Joint-Linker Type Ionic Gels Using Polymerizable Ionic Liquid as a Crosslinker via Thiol-Ene Click Reactions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Gels
2.3. Method of Characterization
3. Results and Discussion
3.1. Fourier Transform Infrared (FTIR) Spectroscopy Analysis
3.2. Mechanical Properties
3.3. Thermal Properties
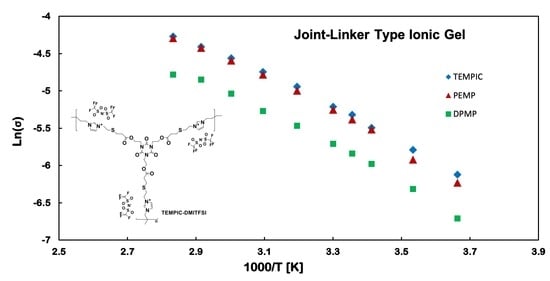
3.4. Conductive Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Miyata, T.; Asami, N.; Okawa, K.; Uragami, T. Rapid response of a poly(acrylamide) hydrogel having a semi-interpenetrating polymer network structure. Polym. Adv. Technol. 2006, 17, 794–797. [Google Scholar] [CrossRef]
- Chirila, T.V.; George, K.A.; Ghafor, W.A.A.; Pas, S.J.; Hill, A.J. Sequential homo-interpenetrating polymer networks of poly(2-hydroxyethyl methacrylate): Synthesis, characterization, and calcium uptake. J. Appl. Polym. Sci. 2012, 126, E455–E466. [Google Scholar] [CrossRef]
- Ahmed, K.; Watanabe, Y.; Higashihara, T.; Arafune, H.; Kamijo, T.; Morinaga, T.; Sato, T.; Makino, M.; Kawakami, M.; Furukawa, H. Investigation of mechanical properties and internal structure of novel ionic double-nework gels and comparison with conventional hydrogels. Microsyst. Technol. 2016, 22, 17–24. [Google Scholar] [CrossRef]
- Susan, M.A.B.H.; Kaneko, T.; Noda, A.; Watanabe, M. Ion gels prepared by in situ radical polymerization of vinyl monomers in an ionic liquid and their characterization as polymer electrolytes. J. Am. Chem. Soc. 2005, 127, 4976–4983. [Google Scholar] [CrossRef] [PubMed]
- Holbrey, J.D.; Seddon, K.R. Ionic liquids. Clean Technol. Environ. Policy 1999, 1, 223–236. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Keim, W. Ionic liquids—New “solutions” for transition metal catalysis. Angew. Chem. 2000, 39, 3772–3789. [Google Scholar] [CrossRef]
- Seddon, K.R. A taste of the future. Nat. Mater. 2003, 2, 363–365. [Google Scholar] [CrossRef]
- Naga, N.; Fujioka, S.; Inose, D.; Ahmed, K.; Nageh, H.; Nakano, T. Synthesis and properties of porous polymers synthesized by Michael addition reactions of multi-functional acrylate, diamine, and dithiol compounds. RSC Adv. 2020, 10, 60–69. [Google Scholar] [CrossRef] [Green Version]
- Naga, N.; Oda, E.; Toyota, A.; Horie, K.; Furukawa, H. Tailored synthesis and fundamental characterization of organic-inorganic hybrid gels by means of a hydrosilylation reaction. Macromol. Chem. Phys. 2006, 207, 627–635. [Google Scholar] [CrossRef]
- Naga, N.; Oda, E.; Toyota, A.; Furukawa, H. Mesh size control of organic-inorganic hybrid gels by means of a hydrosilylation Co-gelation of siloxane or silsesquioxane and α, ω-non-conjugated dienes. Macromol. Chem. Phys. 2007, 208, 2331–2338. [Google Scholar] [CrossRef]
- Ahmed, K.; Naga, N.; Kawakami, M.; Furukawa, H. Development of ionic gels using thiol-based monomers in ionic liquid. Behav. Mech. Multifunct. Mater. Compos. 2016, 980005. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D printing and customized additive manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonards, H.; Engelhardt, S.; Hoffmann, A.; Pongratz, L.; Schriever, S.; Bläsius, J.; Wehner, M.; Gillner, A. Advantages and drawbacks of Thiol-ene based resins for 3D-printing. Proc. SPIE 2015, 9353, 93530F. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Lee, T.Y.; Roper, T. Thiol–enes: Chemistry of the past with promise for the future. J. Polym. Sci. A Polym. Chem. 2004, 42, 5301–5338. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Bowman, C.N. Thiol–ene click chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573. [Google Scholar] [CrossRef]
- Taghavikish, M.; Subianto, S.; Dutta, N.K.; Choudhury, N.R. Facile fabrication of polymerizable ionic liquid based-gel beads via thiol–ene chemistry. ACS Appl. Mater. Interfaces 2015, 7, 17298–17306. [Google Scholar] [CrossRef]
- Rhoades, T.C.; Wistrom, J.C.; Johnson, R.D.; Miller, K.M. Thermal, mechanical and conductive properties of imidazolium-containing thiol-ene poly(ionic liquid) networks. Polymer 2016, 100, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Tibbits, A.C.; Yan, Y.S.; Kloxin, C.J. Covalent incorporation of ionic liquid into ion-conductive networks via thiol-ene photopolymerization. Macromol. Rapid Commun. 2017, 38, 1700113. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, K.; Naga, N.; Kawakami, M.; Furukawa, H. Extremely soft, conductive, and transparent ionic gels by 3D optical printing. Macromol. Chem. Phys. 2018, 1800216. [Google Scholar] [CrossRef]
- Hardwick, L.J.; Saint, J.A.; Lucas, I.T.; Doeff, M.M.; Kostecki, R. FTIR and raman study of the Lix Tiy Mn1 − y O2 ( y = 0 , 0.11 ) cathodes in methylpropyl pyrrolidinium bis(fluoro-sulfonyl)imide, LiTFSI Electrolyte. J. Electrochem. Soc. 2009, 156, A120. [Google Scholar] [CrossRef] [Green Version]
- Fujil, K.; Seki, S.; Fukuda, S.; Kanzaki, R.; Takamuku, T.; Umebayashi, Y.; Ishiguro, S.I. Anion conformation of low-viscosity room-temperature ionic liquid 1-ethyl-3-methylimidazolium bis(fluorosulfonyl) imide. J. Phys. Chem. B 2007, 111, 12829. [Google Scholar] [CrossRef]
- Beran, M.; Příhoda, J.; Žák, Z.; Černík, M. A new route to the syntheses of alkali metal bis(fluorosulfuryl)imides: Crystal structure of LiN(SO2F)2. Polyhedron 2006, 25, 1292. [Google Scholar] [CrossRef]
- Fujii, K.; Seki, S.; Fukuda, S.; Takamuku, T.; Kohara, S.; Kameda, Y.; Umebayashi, Y.; Ishiguro, S.I. Liquid structure and conformation of a low-viscosity ionic liquid, N-methyl-N-propyl-pyrrolidinium bis(fluorosulfonyl) imide studied by high-energy X-ray scattering. J. Mol. Liq. 2008, 143, 64–69. [Google Scholar] [CrossRef]





| Joint Molecule | Monomer Concentration [wt%] | Young’s Modulus [kPa] | Breaking Strain [%] | Breaking Stress [kPa] |
|---|---|---|---|---|
| DPMP | 20 | 352.4 | 29.2 | 80.3 |
| PEMP | 20 | 268.8 | 30.7 | 84.0 |
| TEMPIC | 20 | 71.0 | 33.8 | 89.2 |
| DPMP | 30 | 459.0 | 17.4 | 96.8 |
| PEMP | 30 | 387.1 | 32.5 | 227.8 |
| TEMPIC | 30 | 190.8 | 44.5 | 224.2 |
| Sample | Td [°C] | ||
|---|---|---|---|
| 5 wt % | 50 wt % | Remaining | |
| Pure DAIM TFSI | 372.4 | 422.9 | 475.7 |
| DPMP-DAIM TFSI | 103.8 | 311.7 | 465.3 |
| PEMP-DAIM TFSI | 104.3 | 256.6 | 465.1 |
| TEMPIC-DAIM TFSI | 109.5 | 285.5 | 465.5 |
| Joint Molecule | Monomer Concentration [wt %] | A [S cm−1] | B [K] | T0 [K] | Dapp [10−14 cm2 s−1] | Conductivity at 298 K [mS cm−1] |
|---|---|---|---|---|---|---|
| TEMPIC | 20 | 3.8 | 468.1 | 175.9 | 1.8 | 4.89 |
| PEMP | 20 | 3.6 | 472.3 | 174.2 | 1.8 | 4.60 |
| DPMP | 20 | 2.7 | 530.2 | 165.3 | 1.7 | 2.91 |
| TEMPIC | 30 | 3.1 | 488.7 | 176.5 | 1.2 | 3.14 |
| PEMP | 30 | 3.1 | 494.3 | 177.5 | 1.2 | 2.91 |
| DPMP | 30 | 2.2 | 554.6 | 155.3 | 1.3 | 2.77 |
| DPMP/PC | 30 | 1.8 | 562.0 | 153.5 | - | 2.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, K.; Inagaki, A.; Naga, N. Joint-Linker Type Ionic Gels Using Polymerizable Ionic Liquid as a Crosslinker via Thiol-Ene Click Reactions. Polymers 2020, 12, 2844. https://doi.org/10.3390/polym12122844
Ahmed K, Inagaki A, Naga N. Joint-Linker Type Ionic Gels Using Polymerizable Ionic Liquid as a Crosslinker via Thiol-Ene Click Reactions. Polymers. 2020; 12(12):2844. https://doi.org/10.3390/polym12122844
Chicago/Turabian StyleAhmed, Kumkum, Aoi Inagaki, and Naofumi Naga. 2020. "Joint-Linker Type Ionic Gels Using Polymerizable Ionic Liquid as a Crosslinker via Thiol-Ene Click Reactions" Polymers 12, no. 12: 2844. https://doi.org/10.3390/polym12122844
APA StyleAhmed, K., Inagaki, A., & Naga, N. (2020). Joint-Linker Type Ionic Gels Using Polymerizable Ionic Liquid as a Crosslinker via Thiol-Ene Click Reactions. Polymers, 12(12), 2844. https://doi.org/10.3390/polym12122844