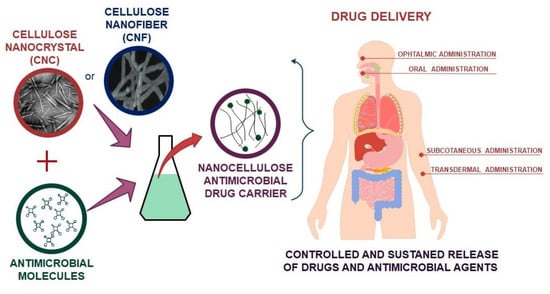
Nanocellulose in Drug Delivery and Antimicrobially Active Materials
Abstract
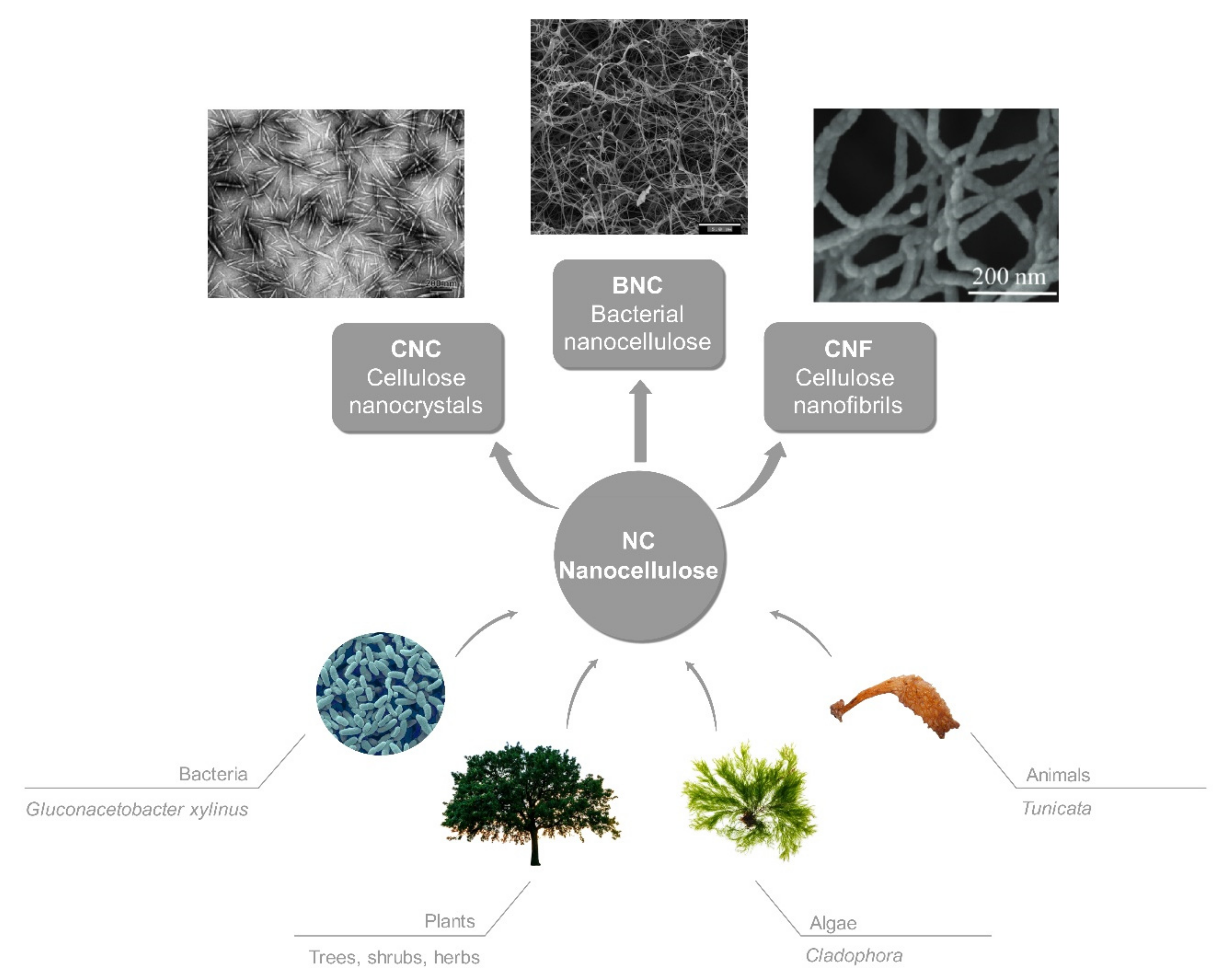
:1. Introduction
2. Application of Nanocellulose in Drug Delivery
2.1. CNCs as a Vehicle for Drug Delivery
2.2. CNFs as a Vehicle for Drug Delivery
2.3. BNC as a Vehicle for Drug Delivery
3. Nanocellulose Based Antimicrobial Hybrids and the Use of Antimicrobials in Drug Delivery
3.1. Inorganic Hybrids
3.2. Organic Hybrids
4. Challenges and Future Prospects
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dufresne, A. Nanocellulose: A new ageless bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Salas, C.; Nypelö, T.; Rodriguez-Abreu, C.; Carrillo, C.; Rojas, O.J. Nanocellulose properties and applications in colloids and interfaces. Curr. Opin. Colloid Interface Sci. 2014, 19, 383–396. [Google Scholar] [CrossRef]
- Moran, J.I.; Alvarez, V.A.; Cyras, V.P.; Vazquez, A. Extraction of cellulose and preparation of nanocellulose from sisal fibers. Cellulose 2008, 15, 149–159. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Echeverria, C.; Sonseca, Á.; Arrieta, M.P.; Fernández-García, M. Bio-Based Polymers with Antimicrobial Properties towards Sustainable Development. Materials 2019, 12, 641. [Google Scholar] [CrossRef] [Green Version]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef] [Green Version]
- Bacakova, L.; Pajorova, J.; Bacakova, M.; Skogberg, A.; Kallio, P.; Kolarova, K.; Svorcik, V. Nanocellulose in Biotechnology and Medicine: Focus on Skin Tissue Engineering and Wound Healing. Preprints 2018. [Google Scholar] [CrossRef]
- Torres, F.G.; Troncoso, O.P.; Lopez, D.; Grande, C.; Gomez, C.M. Reversible stress softening and stress recovery of cellulose networks. Soft Matter 2009, 5, 4185–4190. [Google Scholar] [CrossRef]
- Habibi, Y.; Goffin, A.-L.; Schiltz, N.; Duquesne, E.; Dubois, P.; Dufresne, A. Bionanocomposites based on poly(ε-caprolactone)-grafted cellulose nanocrystals by ring-opening polymerization. J. Mater. Chem. 2008, 18, 5002–5010. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, Z.; Liu, Z.; Kang, H.; Liu, Y. Cellulose nanofibers/polyurethane shape memory composites with fast water-responsivity. J. Mater. Chem. B 2018, 6, 1668–1677. [Google Scholar] [CrossRef]
- Tree. Available online: https://arbordayblog.org/landscapedesign/12-fast-growing-shade-trees/ (accessed on 17 April 2020).
- Cells in Microscopic Detail. Available online: https://66.media.tumblr.com/tumblr_maqu0spvna1qc0noyo1_1280.jpg (accessed on 17 April 2020).
- Styela Clava-Clubbed Tunicate. Available online: http://www.marinespecies.org/photogallery.php?album=669&pic=55030 (accessed on 17 April 2020).
- Cladophora Sericea-Silky Green Branched Weed. Available online: http://www.aphotomarine.com/green_seaweed_cladophora_sericea.html (accessed on 17 April 2020).
- Xu, X.; Liu, F.; Jiang, L.; Zhu, J.Y.; Haagenson, D.; Wiesenborn, D.P. Cellulose Nanocrystals vs. Cellulose Nanofibrils: A Comparative Study on Their Microstructures and Effects as Polymer Reinforcing Agents. ACS Appl. Mater. Interfaces 2013, 5, 2999–3009. [Google Scholar] [CrossRef]
- Mandal, A.; Chakrabarty, D. Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohydr. Polym. 2011, 86, 1291–1299. [Google Scholar] [CrossRef]
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a tiny fiber with huge applications. Curr. Opin. Biotechnol. 2016, 39, 76–88. [Google Scholar] [CrossRef]
- Pierre, G.; Punta, C.; Delattre, C.; Melone, L.; Dubessay, P.; Fiorati, A.; Pastori, N.; Galante, Y.M.; Michaud, P. TEMPO-mediated oxidation of polysaccharides: An ongoing story. Carbohydr. Polym. 2017, 165, 71–85. [Google Scholar] [CrossRef] [Green Version]
- Menon, M.P.; Selvakumar, R.; Kumar, P.S.; Ramakrishna, S. Extraction and modification of cellulose nanofibers derived from biomass for environmental application. RSC Adv. 2017, 7, 42750–42773. [Google Scholar] [CrossRef] [Green Version]
- Shahi, N.; Min, B.; Sapkota, B.; Rangari, V.K. Eco-Friendly Cellulose Nanofiber Extraction from Sugarcane Bagasse and Film Fabrication. Sustainability 2020, 12, 6015. [Google Scholar] [CrossRef]
- Sharma, C.; Bhardwaj, N.K. Bacterial nanocellulose: Present status, biomedical applications and future perspectives. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 104, 109963. [Google Scholar] [CrossRef]
- Khosravi, K.; Koller, M.; Akramzadeh, N.; Mortazavian, A. Bacterial nanocellulose: Biosynthesis and medical application. Biointerface Res. Appl. Chem. 2016, 6, 1511–1516. [Google Scholar]
- Berndt, S.; Wesarg, F.; Wiegand, C.; Kralisch, D.; Mueller, F.A. Antimicrobial porous hybrids consisting of bacterial nanocellulose and silver nanoparticles. Cellulose 2013, 20, 771–783. [Google Scholar] [CrossRef]
- Liyaskina, E.; Revin, V.; Paramonova, E.; Nazarkina, M.; Pestov, N.; Revina, N.; Kolesnikova, S. Nanomaterials from bacterial cellulose for antimicrobial wound dressing. J. Phys. Conf. Ser. 2017, 784, 012034. [Google Scholar] [CrossRef] [Green Version]
- Brown, A.J. XLIII—On an acetic ferment which forms cellulose. J. Chem. Soc. Trans. 1886, 49, 432–439. [Google Scholar] [CrossRef] [Green Version]
- Bacakova, L.; Pajorova, J.; Bacakova, M.; Skogberg, A.; Kallio, P.; Kolarova, K.; Svorcik, V. Versatile Application of Nanocellulose: From Industry to Skin Tissue Engineering and Wound Healing. Nanomaterials 2019, 9, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez Ávila, H.; Schwarz, S.; Feldmann, E.-M.; Mantas, A.; von Bomhard, A.; Gatenholm, P.; Rotter, N. Biocompatibility evaluation of densified bacterial nanocellulose hydrogel as an implant material for auricular cartilage regeneration. Appl. Microbiol. Biotechnol. 2014, 98, 7423–7435. [Google Scholar] [CrossRef]
- Jozala, A.F.; de Lencastre-Novaes, L.C.; Lopes, A.M.; de Carvalho Santos-Ebinuma, V.; Mazzola, P.G.; Pessoa-Jr, A.; Grotto, D.; Gerenutti, M.; Chaud, M.V. Bacterial nanocellulose production and application: A 10-year overview. Appl. Microbiol. Biotechnol. 2016, 100, 2063–2072. [Google Scholar] [CrossRef] [Green Version]
- Habibi, Y. Key advances in the chemical modification of nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542. [Google Scholar] [CrossRef]
- Helenius, G.; Bäckdahl, H.; Bodin, A.; Nannmark, U.; Gatenholm, P.; Risberg, B. In vivo biocompatibility of bacterial cellulose. J. Biomed. Mater. Res. Part A 2006, 76A, 431–438. [Google Scholar] [CrossRef]
- Klemm, D.; Schumann, D.; Kramer, F.; Hessler, N.; Hornung, M.; Schmauder, H.-P.; Marsch, S. Nanocelluloses as innovative polymers in research and application. In Polysaccharides Ii; Klemm, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 205, pp. 49–96. ISBN 978-3-540-37102-1. [Google Scholar]
- Siro, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: From Nature to High Performance Tailored Materials, 2nd ed.; De Gruyter: Berlin, Germany, 2017. [Google Scholar]
- Hossain, A.B.M.S.; Uddin, M.; Nettoor Veettil, V.; Fawzi, M. Nano-cellulose based nano-coating biomaterial dataset using corn leaf biomass: An innovative biodegradable plant Biomaterial. Data Brief 2018, 17, 162–168. [Google Scholar] [CrossRef]
- Rashad, A.; Mohamed Ahmed, S.; Ojansivu, M.; Berstad, K.; Yassin, M.; Kivijärvi, T.; Heggset, E.B.; Syverud, K.; Mustafa, K. Coating 3D Printed Polycaprolactone Scaffolds with Nanocellulose Promotes Growth and Differentiation of Mesenchymal Stem Cells. Biomacromolecules 2018, 19, 4307–4319. [Google Scholar] [CrossRef]
- Korhonen, J.T.; Kettunen, M.; Ras, R.H.A.; Ikkala, O. Hydrophobic Nanocellulose Aerogels as Floating, Sustainable, Reusable, and Recyclable Oil Absorbents. ACS Appl. Mater. Interfaces 2011, 3, 1813–1816. [Google Scholar] [CrossRef]
- Wicklein, B.; Kocjan, A.; Salazar-Alvarez, G.; Carosio, F.; Camino, G.; Antonietti, M.; Bergstrom, L. Thermally insulating and fire-retardant lightweight anisotropic foams based on nanocellulose and graphene oxide. Nat. Nanotechnol. 2015, 10, 277–283. [Google Scholar] [CrossRef]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of Hydrogels and Aerogels Containing Nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Kim, J.-H.; Shim, B.S.; Kim, H.S.; Lee, Y.-J.; Min, S.-K.; Jang, D.; Abas, Z.; Kim, J. Review of nanocellulose for sustainable future materials. Int. J. Precis. Eng. Manuf.-Green Technol. 2015, 2, 197–213. [Google Scholar] [CrossRef] [Green Version]
- Auad, M.L.; Contos, V.S.; Nutt, S.; Aranguren, M.I.; Marcovich, N.E. Characterization of nanocellulose- reinforced shape memory polyurethanes. Polym. Int. 2008, 57, 651–659. [Google Scholar] [CrossRef]
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M. The Future Prospects of Microbial Cellulose in Biomedical Applications. Biomacromolecules 2007, 8, 1–12. [Google Scholar] [CrossRef]
- Kamel, S. Pharmaceutical significance of cellulose: A review. Express Polym. Lett. 2008, 2, 758–778. [Google Scholar] [CrossRef]
- Ullah, H.; Santos, H.A.; Khan, T. Applications of bacterial cellulose in food, cosmetics and drug delivery. Cellulose 2016, 23, 2291–2314. [Google Scholar] [CrossRef]
- Mozafari, M.R. (Ed.) Nanocarrier Technologies: Frontiers of Nanotherapy; Springer Netherlands: Dordrecht, The Netherlands, 2006; ISBN 978-1-4020-5040-4. [Google Scholar]
- Pachuau, L. Application of Nanocellulose for Controlled Drug Delivery. In Nanocellulose and Nanohydrogel Matrices; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 1–19. ISBN 978-3-527-80383-5. [Google Scholar]
- Moscovici, M.; Hlevca, C.; Casarica, A.; Pavaloiu, R.-D. Nanocellulose and Nanogels as Modern Drug Delivery Systems. In Nanocellulose and Nanohydrogel Matrices; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 209–269. ISBN 978-3-527-80383-5. [Google Scholar]
- Andresen, M.; Stenstad, P.; Møretrø, T.; Langsrud, S.; Syverud, K.; Johansson, L.-S.; Stenius, P. Nonleaching Antimicrobial Films Prepared from Surface-Modified Microfibrillated Cellulose. Biomacromolecules 2007, 8, 2149–2155. [Google Scholar] [CrossRef]
- Takahashi, H.; Caputo, G.A.; Vemparala, S.; Kuroda, K. Synthetic Random Copolymers as a Molecular Platform To Mimic Host-Defense Antimicrobial Peptides. Bioconjug. Chem. 2017, 28, 1340–1350. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Lin, N.; Tang, J.; Dufresne, A.; Tam, M.K.C. (Eds.) Advanced Functional Materials from Nanopolysaccharides; Springer Series in Biomaterials Science and Engineering; Springer Singapore: Singapore, 2019; ISBN 9789811509124. [Google Scholar]
- Sun, B.; Hou, Q.; Liu, Z.; Ni, Y. Sodium periodate oxidation of cellulose nanocrystal and its application as a paper wet strength additive. Cellulose 2015, 22, 1135–1146. [Google Scholar] [CrossRef]
- Salam, A.; Lucia, L.A.; Jameel, H. Fluorine-based surface decorated cellulose nanocrystals as potential hydrophobic and oleophobic materials. Cellulose 2015, 22, 397–406. [Google Scholar] [CrossRef]
- Huang, J.-L.; Li, C.-J.; Gray, D.G. Functionalization of cellulose nanocrystal films via “thiol–ene” click reaction. RSC Adv. 2014, 4, 6965–6969. [Google Scholar] [CrossRef]
- Hemraz, U.D.; Campbell, K.A.; Burdick, J.S.; Ckless, K.; Boluk, Y.; Sunasee, R. Cationic Poly(2-aminoethylmethacrylate) and Poly(N-(2-aminoethylmethacrylamide) Modified Cellulose Nanocrystals: Synthesis, Characterization, and Cytotoxicity. Biomacromolecules 2015, 16, 319–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorfi, M.; Foster, E.J. Recent advances in nanocellulose for biomedical applications. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Čolić, M.; Tomić, S.; Bekić, M. Immunological aspects of nanocellulose. Immunol. Lett. 2020, 222, 80–89. [Google Scholar] [CrossRef]
- Čolić, M.; Mihajlović, D.; Mathew, A.; Naseri, N.; Kokol, V. Cytocompatibility and immunomodulatory properties of wood based nanofibrillated cellulose. Cellulose 2015, 22, 763–778. [Google Scholar] [CrossRef]
- Tomić, S.; Kokol, V.; Mihajlović, D.; Mirčić, A.; Čolić, M. Native cellulose nanofibrills induce immune tolerance in vitro by acting on dendritic cells. Sci. Rep. 2016, 6, 31618. [Google Scholar] [CrossRef]
- Tomić, S.; Ilić, N.; Kokol, V.; Gruden-Movsesijan, A.; Mihajlović, D.; Bekić, M.; Sofronić-Milosavljević, L.; Čolić, M.; Vučević, D. Functionalization-dependent effects of cellulose nanofibrils on tolerogenic mechanisms of human dendritic cells. Int. J. Nanomed. 2018, 13, 6941–6960. [Google Scholar] [CrossRef] [Green Version]
- Namvar, F.; Jawaid, M.; Tanir, P.M.; Mohamad, R.; Azizi, S.; Khodavandi, A.; Rahman, H.S.; Nayeri, M.D. Potential Use of Plant Fibres and their Composites for Biomedical Applications. BioResources 2014, 9, 5688–5706. [Google Scholar] [CrossRef]
- Pickering, K.L.; Efendy, M.G.A.; Le, T.M. A review of recent developments in natural fibre composites and their mechanical performance. Compos. Part A Appl. Sci. Manuf. 2016, 83, 98–112. [Google Scholar] [CrossRef] [Green Version]
- Cheung, H.; Ho, M.; Lau, K.; Cardona, F.; Hui, D. Natural fibre-reinforced composites for bioengineering and environmental engineering applications. Compos. Part B Eng. 2009, 40, 655–663. [Google Scholar] [CrossRef]
- Jia, B.; Li, Y.; Yang, B.; Xiao, D.; Zhang, S.; Rajulu, A.V.; Kondo, T.; Zhang, L.; Zhou, J. Effect of microcrystal cellulose and cellulose whisker on biocompatibility of cellulose-based electrospun scaffolds. Cellulose 2013, 20, 1911–1923. [Google Scholar] [CrossRef]
- Shi, Z.; Phillips, G.O.; Yang, G. Nanocellulose electroconductive composites. Nanoscale 2013, 5, 3194–3201. [Google Scholar] [CrossRef]
- Li, J.; Cha, R.; Mou, K.; Zhao, X.; Long, K.; Luo, H.; Zhou, F.; Jiang, X. Nanocellulose-Based Antibacterial Materials. Adv. Healthc. Mater. 2018, 7, 1800334. [Google Scholar] [CrossRef]
- Maneerung, T.; Tokura, S.; Rujiravanit, R. Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohydr. Polym. 2008, 72, 43–51. [Google Scholar] [CrossRef]
- Luan, J.; Wu, J.; Zheng, Y.; Song, W.; Wang, G.; Guo, J.; Ding, X. Impregnation of silver sulfadiazine into bacterial cellulose for antimicrobial and biocompatible wound dressing. Biomed. Mater. 2012, 7, 065006. [Google Scholar] [CrossRef]
- Liu, C.; Yang, D.; Wang, Y.; Shi, J.; Jiang, Z. Fabrication of antimicrobial bacterial cellulose–Ag/AgCl nanocomposite using bacteria as versatile biofactory. J. Nanopart. Res. 2012, 14, 1084. [Google Scholar] [CrossRef]
- Xiong, R.; Lu, C.; Zhang, W.; Zhou, Z.; Zhang, X. Facile synthesis of tunable silver nanostructures for antibacterial application using cellulose nanocrystals. Carbohydr. Polym. 2013, 95, 214–219. [Google Scholar] [CrossRef]
- Fortunati, E.; Rinaldi, S.; Peltzer, M.; Bloise, N.; Visai, L.; Armentano, I.; Jiménez, A.; Latterini, L.; Kenny, J.M. Nano-biocomposite films with modified cellulose nanocrystals and synthesized silver nanoparticles. Carbohydr. Polym. 2014, 101, 1122–1133. [Google Scholar] [CrossRef] [Green Version]
- Gan, I.; Chow, W.S. Antimicrobial poly(lactic acid)/cellulose bionanocomposite for food packaging application: A review. Food Packaging and Shelf Life 2018, 17, 150–161. [Google Scholar] [CrossRef]
- Jipa, I.M.; Stoica-Guzun, A.; Stroescu, M. Controlled release of sorbic acid from bacterial cellulose based mono and multilayer antimicrobial films. LWT 2012, 47, 400–406. [Google Scholar] [CrossRef]
- Fernandes, S.C.M.; Sadocco, P.; Alonso-Varona, A.; Palomares, T.; Eceiza, A.; Silvestre, A.J.D.; Mondragon, I.; Freire, C.S.R. Bioinspired Antimicrobial and Biocompatible Bacterial Cellulose Membranes Obtained by Surface Functionalization with Aminoalkyl Groups. ACS Appl. Mater. Interfaces 2013, 5, 3290–3297. [Google Scholar] [CrossRef]
- Butchosa, N.; Brown, C.; Larsson, P.T.; Berglund, L.A.; Bulone, V.; Zhou, Q. Nanocomposites of bacterial cellulose nanofibers and chitin nanocrystals: Fabrication, characterization and bactericidal activity. Green Chem. 2013, 15, 3404–3413. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, P.; Zhan, Y.; Shi, X.; Lin, J.; Du, Y.; Li, X.; Deng, H. Pectin/lysozyme bilayers layer-by-layer deposited cellulose nanofibrous mats for antibacterial application. Carbohydr. Polym. 2015, 117, 687–693. [Google Scholar] [CrossRef]
- Shao, W.; Liu, H.; Liu, X.; Wang, S.; Zhang, R. Anti-bacterial performances and biocompatibility of bacterial cellulose/graphene oxide composites. RSC Adv. 2014, 5, 4795–4803. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, L.; Cao, Z.; Deng, Y.; Liu, L.; Fong, H.; Sun, Y. Electrospun composite nanofiber fabrics containing uniformly dispersed antimicrobial agents as an innovative type of polymeric materials with superior antimicrobial efficacy. ACS Appl. Mater. Interfaces 2010, 2, 952–956. [Google Scholar] [CrossRef]
- Gomez-Orellana, I. Strategies to improve oral drug bioavailability. Expert Opin. Drug Deliv. 2005, 2, 419–433. [Google Scholar] [CrossRef]
- Boddupalli, B.M.; Mohammed, Z.N.K.; Nath, R.A.; Banji, D. Mucoadhesive drug delivery system: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 381–387. [Google Scholar] [CrossRef] [Green Version]
- Smart, J.D. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 2005, 57, 1556–1568. [Google Scholar] [CrossRef]
- Shaikh, R.; Raj Singh, T.R.; Garland, M.J.; Woolfson, A.D.; Donnelly, R.F. Mucoadhesive drug delivery systems. J. Pharm. Bioallied Sci. 2011, 3, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Verma, R.; Garcia-Contreras, L. Inhalation drug delivery devices: Technology update. Med. Devices 2015, 8, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Peterfreund, R.A.; Philip, J.H. Critical parameters in drug delivery by intravenous infusion. Expert Opin. Drug Deliv. 2013, 10, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Van Smeden, J.; Janssens, M.; Gooris, G.S.; Bouwstra, J.A. The important role of stratum corneum lipids for the cutaneous barrier function. Biochim. Biophys. Acta 2014, 1841, 295–313. [Google Scholar] [CrossRef]
- Maher, S.; Brayden, D.J.; Casettari, L.; Illum, L. Application of Permeation Enhancers in Oral Delivery of Macromolecules: An Update. Pharmaceutics 2019, 11, 41. [Google Scholar] [CrossRef] [Green Version]
- Lane, M.E. Skin penetration enhancers. Int. J. Pharm. 2013, 447, 12–21. [Google Scholar] [CrossRef]
- Tran, M.; Wang, C. Semi-solid materials for controlled release drug formulation: Current status and future prospects. Front. Chem. Sci. Eng. 2014, 8, 225–232. [Google Scholar] [CrossRef]
- Ioelovich, M. Chapter 9 - Nanocellulose—Fabrication, structure, properties, and application in the area of care and cure. In Fabrication and Self-Assembly of Nanobiomaterials; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 243–288. ISBN 978-0-323-41533-0. [Google Scholar]
- Börjesson, M.; Westman, G. Crystalline Nanocellulose—Preparation, Modification, and Properties. Cellulose: Fundamental Aspects and Current Trends; IntechOpen: London, UK, 2015. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.; Hou, J.; Chang, P.R.; Huang, J. Structure and Properties of Cellulose Nanocrystals. In Nanocellulose: From Fundamentals to Advanced Materials; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 21–52. ISBN 978-3-527-80743-7. [Google Scholar]
- Daud, J.B.; Lee, K.-Y. Surface Modification of Nanocellulose. In Handbook of Nanocellulose and Cellulose Nanocomposites; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 101–122. ISBN 978-3-527-68997-2. [Google Scholar]
- Islam, M.T.; Alam, M.M.; Zoccola, M. Review on modification of nanocellulose for application in composites. Int. J. Inov. Res. Sci. Eng. Technol. 2013, 2, 5444–5451. [Google Scholar]
- Tan, T.H.; Lee, H.V.; Yehya Dabdawb, W.A.; Hamid, S.B.B.O.A.A. Chapter 5 - A review of nanocellulose in the drug-delivery system. In Materials for Biomedical Engineering; Holban, A.-M., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 131–164. ISBN 978-0-12-816913-1. [Google Scholar]
- Kolakovic, R.; Peltonen, L.; Laukkanen, A.; Hirvonen, J.; Laaksonen, T. Nanofibrillar cellulose films for controlled drug delivery. Eur. J. Pharm. Biopharm. 2012, 82, 308–315. [Google Scholar] [CrossRef]
- Salimi, S.; Sotudeh-Gharebagh, R.; Zarghami, R.; Chan, S.Y.; Yuen, K.H. Production of Nanocellulose and Its Applications in Drug Delivery: A Critical Review. ACS Sustain. Chem. Eng. 2019, 7, 15800–15827. [Google Scholar] [CrossRef]
- Plackett, D.V.; Letchford, K.; Jackson, J.K.; Burt, H.M. A review of nanocellulose as a novel vehicle for drug delivery. Nord. Pulp Paper Res. J. 2014, 29, 105–118. [Google Scholar] [CrossRef]
- Kiliona, K.P.S.; Lwal, A.L.J.; Tao, H.; Lin, N. Surface Modification with Grafting Functional Molecules on Nanopolysaccharides. In Advanced Functional Materials from Nanopolysaccharides; Lin, N., Tang, J., Dufresne, A., Tam, M.K.C., Eds.; Springer Series in Biomaterials Science and Engineering; Springer: Singapore, 2019; pp. 55–85. ISBN 9789811509131. [Google Scholar]
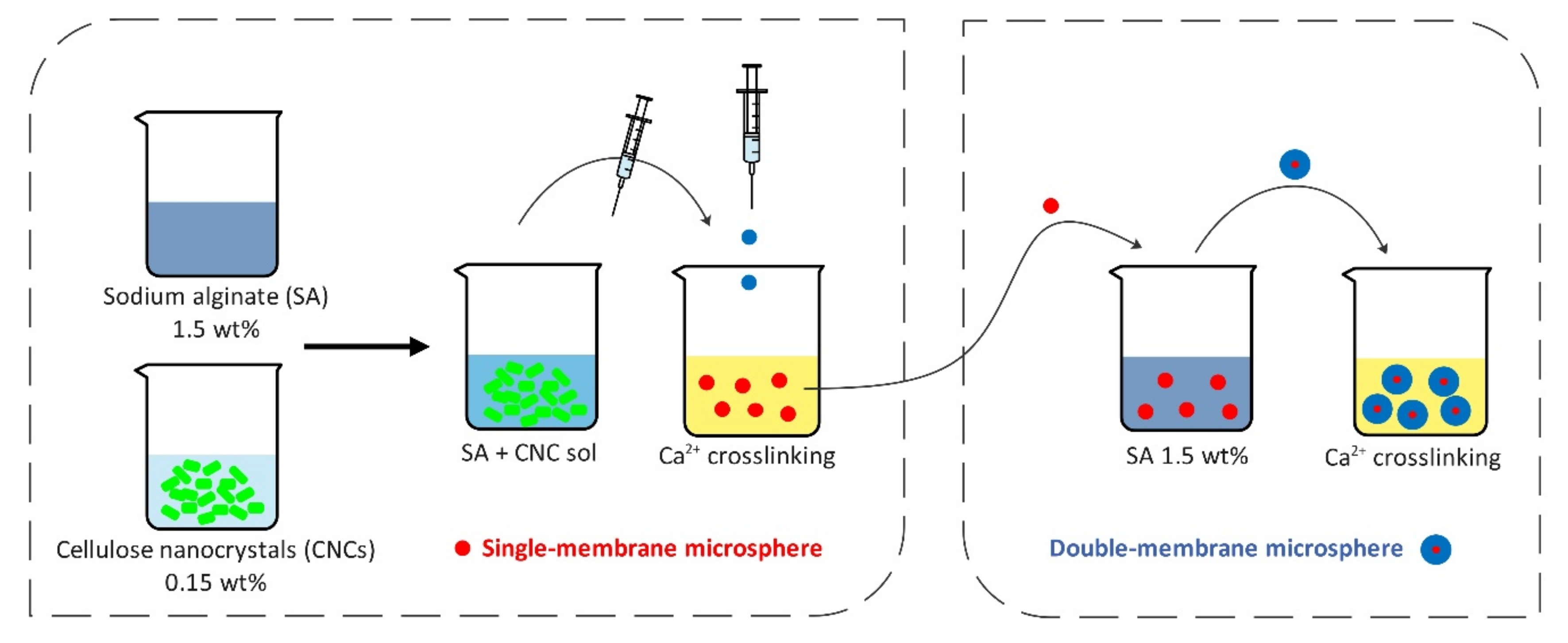
- Lin, N.; Gèze, A.; Wouessidjewe, D.; Huang, J.; Dufresne, A. Biocompatible Double-Membrane Hydrogels from Cationic Cellulose Nanocrystals and Anionic Alginate as Complexing Drugs Codelivery. ACS Appl. Mater. Interfaces 2016, 8, 6880–6889. [Google Scholar] [CrossRef]
- Ndong Ntoutoume, G.M.A.; Granet, R.; Mbakidi, J.P.; Brégier, F.; Léger, D.Y.; Fidanzi-Dugas, C.; Lequart, V.; Joly, N.; Liagre, B.; Chaleix, V.; et al. Development of curcumin–cyclodextrin/cellulose nanocrystals complexes: New anticancer drug delivery systems. Bioorganic Med. Chem. Lett. 2016, 26, 941–945. [Google Scholar] [CrossRef]
- You, J.; Cao, J.; Zhao, Y.; Zhang, L.; Zhou, J.; Chen, Y. Improved Mechanical Properties and Sustained Release Behavior of Cationic Cellulose Nanocrystals Reinforeced Cationic Cellulose Injectable Hydrogels. Biomacromolecules 2016, 17, 2839–2848. [Google Scholar] [CrossRef] [PubMed]
- Golshan, M.; Salami-Kalajahi, M.; Roghani-Mamaqani, H.; Mohammadi, M. Poly(propylene imine) dendrimer-grafted nanocrystalline cellulose: Doxorubicin loading and release behavior. Polymer 2017, 117, 287–294. [Google Scholar] [CrossRef] [Green Version]
- Gorgieva, S.; Vivod, V.; Maver, U.; Gradišnik, L.; Dolenšek, J.; Kokol, V. Internalization of (bis)phosphonate-modified cellulose nanocrystals by human osteoblast cells. Cellulose 2017, 24, 4235–4252. [Google Scholar] [CrossRef] [Green Version]
- Supramaniam, J.; Adnan, R.; Mohd Kaus, N.H.; Bushra, R. Magnetic nanocellulose alginate hydrogel beads as potential drug delivery system. Int. J. Biol. Macromol. 2018, 118, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Tummala, G.K. Hydrogels of Poly(vinyl alcohol) and Nanocellulose for Ophthalmic Applications: Synthesis, Characterization, Biocompatibility and Drug Delivery Studies. Ph.D. Thesis, Acta Universitatis Upsaliensis, Uppsala, Sweden, 2018. [Google Scholar]
- Md Abu, T.; Zahan, K.A.; Rajaie, M.A.; Leong, C.R.; Ab Rashid, S.; Mohd Nor Hamin, N.S.; Tan, W.N.; Tong, W.Y. Nanocellulose as drug delivery system for honey as antimicrobial wound dressing. Mater. Today Proc. 2020, 31, 14–17. [Google Scholar] [CrossRef]
- Zainuddin, N.; Ahmad, I.; Kargarzadeh, H.; Ramli, S. Hydrophobic kenaf nanocrystalline cellulose for the binding of curcumin. Carbohydr. Polym. 2017, 163, 261–269. [Google Scholar] [CrossRef]
- Tong, W.Y.; bin Abdullah, A.Y.K.; binti Rozman, N.A.S.; bin Wahid, M.I.A.; Hossain, M.S.; Ring, L.C.; Lazim, Y.; Tan, W.-N. Antimicrobial wound dressing film utilizing cellulose nanocrystal as drug delivery system for curcumin. Cellulose 2018, 25, 631–638. [Google Scholar] [CrossRef]
- Udeni Gunathilake, T.M.S.; Ching, Y.C.; Chuah, C.H. Enhancement of Curcumin Bioavailability Using Nanocellulose Reinforced Chitosan Hydrogel. Polymers 2017, 9, 64. [Google Scholar] [CrossRef] [Green Version]
- Sampath Udeni Gunathilake, T.M.; Ching, Y.C.; Chuah, C.H.; Illias, H.A.; Ching, K.Y.; Singh, R.; Nai-Shang, L. Influence of a nonionic surfactant on curcumin delivery of nanocellulose reinforced chitosan hydrogel. Int. J. Biol. Macromol. 2018, 118, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Ching, Y.C.; Gunathilake, T.M.S.U.; Chuah, C.H.; Ching, K.Y.; Singh, R.; Liou, N.-S. Curcumin/Tween 20-incorporated cellulose nanoparticles with enhanced curcumin solubility for nano-drug delivery: Characterization and in vitro evaluation. Cellulose 2019, 26, 5467–5481. [Google Scholar] [CrossRef]
- Li, N.; Zhang, H.; Xiao, Y.; Huang, Y.; Xu, M.; You, D.; Lu, W.; Yu, J. Fabrication of Cellulose-Nanocrystal-Based Folate Targeted Nanomedicine via Layer-by-Layer Assembly with Lysosomal pH-Controlled Drug Release into the Nucleus. Biomacromolecules 2019, 20, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Hivechi, A.; Bahrami, S.H.; Siegel, R.A. Drug release and biodegradability of electrospun cellulose nanocrystal reinforced polycaprolactone. Mater. Sci. Eng. C 2019, 94, 929–937. [Google Scholar] [CrossRef]
- Löbmann, K.; Svagan, A.J. Cellulose nanofibers as excipient for the delivery of poorly soluble drugs. Int. J. Pharm. 2017, 533, 285–297. [Google Scholar] [CrossRef]
- Svagan, A.J.; Benjamins, J.-W.; Al-Ansari, Z.; Shalom, D.B.; Müllertz, A.; Wågberg, L.; Löbmann, K. Solid cellulose nanofiber based foams—Towards facile design of sustained drug delivery systems. J. Control. Release 2016, 244, 74–82. [Google Scholar] [CrossRef]
- Löbmann, K.; Wohlert, J.; Müllertz, A.; Wågberg, L.; Svagan, A.J. Cellulose Nanopaper and Nanofoam for Patient-Tailored Drug Delivery. Adv. Mater. Interfaces 2017, 4, 1600655. [Google Scholar] [CrossRef]
- Bhandari, J.; Mishra, H.; Mishra, P.K.; Wimmer, R.; Ahmad, F.J.; Talegaonkar, S. Cellulose nanofiber aerogel as a promising biomaterial for customized oral drug delivery. Int. J. Nanomed. 2017, 12, 2021–2031. [Google Scholar] [CrossRef] [Green Version]
- Paukkonen, H.; Ukkonen, A.; Szilvay, G.; Yliperttula, M.; Laaksonen, T. Hydrophobin-nanofibrillated cellulose stabilized emulsions for encapsulation and release of BCS class II drugs. Eur. J. Pharm. Sci. 2017, 100, 238–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svagan, A.J.; Müllertz, A.; Löbmann, K. Floating solid cellulose nanofibre nanofoams for sustained release of the poorly soluble model drug furosemide. J. Pharm. Pharmacol. 2017, 69, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, A.; Tahami, S.; Nejad, P.A. Preparation and characterization of Fe3O4-Ag2O quantum dots decorated cellulose nanofibers as a carrier of anticancer drugs for skin cancer. J. Photochem. Photobiol. B Biol. 2017, 175, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Paukkonen, H.; Kunnari, M.; Laurén, P.; Hakkarainen, T.; Auvinen, V.-V.; Oksanen, T.; Koivuniemi, R.; Yliperttula, M.; Laaksonen, T. Nanofibrillar cellulose hydrogels and reconstructed hydrogels as matrices for controlled drug release. Int. J. Pharm. 2017, 532, 269–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, T.; Pei, Y.; Tang, K.; He, X.; Huang, J.; Wang, F. Mechanical and drug release properties of alginate beads reinforced with cellulose. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Poonguzhali, R.; Khaleel Basha, S.; Sugantha Kumari, V. Synthesis of alginate/nanocellulose bionanocomposite for in vitro delivery of ampicillin. Polym. Bull. 2018, 75, 4165–4173. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Sui, Y.; Liu, C.; Liu, C.; Wu, M.; Li, B.; Li, Y. A physically crosslinked polydopamine/nanocellulose hydrogel as potential versatile vehicles for drug delivery and wound healing. Carbohydr. Polym. 2018, 188, 27–36. [Google Scholar] [CrossRef]
- Sarkar, G.; Orasugh, J.T.; Saha, N.R.; Roy, I.; Bhattacharyya, A.; Chattopadhyay, A.K.; Rana, D.; Chattopadhyay, D. Cellulose nanofibrils/chitosan based transdermal drug delivery vehicle for controlled release of ketorolac tromethamine. New J. Chem. 2017, 41, 15312–15319. [Google Scholar] [CrossRef]
- Orasugh, J.T.; Saha, N.R.; Rana, D.; Sarkar, G.; Mollick, M.M.R.; Chattoapadhyay, A.; Mitra, B.C.; Mondal, D.; Ghosh, S.K.; Chattopadhyay, D. Jute cellulose nano-fibrils/hydroxypropylmethylcellulose nanocomposite: A novel material with potential for application in packaging and transdermal drug delivery system. Ind. Crops Prod. 2018, 112, 633–643. [Google Scholar] [CrossRef]
- Auvinen, V.-V.; Virtanen, J.; Merivaara, A.; Virtanen, V.; Laurén, P.; Tuukkanen, S.; Laaksonen, T. Modulating sustained drug release from nanocellulose hydrogel by adjusting the inner geometry of implantable capsules. J. Drug Deliv. Sci. Technol. 2020, 57, 101625. [Google Scholar] [CrossRef]
- Gun’ko, V.M.; Savina, I.N.; Mikhalovsky, S.V. Cryogels: Morphological, structural and adsorption characterisation. Adv. Colloid Interface Sci. 2013, 187–188, 1–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Wang, Y.; Zhang, L.; Xu, Z.; Dai, H.; Wu, W. Nanocellulose/Gelatin Composite Cryogels for Controlled Drug Release. ACS Sustain. Chem. Eng. 2019, 7, 6381–6389. [Google Scholar] [CrossRef]
- Meneguin, A.B.; Ferreira Cury, B.S.; dos Santos, A.M.; Franco, D.F.; Barud, H.S.; da Silva Filho, E.C. Resistant starch/pectin free-standing films reinforced with nanocellulose intended for colonic methotrexate release. Carbohydr. Polym. 2017, 157, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Fiorati, A.; Turco, G.; Travan, A.; Caneva, E.; Pastori, N.; Cametti, M.; Punta, C.; Melone, L. Mechanical and Drug Release Properties of Sponges from Cross-linked Cellulose Nanofibers. ChemPlusChem 2017, 82, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Fiorati, A.; Contessi Negrini, N.; Baschenis, E.; Altomare, L.; Faré, S.; Giacometti Schieroni, A.; Piovani, D.; Mendichi, R.; Ferro, M.; Castiglione, F.; et al. TEMPO-Nanocellulose/Ca2+ Hydrogels: Ibuprofen Drug Diffusion and In Vitro Cytocompatibility. Materials 2020, 13, 183. [Google Scholar] [CrossRef] [Green Version]
- Alkhatib, Y.; Dewaldt, M.; Moritz, S.; Nitzsche, R.; Kralisch, D.; Fischer, D. Controlled extended octenidine release from a bacterial nanocellulose/Poloxamer hybrid system. Eur. J. Pharm. Biopharm. 2017, 112, 164–176. [Google Scholar] [CrossRef]
- Medhi, P.; Olatunji, O.; Nayak, A.; Uppuluri, C.T.; Olsson, R.T.; Nalluri, B.N.; Das, D.B. Lidocaine-loaded fish scale-nanocellulose biopolymer composite microneedles. AAPS PharmSciTech 2017, 18, 1488–1494. [Google Scholar] [CrossRef]
- Saïdi, L.; Vilela, C.; Oliveira, H.; Silvestre, A.J.D.; Freire, C.S.R. Poly(N-methacryloyl glycine)/nanocellulose composites as pH-sensitive systems for controlled release of diclofenac. Carbohydr. Polym. 2017, 169, 357–365. [Google Scholar] [CrossRef]
- Ratnayake, W.M.K.M.; Damunupola, J.W.; Rajapakse, S.; Jayasundera, A.C.A. Nanocellulose-Protein Matrices: A Model System for Controlled Drug Delivery. In Proceedings of the 5th International Conference on Nanoscience and Nanotechnology, Dubai, UAE, 16 October 2017; 2018; Volume 5, pp. 1–13. [Google Scholar]
- Fey, C.; Betz, J.; Rosenbaum, C.; Kralisch, D.; Vielreicher, M.; Friedrich, O.; Metzger, M.; Zdzieblo, D. Bacterial nanocellulose as novel carrier for intestinal epithelial cells in drug delivery studies. Mater. Sci. Eng. C 2020, 109, 110613. [Google Scholar] [CrossRef]
- Silva, N.H.C.S.; Mota, J.P.; Santos de Almeida, T.; Carvalho, J.P.F.; Silvestre, A.J.D.; Vilela, C.; Rosado, C.; Freire, C.S.R. Topical Drug Delivery Systems Based on Bacterial Nanocellulose: Accelerated Stability Testing. Int. J. Mol. Sci. 2020, 21, 1262. [Google Scholar] [CrossRef] [Green Version]
- Abba, M.; Ibrahim, Z.; Chong, C.S.; Zawawi, N.A.; Kadir, M.R.A.; Yusof, A.H.M.; Razak, S.I.A. Transdermal Delivery of Crocin Using Bacterial Nanocellulose Membrane. Fibers Polym. 2019, 20, 2025–2031. [Google Scholar] [CrossRef]
- Alavizadeh, S.H.; Hosseinzadeh, H. Bioactivity assessment and toxicity of crocin: A comprehensive review. Food Chem. Toxicol. 2014, 64, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Knapp, J.S.; Guthrie, J.T.; Perrier, S. Antibacterial cellulose fiber via RAFT surface graft polymerization. Biomacromolecules 2008, 9, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Yan, T.; Du, J.; He, F.; Luo, H.; Wan, Y. Introduction of broad spectrum antibacterial properties to bacterial cellulose nanofibers via immobilising ε-polylysine nanocoatings. Food Hydrocoll. 2014, 36, 204–211. [Google Scholar] [CrossRef]
- Panchal, P.; Ogunsona, E.; Mekonnen, T. Trends in Advanced Functional Material Applications of Nanocellulose. Processes 2019, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Appendini, P.; Hotchkiss, J.H. Review of antimicrobial food packaging. Innov. Food Sci. Emerg. Technol. 2002, 3, 113–126. [Google Scholar] [CrossRef]
- Gudikandula, K.; Maringanti, S.C. Synthesis of silver nanoparticles by chemical and biological methods and their antimicrobial properties. J. Exp. Nanosci. 2016, 11, 714–721. [Google Scholar] [CrossRef]
- Panyala, N.R.; Pena, E.M.; Havel, J. Silver or silver nanoparticles: A hazardous threat to the environment and human health? J. Appl. Biomed. 2008, 6, 117–129. [Google Scholar] [CrossRef] [Green Version]
- Cho, K.-H.; Park, J.-E.; Osaka, T.; Park, S.-G. The study of antimicrobial activity and preservative effects of nanosilver ingredient. Electrochim. Acta 2005, 51, 956–960. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, K.-Y.; Byeon, J.H.; Park, J.-H.; Ji, J.H.; Bae, G.N.; Hwang, J. Antimicrobial Characteristics of Silver Aerosol Nanoparticles against Bacillus subtilis Bioaerosols. Environ. Eng. Sci. 2008, 25, 289–294. [Google Scholar] [CrossRef] [Green Version]
- Shrivastava, S.; Bera, T.; Roy, A.; Singh, G.; Ramachandrarao, P.; Dash, D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 2007, 18, 225103. [Google Scholar] [CrossRef]
- Panáček, A.; Kvítek, L.; Prucek, R.; Kolář, M.; Večeřová, R.; Pizúrová, N.; Sharma, V.K.; Nevěčná, T.; Zbořil, R. Silver Colloid Nanoparticles: Synthesis, Characterization, and Their Antibacterial Activity. J. Phys. Chem. B 2006, 110, 16248–16253. [Google Scholar] [CrossRef]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef]
- Barud, H.; Regiani, T.; Marques, R.; Lustri, W.; Messaddeq, Y.; Ribeiro, S. Antimicrobial Bacterial Cellulose-Silver Nanoparticles Composite Membranes. J. Nanomater. 2011, 2011, 721631. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Xie, J.; Hong, F.; Cao, Z.; Yang, X. Antimicrobial activity of silver nanoparticle impregnated bacterial cellulose membrane: Effect of fermentation carbon sources of bacterial cellulose. Carbohydr. Polym. 2012, 87, 839–845. [Google Scholar] [CrossRef]
- Amini, E.; Azadfallah, M.; Layeghi, M.; Talaei-Hassanloui, R. Silver-nanoparticle-impregnated cellulose nanofiber coating for packaging paper. Cellulose 2016, 23, 557–570. [Google Scholar] [CrossRef]
- Mohite, B.V.; Patil, S.V. In situ development of nanosilver-impregnated bacterial cellulose for sustainable released antimicrobial wound dressing. J. Appl. Biomater. Funct. Mater. 2016, 14, e53–e58. [Google Scholar] [CrossRef] [Green Version]
- Sygnatowicz, M.; Keyshar, K.; Tiwari, A. Antimicrobial properties of silver-doped hydroxyapatite nano-powders and thin films. JOM 2010, 62, 65–70. [Google Scholar] [CrossRef]
- Joshy, M.I.A.; Elayaraja, K.; Sakthivel, N.; Chandra, V.S.; Shanthini, G.M.; Kalkura, S.N. Freeze dried cross linking free biodegradable composites with microstructures for tissue engineering and drug delivery application. Mater. Sci. Eng. C 2013, 33, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Dubnika, A.; Loca, D.; Rudovica, V.; Parekh, M.B.; Berzina-Cimdina, L. Functionalized silver doped hydroxyapatite scaffolds for controlled simultaneous silver ion and drug delivery. Ceram. Int. 2017, 43, 3698–3705. [Google Scholar] [CrossRef]
- Yadollahi, M.; Farhoudian, S.; Namazi, H. One-pot synthesis of antibacterial chitosan/silver bio-nanocomposite hydrogel beads as drug delivery systems. Int. J. Biol. Macromol. 2015, 79, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, S.I.; Kwon, I.B.; Kim, M.H.; Lim, J.I. Simple fabrication of silver hybridized porous chitosan-based patch for transdermal drug-delivery system. Mater. Lett. 2013, 95, 48–51. [Google Scholar] [CrossRef]
- Tran, C.; Prosenc, F.; Franko, M.; Benzi, G. One-Pot Synthesis of Biocompatible Silver Nanoparticle Composites from Cellulose and Keratin: Characterization and Antimicrobial Activity. ACS Appl. Mater. Interfaces 2016, 8, 34791–34801. [Google Scholar] [CrossRef] [Green Version]
- Dallas, P.; Sharma, V.K.; Zboril, R. Silver polymeric nanocomposites as advanced antimicrobial agents: Classification, synthetic paths, applications, and perspectives. Adv. Colloid Interface Sci. 2011, 166, 119–135. [Google Scholar] [CrossRef]
- Lizundia, E.; Goikuria, U.; Vilas, J.L.; Cristofaro, F.; Bruni, G.; Fortunati, E.; Armentano, I.; Visai, L.; Torre, L. Metal Nanoparticles Embedded in Cellulose Nanocrystal Based Films: Material Properties and Post-use Analysis. Biomacromolecules 2018, 19, 2618–2628. [Google Scholar] [CrossRef]
- Rasmussen, J.W.; Martinez, E.; Louka, P.; Wingett, D.G. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin. Drug Deliv. 2010, 7, 1063–1077. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Q.; Hein, S.; Misra, R.D.K. New generation of chitosan-encapsulated ZnO quantum dots loaded with drug: Synthesis, characterization and in vitro drug delivery response. Acta Biomater. 2010, 6, 2732–2739. [Google Scholar] [CrossRef]
- Barick, K.C.; Nigam, S.; Bahadur, D. Nanoscale assembly of mesoporous ZnO: A potential drug carrier. J. Mater. Chem. 2010, 20, 6446–6452. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, C.; Chen, B.; Wang, X. Daunorubicin-TiO2 nanocomposites as a “smart” pH-responsive drug delivery system. Int. J. Nanomed. 2012, 7, 235–242. [Google Scholar] [CrossRef]
- Xiong, H.-M. ZnO Nanoparticles Applied to Bioimaging and Drug Delivery. Adv. Mater. 2013, 25, 5329–5335. [Google Scholar] [CrossRef] [PubMed]
- El-Wakil, N.A.; Hassan, E.A.; Abou-Zeid, R.E.; Dufresne, A. Development of wheat gluten/nanocellulose/titanium dioxide nanocomposites for active food packaging. Carbohydr. Polym. 2015, 124, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Galkina, O.L.; Önneby, K.; Huang, P.; Ivanov, V.K.; Agafonov, A.V.; Seisenbaeva, G.A.; Kessler, V.G. Antibacterial and photochemical properties of cellulose nanofiber–titania nanocomposites loaded with two different types of antibiotic medicines. J. Mater. Chem. B 2015, 3, 7125–7134. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Das, G.; Aidew, L.; Buragohain, A.K.; Karak, N. Copper–copper oxide coated nanofibrillar cellulose: A promising biomaterial. RSC Adv. 2013, 3, 14997–15004. [Google Scholar] [CrossRef]
- Lefatshe, K.; Muiva, C.M.; Kebaabetswe, L.P. Extraction of nanocellulose and in-situ casting of ZnO/cellulose nanocomposite with enhanced photocatalytic and antibacterial activity. Carbohydr. Polym. 2017, 164, 301–308. [Google Scholar] [CrossRef]
- Martins, N.C.T.; Freire, C.S.R.; Neto, C.P.; Silvestre, A.J.D.; Causio, J.; Baldi, G.; Sadocco, P.; Trindade, T. Antibacterial paper based on composite coatings of nanofibrillated cellulose and ZnO. Colloids Surf. A Physicochem. Eng. Asp. 2013, 417, 111–119. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Khattak, W.A.; Ullah, M.W.; Khan, S.; Park, J.K. Synthesis of regenerated bacterial cellulose-zinc oxide nanocomposite films for biomedical applications. Cellulose 2014, 21, 433–447. [Google Scholar] [CrossRef]
- Katepetch, C.; Rujiravanit, R.; Tamura, H. Formation of nanocrystalline ZnO particles into bacterial cellulose pellicle by ultrasonic-assisted in situ synthesis. Cellulose 2013, 20, 1275–1292. [Google Scholar] [CrossRef]
- Mirtalebi, S.S.; Almasi, H.; Alizadeh Khaledabad, M. Physical, morphological, antimicrobial and release properties of novel MgO-bacterial cellulose nanohybrids prepared by in-situ and ex-situ methods. Int. J. Biol. Macromol. 2019, 128, 848–857. [Google Scholar] [CrossRef]
- Wang, J.; Vermerris, W. Antimicrobial Nanomaterials Derived from Natural Products—A Review. Materials 2016, 9, 255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilela, C.; Moreirinha, C.; Domingues, E.M.; Figueiredo, F.M.L.; Almeida, A.; Freire, C.S.R. Antimicrobial and Conductive Nanocellulose-Based Films for Active and Intelligent Food Packaging. Nanomaterials 2019, 9, 980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Wu, Q.; Zhang, X.; Ren, S.; Lei, T.; Li, W.; Xu, G.; Zhang, Q. Nanocellulose films with combined cellulose nanofibers and nanocrystals: Tailored thermal, optical and mechanical properties. Cellulose 2018, 25, 1103–1115. [Google Scholar] [CrossRef]
- Halib, N.; Ahmad, I. Nanocellulose: Insight into health and medical applications. In Handbook of Ecomaterials; Springer International Publishing: Cham, Switzerland, 2019; pp. 1345–1363. [Google Scholar] [CrossRef]
- Bielecki, S.; Kalinowska, H.; Krystynowicz, A.; Kubiak, K.; Kołodziejczyk, M.; De Groeve, M. Wound dressings and cosmetic materials from bacterial nanocellulose. In Bacterial NanoCellulose: A Sophisticated Multifunctional Material; CRC Press: Boca Raton, FL, USA, 2016; pp. 157–174. [Google Scholar]
- Ioelovich, M.; Figovsky, O. Nano-cellulose as Promising Biocarrier. J. Mater. Res. 2008, 47–50, 1286–1289. [Google Scholar] [CrossRef]
- Ludwicka, K.; Jedrzejczak-Krzepkowska, M.; Kubiak, K.; Kolodziejczyk, M.; Pankiewicz, T.; Bielecki, S. Chapter 9 - Medical and Cosmetic Applications of Bacterial NanoCellulose. In Bacterial Nanocellulose; Gama, M., Dourado, F., Bielecki, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 145–165. ISBN 978-0-444-63458-0. [Google Scholar]
- Dumanli, A.G. Nanocellulose and its Composites for Biomedical Applications. Curr. Med. Chem. 2017, 24, 512–528. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Yang, G.; Hong, F. Preparation and evaluation of a kind of bacterial cellulose dry films with antibacterial properties. Carbohydr. Polym. 2011, 84, 533–538. [Google Scholar] [CrossRef]
- Wiegand, C.; Moritz, S.; Hessler, N.; Kralisch, D.; Wesarg, F.; Müller, F.A.; Fischer, D.; Hipler, U.-C. Antimicrobial functionalization of bacterial nanocellulose by loading with polihexanide and povidone-iodine. J. Mater. Sci. Mater. Med. 2015, 26, 245. [Google Scholar] [CrossRef]
- Okabe, K.; Kimura, H.; Okabe, J.; Kato, A.; Shimizu, H.; Ueda, T.; Shimada, S.; Ogura, Y. Effect of Benzalkonium Chloride on Transscleral Drug Delivery. Investig. Ophthalmol. Vis. Sci. 2005, 46, 703–708. [Google Scholar] [CrossRef] [Green Version]
- Johannsdottir, S.; Jansook, P.; Stefansson, E.; Kristinsdottir, I.M.; Asgrimsdottir, G.M.; Loftsson, T. Topical drug delivery to the posterior segment of the eye: The effect of benzalkonium chloride on topical dexamethasone penetration into the eye in vivo. J. Drug Deliv. Sci. Technol. 2018, 48, 125–127. [Google Scholar] [CrossRef]
- Lam, H.T.; Le-Vinh, B.; Phan, T.N.Q.; Bernkop-Schnürch, A. Self-emulsifying drug delivery systems and cationic surfactants: Do they potentiate each other in cytotoxicity? J. Pharm. Pharmacol. 2019, 71, 156–166. [Google Scholar] [CrossRef]
- Garcia-Fernandez, M.J.; Brackman, G.; Coenye, T.; Concheiro, A.; Alvarez-Lorenzo, C. Antiseptic cyclodextrin-functionalized hydrogels and gauzes for loading and delivery of benzalkonium chloride. Biofouling 2013, 29, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I.; Badawy, M.E.-T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Abral, H.; Ariksa, J.; Mahardika, M.; Handayani, D.; Aminah, I.; Sandrawati, N.; Pratama, A.B.; Fajri, N.; Sapuan, S.M.; Ilyas, R.A. Transparent and antimicrobial cellulose film from ginger nanofiber. Food Hydrocoll. 2020, 98, 105266. [Google Scholar] [CrossRef]
- Zhang, M.; Xiao, B.; Wang, H.; Han, M.K.; Zhang, Z.; Viennois, E.; Xu, C.; Merlin, D. Edible Ginger-derived Nano-lipids Loaded with Doxorubicin as a Novel Drug-delivery Approach for Colon Cancer Therapy. Mol. Ther. 2016, 24, 1783–1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baskar, V.; Selvakumar, K. Study on Improving Bioavailability ratio of anti-inflammatory compound from ginger through nano transdermal drug delivery. Asian J. Pharm. Amp Clin. Res. 2012, 5, 241–246. [Google Scholar]
- Naghsh, F. Nano drug delivery Study of Anticancer Properties on Ginger using QM/MM Methods. Orient. J. Chem. 2015, 31, 465–478. [Google Scholar] [CrossRef] [Green Version]
- Helander, I.M.; Nurmiaho-Lassila, E.-L.; Ahvenainen, R.; Rhoades, J.; Roller, S. Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria. Int. J. Food Microbiol. 2001, 71, 235–244. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [Green Version]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef]
- Sannan, T.; Kurita, K.; Iwakura, Y. Studies on chitin, 2. Effect of deacetylation on solubility. Die Makromol. Chem. 1976, 177, 3589–3600. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Aljaeid, B.M. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Dev. Ther. 2016, 10, 483–507. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E. Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohydr. Polym. 2018, 199, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quiñones, J.P.; Peniche, H.; Peniche, C. Chitosan Based Self-Assembled Nanoparticles in Drug Delivery. Polymers 2018, 10, 235. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, A. The use of mucoadhesive polymers in ocular drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1595–1639. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [Green Version]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Kravanja, G.; Primožič, M.; Knez, Ž.; Leitgeb, M. Chitosan-based (Nano)materials for Novel Biomedical Applications. Molecules 2019, 24, 1960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaliva, M.; Georgopoulou, A.; Dragatogiannis, D.A.; Charitidis, C.A.; Chatzinikolaidou, M.; Vamvakaki, M. Biodegradable Chitosan-graft-Poly(l-lactide) Copolymers For Bone Tissue Engineering. Polymers 2020, 12, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felt, O.; Buri, P.; Gurny, R. Chitosan: A unique polysaccharide for drug delivery. Drug Dev. Ind. Pharm. 1998, 24, 979–993. [Google Scholar] [CrossRef] [PubMed]
- Parhi, R. Drug delivery applications of chitin and chitosan: A review. Environ. Chem. Lett. 2020, 18, 577–594. [Google Scholar] [CrossRef]
- Naskar, S.; Kuotsu, K.; Sharma, S. Chitosan-based nanoparticles as drug delivery systems: A review on two decades of research. J. Drug Target. 2019, 27, 379–393. [Google Scholar] [CrossRef]
- Tome, L.C.; Fernandes, S.C.M.; Perez, D.S.; Sadocco, P.; Silvestre, A.J.D.; Pascoal Neto, C.; Marrucho, I.M.; Freire, C.S.R. The role of nanocellulose fibers, starch and chitosan on multipolysaccharide based films. Cellulose 2013, 20, 1807–1818. [Google Scholar] [CrossRef]
- Devlieghere, F.; Vermeulen, A.; Debevere, J. Chitosan: Antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food Microbiol. 2004, 21, 703–714. [Google Scholar] [CrossRef]
- Goy, R.C.; de Britto, D.; Assis, O.B.G. A review of the antimicrobial activity of chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, L.; Zhang, Q.; Hong, F.F. Using In situ Dynamic Cultures to Rapidly Biofabricate Fabric-Reinforced Composites of Chitosan/Bacterial Nanocellulose for Antibacterial Wound Dressings. Front. Microbiol. 2016, 7, 260. [Google Scholar] [CrossRef] [Green Version]
- Sampath, U.G.T.M.; Ching, Y.C.; Chuah, C.H.; Singh, R.; Lin, P.-C. Preparation and characterization of nanocellulose reinforced semi-interpenetrating polymer network of chitosan hydrogel. Cellulose 2017, 24, 2215–2228. [Google Scholar] [CrossRef]
- Poonguzhali, R.; Basha, S.K.; Kumari, V.S. Synthesis and characterization of chitosan-PVP-nanocellulose composites for in-vitro wound dressing application. Int. J. Biol. Macromol. 2017, 105, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.P.; Phani, A.R.; Prasad, R.G.S.V.; Sanganal, J.S.; Manali, N.; Gupta, R.; Rashmi, N.; Prabhakara, G.S.; Salins, C.P.; Sandeep, K.; et al. Polyvinylpyrrolidone oral films of enrofloxacin: Film characterization and drug release. Int. J. Pharm. 2014, 471, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Kadajji, V.G.; Betageri, G.V. Water Soluble Polymers for Pharmaceutical Applications. Polymers 2011, 3, 1972–2009. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.-D.; Wu, I.-D.; Chang, F.-C. The interaction behavior of polymer electrolytes composed of poly(vinyl pyrrolidone) and lithium perchlorate (LiClO4). Polymer 2001, 42, 555–562. [Google Scholar] [CrossRef]
- Hasan, A.; Waibhaw, G.; Tiwari, S.; Dharmalingam, K.; Shukla, I.; Pandey, L.M. Fabrication and characterization of chitosan, polyvinylpyrrolidone, and cellulose nanowhiskers nanocomposite films for wound healing drug delivery application. J. Biomed. Mater. Res. Part A 2017, 105, 2391–2404. [Google Scholar] [CrossRef]
- Moore, A. Antimicrobial Tissue Paper Could Help Fight the Spread of COVID-19. College of Natural Resources News, 30 March 2020. [Google Scholar]
- Patel, M. Nanoparticle-based Antimicrobial Paper as Spread-breaker for Coronavirus. Pap. Technol. Int. 2020, 62, 20–25. [Google Scholar]
- Tyagi, P.; Mathew, R.; Opperman, C.; Jameel, H.; Gonzalez, R.; Lucia, L.; Hubbe, M.; Pal, L. High-Strength Antibacterial Chitosan-Cellulose Nanocrystal Composite Tissue Paper. Langmuir 2019, 35, 104–112. [Google Scholar] [CrossRef]
- Patel, M. Antimicrobial Paper Embedded with Nanoparticles as Spread-Breaker for Corona Virus. J. Environ. Life Sci. 2020, 6, 001–012. [Google Scholar]
- Sundaram, J.; Pant, J.; Goudie, M.J.; Mani, S.; Handa, H. Antimicrobial and Physicochemical Characterization of Biodegradable, Nitric Oxide-Releasing Nanocellulose-Chitosan Packaging Membranes. J. Agric. Food Chem. 2016, 64, 5260–5266. [Google Scholar] [CrossRef]
- Fang, F.C. Antimicrobial actions of nitric oxide. Nitric Oxide 2012, 27, S10. [Google Scholar] [CrossRef]
- Brisbois, E.J.; Kim, M.; Wang, X.; Mohammed, A.; Major, T.C.; Wu, J.; Brownstein, J.; Xi, C.; Handa, H.; Bartlett, R.H.; et al. Improved Hemocompatibility of Multilumen Catheters via Nitric Oxide (NO) Release from S-Nitroso-N-acetylpenicillamine (SNAP) Composite Filled Lumen. ACS Appl. Mater. Interfaces 2016, 8, 29270–29279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sogut, E.; Seydim, A.C. Development of Chitosan and Polycaprolactone based active bilayer films enhanced with nanocellulose and grape seed extract. Carbohydr. Polym. 2018, 195, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Furiga, A.; Lonvaud-Funel, A.; Badet, C. In vitro study of antioxidant capacity and antibacterial activity on oral anaerobes of a grape seed extract. Food Chem. 2009, 113, 1037–1040. [Google Scholar] [CrossRef]
- Benoit, M.-A.; Baras, B.; Gillard, J. Preparation and characterization of protein-loaded poly(ε-caprolactone) microparticles for oral vaccine delivery. Int. J. Pharm. 1999, 184, 73–84. [Google Scholar] [CrossRef]
- Pitt, C.G.; Jeffcoat, A.R.; Zweidinger, R.A.; Schindler, A. Sustained drug delivery systems. I. The permeability of poly(ϵ-caprolactone), poly(DL-lactic acid), and their copolymers. J. Biomed. Mater. Res. 1979, 13, 497–507. [Google Scholar] [CrossRef]
- Sahoo, S.; Sasmal, A.; Nanda, R.; Phani, A.R.; Nayak, P.L. Synthesis of chitosan–polycaprolactone blend for control delivery of ofloxacin drug. Carbohydr. Polym. 2010, 79, 106–113. [Google Scholar] [CrossRef]
- Roemhild, K.; Wiegand, C.; Hipler, U.-C.; Heinze, T. Novel bioactive amino-functionalized cellulose nanofibers. Macromol. Rapid Commun. 2013, 34, 1767–1771. [Google Scholar] [CrossRef]
- Saini, S.; Belgacem, M.N.; Salon, M.-C.B.; Bras, J. Non leaching biomimetic antimicrobial surfaces via surface functionalisation of cellulose nanofibers with aminosilane. Cellulose 2016, 23, 795–810. [Google Scholar] [CrossRef]
- He, W.; Zhang, Z.; Zheng, Y.; Qiao, S.; Xie, Y.; Sun, Y.; Qiao, K.; Feng, Z.; Wang, X.; Wang, J. Preparation of aminoalkyl-grafted bacterial cellulose membranes with improved antimicrobial properties for biomedical applications. J. Biomed. Mater. Res. A 2020, 108, 1086–1098. [Google Scholar] [CrossRef]
- Weishaupt, R.; Heuberger, L.; Siqueira, G.; Gutt, B.; Zimmermann, T.; Maniura-Weber, K.; Salentinig, S.; Faccio, G. Enhanced Antimicrobial Activity and Structural Transitions of a Nanofibrillated Cellulose–Nisin Biocomposite Suspension. ACS Appl. Mater. Interfaces 2018, 10, 20170–20181. [Google Scholar] [CrossRef]
- dos Santos, C.A.; dos Santos, G.R.; Soeiro, V.S.; dos Santos, J.R.; de Araujo Rebelo, M.; Chaud, M.V.; Gerenutti, M.; Grotto, D.; Pandit, R.; Rai, M.; et al. Bacterial nanocellulose membranes combined with nisin: A strategy to prevent microbial growth. Cellulose 2018, 25, 6681–6689. [Google Scholar] [CrossRef]
- Ugurlu, T.; Turkoglu, M.; Gurer, U.S.; Akarsu, B.G. Colonic delivery of compression coated nisin tablets using pectin/HPMC polymer mixture. Eur. J. Pharm. Biopharm. 2007, 67, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Correia, R.C.; Jozala, A.F.; Martins, K.F.; Penna, T.C.V.; de Rezende Duek, E.A.; de Oliveira Rangel-Yagui, C.; Lopes, A.M. Poly(lactic-co-glycolic acid) matrix incorporated with nisin as a novel antimicrobial biomaterial. World J. Microbiol. Biotechnol. 2015, 31, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.M.; Gwak, J.W.; Kamarajan, P.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. Biomedical applications of nisin. J. Appl. Microbiol. 2016, 120, 1449–1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurer, H.R. Bromelain: Biochemistry, pharmacology and medical use. Cell. Mol. Life Sci. 2001, 58, 1234–1245. [Google Scholar] [CrossRef] [PubMed]
- Cherian, B.M.; Leão, A.L.; de Souza, S.F.; Thomas, S.; Pothan, L.A.; Kottaisamy, M. Isolation of nanocellulose from pineapple leaf fibres by steam explosion. Carbohydr. Polym. 2010, 81, 720–725. [Google Scholar] [CrossRef]
- Ataide, J.A.; de Carvalho, N.M.; de Araújo Rebelo, M.; Chaud, M.V.; Grotto, D.; Gerenutti, M.; Rai, M.; Mazzola, P.G.; Jozala, A.F. Bacterial Nanocellulose Loaded with Bromelain: Assessment of Antimicrobial, Antioxidant and Physical-Chemical Properties. Sci Rep. 2017, 7, 18031. [Google Scholar] [CrossRef]
- Bagga, P.; Ansari, T.M.; Siddiqui, H.H.; Syed, A.; Bahkali, A.H.; Rahman, M.A.; Khan, M.S. Bromelain capped gold nanoparticles as the novel drug delivery carriers to aggrandize effect of the antibiotic levofloxacin. EXCLI J. 2016, 15, 772–780. [Google Scholar] [CrossRef]
- Nasiri, R.; Hamzehalipour Almaki, J.; Idris, A.; Nasiri, M.; Irfan, M.; Majid, F.A.A.; Rashidi Nodeh, H.; Hasham, R. Targeted delivery of bromelain using dual mode nanoparticles: Synthesis, physicochemical characterization, in vitro and in vivo evaluation. RSC Adv. 2017, 7, 40074–40094. [Google Scholar] [CrossRef] [Green Version]
- Bhatnagar, P.; Patnaik, S.; Srivastava, A.K.; Mudiam, M.K.R.; Shukla, Y.; Panda, A.K.; Pant, A.B.; Kumar, P.; Gupta, K.C. Anti-cancer activity of bromelain nanoparticles by oral administration. J. Biomed. Nanotechnol. 2014, 10, 3558–3575. [Google Scholar] [CrossRef]
- Ahluwalia, A.K.; Sekhon, B.S. Enzybiotics: A promising approach to fight infectious diseases and an upcoming need for future. J. Pharm. Educ. Res. 2012, 3, 42–51. [Google Scholar]
- Sampaio, L.M.P.; Padrao, J.; Faria, J.; Silva, J.P.; Silva, C.J.; Dourado, F.; Zille, A. Laccase immobilization on bacterial nanocellulose membranes: Antimicrobial, kinetic and stability properties. Carbohydr. Polym. 2016, 145, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Chen, T.; Lim, J.; Gu, F.; Fang, F.; Cheng, L.; Campanella, O.H.; Hamaker, B.R. Acid gelation of soluble laccase-crosslinked corn bran arabinoxylan and possible gel formation mechanism. Food Hydrocoll. 2019, 92, 1–9. [Google Scholar] [CrossRef]
- Raghavendra, G.M.; Jayaramudu, T.; Varaprasad, K.; Ramesh, S.; Raju, K.M. Microbial resistant nanocurcumin-gelatin-cellulose fibers for advanced medical applications. RSC Adv. 2013, 4, 3494–3501. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The Indian solid gold. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Aggarwal, B.B., Surh, Y.-J., Shishodia, S., Eds.; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 2007; pp. 1–75. ISBN 978-0-387-46401-5. [Google Scholar]
- Shaikh, J.; Ankola, D.D.; Beniwal, V.; Singh, D.; Kumar, M.N.V.R. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur. J. Pharm. Sci. 2009, 37, 223–230. [Google Scholar] [CrossRef]
- Sanoj Rejinold, N.; Sreerekha, P.R.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. Biocompatible, biodegradable and thermo-sensitive chitosan-g-poly (N-isopropylacrylamide) nanocarrier for curcumin drug delivery. Int. J. Biol. Macromol. 2011, 49, 161–172. [Google Scholar] [CrossRef]
- Sun, D.; Zhuang, X.; Xiang, X.; Liu, Y.; Zhang, S.; Liu, C.; Barnes, S.; Grizzle, W.; Miller, D.; Zhang, H.G. A Novel Nanoparticle Drug Delivery System: The Anti-inflammatory Activity of Curcumin Is Enhanced When Encapsulated in Exosomes. Mol. Ther. 2010, 18, 1606–1614. [Google Scholar] [CrossRef]
- Pham, X.N.; Nguyen, T.P.; Pham, T.N.; Tran, T.T.N.; Tran, T.V.T. Synthesis and characterization of chitosan-coated magnetite nanoparticles and their application in curcumin drug delivery. Adv. Nat. Sci Nanosci. Nanotechnol. 2016, 7, 045010. [Google Scholar] [CrossRef]
- Gupta, M.; Chauhan, D.N.; Sharma, V.; Chauhan, N.S. Novel Drug Delivery Systems for Phytoconstituents; CRC Press: Boca Raton, FL, USA, 2019; ISBN 978-1-351-05762-2. [Google Scholar]
- Sipponen, A.; Laitinen, K. Antimicrobial properties of natural coniferous rosin in the European Pharmacopoeia challenge test. APMIS 2011, 119, 720–724. [Google Scholar] [CrossRef]
- De Castro, D.O.; Bras, J.; Gandini, A.; Belgacem, N. Surface grafting of cellulose nanocrystals with natural antimicrobial rosin mixture using a green process. Carbohydr. Polym. 2016, 137, 1–8. [Google Scholar] [CrossRef]
- El-Sayed Abdel-Raouf, M.; Mahmoud Abdul-Raheim, A.-R. Rosin: Chemistry, Derivatives, and Applications: A review. BAOJ Chem. 2018, 4, 039. [Google Scholar]
- Yadav, B.K.; Gidwani, B.; Vyas, A. Rosin: Recent advances and potential applications in novel drug delivery system. J. Bioact. Compat. Polym. 2016, 31, 111–126. [Google Scholar] [CrossRef]
- Uddin, K.M.A.; Orelma, H.; Mohammadi, P.; Borghei, M.; Laine, J.; Linder, M.; Rojas, O.J. Retention of lysozyme activity by physical immobilization in nanocellulose aerogels and antibacterial effects. Cellulose 2017, 24, 2837–2848. [Google Scholar] [CrossRef]
- Barbiroli, A.; Bonomi, F.; Capretti, G.; Iametti, S.; Manzoni, M.; Piergiovanni, L.; Rollini, M. Antimicrobial activity of lysozyme and lactoferrin incorporated in cellulose-based food packaging. Food Control 2012, 26, 387–392. [Google Scholar] [CrossRef] [Green Version]
- Abeyrathne, E.D.N.S.; Lee, H.Y.; Ahn, D.U. Egg white proteins and their potential use in food processing or as nutraceutical and pharmaceutical agents—A review. Poult. Sci. 2013, 92, 3292–3299. [Google Scholar] [CrossRef]
- Alves, M.; Vieira, N.S.M.; Rebelo, L.P.N.; Araújo, J.M.M.; Pereiro, A.B.; Archer, M. Fluorinated ionic liquids for protein drug delivery systems: Investigating their impact on the structure and function of lysozyme. Int. J. Pharm. 2017, 526, 309–320. [Google Scholar] [CrossRef]
- Wu, T.; Huang, J.; Jiang, Y.; Hu, Y.; Ye, X.; Liu, D.; Chen, J. Formation of hydrogels based on chitosan/alginate for the delivery of lysozyme and their antibacterial activity. Food Chem. 2018, 240, 361–369. [Google Scholar] [CrossRef]
- Marchese, A.; Barbieri, R.; Sanches-Silva, A.; Daglia, M.; Nabavi, S.F.; Jafari, N.J.; Izadi, M.; Ajami, M.; Nabavi, S.M. Antifungal and antibacterial activities of allicin: A review. Trends Food Sci. Technol. 2016, 52, 49–56. [Google Scholar] [CrossRef]
- Jamindar, D.; Patidar, N.; Jain, S. Formulation and characterization of allicin-amphotericin-b liposomal gel for the treatment of fungal infections. J. Drug Deliv. Ther. 2017, 7, 69–70. [Google Scholar] [CrossRef]
- Jebali, A.; Hekmatimoghaddam, S.; Behzadi, A.; Rezapor, I.; Mohammadi, B.H.; Jasemizad, T.; Yasini, S.A.; Javadzadeh, M.; Amiri, A.; Soltani, M.; et al. Antimicrobial activity of nanocellulose conjugated with allicin and lysozyme. Cellulose 2013, 20, 2897–2907. [Google Scholar] [CrossRef]
- Jafary, R.; Mehrizi, M.K.; Hekmatimoghaddam, S.H.; Jebali, A. Antibacterial property of cellulose fabric finished by allicin-conjugated nanocellulose. J. Text. Inst. 2015, 106, 683–689. [Google Scholar] [CrossRef]
- Atef, M.; Rezaei, M.; Behrooz, R. Characterization of physical, mechanical, and antibacterial properties of agar-cellulose bionanocomposite films incorporated with savory essential oil. Food Hydrocoll. 2015, 45, 150–157. [Google Scholar] [CrossRef]
- Deans, S.G.; Svoboda, K.P. Antibacterial activity of summer savory (Satureja hortensis L) essential oil and its constituents. J. Hortic. Sci. 1989, 64, 205–210. [Google Scholar] [CrossRef]
- Feyzioglu, G.C.; Tornuk, F. Development of chitosan nanoparticles loaded with summer savory (Satureja hortensis L.) essential oil for antimicrobial and antioxidant delivery applications. LWT 2016, 70, 104–110. [Google Scholar] [CrossRef]
- Wojtyczka, R.D.; Kępa, M.; Idzik, D.; Kubina, R.; Kabała-Dzik, A.; Dziedzic, A.; Wąsik, T.J. In Vitro Antimicrobial Activity of Ethanolic Extract of Polish Propolis against Biofilm Forming Staphylococcus epidermidis Strains. Evid.Based Complement. Alternat. Med. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- da Silva Barud, H.; de Araújo Júnior, A.M.; Saska, S.; Mestieri, L.B.; Campos, J.A.D.B.; de Freitas, R.M.; Ferreira, N.U.; Nascimento, A.P.; Miguel, F.G.; de Oliveira Lima Leite Vaz, M.M.; et al. Antimicrobial Brazilian Propolis (EPP-AF) Containing Biocellulose Membranes as Promising Biomaterial for Skin Wound Healing. Evid. Based Complement. Alternat. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Al-Waili, N.; Al-Ghamdi, A.; Ansari, M.J.; Al-Attal, Y.; Salom, K. Synergistic effects of honey and propolis toward drug multi-resistant Staphylococcus aureus, Escherichia coli and Candida albicans isolates in single and polymicrobial cultures. Int. J. Med. Sci. 2012, 9, 793–800. [Google Scholar] [CrossRef] [Green Version]
- Dantas, T.N.C.; Silva, H.S.R.C.; Dantas Neto, A.A.; Marcucci, M.C.; Maciel, M.A.M. Development of a new propolis microemulsion system for topical applications. Rev. Bras. Farmacogn. 2010, 20, 368–375. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, E.T.; Abo-Salem, O.M.; Osman, A. The Influence of Egyptian Propolis on Induced Burn Wound Healing in Diabetic Rats; Antibacterial Mechanism. Sci. J. Med. Clin. Trials 2011, 1. [Google Scholar] [CrossRef]
- Berretta, A.A.; Nascimento, A.P.; Bueno, P.C.P.; de Oliveira Lima Leite Vaz, M.M.; Marchetti, J.M. Propolis Standardized Extract (EPP-AF®), an Innovative Chemically and Biologically Reproducible Pharmaceutical Compound for Treating Wounds. Int. J. Biol. Sci. 2012, 8, 512–521. [Google Scholar] [CrossRef]
- Balata, G.; Nahas, H.M.E.; Radwan, S. Propolis organogel as a novel topical delivery system for treating wounds. Drug Deliv. 2014, 21, 55–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rassu, G.; Cossu, M.; Langasco, R.; Carta, A.; Cavalli, R.; Giunchedi, P.; Gavini, E. Propolis as lipid bioactive nano-carrier for topical nasal drug delivery. Colloids Surf. B Biointerfaces 2015, 136, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Fan, L.; Ma, L.; Wang, Y.; Lin, S.; Yu, F.; Pan, X.; Luo, G.; Zhang, D.; Wang, H. Green electrospun Manuka honey/silk fibroin fibrous matrices as potential wound dressing. Mater. Des. 2017, 119, 76–84. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.H. 14 - Drug delivery dressings. In Advanced Wound Repair Therapies; Farrar, D., Ed.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Cambridge, UK, 2011; pp. 361–394. ISBN 978-1-84569-700-6. [Google Scholar]
- Tenci, M.; Rossi, S.; Bonferoni, M.C.; Sandri, G.; Boselli, C.; Di Lorenzo, A.; Daglia, M.; Icaro Cornaglia, A.; Gioglio, L.; Perotti, C.; et al. Particulate systems based on pectin/chitosan association for the delivery of manuka honey components and platelet lysate in chronic skin ulcers. Int. J. Pharm. 2016, 509, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Subash Babu, P.; Prabuseenivasan, S.; Ignacimuthu, S. Cinnamaldehyde—A potential antidiabetic agent. Phytomedicine 2007, 14, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Sanla-Ead, N.; Jangchud, A.; Chonhenchob, V.; Suppakul, P. Antimicrobial Activity of Cinnamaldehyde and Eugenol and Their Activity after Incorporation into Cellulose-based Packaging Films. Packag. Technol. Sci. 2012, 25, 7–17. [Google Scholar] [CrossRef]
- Kenawy, E.; Omer, A.M.; Tamer, T.M.; Elmeligy, M.A.; Eldin, M.S.M. Fabrication of biodegradable gelatin/chitosan/cinnamaldehyde crosslinked membranes for antibacterial wound dressing applications. Int. J. Biol. Macromol. 2019, 139, 440–448. [Google Scholar] [CrossRef]
- Bang, K.-H.; Kim, K.S. Development of trans-cinnamaldehyde self-microemulsifying drug delivery system (SMEDDS) with superior stability. J. Korea Acad.-Ind. Coop. Soc. 2019, 20, 555–562. [Google Scholar] [CrossRef]
- Patole, V.C.; Chaudhari, S.P.; Pandit, A.P.; Lokhande, P.P. Thymol and eugenol loaded chitosan dental film for treatment of periodontitis. Indian Drugs 2019, 54, 51–58. [Google Scholar]
- Esmaeili, F.; Rajabnejhad, S.; Partoazar, A.R.; Mehr, S.E.; Faridi-Majidi, R.; Sahebgharani, M.; Syedmoradi, L.; Rajabnejhad, M.R.; Amani, A. Anti-inflammatory effects of eugenol nanoemulsion as a topical delivery system. Pharm. Dev. Technol. 2016, 21, 887–893. [Google Scholar] [CrossRef] [PubMed]
- El Khayat, N.W.; Donia, A.A.; Mady, O.Y.; El Maghraby, G.M. Optimization of eugenol microemulsion for transdermal delivery of indomethacin. J. Drug Deliv. Sci. Technol. 2018, 48, 311–318. [Google Scholar] [CrossRef]
- Silva, S.S.; Caridade, S.G.; Mano, J.F.; Reis, R.L. Effect of crosslinking in chitosan/aloe vera-based membranes for biomedical applications. Carbohydr. Polym. 2013, 98, 581–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godinho, J.F.; Berti, F.V.; Müller, D.; Rambo, C.R.; Porto, L.M. Incorporation of Aloe vera extracts into nanocellulose during biosynthesis. Cellulose 2016, 23, 545–555. [Google Scholar] [CrossRef]
- Saibuatong, O.; Phisalaphong, M. Novo aloe vera–bacterial cellulose composite film from biosynthesis. Carbohydr. Polym. 2010, 79, 455–460. [Google Scholar] [CrossRef]
- Gupta, B.; Agarwal, R.; Alam, S. Aloe Vera Loaded Poly(Vinyl Alcohol)–Poly(Ethylene Oxide)-Carboxymethyl Cellulose-Polyester Nonwoven Membranes. J. Biomater. Tissue Eng. 2013, 3, 503–511. [Google Scholar] [CrossRef]
- Laux, A.; Gouws, C.; Hamman, J.H. Aloe vera gel and whole leaf extract: Functional and versatile excipients for drug delivery? Expert Opin. Drug Deliv. 2019, 16, 1283–1285. [Google Scholar] [CrossRef] [Green Version]
- Jacob, J.; Haponiuk, J.T.; Thomas, S.; Peter, G.; Gopi, S. Use of Ginger Nanofibers for the Preparation of Cellulose Nanocomposites and Their Antimicrobial Activities. Fibers 2018, 6, 79. [Google Scholar] [CrossRef] [Green Version]
- Jacob, J.; Peter, G.; Thomas, S.; Haponiuk, J.T.; Gopi, S. Chitosan and polyvinyl alcohol nanocomposites with cellulose nanofibers from ginger rhizomes and its antimicrobial activities. Int. J. Biol. Macromol. 2019, 129, 370–376. [Google Scholar] [CrossRef]
- Dehnad, D.; Mirzaei, H.; Emam-Djomeh, Z.; Jafari, S.-M.; Dadashi, S. Thermal and antimicrobial properties of chitosan–nanocellulose films for extending shelf life of ground meat. Carbohydr. Polym. 2014, 109, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, M.; Salaberria, A.M.; Andres, M.A.; Kaya, M.; Gunyakti, A.; Labidi, J. Utilization of flax (Linum usitatissimum) cellulose nanocrystals as reinforcing material for chitosan films. Int. J. Biol. Macromol. 2017, 104, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Sillard, C.; Belgacem, M.N.; Bras, J. Nisin anchored cellulose nanofibers for long term antimicrobial active food packaging. RSC Adv. 2016, 6, 12422–12430. [Google Scholar] [CrossRef]
- Salmieri, S.; Islam, F.; Khan, R.A.; Hossain, F.M.; Ibrahim, H.M.M.; Miao, C.; Hamad, W.Y.; Lacroix, M. Antimicrobial nanocomposite films made of poly(lactic acid)-cellulose nanocrystals (PLA-CNC) in food applications: Part A—Effect of nisin release on the inactivation of Listeria monocytogenes in ham. Cellulose 2014, 21, 1837–1850. [Google Scholar] [CrossRef]
- Tangsatianpan, V.; Torgbo, S.; Sukyai, P. Release Kinetic Model and Antimicrobial Activity of Freeze-Dried Curcumin-loaded Bacterial Nanocellulose Composite. Polym. Sci. Ser. A 2020. [Google Scholar] [CrossRef]
- de Castro, D.O.; Tabary, N.; Martel, B.; Gandini, A.; Belgacem, N.; Bras, J. Controlled release of carvacrol and curcumin: Bio-based food packaging by synergism action of TEMPO-oxidized cellulose nanocrystals and cyclodextrin. Cellulose 2018, 25, 1249–1263. [Google Scholar] [CrossRef]
- Niu, X.; Liu, Y.; Song, Y.; Han, J.; Pan, H. Rosin modified cellulose nanofiber as a reinforcing and co-antimicrobial agents in polylactic acid /chitosan composite film for food packaging. Carbohydr. Polym. 2018, 183, 102–109. [Google Scholar] [CrossRef]
- Bayazidi, P.; Almasi, H.; Asl, A.K. Immobilization of lysozyme on bacterial cellulose nanofibers: Characteristics, antimicrobial activity and morphological properties. Int. J. Biol. Macromol. 2018, 107, 2544–2551. [Google Scholar] [CrossRef]







| Co-Carrier | Modification Agent | Carrier Form | Model Drug | Possible Application | Ref. |
|---|---|---|---|---|---|
| Sodium alginate | CaCl2, EPTMAC, PEI | Hydrogel | Ceftazidime hydrate and epidermal growth factor human | Codelivery of drugs in the oral administration and wound dressing | [99] |
| Cyclodextrin | - | Complexes | Curcumin | Anticancer drug delivery systems | [100] |
| Quaternized cellulose | CHPTAC, β-GP | Hydrogel | Doxorubicin | Localized and sustained drug delivery depot systems for anticancer therapy | [101] |
| - | APTES, PPI-dendrimer, FA | Lyophilized NPs | Doxorubicin | Delivery of anticancer drug | [102] |
| - | Aln, ApA | Dispersion | -- | Bone therapied and theranostics | [103] |
| Sodium alginate | - | Hydrogel | Ibuprofen | Drug carrier | [104] |
| PVA | - | Hydrogel lenses | Chitosan–poly(acrylic acid) NPs | Ophthalmic use as a drug carrier and as cornea regeneration implant | [105] |
| - | PVP | Film | Honey | Wound dressing for the treatment of chronic wounds | [106] |
| - | CTAB | Suspension | Curcumin | Drug carrier for hydrophobic drugs | [107] |
| - | PVA | Film | Curcumin | Antimicrobial drug delivery in a diabetic wound dressing | [108] |
| Chitosan | Tween 20, GA | Hydrogel | Curcumin | Drug delivery of curcumin | [110] |
| - | Tween 20 | Solution | Curcumin | Delivery of curcumin to the stomach and upper intestinal tract | [111] |
| FO and PEI | - | Lyophilized hybrids | Doxorubicin | Layer-by-layer assembly with lysosomal pH-controlled drug release into the nucleus | [112] |
| PCL | - | Nanofibers | Tetracycline | Drug delivery system | [113] |
| Co-Carrier | Modification Agent | Carrier Form | Model Drug | Possible Application | Ref. |
|---|---|---|---|---|---|
| - | LA | Dry foams and films | Riboflavin | Gastro retentive drug delivery | [115] |
| - | - | Nanopapers and nanofoams | Indomethacin | Fast and intermediate release profiles for drug delivery | [116] |
| - | - | Aerogels | Bendamustine hydrochloride | Carriers for oral drug delivery | [117] |
| HFBII | - | Emulsion | Naproxen and ibuprofen | Drug delivery applications via the oral route | [118] |
| - | GTMAC | Nanofoam | Furosemide | Prolonged drug delivery in the upper part of the gastrointestinal tract | [119] |
| Fe3O4 -Ag2O quantum dots | - | Powder | Etoposide and Methotrexate | Carrier of anticancer drugs for skin cancer | [120] |
| - | FITIC-DEX, lysozyme, and BSA | Hydrogel | Metronidazole, nadolol, and ketoprofen | Controlled delivery of several types of molecules | [121] |
| Sodium alginate | - | Beads (dried hydrogel) | Metformin hydrochloride | Drug carrier | [122] |
| Sodium alginate | - | Film | Ampicillin | Supporting material, drug delivery system for Tissue Engineering | [123] |
| PDA | calcium ion | Hydrogel | Tetracycline hydrochloride | Drug delivery vehicle | [124] |
| Chitosan | - | Film | Ketorolac tromethamine | Transdermal delivery systems | [125] |
| HPMC | - | Film | Ketorolac tromethamine | Food packaging and transdermal drug delivery applications | [126] |
| PLA | - | Hydrogel | Metoprolol and nadolol | Modulating sustained drug release | [127] |
| Gelatin | Dialdehyde starch | Cryogel | 5-fluorouracil | Carrier for controlled drug release | [129] |
| Starch/pectin | Glycerin | Film | Methotrexate | Colonic drug release | [130] |
| - | - | Aerogel | Ibuprofen and amoxicillin | Drug delivery vehicle | [131] |
| - | - | Hydrogel | Ibuprofen | Controlled drug release system | [132] |
| Co-Carrier | Modification Agent | Carrier Form | Model Drug | Possible Application | Ref. |
|---|---|---|---|---|---|
| Starch/pectin | Glycerin | Film | Methotrexate | Colonic drug release | [130] |
| Poloxamer | - | Gel and micelle | Octenidine | Long-term dermal wound treatment and drug delivery | [133] |
| Collagen | - | Film | Lidocaine | Biodegradable microneedles for transdermal drug delivery | [134] |
| PMGly | MGly, AAPH, and MBA | Membranes | Diclofenac | Drug carriers for dermal and oral drug delivery | [135] |
| - | - | Hydrogel | BSA | Carrier for controlled delivery of proteins | [136] |
| - | - | Scaffolds | Caco-2-cells | Caco-2-based in vitro models of the human intestine | [137] |
| - | Glycerol | Membranes | Caffeine, ibuprofen, lidocaine and diclofenac | Topical drug delivery systems | [138] |
| - | - | Film | Crocin | Transdermal delivery of crocin | [139] |
| Type of NC | Antimicrobial Agent/ Compound | Hybrid Form | Synthesis of Hybrids | Microbial Growth Inhibition | Ref. |
|---|---|---|---|---|---|
| BNC | AgNPs | Porous hybrids | Immobilization on the top and bottom surfaces of BNC by chemical interactions | E. coli, S. aureus | [23] |
| CNF | AgNPs | Coated papers | Impregnation of CNF with AgNPs’ solution | E. coli, S. aureus | [156] |
| BNC | AgNPs | Nanocomposites | Classical Tollens reaction and reduction of AgNO3 with NaBH4 | S. aureus | [157] |
| CNC | ZnO | Films | Evaporation-induced self-assembly in aqueous solution | E. coli, S. aureus | [165] |
| TiO2 | |||||
| Ag2O | |||||
| CNC/WG | TiO2 | Films | TiO2 NPs were added to the WG/CNC suspension | E. coli, S. cerevisiae, S. aureus | [171] |
| CNF | TiO2 | Nanocomposites | Cross-linking of TiNPs with BTCA and SHP | E. coli, S. aureus | [172] |
| CNF | CuO | Freeze-dried nanofibrils | “Green” reductive technique using an alcoholic extract of Terminalia chebula fruit | C. albicans, E. coli, S. aureus | [173] |
| CNC | ZnO | Nanocomposites | In situ solution casting technique | E. coli, S. aureus | [174] |
| CNF | ZnO | Paper sheets | Electrostatic assembly in aqueous medium using polyelectrolytes as macromolecular linkers | B. cereus, K. pneumoniae, S. aureus | [175] |
| BNC | ZnO | Films | Powdered BNC was dissolved in NMMO, and ZnO NPs were mixed into the BNC solution; | E. coli | [176] |
| BNC | ZnO | Sheets | Ultrasonic-assisted in situ synthesis | E. coli, S. aureus | [177] |
| BNC | MgO | Membranes | In situ: Sonochemical and wet chemical methods Ex situ: Immersion of BNC pellicles into a MgO dispersion | E. coli, S. aureus | [178] |
| Type of NC | Antimicrobial Agent (Additional Compound) | Hybrid Form | Synthesis of Hybrids | Microbial Growth Inhibition | Ref. |
|---|---|---|---|---|---|
| BNC | Benzalkonium chloride | Dry film | Immersion of BNC film into BZC solution | B. subtilis, S. aureus, S. typhimurium | [187] |
| BNC | Polyhexanide, povidone-iodine | Fleeces | Immersion of BNC samples in PI or PHMB | S. aureus | [188] |
| CNF from ginger | - | Film | Preparation of film with chemicals (alkalization, bleaching, acid hydrolysis) and ultrasonication | B. subtilis, C. albicans, E. coli, P. aeruginosa, S. aureus | [194] |
| CNF from ginger | - | Nanocomposites | Solvent-casting method reinforcement using PS and TS | B. cereus, E. coli, S. aureus, S. typhimurium | [304] |
| CNF from ginger | (Chitosan, PVA) | Nanocomposites | Solvent-casting method | B. cereus, E. coli, S. aureus, S. typhimurium | [305] |
| BNC | Chitosan | Hydrogel | Adding CS in culture media during bacterial cultivation | E. coli, S. aureus | [220] |
| NC | Chitosan | Nanocomposites | Mixing of chitosan solution, NC solution and glycerol solution | E. coli, S. aureus, S. enteritidis | [306] |
| CNC | Chitosan | Films | Flax CNC incorporated in CS film solution by following the solution casting method | E. coli, E. faecalis, L. monocytogenes, P. aeruginosa, S. aureus | [307] |
| CNC | Chitosan (PVP) | Film | Solution casting method | P. aeruginosa, S. aureus | [222] |
| CNC | Chitosan, PCL, grape seed extract | Film | Casting method for preparation of CS films with GSE and NC, the addition of PCL was achieved with coating and compression molding method for PCL | E. coli, L. monocytogenes | [234] |
| CNF | Chitosan, SNAP | Membrane | Encapsulation of SNAP in dispersed CS and mixed with CNFs | E. faecalis, L. monocytogenes, S. aureus | [231] |
| BNC | APS | Membrane | Surface functionalization with aminoalkyl groups | E. coli, S. aureus | [73] |
| BNC | Nisin, EDTA | Membrane | Immersion of BNC membranes into nisin solution with or without EDTA | E. coli, S. aureus | [243] |
| CNF | Nisin | Film | Immobilization of nisin on CNF using the coupling agent (EDC-NHS) | B. subtilis, S. aureus | [308] |
| CNC | Nisin (PLA) | Films | PLA-CNC films were treated with nisin by adsorption/diffusion coating method | L. monocytogenes | [309] |
| BNC | Bromelain | Membrane | Submersion of BNC into a BL solution | E. coli, P. aeruginosa, S. aureus | [249] |
| BNC | Laccase | Membrane | Physical enzyme immobilization: immersion of BNC into a laccase preparation | E. coli, S. aureus | [254] |
| CNC | Curcumin | Film | CNF suspended in PVA solution, and then curcumin was added | B. coagulans, C. albicans, E. coli, P. mirabilis, S. aureus, Streptococcus sp. | [108] |
| BNC | Freeze-dried curcumin | Membrane | Immersion method | E. coli, S. aureus | [310] |
| CNC | Carvacrol, curcumin (βCD, HPβCD) | Film | TOCNC-COONa and TOCNC-COOH were modified with βCD and HPβCD; Curcumin and carvacrol were entrapped by the attached HPβCD | B. subtilis | [311] |
| CNC | Rosin | Film | Esterification on CNC using rosin as the grafting agent and reaction solvent | B. subtilis, E. coli | [264] |
| CNF | Rosin, (PLA/chitosan) | Film | CNF modified by rosin by the SolReact process and then used as a reinforcement filler within the PLA matrix; the film was further coated with CS | B. subtilis, E. coli | [312] |
| CNF | Lysozyme | Aerogels | Physical immobilization of lysozyme: Mixing of the enzyme with CNFs followed by lyophilization | E. coli, S. aureus | [267] |
| BNC | Lysozyme | Dispersion | Physical absorption method to immobilize lysozyme onto BC nanofibers | A. niger, E. coli, L. monocytogenes, S. aureus, S. cerevisiae, Y. enterocolitica | [313] |
| CNC | Allicin | Nanocomposites | Modification of CNC with CA, further conjugation with allicin by a carbodiimide (EDC) cross-linker; | A. niger, C. albicans, E. coli, S. aureus | [274] |
| Lysozyme | Coating of CNC with BSA and conjugated with lysozyme by the EDC method | ||||
| NC | Allicin | Fabric | Cellulose fabrics modified by APTES and conjugated with allicin-conjugated NC (EDC method) | S. aureus | [275] |
| CNC | Savory essential oil (agar) | Film | CNC suspension was dispersed in an AG solution; Tween 80 was added as the emulsifier, then SEO was added to the mixture | B. cereus, E. coli, L. monocytogenes, S. aureus | [276] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kupnik, K.; Primožič, M.; Kokol, V.; Leitgeb, M. Nanocellulose in Drug Delivery and Antimicrobially Active Materials. Polymers 2020, 12, 2825. https://doi.org/10.3390/polym12122825
Kupnik K, Primožič M, Kokol V, Leitgeb M. Nanocellulose in Drug Delivery and Antimicrobially Active Materials. Polymers. 2020; 12(12):2825. https://doi.org/10.3390/polym12122825
Chicago/Turabian StyleKupnik, Kaja, Mateja Primožič, Vanja Kokol, and Maja Leitgeb. 2020. "Nanocellulose in Drug Delivery and Antimicrobially Active Materials" Polymers 12, no. 12: 2825. https://doi.org/10.3390/polym12122825
APA StyleKupnik, K., Primožič, M., Kokol, V., & Leitgeb, M. (2020). Nanocellulose in Drug Delivery and Antimicrobially Active Materials. Polymers, 12(12), 2825. https://doi.org/10.3390/polym12122825