Chemical Hydrogels Bearing Thiazolium Groups with a Broad Spectrum of Antimicrobial Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Hydrogels
2.3. Characterization Techniques
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klouda, L. Thermoresponsive hydrogels in biomedical applications A seven-year update. Eur. J. Pharm. Biopharm. 2015, 97, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Teo, A.J.T.; Mishra, A.; Park, I.; Kim, Y.J.; Park, W.T.; Yoon, Y.J. Polymeric Biomaterials for Medical Implants and Devices. ACS Biomater. Sci. Eng. 2016, 2, 454–472. [Google Scholar] [CrossRef]
- Palza, H.; Zapata, P.A.; Angulo-Pineda, C. Electroactive Smart Polymers for Biomedical Applications. Materials 2019, 12, 277. [Google Scholar]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive polymers for biomedical applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Li, Y.; Han, Y.; Wang, X.; Peng, J.; Xu, Y.; Chang, J. Multifunctional Hydrogels Prepared by Dual Ion Cross-Linking for Chronic Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 16054–16062. [Google Scholar] [CrossRef]
- Ergene, C.; Yasuhara, K.; Palermo, E.F. Biomimetic antimicrobial polymers: Recent advances in molecular design. Polym. Chem. 2018, 9, 2407–2427. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Boyer, C.; Nebhani, L.; Wong, E.H.H. Highly Bactericidal Macroporous Antimicrobial Polymeric Gel for Point-of-Use Water Disinfection. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Harvey, A.C.; Madsen, J.; Douglas, C.W.I.; MacNeil, S.; Armes, S.P. Antimicrobial Graft Copolymer Gels. Biomacromolecules 2016, 17, 2710–2718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malmsten, M. Antimicrobial and antiviral hydrogels. Soft Matter 2011, 7, 8725–8736. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A. Antimicrobial Polymeric Gels; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; ISBN 9780081021798. [Google Scholar]
- Muñoz-Bonilla, A.; Fernández-García, M. The roadmap of antimicrobial polymeric materials in macromolecular nanotechnology. Eur. Polym. J. 2015, 65. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Fernández-García, M. Poly(ionic liquid)s as antimicrobial materials. Eur. Polym. J. 2018, 105. [Google Scholar] [CrossRef]
- Alvarez-Paino, M.; Munoz-Bonilla, A.; Fernandez-Garcia, M.; Álvarez-Paino, M.; Muñoz-Bonilla, A.; Fernández-García, M. Antimicrobial Polymers in the Nano-World. Nanomaterials 2017, 7, 48. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Bonilla, A.; Echeverria, C.; Sonseca, Á.; Arrieta, M.P.; Fernández-García, M. Bio-Based Polymers with Antimicrobial Properties towards Sustainable Development. Materials 2019, 12, 641. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, K.; Caputo, G.A.; DeGrado, W.F. The Role of Hydrophobicity in the Antimicrobial and Hemolytic Activities of Polymethacrylate Derivatives. Chem. A Eur. J. 2009, 15, 1123–1133. [Google Scholar] [CrossRef] [Green Version]
- Palermo, E.F.; Sovadinova, I.; Kuroda, K. Structural determinants of antimicrobial activity and biocompatibility in membrane-disrupting methacrylamide random copolymers. Biomacromolecules 2009, 10, 3098–3107. [Google Scholar] [CrossRef]
- Palermo, E.F.; Kuroda, K. Chemical Structure of Cationic Groups in Amphiphilic Polymethacrylates Modulates the Antimicrobial and Hemolytic Activities. Biomacromolecules 2009, 10, 1416–1428. [Google Scholar] [CrossRef]
- Palermo, E.F.; Kuroda, K. Structural determinants of antimicrobial activity in polymers which mimic host defense peptides. Appl. Microbiol. Biotechnol. 2010, 87, 1605–1615. [Google Scholar] [CrossRef]
- Takahashi, H.; Palermo, E.F.; Yasuhara, K.; Caputo, G.A.; Kuroda, K. Molecular design, structures, and activity of antimicrobial peptide-mimetic polymers. Macromol. Biosci. 2013, 13, 1285–1299. [Google Scholar] [CrossRef] [Green Version]
- Pavlukhina, S.; Lu, Y.; Patimetha, A.; Libera, M.; Sukhishvili, S. Polymer Multilayers with pH-Triggered Release of Antibacterial Agents. Biomacromolecules 2010, 11, 3448–3456. [Google Scholar] [CrossRef] [PubMed]
- Andrews, M.A.; Figuly, G.D.; Chapman, J.S.; Hunt, T.W.; Glunt, C.D.; Rivenbark, J.A.; Chenault, H.K. Antimicrobial hydrogels formed by crosslinking polyallylamine with aldaric acid derivatives. J. Appl. Polym. Sci. 2011, 119, 3244–3252. [Google Scholar] [CrossRef]
- Hamzé, A.; Rubi, E.; Arnal, P.; Boisbrun, M.; Carcel, C.; Salom-Roig, X.; Maynadier, M.; Wein, S.; Vial, H.; Le Calas, M. Brief Articles Mono-and Bis-Thiazolium Salts Have Potent Antimalarial Activity. J. Med. Chem. 2005, 48, 3639–3643. [Google Scholar] [CrossRef] [PubMed]
- Caldarelli, S.A.; El Fangour, S.; Wein, S.; Van Tran Ba, C.; Périgaud, C.; Pellet, A.; Vial, H.J.; Peyrottes, S. New bis-thiazolium analogues as potential antimalarial agents: Design, synthesis, and biological evaluation. J. Med. Chem. 2013, 56, 496–509. [Google Scholar] [CrossRef]
- Shiradkar, M.; Suresh Kumar, G.V.; Dasari, V.; Tatikonda, S.; Akula, K.C.; Shah, R. Clubbed triazoles: A novel approach to antitubercular drugs. Eur. J. Med. Chem. 2007, 42, 807–816. [Google Scholar] [CrossRef]
- Wang, M.W.; Zhu, H.H.; Wang, P.Y.; Zeng, D.; Wu, Y.Y.; Liu, L.W.; Wu, Z.B.; Li, Z.; Yang, S. Synthesis of Thiazolium-Labeled 1,3,4-Oxadiazole Thioethers as Prospective Antimicrobials: In Vitro and in Vivo Bioactivity and Mechanism of Action. J. Agric. Food Chem. 2019, 67, 12696–12708. [Google Scholar] [CrossRef]
- Tejero, R.; López, D.; López-Fabal, F.; Gómez-Garcés, J.L.; Fernández-García, M. Antimicrobial polymethacrylates based on quaternized 1,3-thiazole and 1,2,3-triazole side-chain groups. Polym. Chem. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Tejero, R.; López, D.; López-Fabal, F.; Gómez-Garcés, J.L.; Fernández-García, M. High efficiency antimicrobial thiazolium and triazolium side-chain polymethacrylates obtained by controlled alkylation of the corresponding azole derivatives. Biomacromolecules 2015, 16. [Google Scholar] [CrossRef]
- Tejero, R.; Gutiérrez, B.; López, D.; López-Fabal, F.; Gómez-Garcés, J.L.; Fernández-García, M. Copolymers of acrylonitrile with quaternizable thiazole and triazole side-chain methacrylates as potent antimicrobial and hemocompatible systems. Acta Biomater. 2015, 25. [Google Scholar] [CrossRef]
- Alvarez-Paino, M.; Juan-Rodríguez, R.; Cuervo-Rodríguez, R.; Tejero, R.; López, D.; López-Fabal, F.; Gómez-Garcés, J.L.; Muñoz-Bonilla, A.; Fernández-García, M. Antimicrobial films obtained from latex particles functionalized with quaternized block copolymers. Colloids Surf. B Biointerfaces 2016, 140. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; López, D.; Fernández-García, M. Providing Antibacterial Activity to Poly(2-Hydroxy Ethyl Methacrylate) by Copolymerization with a Methacrylic Thiazolium Derivative. Int. J. Mol. Sci. 2018, 19, 4120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tejero, R.; Gutiérrez, B.; López, D.; López-Fabal, F.; Gómez-Garcés, J.L.J.L.; Muñoz-Bonilla, A.; Fernández-García, M. Tailoring macromolecular structure of cationic polymers towards efficient contact active antimicrobial surfaces. Polymers 2018, 10, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuervo-Rodríguez, R.; López-Fabal, F.; Gómez-Garcés, J.L.; Muñoz-Bonilla, A.; Fernández-García, M. Contact Active Antimicrobial Coatings Prepared by Polymer Blending. Macromol. Biosci. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, A.; Echeverria, C.; San Martin, M.; Cuervo-Rodriguez, R.; Fernandez-Garcia, M.; Munoz-Bonilla, A. Porous Microstructured Surfaces with pH-Triggered Antibacterial Properties. Macromol. Biosci. 2019, 19, e1900127. [Google Scholar] [CrossRef]
- Echeverría, C.; Muñoz-Bonilla, A.; Cuervo-Rodríguez, R.; López, D.; Fernández-García, M. Antibacterial PLA Fibers Containing Thiazolium Groups as Wound Dressing Materials. ACS Appl. Bio Mater. 2019, 2, 4714–4719. [Google Scholar] [CrossRef]
- ASTM E2149-01, Standard Test Method for Determining the Antimicrobial Activity of Immobilized Antimicrobial Agents Under Dynamic Contact Conditions (Withdrawn 2010); ASTM International: West Conshohocken, PA, USA, 2001; Available online: www.astm.org (accessed on 1 November 2020).
- Vargün, E.; Usanmaz, A. Degradation of Poly(2-hydroxyethyl methacrylate) Obtained by Radiation in Aqueous Solution. J. Macromol. Sci. Part A 2010, 47, 882–891. [Google Scholar] [CrossRef]
- Fernández-García, M.; Torrado, M.F.F.; Martínez, G.; Sánchez-Chaves, M.; Madruga, E.L. Free radical copolymerization of 2-hydroxyethyl methacrylate with butyl methacrylate: Determination of monomer reactivity ratios and glass transition temperatures. Polymer 2000, 41, 8001–8008. [Google Scholar] [CrossRef]
- Demirelli, K.; Coşkun, M.; Kaya, E. A detailed study of thermal degradation of poly(2-hydroxyethyl methacrylate). Polym. Degrad. Stab. 2001, 72, 75–80. [Google Scholar] [CrossRef]
- Çaykara, T.; Özyürek, C.; Kantoğlu, Ö. Investigation of thermal behavior of poly(2-hydroxyethyl methacrylate-co-itaconic acid) networks. J. Appl. Polym. Sci. 2006, 103, 1602–1607. [Google Scholar] [CrossRef]
- Sanna, R.; Alzari, V.; Nuvoli, D.; Scognamillo, S.; Marceddu, S.; Mariani, A. Polymer hydrogels of 2-hydroxyethyl acrylate and acrylic acid obtained by frontal polymerization. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 1515–1520. [Google Scholar] [CrossRef]
- Cuervo-Rodríguez, R.; Muñoz-Bonilla, A.; Araujo, J.; Echeverría, C.; Fernández-García, M. Influence of side chain structure on the thermal and antimicrobial properties of cationic methacrylic polymers. Eur. Polym. J. 2019, 117, 86–93. [Google Scholar] [CrossRef]

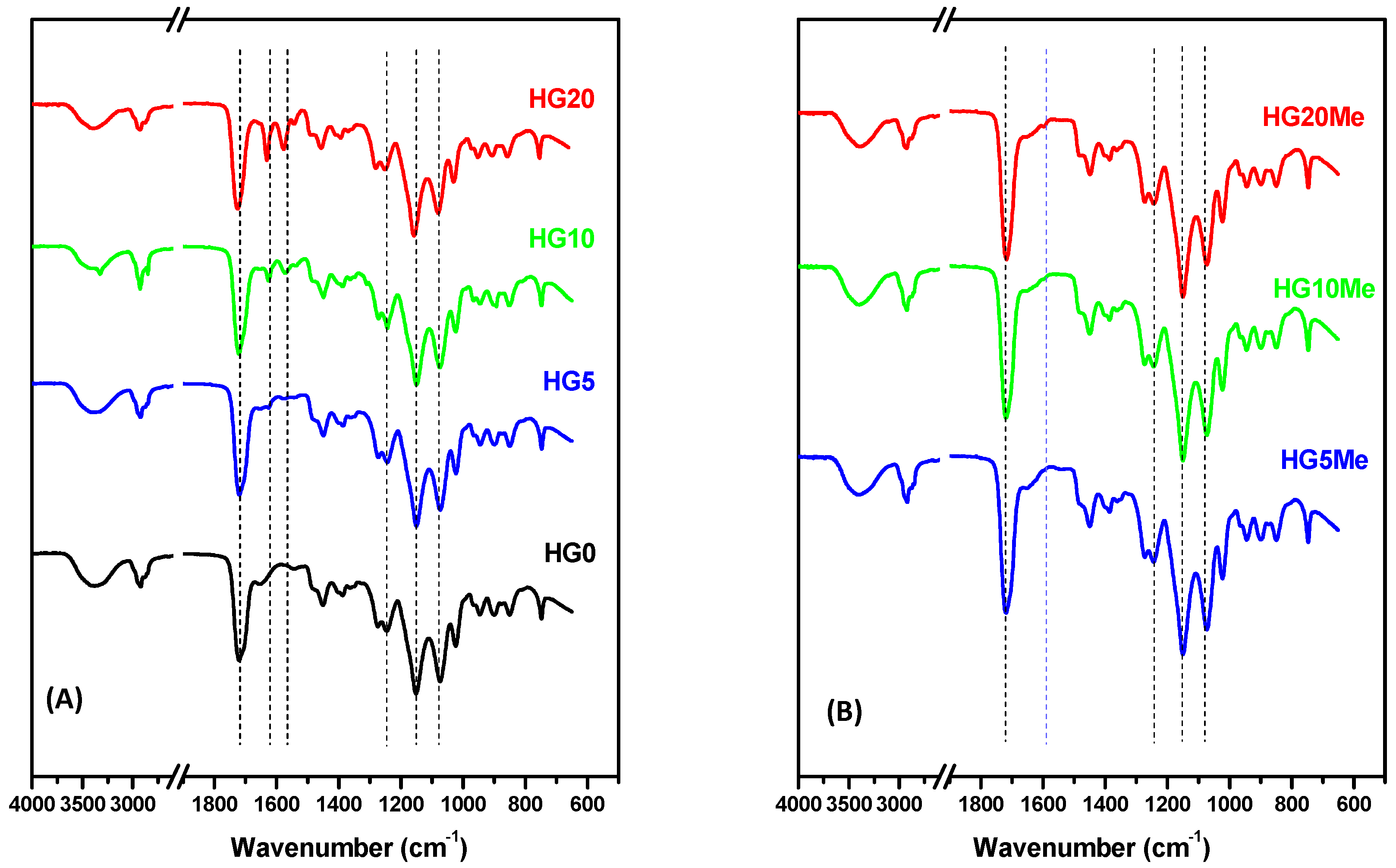
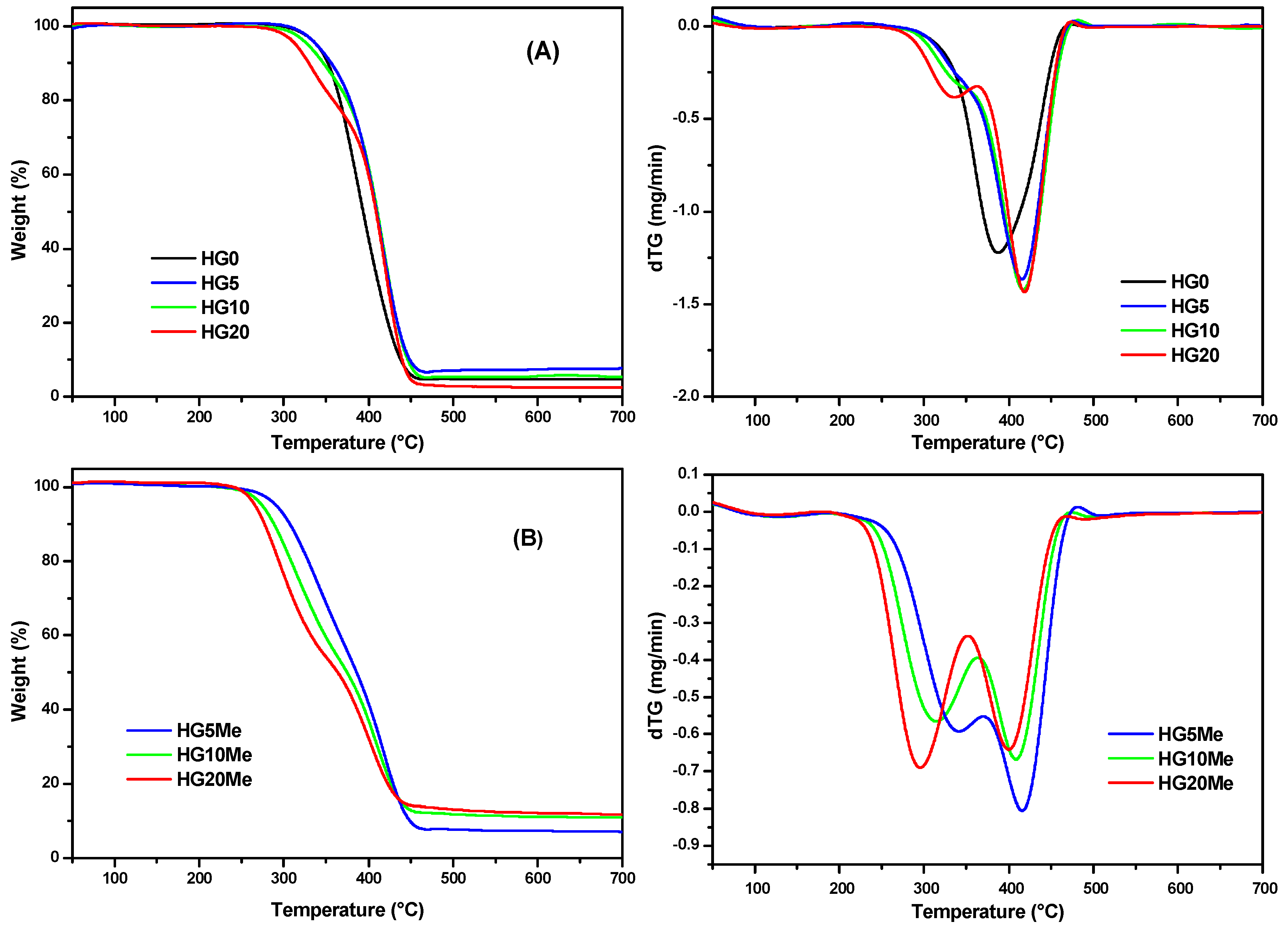

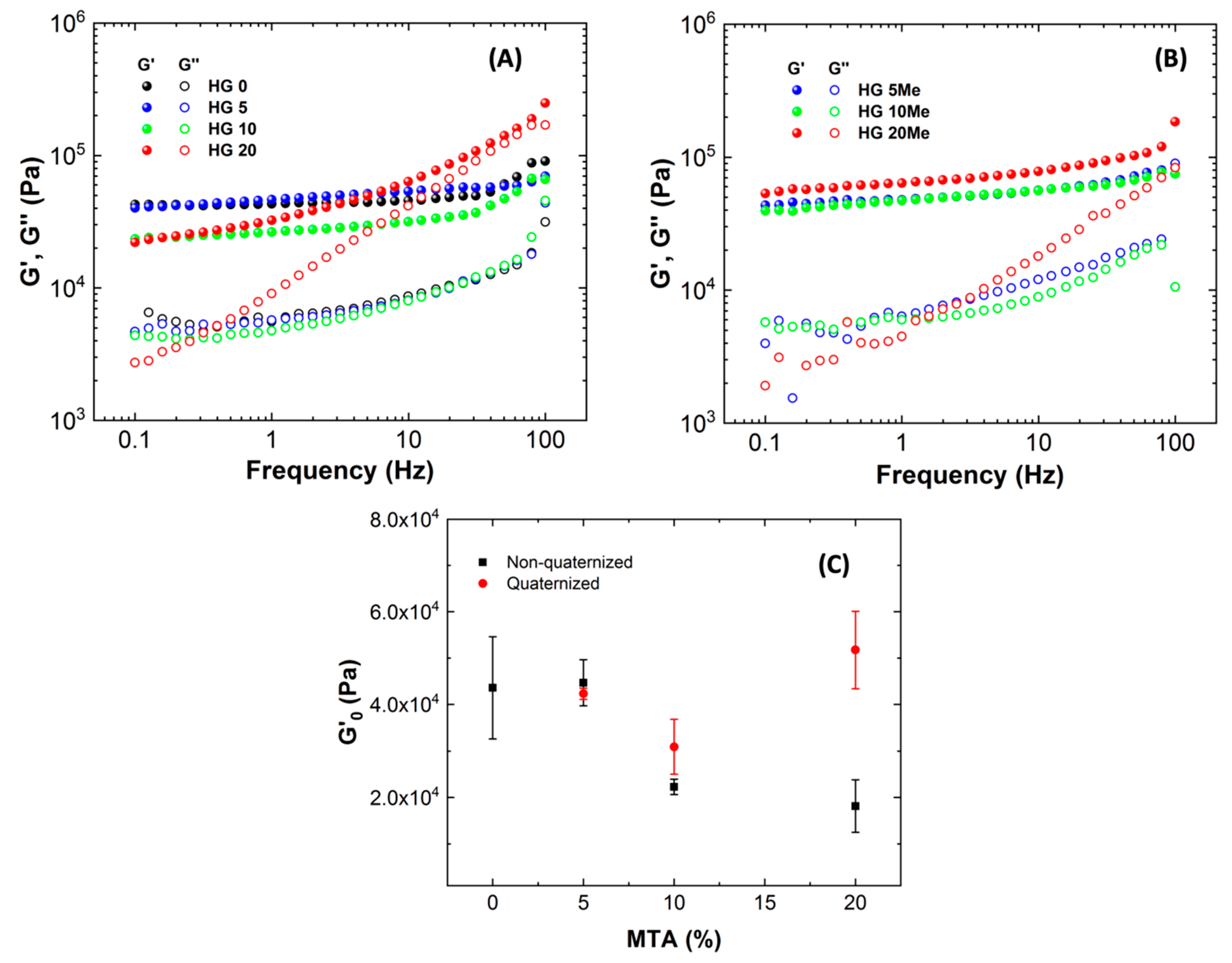

| Hydrogels | HEMA | PEGDA | ACPA | MTA |
|---|---|---|---|---|
| HG0 | 81.70 | 16.34 | 1.96 | 0 |
| HG5 | 78.49 | 15.70 | 1.88 | 3.92 |
| HG10 | 75.53 | 15.11 | 1.81 | 7.55 |
| HG20 | 70.2 | 14.01 | 1.69 | 14.1 |
| Hydrogels | T0 (°C) | Tmax1 (°C) | Tmax2 (°C) | Residue (%) |
|---|---|---|---|---|
| HG0 | 317 | 388 | 4.7 | |
| HG5 HG5Me | 317 287 | 336 * 342 | 416 416 | 7.6 7.3 |
| HG10 HG10Me | 305 269 | 335 * 314 | 418 409 | 5.5 11.1 |
| HG20 HG20Me | 305 250 | 334 296 | 418 400 | 2.5 12.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Bonilla, A.; Zagora, J.; Plachá, D.; Echeverría, C.; Chiloeches, A.; Fernández-García, M. Chemical Hydrogels Bearing Thiazolium Groups with a Broad Spectrum of Antimicrobial Behavior. Polymers 2020, 12, 2853. https://doi.org/10.3390/polym12122853
Muñoz-Bonilla A, Zagora J, Plachá D, Echeverría C, Chiloeches A, Fernández-García M. Chemical Hydrogels Bearing Thiazolium Groups with a Broad Spectrum of Antimicrobial Behavior. Polymers. 2020; 12(12):2853. https://doi.org/10.3390/polym12122853
Chicago/Turabian StyleMuñoz-Bonilla, Alexandra, Jakub Zagora, Daniela Plachá, Coro Echeverría, Alberto Chiloeches, and Marta Fernández-García. 2020. "Chemical Hydrogels Bearing Thiazolium Groups with a Broad Spectrum of Antimicrobial Behavior" Polymers 12, no. 12: 2853. https://doi.org/10.3390/polym12122853
APA StyleMuñoz-Bonilla, A., Zagora, J., Plachá, D., Echeverría, C., Chiloeches, A., & Fernández-García, M. (2020). Chemical Hydrogels Bearing Thiazolium Groups with a Broad Spectrum of Antimicrobial Behavior. Polymers, 12(12), 2853. https://doi.org/10.3390/polym12122853









