V.; Takeshima, H.; Satoh, K.; Kamigaito, M.; Semsarilar, M.; Negrell, C.; Lacroix-Desmazes, P.; Caillol, S. Ferulic acid-based reactive core–shell latex by seeded emulsion polymerization. Polym. Chem. 2019, 10, 3116–3126. [Google Scholar] [CrossRef]
- Mock, E.B.; De Bruyn, H.; Hawkett, B.S.; Gilbert, R.G.; Zukoski, C.F. Synthesis of anisotropic nanoparticles by seeded emulsion polymerization. Langmuir 2006, 22, 4037–4043. [Google Scholar] [CrossRef]
- Wichaita, W.; Polpanich, D.; Suteewong, T.; Tangboriboonrat, P. Hollow core-shell particles via NR latex seeded emulsion polymerization. Polymer 2016, 99, 324–331. [Google Scholar] [CrossRef]
- Lyu, S.; Untereker, D. Degradability of polymers for implantable biomedical devices. Int. J. Mol. Sci. 2009, 10, 4033–4065. [Google Scholar] [CrossRef]
- Hsu, S.L. Poly(methyl methacrylate). In Polymer Data Handbook; Mark, J.E., Ed.; Oxford University Press, Inc.: Oxford, UK, 1999; pp. 655–657. [Google Scholar]
- Neto, W.S.; Dutra, G.V.S.; Jensen, A.T.; Araújo, O.A.; Garg, V.; Oliveira, A.C.; Valadares, L.F.; Souza, F.G.; Machado, F. Superparamagnetic nanoparticles stabilized with free-radical polymerizable oleic acid-based coating. J. Alloys Compd. 2018, 739, 1025–1036. [Google Scholar] [CrossRef]
- Jensen, A.T.; Sayer, C.; Araújo, P.H.H.; Machado, F. Emulsion copolymerization of styrene and acrylated methyl oleate. Eur. J. Lipid Sci. Technol. 2014, 116, 37–43. [Google Scholar] [CrossRef]
- Tang, P.L.; Sudol, E.D.; Adams, M.; El-Aasser, M.S.; Asua, J.M. Seeded emulsion polymerization of n-butyl acrylate utilizing miniemulsions. J. Appl. Polym. Sci. 1991, 42, 2019–2028. [Google Scholar] [CrossRef]
- Lobato, N.C.C.; Mansur, M.B.; Ferreira, A.M. Characterization and Chemical Stability of Hydrophilic and Hydrophobic Magnetic Nanoparticles. Mater. Res. 2017, 20, 736–746. [Google Scholar] [CrossRef]
- Araújo-Neto, R.P.; Silva-Freitas, E.L.; Carvalho, J.F.; Pontes, T.R.F.; Silva, K.L.; Damasceno, I.H.M.; Egito, E.S.T.; Dantas, A.L.; Morales, M.A.; Carriço, A.S. Monodisperse sodium oleate coated magnetite high susceptibility nanoparticles for hyperthermia applications. J. Magn. Magn. Mater. 2014, 364, 72–79. [Google Scholar] [CrossRef]
- Feuser, P.E.; Bubniak, L.d.S.; Silva, M.C.d.S.; Viegas, A.d.C.; Castilho Fernandes, A.; Ricci-Junior, E.; Nele, M.; Tedesco, A.C.; Sayer, C.; de Araújo, P.H.H. Encapsulation of magnetic nanoparticles in poly(methyl methacrylate) by miniemulsion and evaluation of hyperthermia in U87MG cells. Eur. Polym. J. 2015, 68, 355–365. [Google Scholar] [CrossRef]
- Klencsár, Z.; Ábrahám, A.; Szabó, L.; Szabó, E.G.; Stichleutner, S.; Kuzmann, E.; Homonnay, Z.; Tolnai, G. The effect of preparation conditions on magnetite nanoparticles obtained via chemical co-precipitation. Mater. Chem. Phys. 2019, 223, 122–132. [Google Scholar] [CrossRef]
- Dutra, G.V.S.; Araújo, O.A.; Neto, W.S.; Garg, V.K.; Oliveira, A.C.; Júnior, A.F. Obtaining superhydrophopic magnetic nanoparticles applicable in the removal of oils on aqueous surface. Mater. Chem. Phys. 2017, 200, 204–216. [Google Scholar] [CrossRef]
- Li, Q.; Kartikowati, C.W.; Horie, S.; Ogi, T.; Iwaki, T.; Okuyama, K. Correlation between particle size/domain structure and magnetic properties of highly crystalline Fe3O4 nanoparticles. Sci. Rep. 2017, 7, 9894–9900. [Google Scholar] [CrossRef]
- Wahajuddin; Arora, S. Superparamagnetic iron oxide nanoparticles: Magnetic nanoplatforms as drug carriers. Int. J. Nanomed. 2012, 7, 3445–3471. [Google Scholar] [CrossRef]
- Islam, N.; Haldorai, Y.; Nguyen, V.H.; Shim, J.-J. Synthesis of poly(vinyl pivalate) by atom transfer radical polymerization in supercritical carbon dioxide. Eur. Polym. J. 2014, 61, 93–104. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.A. Introduction to Spectroscopy; Cengage Learning: Boston, MA, USA, 2008. [Google Scholar]
- Lei, W.; Liu, Y.; Si, X.; Xu, J.; Du, W.; Yang, J.; Zhou, T.; Lin, J. Synthesis and magnetic properties of octahedral Fe3O4 via a one-pot hydrothermal route. Phys. Lett. A 2017, 381, 314–318. [Google Scholar] [CrossRef]
- Qiu, G.; Wang, Q.; Wang, C.; Lau, W.; Guo, Y. Polystyrene/Fe3O4 magnetic emulsion and nanocomposite prepared by ultrasonically initiated miniemulsion polymerization. Ultrason. Sonochem. 2007, 14, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Brandrup, J.; Immergut, E.H.; Grulke, E.A. Polymer Handbook, 4th ed.; Wiley–Blackwell: New York, NY, USA, 1999. [Google Scholar]
- Barandiaran, I.; Kortaberria, G. Magnetic nanocomposites based on poly(styrene-b-butadiene-b-methyl methacrylate) and modified Fe2O3 nanoparticles. Eur. Polym. J. 2016, 78, 340–351. [Google Scholar] [CrossRef]
- Norakankorn, C.; Pan, Q.; Rempel, G.L.; Kiatkamjornwong, S. Synthesis of poly(methyl methacrylate) nanoparticles initiated by azobisisobutyronitrile using a differential microemulsion polymerization technique. J. Appl. Polym. Sci. 2009, 113, 375–382. [Google Scholar] [CrossRef]
- Billmeyer, F.W. Textbook of Polymer Science; Wiley-Interscience: New York, NY, USA, 1984. [Google Scholar]
- Jensen, A.T.; Oliveira, A.C.C.; Gonçalves, S.B.; Gambetta, R.; Machado, F. Evaluation of the emulsion copolymerization of vinyl pivalate and methacrylated methyl oleate. J. Appl. Polym. Sci. 2016, 133, 44129–44139. [Google Scholar] [CrossRef]
- Feuser, P.E.; Bubniak, L.S.; Bodack, C.N.; Valério, A.; Santos-Silva, M.C.; Ricci-Júnior, E.; Sayer, C.; Araujo, P.H.H. In vitro cytotoxicity of poly(methyl methacrylate) nanoparticles and nanocapsules obtained by miniemulsion polymerization for drug delivery application. J. Nanosci. Nanotechnol. 2016, 16, 7669–7676. [Google Scholar] [CrossRef]
- Guindani, C.; Feuser, P.E.; Cordeiro, A.P.; de Meneses, A.C.; Possato, J.C.; da Silva Abel, J.; Machado-de-Ávila, R.A.; Sayer, C.; de Araújo, P.H.H. Bovine serum albumin conjugation on poly(methyl methacrylate) nanoparticles for targeted drug delivery applications. J. Drug Deliv. Sci. Technol. 2020, 56, 101490. [Google Scholar] [CrossRef]
- Mendes, A.N.; Filgueiras, L.A.; Siqueira, M.R.P.; Barbosa, G.M.; Holandino, C.; de Lima Moreira, D.; Pinto, J.C.; Nele, M. Encapsulation of Piper cabralanum (Piperaceae) nonpolar extract in poly(methyl methacrylate) by miniemulsion and evaluation of increase in the effectiveness of antileukemic activity in K562 cells. Int. J. Nanomed. 2017, 12, 8363–8373. [Google Scholar] [CrossRef]
- Shanmugam, S.; Gopal, B. Antimicrobial and cytotoxicity evaluation of aliovalent substituted hydroxyapatite. Appl. Surf. Sci. 2014, 303, 277–281. [Google Scholar] [CrossRef]
- Morozov, A.G.; Razborov, D.A.; Egiazaryan, T.A.; Baten’kin, M.A.; Aleynik, D.Y.; Egorikhina, M.N.; Rubtsova, Y.P.; Charikova, I.N.; Chesnokov, S.A.; Fedushkin, I.L. In Vitro Study of Degradation Behavior, Cytotoxicity, and Cell Adhesion of the Atactic Polylactic Acid for Biomedical Purposes. J. Polym. Environ. 2020, 28, 2652–2660. [Google Scholar] [CrossRef]
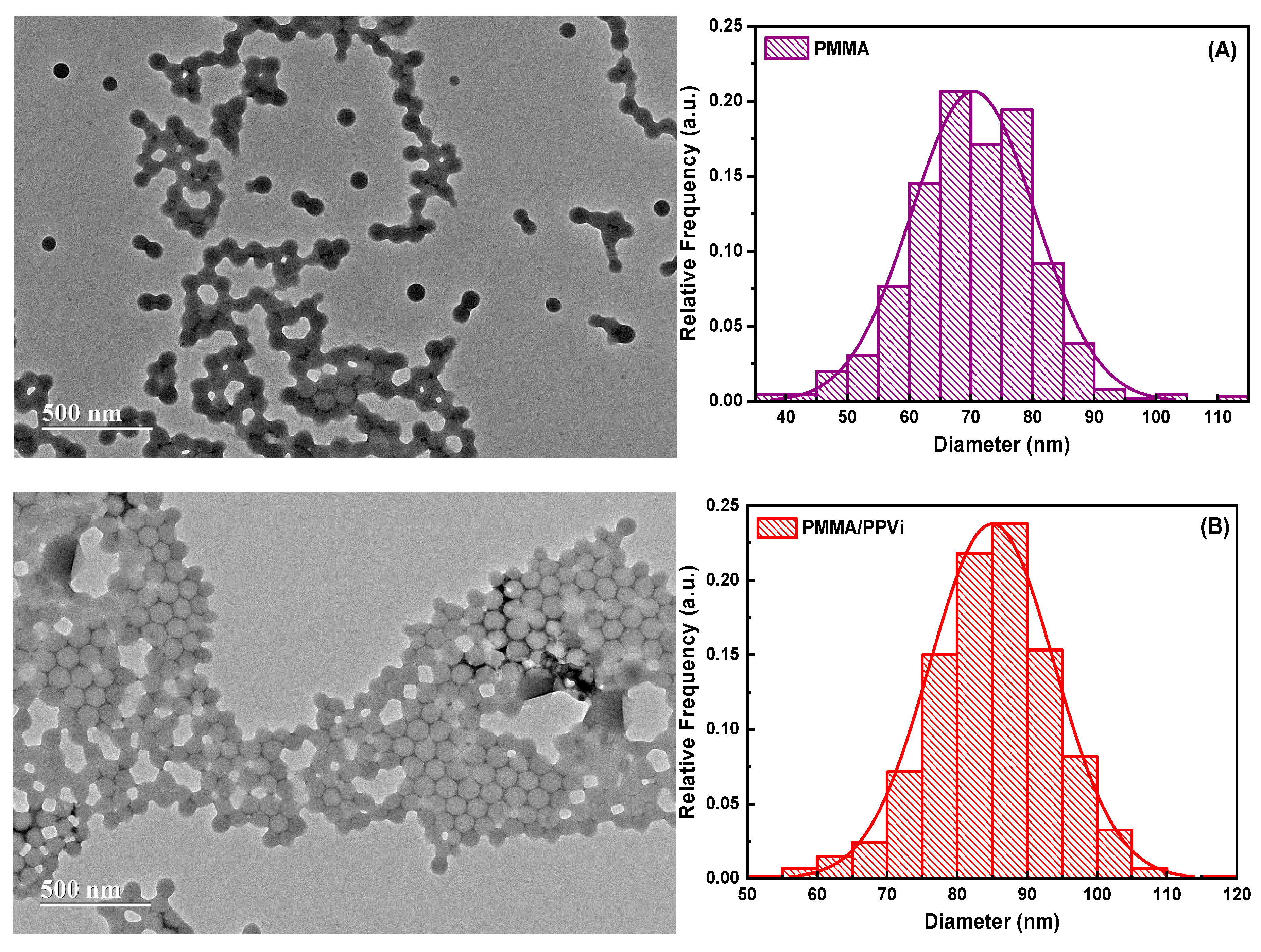
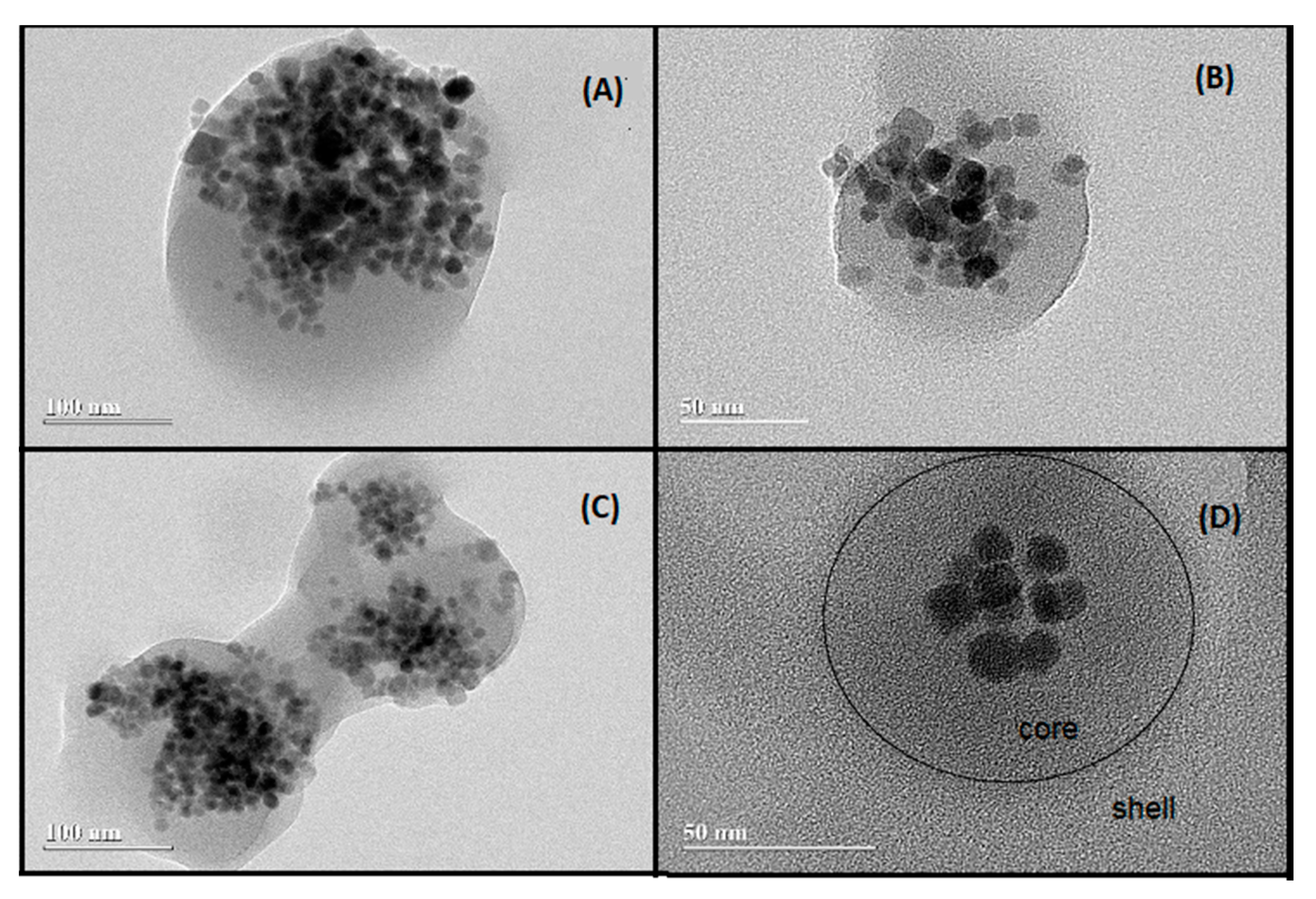
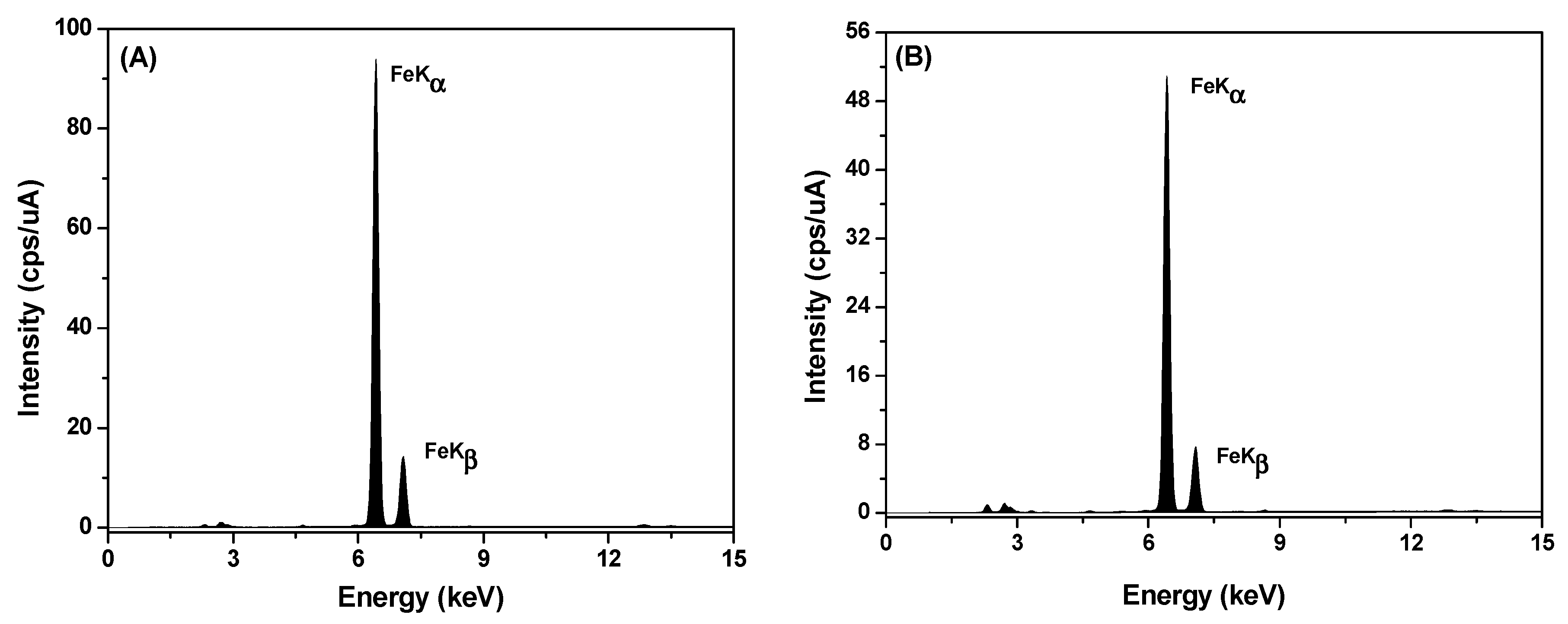

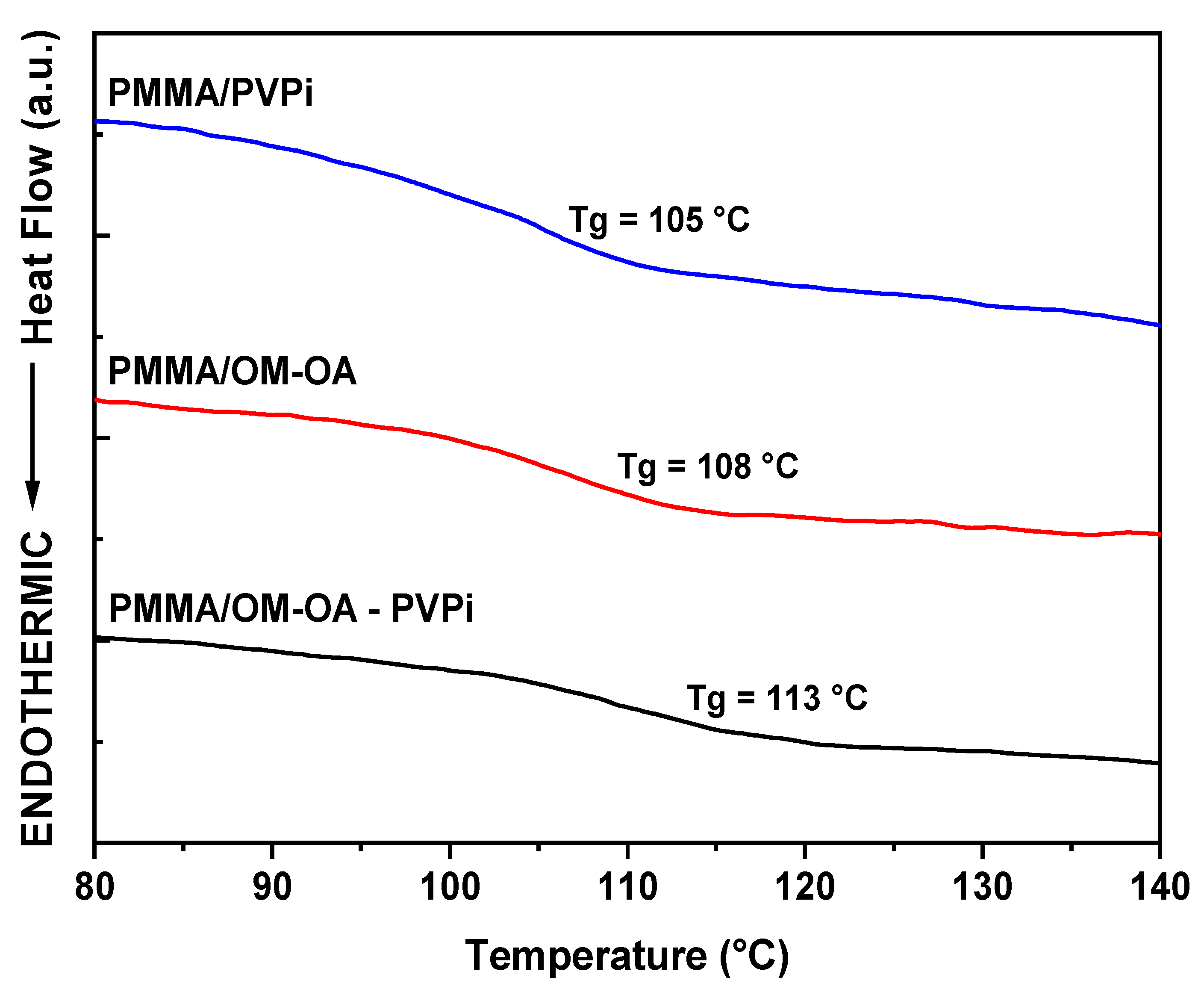

| Wavenumbers (cm−1) | Vibrational Assignments |
|---|---|
| 2995–2852 | Asymmetrical and symmetrical stretch C-H sp3 |
| 1731 | Stretch C=O of ester |
| 1489 and 1446 | Angular deformation of methylene groups (–CH2) |
| 1381 and 1365 | Symmetrical angular deformation –CH3 of tert-butyl groups |
| 1272 and 1149 | Stretch C-O of ester |
| 985 | Out of plane angular deformation C-H |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Resende, G.; Dutra, G.V.S.; Neta, M.S.B.; Araújo, O.A.; Chaves, S.B.; Machado, F. Well Defined Poly(Methyl Methacrylate)-Fe3O4/Poly(Vinyl Pivalate) Core–Shell Superparamagnetic Nanoparticles: Design and Evaluation of In Vitro Cytotoxicity Activity Against Cancer Cells. Polymers 2020, 12, 2868. https://doi.org/10.3390/polym12122868
Resende G, Dutra GVS, Neta MSB, Araújo OA, Chaves SB, Machado F. Well Defined Poly(Methyl Methacrylate)-Fe3O4/Poly(Vinyl Pivalate) Core–Shell Superparamagnetic Nanoparticles: Design and Evaluation of In Vitro Cytotoxicity Activity Against Cancer Cells. Polymers. 2020; 12(12):2868. https://doi.org/10.3390/polym12122868
Chicago/Turabian StyleResende, Graciane, Gabriel V. S. Dutra, Maria S. B. Neta, Olacir A. Araújo, Sacha B. Chaves, and Fabricio Machado. 2020. "Well Defined Poly(Methyl Methacrylate)-Fe3O4/Poly(Vinyl Pivalate) Core–Shell Superparamagnetic Nanoparticles: Design and Evaluation of In Vitro Cytotoxicity Activity Against Cancer Cells" Polymers 12, no. 12: 2868. https://doi.org/10.3390/polym12122868
APA StyleResende, G., Dutra, G. V. S., Neta, M. S. B., Araújo, O. A., Chaves, S. B., & Machado, F. (2020). Well Defined Poly(Methyl Methacrylate)-Fe3O4/Poly(Vinyl Pivalate) Core–Shell Superparamagnetic Nanoparticles: Design and Evaluation of In Vitro Cytotoxicity Activity Against Cancer Cells. Polymers, 12(12), 2868. https://doi.org/10.3390/polym12122868