Review on Nanocrystalline Cellulose in Bone Tissue Engineering Applications
Abstract
1. Introduction
2. Bone Structure and Its Properties
3. Cellulose
3.1. Bacterial Cellulose
3.2. Fibrillated Cellulose
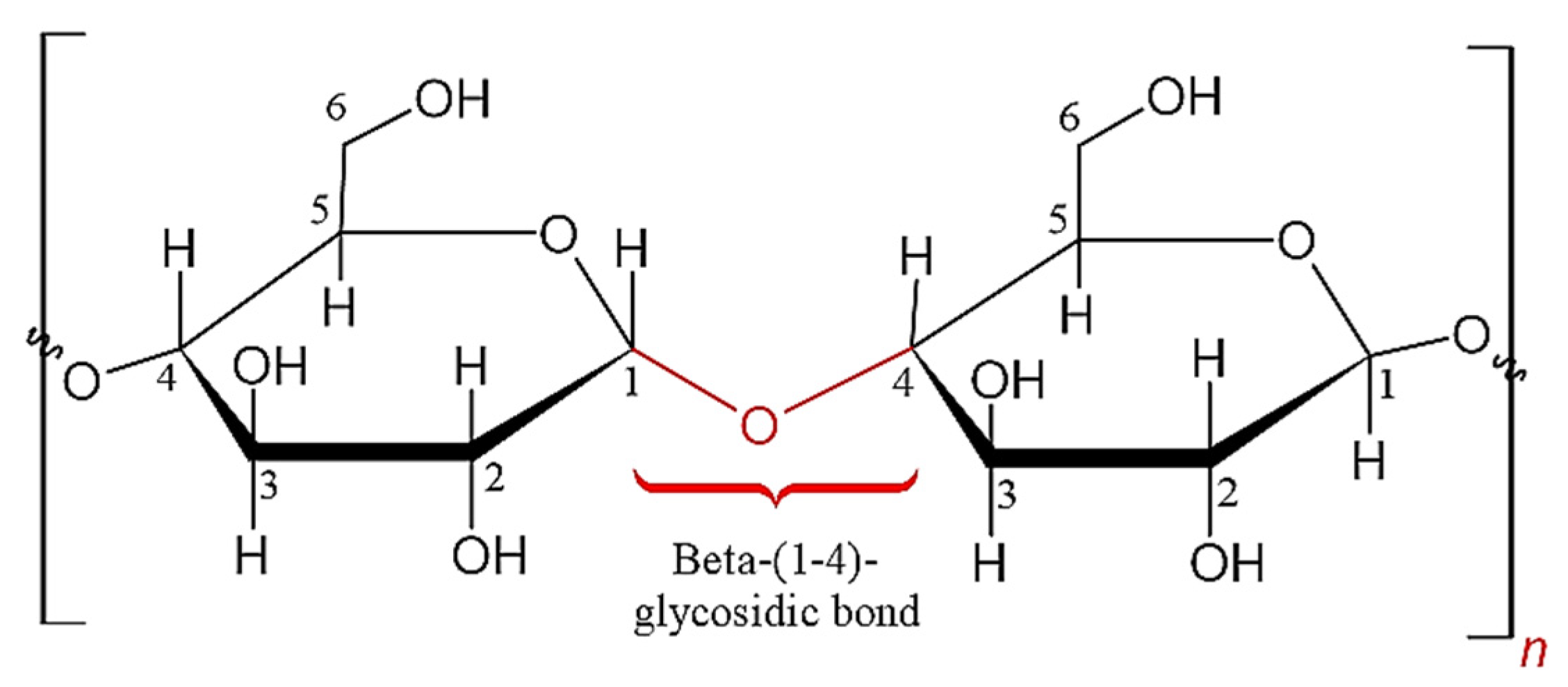
3.3. Crystalline Cellulose
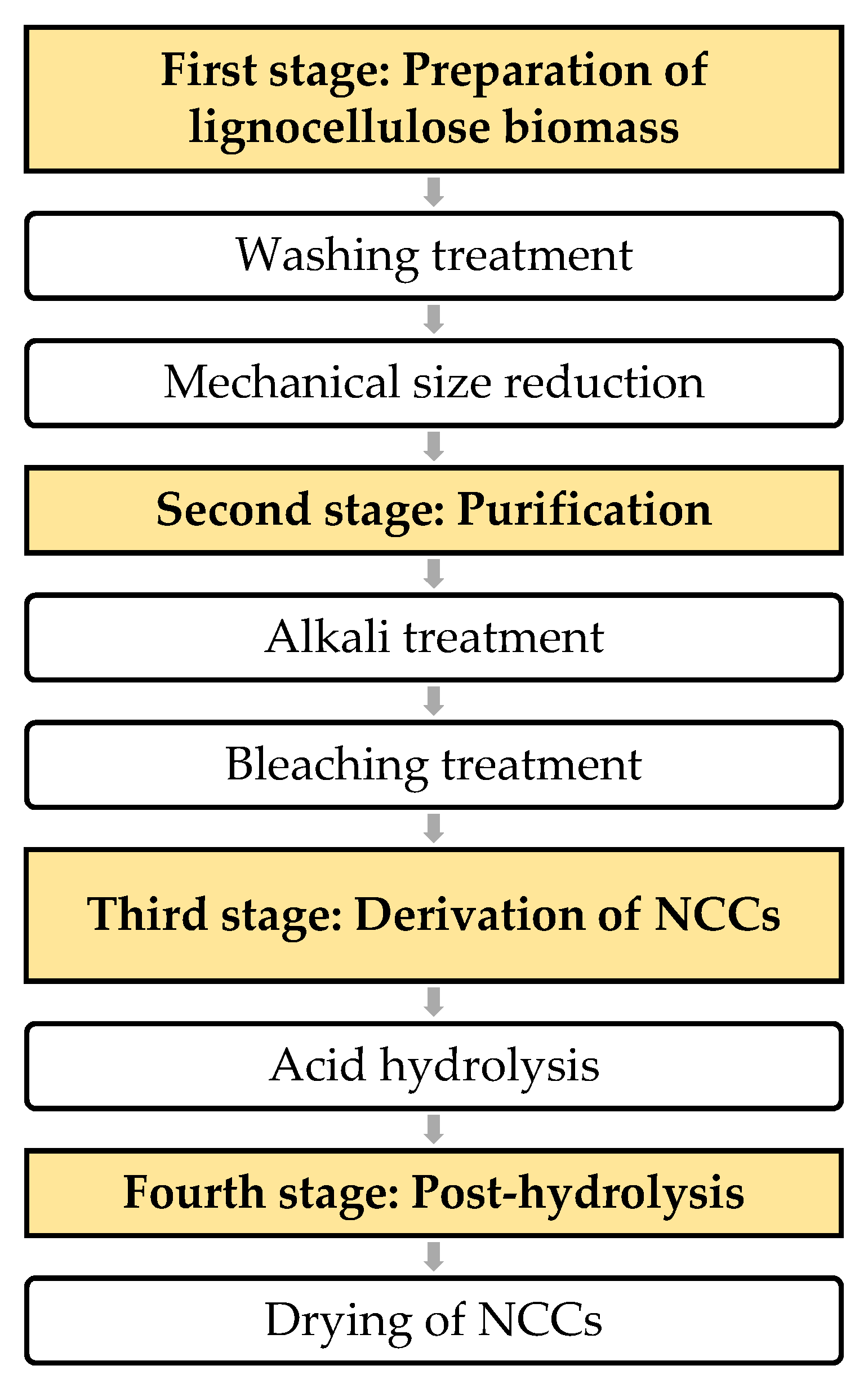
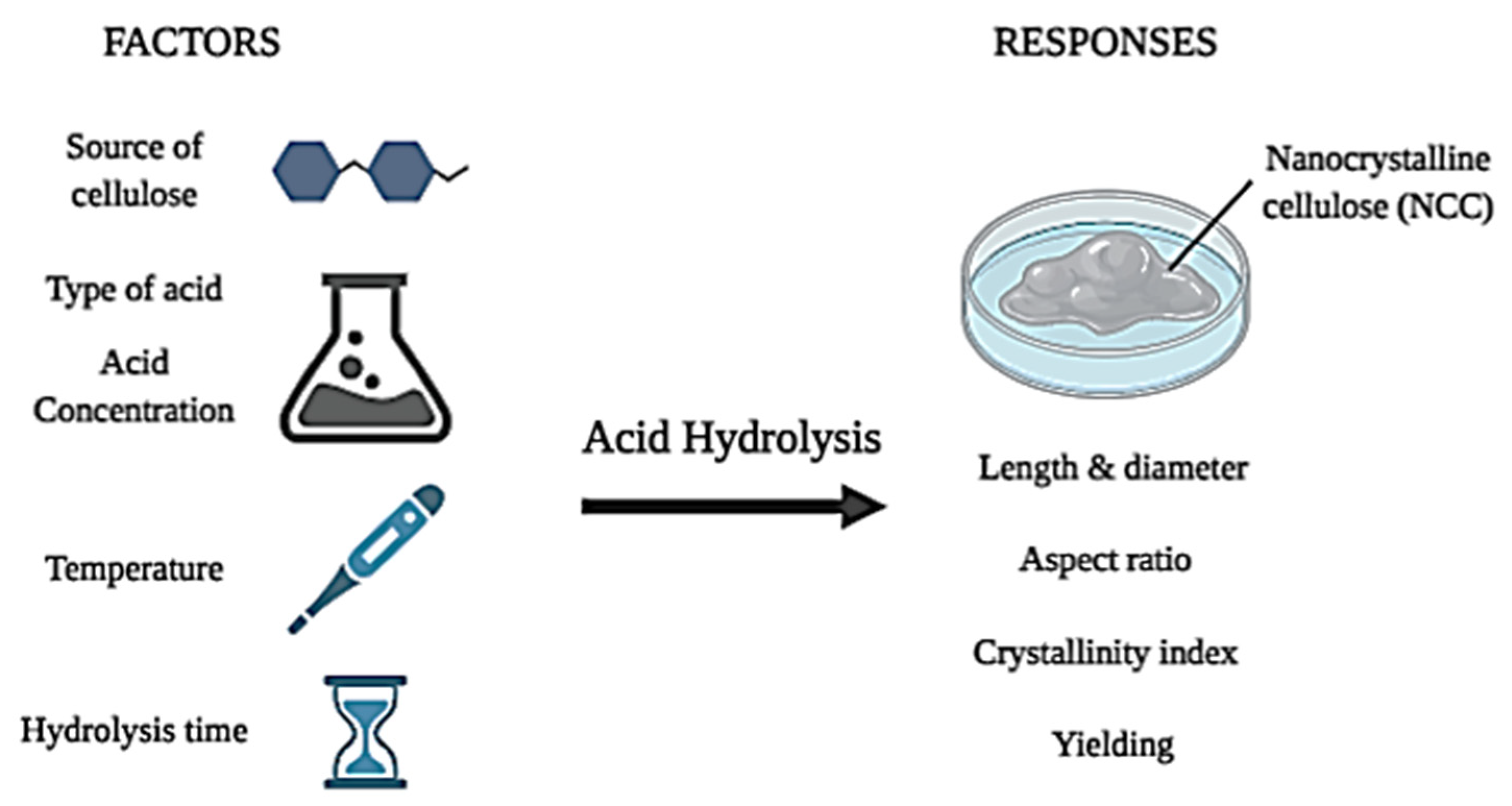
3.4. Derivation of Nanocrystalline Cellulose
4. Contribution of Nanocrystalline Cellulose in Bone Tissue Engineering
- biomaterials with suitable additives and modification, and
- fabrication process.
- mechanical properties:
- biocompatibility;
- biodegradability; and
- morphology of scaffold; this effect will not be discussed in this review.
4.1. Mechanical Properties
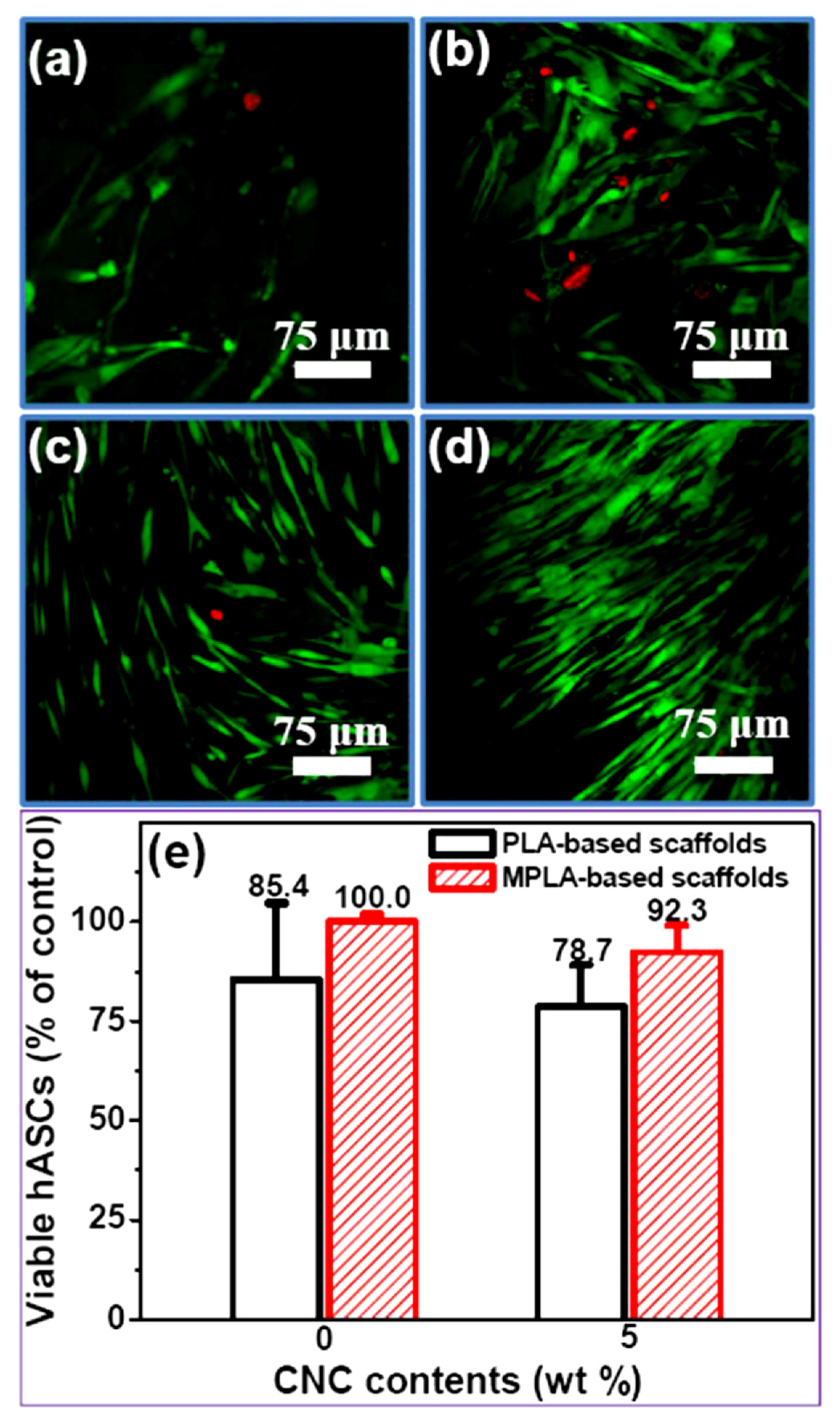
4.2. Biocompatibility Properties
4.3. Biodegradability
5. Challenge in Future Development of NCC
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| °C | Degree celsius |
| BC | Bacterial cellulose |
| BNC | Bacteria nanocellulose |
| CC | Crystalline cellulose |
| CNC | Cellulose nanocrystal |
| DIW | Deionized water |
| DW | Distilled water |
| E | Young’s modulus |
| ECM | Extracellular matrix |
| FC | Fibrillated cellulose |
| GPA | Giga pascals |
| H2SO4 | Sulfuric acid |
| HA | Hydroxyapatite |
| hASCs | Human adipose stem cells |
| HBr | Hydrobromic acid |
| HCl | Hydrochloric acid |
| hMSCs | Human mesenchymal stem cells |
| HNO3 | Nitric acid |
| KOH | Potassium hydroxide |
| LB | Lignocellulosic biomass |
| M058K | Glioblastoma multiforme cell line |
| MC3T3 | Osteoblast precursor cell line |
| MCC | Microcrystalline cellulose |
| MFC | Microfibrillated cellulose |
| MG63 | Human osteosarcoma cell line |
| MPa | Mega pascals |
| NaOH | Sodium hydroxide |
| NCC | Nanocrystalline cellulose |
| NFC | Nanofibrillated cellulose |
| n-HA | Nano-hydroxyapatite |
| NWC | Nanowhisker cellulose |
| OVA | Ovalbumin |
| PBS | Phosphate buffered saline |
| PCL | Ε-poly(caprolactone) |
| PEG | Poly(ethylene glycol) |
| PLA | Poly(lactic acid) |
| PLLA | Poly(l-lactide acid) |
| PPF | Poly(propylene fumarate) |
| PVA | Poly(vinyl alcohol) |
| SBF | Simulated body fluid |
| TE | Tissue engineering |
References
- De Mori, A.; Peña Fernández, M.; Blunn, G.; Tozzi, G.; Roldo, M. 3D Printing and Electrospinning of Composite Hydrogels for Cartilage and Bone Tissue Engineering. Polymers 2018, 10, 285. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zuo, R.; Long, H.; Wang, Y.; Zhang, Y.; Sun, C.; Luo, G.; Zhang, Y.; Li, C.; Zhou, Y.; et al. Advances in the Masquelet technique: Myeloid-derived suppressor cells promote angiogenesis in PMMA-induced membranes. Acta Biomater. 2020, 108, 223–236. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, J.Y.; Pang, X.L.; Zhao, M.; Wang, B.Q.; Yang, L.L.; Wan, H.S.; Wu, J.B.; Fu, S.Z. Magnetic nanoparticle-loaded electrospun polymeric nanofibers for tissue engineering. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 73, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Aldemir Dikici, B.; Dikici, S.; Reilly, G.C.; MacNeil, S.; Claeyssens, F. A Novel Bilayer Polycaprolactone Membrane for Guided Bone Regeneration: Combining Electrospinning and Emulsion Templating. Materials 2019, 12, 2643. [Google Scholar] [CrossRef]
- Lee, H.-S.; Byun, S.-H.; Cho, S.-W.; Yang, B.-E. Past, Present, and Future of Regeneration Therapy in Oral and Periodontal Tissue: A Review. Appl. Sci. 2019, 9, 1046. [Google Scholar] [CrossRef]
- Nurulhuda, A.; Izman, S.; Ngadiman, N.H.A. Fabrication PEGDA/ANFs Biomaterial as 3D Tissue Engineering Scaffold by DLP 3D Printing Tecshnology. Int. J. Eng. Adv. Technol. 2019, 8, 751–758. [Google Scholar] [CrossRef]
- Berthiaume, F.; Maguire, T.J.; Yarmush, M.L. Tissue Engineering and Regenerative Medicine: History, Progress, and Challenges. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 403–430. [Google Scholar] [CrossRef]
- Yang, E.; Miao, S.; Zhong, J.; Zhang, Z.; Mills, D.K.; Zhang, L.G. Bio-Based Polymers for 3D Printing of Bioscaffolds. Polym. Rev. 2018, 58, 668–687. [Google Scholar] [CrossRef]
- Arcaute, K.; Mann, B.K.; Wicker, R.B. Stereolithography of Three-Dimensional Bioactive Poly(Ethylene Glycol) Constructs with Encapsulated Cells. Ann. Biomed. Eng. 2006, 34, 1429–1441. [Google Scholar] [CrossRef]
- Hasan, A.; Morshed, M.; Memic, A.; Hassan, S.; Webster, T.; Marei, H. Nanoparticles in tissue engineering: Applications, challenges and prospects. Int. J. Nanomed. 2018, 13, 5637–5655. [Google Scholar] [CrossRef] [PubMed]
- Elomaa, L.; Pan, C.-C.; Shanjani, Y.; Malkovskiy, A.; Seppälä, J.V.; Yang, Y. Three-dimensional fabrication of cell-laden biodegradable poly(ethylene glycol-co-depsipeptide) hydrogels by visible light stereolithography. J. Mater. Chem. B 2015, 3, 8348–8358. [Google Scholar] [CrossRef] [PubMed]
- Torgbo, S.; Sukyai, P. Bacterial cellulose-based scaffold materials for bone tissue engineering. Appl. Mater. Today 2018, 11, 34–49. [Google Scholar] [CrossRef]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Hickey, R.J.; Pelling, A.E. Cellulose Biomaterials for Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7, 45. [Google Scholar] [CrossRef]
- Shi, C.; Yuan, Z.; Han, F.; Zhu, C.; Li, B. Polymeric biomaterials for bone regeneration. Ann. Jt. 2016, 1, 27. [Google Scholar] [CrossRef]
- Mi, H.-Y.; Salick, M.R.; Jing, X.; Jacques, B.R.; Crone, W.C.; Peng, X.-F.; Turng, L.-S. Characterization of thermoplastic polyurethane/polylactic acid (TPU/PLA) tissue engineering scaffolds fabricated by microcellular injection molding. Mater. Sci. Eng. C 2013, 33, 4767–4776. [Google Scholar] [CrossRef]
- Ronca, A.; Ambrosio, L.; Grijpma, D.W. Design of porous three-dimensional PDLLA/nano-hap composite scaffolds using stereolithography. J. Appl. Biomater. Funct. Mater. 2012, 10, 249–258. [Google Scholar] [CrossRef]
- Flauzino Neto, W.P.; Silvério, H.A.; Dantas, N.O.; Pasquini, D. Extraction and characterization of cellulose nanocrystals from agro-industrial residue—Soy hulls. Ind. Crops Prod. 2013, 42, 480–488. [Google Scholar] [CrossRef]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and application. Carbon Resour. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Nemati, S.; Kim, S.-J.; Shin, Y.M.; Shin, H. Current progress in application of polymeric nanofibers to tissue engineering. Nano Converg. 2019, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Barui, S.; Chatterjee, S.; Mandal, S.; Kumar, A.; Basu, B. Microstructure and compression properties of 3D powder printed Ti-6Al-4V scaffolds with designed porosity: Experimental and computational analysis. Mater. Sci. Eng. C 2017, 70, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Hassanajili, S.; Karami-Pour, A.; Oryan, A.; Talaei-Khozani, T. Preparation and characterization of PLA/PCL/HA composite scaffolds using indirect 3D printing for bone tissue engineering. Mater. Sci. Eng. C 2019, 104, 1009960. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef]
- Elomaa, L.; Teixeira, S.; Hakala, R.; Korhonen, H.; Grijpma, D.W.; Seppälä, J.V. Preparation of poly(ε-caprolactone)-based tissue engineering scaffolds by stereolithography. Acta Biomater. 2011, 7, 3850–3856. [Google Scholar] [CrossRef]
- Lee, K.-W.; Wang, S.; Fox, B.C.; Ritman, E.L.; Yaszemski, M.J.; Lu, L. Poly(propylene fumarate) Bone Tissue Engineering Scaffold Fabrication Using Stereolithography: Effects of Resin Formulations and Laser Parameters. Biomacromolecules 2007, 8, 1077–1084. [Google Scholar] [CrossRef]
- Kumar, A.; Negi, Y.S.; Choudhary, V.; Bhardwaj, N.K. Microstructural and mechanical properties of porous biocomposite scaffolds based on polyvinyl alcohol, nano-hydroxyapatite and cellulose nanocrystals. Cellulose 2014, 21, 3409–3426. [Google Scholar] [CrossRef]
- Kumar, A.; Han, S.S. PVA-based hydrogels for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2016, 66, 159–182. [Google Scholar] [CrossRef]
- Oropallo, W.; Piegl, L.A. Ten challenges in 3D printing. Eng. Comput. 2015, 32, 135–148. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Q.; Yue, Y.; Zhang, Q. Application of rod-shaped cellulose nanocrystals in polyacrylamide hydrogels. J. Colloid Interface Sci. 2011, 353, 116–123. [Google Scholar] [CrossRef]
- Kumar, S.; Hofmann, M.; Steinmann, B.; Foster, E.J.; Weder, C. Reinforcement of Stereolithographic Resins for Rapid Prototyping with Cellulose Nanocrystals. ACS Appl. Mater. Interfaces 2012, 4, 5399–5407. [Google Scholar] [CrossRef] [PubMed]
- Hammonds, R.L. The Advancement of Bacterial Cellulose As A Bone and Vascular Scaffolds. Ph.D. Thesis, University of Tennessee, Knoxville, TN, USA, 2013. [Google Scholar]
- Dugan, J.M.; Gough, J.E.; Eichhorn, S.J. Bacterial cellulose scaffolds and cellulose nanowhiskers for tissue engineering. Nanomedicine 2013, 8, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Favi, P.M.; Benson, R.S.; Neilsen, N.R.; Hammonds, R.L.; Bates, C.C.; Stephens, C.P.; Dhar, M.S. Cell proliferation, viability, and in vitro differentiation of equine mesenchymal stem cells seeded on bacterial cellulose hydrogel scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
- Jorfi, M.; Foster, E.J. Recent advances in nanocellulose for biomedical applications. J. Appl. Polym. Sci. 2015, 132, 1–19. [Google Scholar] [CrossRef]
- Dorozhkin, S. Calcium Orthophosphates in Nature, Biology and Medicine. Materials 2009, 2, 399–498. [Google Scholar] [CrossRef]
- Oliveira, T.C.; Gomes, M.S.; Gomes, A.C. The Crossroads between Infection and Bone Loss. Microorganisms 2020, 8, 1765. [Google Scholar] [CrossRef]
- Wu, T.; Yu, S.; Chen, D.; Wang, Y. Bionic Design, Materials and Performance of Bone Tissue Scaffolds. Materials 2017, 10, 1187. [Google Scholar] [CrossRef]
- Roohani-Esfahani, S.-I.; Newman, P.; Zreiqat, H. Design and Fabrication of 3D printed Scaffolds with a Mechanical Strength Comparable to Cortical Bone to Repair Large Bone Defects. Sci. Rep. 2016, 6, 19468. [Google Scholar] [CrossRef]
- Pilia, M.; Guda, T.; Appleford, M. Development of Composite Scaffolds for Load-Bearing Segmental Bone Defects. BioMed Res. Int. 2013, 2013, 1–15. [Google Scholar] [CrossRef]
- Leng, H.; Reyes, M.J.; Dong, X.N.; Wang, X. Effect of age on mechanical properties of the collagen phase in different orientations of human cortical bone. Bone 2013, 55, 288–291. [Google Scholar] [CrossRef]
- Domingues, R.M.A.; Gomes, M.E.; Reis, R.L. The Potential of Cellulose Nanocrystals in Tissue Engineering Strategies. Biomacromolecules 2014, 15, 2327–2346. [Google Scholar] [CrossRef] [PubMed]
- Shelke, N.B.; James, R.; Laurencin, C.T.; Kumbar, S.G. Polysaccharide biomaterials for drug delivery and regenerative engineering. Polym. Adv. Technol. 2014, 25, 448–460. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Plants and plant-based polymers as scaffolds for tissue engineering. Green Chem. 2019, 21, 4839–4867. [Google Scholar] [CrossRef]
- Xu, K.; Liu, C.; Kang, K.; Zheng, Z.; Wang, S.; Tang, Z.; Yang, W. Isolation of nanocrystalline cellulose from rice straw and preparation of its biocomposites with chitosan: Physicochemical characterization and evaluation of interfacial compatibility. Compos. Sci. Technol. 2018, 154, 8–17. [Google Scholar] [CrossRef]
- Luo, W.; Cheng, L.; Yuan, C.; Wu, Z.; Yuan, G.; Hou, M.; Chen, J.Y.; Luo, C.; Li, W. Preparation, characterization and evaluation of cellulose nanocrystal/poly(lactic acid) in situ nanocomposite scaffolds for tissue engineering. Int. J. Biol. Macromol. 2019, 134, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, A.; Rigotti, D.; Nguyen, V.D.H.; Pegoretti, A. Polyvinyl alcohol reinforced with crystalline nanocellulose for 3D printing application. Mater. Today Commun. 2018, 15, 236–244. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ioelovich, M.; Ahmad, I.; Thomas, S.; Dufresne, A. Methods for Extraction of Nanocellulose from Various Sources. In Handbook of Nanocellulose and Cellulose Nanocomposites; Wiley: Hoboken, NJ, USA, 2017; pp. 1–49. [Google Scholar] [CrossRef]
- Shaheen, T.I.; Montaser, A.S.; Li, S. Effect of cellulose nanocrystals on scaffolds comprising chitosan, alginate and hydroxyapatite for bone tissue engineering. Int. J. Biol. Macromol. 2019, 121, 814–821. [Google Scholar] [CrossRef]
- Nascimento, D.M.; Almeida, J.S.; Dias, A.F.; Figueirêdo, M.C.B.; Morais, J.P.S.; Feitosa, J.P.A.; Rosa, M.d.F. A novel green approach for the preparation of cellulose nanowhiskers from white coir. Carbohydr. Polym. 2014, 110, 456–463. [Google Scholar] [CrossRef]
- Hebeish, A.; Farag, S.; Sharaf, S.; Rabie, A.M.; Shaheen, T.I. Modulation of the Nanostructural Characteristics of Cellulose Nanowhiskers via Sulfuric Acid Concentration. Egypt. J. Chem. 2013, 56, 271–289. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Yan, H.; Chen, X.; Feng, M.; Shi, Z.; Zhang, D.; Lin, Q. Layer-by-layer assembly of 3D alginate-chitosan-gelatin composite scaffold incorporating bacterial cellulose nanocrystals for bone tissue engineering. Mater. Lett. 2017, 209, 492–496. [Google Scholar] [CrossRef]
- Dinh Vu, N.; Thi Tran, H.; Duy Nguyen, T. Characterization of Polypropylene Green Composites Reinforced by Cellulose Fibers Extracted from Rice Straw. Int. J. Polym. Sci. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Mohan, D.J.; Kang, I.-A.; Doh, G.-H.; Lee, S.; Han, S.O. Nanocellulose reinforced PVA composite films: Effects of acid treatment and filler loading. Fibers Polym. 2009, 10, 77–82. [Google Scholar] [CrossRef]
- Mtibe, A.; Linganiso, L.Z.; Mathew, A.P.; Oksman, K.; John, M.J.; Anandjiwala, R.D. A comparative study on properties of micro and nanopapers produced from cellulose and cellulose nanofibres. Carbohydr. Polym. 2015, 118, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.-M.; Sin, L.T.; Tee, T.-T.; Bee, S.-T.; Hui, D.; Low, C.-Y.; Rahmat, A.R. Extraction of cellulose nanocrystals from plant sources for application as reinforcing agent in polymers. Compos. Part B Eng. 2015, 75, 176–200. [Google Scholar] [CrossRef]
- Watkins, D.; Nuruddin, M.; Hosur, M.; Tcherbi-Narteh, A.; Jeelani, S. Extraction and characterization of lignin from different biomass resources. J. Mater. Res. Technol. 2015, 4, 26–32. [Google Scholar] [CrossRef]
- Lu, P.; Hsieh, Y.-L. Preparation and characterization of cellulose nanocrystals from rice straw. Carbohydr. Polym. 2012, 87, 564–573. [Google Scholar] [CrossRef]
- Sunasee, R.; Hemraz, U. Synthetic Strategies for the Fabrication of Cationic Surface-Modified Cellulose Nanocrystals. Fibers 2018, 6, 15. [Google Scholar] [CrossRef]
- Chen, D.; Lawton, D.; Thompson, M.R.; Liu, Q. Biocomposites reinforced with cellulose nanocrystals derived from potato peel waste. Carbohydr. Polym. 2012, 90, 709–716. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ahmad, I.; Abdullah, I.; Dufresne, A.; Zainudin, S.Y.; Sheltami, R.M. Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose 2012, 19, 855–866. [Google Scholar] [CrossRef]
- Nur Hanani, A.S.; Zuliahani, A.; Nawawi, W.I.; Razif, N.; Rozyanty, A.R. The Effect of Various Acids on Properties of Microcrystalline Cellulose (MCC) Extracted from Rice Husk (RH). In Proceedings of the IOP Conference Series: Materials Science and Engineering, Bali, Indonesia, 1–3 April 2017; pp. 1–9. [Google Scholar]
- Mahsut Dinçel, Y. Bone Graft Types. In Bone Grafting—Recent Advances with Special References to Cranio-Maxillofacial Surgery; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Dinh Vu, N.; Thi Tran, H.; Bui, N.D.; Duc Vu, C.; Viet Nguyen, H. Lignin and Cellulose Extraction from Vietnam’s Rice Straw Using Ultrasound-Assisted Alkaline Treatment Method. Int. J. Polym. Sci. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Szymańska-Chargot, M.; Chylińska, M.; Gdula, K.; Kozioł, A.; Zdunek, A. Isolation and Characterization of Cellulose from Different Fruit and Vegetable Pomaces. Polymers 2017, 9, 495. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.K.; Tiwari, A.P.; Pant, H.R.; Shrestha, B.K.; Kim, H.J.; Park, C.H.; Kim, C.S. In Situ Generation of Cellulose Nanocrystals in Polycaprolactone Nanofibers: Effects on Crystallinity, Mechanical Strength, Biocompatibility, and Biomimetic Mineralization. ACS Appl. Mater. Interfaces 2015, 7, 19672–19683. [Google Scholar] [CrossRef] [PubMed]
- Osorio, D.A.; Lee, B.E.J.; Kwiecien, J.M.; Wang, X.; Shahid, I.; Hurley, A.L.; Cranston, E.D.; Grandfield, K. Cross-linked cellulose nanocrystal aerogels as viable bone tissue scaffolds. Acta Biomater. 2019, 87, 152–165. [Google Scholar] [CrossRef]
- Nuruddin, M.; Chowdhury, A.; Haque, S.A.; Rahman, M.; Farhad, S.F.; Jahan, M.S.; Quaiyyum, A. Extraction and Characterization of Cellulose Microfibrils from Agricultural Wastes in an Integrated Biorefinery Initiative. Cell Chem. Technol. 2011, 45, 347–354. [Google Scholar]
- Ratanakamnuan, U.; Ninsin, Y.Y. Synthesis of Rice Straw Cellulose Ester for Use as Biodegradable Plastic Film. Adv. Mater. Res. 2012, 488–489, 980–984. [Google Scholar] [CrossRef]
- Li, V.C.-F.; Dunn, C.K.; Zhang, Z.; Deng, Y.; Qi, H.J. Direct Ink Write (DIW) 3D Printed Cellulose Nanocrystal Aerogel Structures. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Hong, J.K. Bioactive Cellulose Nanocrystal Reinforced 3D Printable Poly(ε-caprolactone) Nanocomposite for Bone Tissue Engineering. Ph.D. Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2015. [Google Scholar]
- Alemán-Domínguez, M.E.; Giusto, E.; Ortega, Z.; Tamaddon, M.; Benítez, A.N.; Liu, C. Three-dimensional printed polycaprolactone-microcrystalline cellulose scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 521–528. [Google Scholar] [CrossRef]
- Herdocia-Lluberes, C.S.; Laboy-López, S.; Morales, S.; Gonzalez-Robles, T.J.; González-Feliciano, J.A.; Nicolau, E. Evaluation of Synthesized Nanohydroxyapatite-Nanocellulose Composites as Biocompatible Scaffolds for Applications in Bone Tissue Engineering. J. Nanomater. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Tran, T.T.; Hamid, Z.A.; Cheong, K.Y. A Review of Mechanical Properties of Scaffold in Tissue Engineering: Aloe Vera Composites. J. Phys. Conf. Ser. 2018, 1082, 012080. [Google Scholar] [CrossRef]
- Balagangadharan, K.; Dhivya, S.; Selvamurugan, N. Chitosan based nanofibers in bone tissue engineering. Int. J. Biol. Macromol. 2017, 104, 1372–1382. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Shi, Q.; Guo, W.; Terrell, L.; Qureshi, A.T.; Hayes, D.J.; Wu, Q. Electrospun Bio-Nanocomposite Scaffolds for Bone Tissue Engineering by Cellulose Nanocrystals Reinforcing Maleic Anhydride Grafted PLA. ACS Appl. Mater. Interfaces 2013, 5, 3847–3854. [Google Scholar] [CrossRef]
- Zhang, C.; Salick, M.R.; Cordie, T.M.; Ellingham, T.; Dan, Y.; Turng, L.-S. Incorporation of poly(ethylene glycol) grafted cellulose nanocrystals in poly(lactic acid) electrospun nanocomposite fibers as potential scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2015, 49, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Charles-Harris, M.; del Valle, S.; Hentges, E.; Bleuet, P.; Lacroix, D.; Planell, J.A. Mechanical and structural characterisation of completely degradable polylactic acid/calcium phosphate glass scaffolds. Biomaterials 2007, 28, 4429–4438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Vijayavenkataraman, S.; Lu, W.F.; Fuh, J.Y.H. A review on the use of computational methods to characterize, design, and optimize tissue engineering scaffolds, with a potential in 3D printing fabrication. J. Biomed. Mater. Res. B 2019, 107, 1329–1351. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, S.; El Sawi, I.; Bagheri, Z.S.; Turcotte, G.; Bougherara, H. Fabrication and characterization of novel biomimetic PLLA/cellulose/hydroxyapatite nanocomposite for bone repair applications. Mater. Sci. Eng. C-Mater. Biol. Appl. 2014, 39, 120–125. [Google Scholar] [CrossRef]
- Li, V. 3D Printing Structured Nanocelluloses and Their Composites: Printability, Structures, and Properties. Ph.D. Thesis, Georgia Institute of Technology, Atlanta, GA, USA, 2019. [Google Scholar]
- Kumar, A.; Negi, Y.S.; Choudhary, V.; Bhardwaj, N.K. Fabrication of poly (vinyl alcohol)/ovalbumin/cellulose nanocrystals/nanohydroxyapatite based biocomposite scaffolds. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 191–201. [Google Scholar] [CrossRef]
- Chen, X.M.; Zhou, R.M.; Chen, B.; Chen, J.T. Nanohydroxyapatite/cellulose nanocrystals/silk fibroin ternary scaffolds for rat calvarial defect regeneration. RSC Adv. 2016, 6, 35684–35691. [Google Scholar] [CrossRef]
- Niamsap, T.; Lam, N.T.; Sukyai, P. Production of hydroxyapatite-bacterial nanocellulose scaffold with assist of cellulose nanocrystals. Carbohydr. Polym. 2019, 205, 159–166. [Google Scholar] [CrossRef]
- Fallahiarezoudar, E.; Ahmadipourroudposht, M.; Yusof, N.M.; Idris, A.; Ngadiman, N.H.A. 3D Biofabrication of Thermoplastic Polyurethane (TPU)/Poly-l-lactic Acid (PLLA) Electrospun Nanofibers Containing Maghemite (gamma-Fe(2)O(3)) for Tissue Engineering Aortic Heart Valve. Polymers 2017, 9, 584. [Google Scholar] [CrossRef]
- Kamboj, N.; Kazantseva, J.; Rahmani, R.; Rodríguez, M.A.; Hussainova, I. Selective laser sintered bio-inspired silicon-wollastonite scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2020, 116, 111223. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.; Male, K.B.; Chong, J.H.; Leung, A.C.W.; Luong, J.H.T. Applications of functionalized and nanoparticle-modified nanocrystalline cellulose. Trends Biotechnol. 2012, 30, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Martson, M.; Viljanto, J.; Hurme, T.; Laippala, P.; Saukko, P. Is cellulose sponge degradable or stable as implantation material? An in vivo subcutaneous study in the rat. Biomaterials 1999, 20, 1989–1995. [Google Scholar] [CrossRef]
- Huang, C.; Bhagia, S.; Hao, N.J.; Meng, X.Z.; Liang, L.N.; Yong, Q.; Ragauskas, A.J. Biomimetic composite scaffold from an in situ hydroxyapatite coating on cellulose nanocrystals. RSC Adv. 2019, 9, 5786–5793. [Google Scholar] [CrossRef]


| Type of Cellulose Particles | Sources | Derivation Methods | Particles Size | Crystallinity Index (%) | Ref. | ||
|---|---|---|---|---|---|---|---|
| ℓ (µm) | W (nm) | ||||||
| Bacterial cellulose (BC) | Bacterial nanocellulose (BNC) | Low molecular weight sugars and alcohols | Bacterial synthesis with the presence of Gluconacetobacter xylinus | >1 | 30–50 | 65–79 | [13,32,48] |
| Fibrillated cellulose (FC) | Microfibrillated cellulose (MFC) | Wood pulp, potato peel, sugar beet, hemp | Mechanical disintegration produced by high pressure and/or shearing forces of mechanical fibrillated after pretreatment | 0.5–50 | 10–100 | 51–69 | [49] |
| Nanofibrillated cellulose (NFC) | 0.5–2 | 4–20 | - | [50] | |||
| Crystalline cellulose (CC) | Microcrystalline cellulose (MCC) | Cotton, softwood pulp, rice husk, rice straw, wheat straw, empty fruit brunch, ramie, corn stalk, some form of algae and bacteria | Purified cellulosic fibers undergo chemical (acid) hydrolysis after complete dissolution of the non-crystalline fraction | 10–50 | 10–20 | 80–85 | [51] |
| Nanocrystalline cellulose (NCC) | 0.05–0.5 | 3–5 | 54–90 | [45,52] | |||
| Cellulose Sources | Polymer | Additives/Modification | Material Composition | Fabrication Method | Mechanical Properties (MPa) | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| σT | E | σC | E | ||||||
| Cotton | PLA | PLA grafted maleic anhydride (MPLA) | MPLA | Electrospinning | 1.6 ± 0.4 | 7.8 ± 3.1 | - | - | [77] |
| MPLA/NCC-1 | 4.8 ± 0.8 | 77.2 ± 7.8 | |||||||
| MPLA/NCC-2 | 4.9 ± 1.0 | 87.4 ± 8.0 | |||||||
| MPLA/NCC-5 | 10.8 ± 1.7 | 135.1 ± 10.4 | |||||||
| PLA/NCC-5 | 6.3 ± 1.2 | 125.6 ± 9.9 | |||||||
| Cotton | PLA | Add SnCl2·H2O; p-TSA | PLA | Freeze-drying/lyophilization | - | - | - | 19 | [46] |
| PLA/NCC-0.2 | 25 | ||||||||
| PLA/NCC-0.4 | 38 | ||||||||
| PLA/NCC-0.6 | 65 | ||||||||
| PLA/NCC-0.8 | 89 | ||||||||
| Pine wood | PLA | NCC grafted with peg (CNC-g-PEG) | PLA | Electrospinning | 2.8 ± 0.4 | - | - | - | [78] |
| PLA/NCC-1 | 2.8 ± 0.5 | ||||||||
| PLA/NCC-5 | 2.3 ± 0.5 | ||||||||
| PLA/NCC-g-PEG-1 | 3.5 ± 0.2 | ||||||||
| PLA/NCC-g-PEG-5 | 4.7 ± 0.3 | ||||||||
| PLA/NCC-g-PEG-10 | 2.8 ± 0.3 | ||||||||
| Sugarcane bagasse | PVA | Incorporated with n-HA | PVA | Freeze-drying | - | - | 0.4 | 0.32 | [27] |
| PVA/n-HA | 0.85 | 4.68 | |||||||
| PVA/n-HA/NCC-2 | 1.39 | 10.67 | |||||||
| PVA/n-HA/NCC-4 | 1.4 | 10.1 | |||||||
| PVA/n-HA/NCC-6 | 1.48 | 13.41 | |||||||
| PVA/n-HA/NCC-8 | 1.6 | 14.5 | |||||||
| PVA/n-HA/NCC-10 | 2.09 | 16.01 | |||||||
| Commercialized purified cellulose | PVA | Incorporated with ovalbumin (OVA) and n-HA and cross-linked with glutaraldehyde Composition: PVA/OVA/NCC/n-HA | PVA/OVA/NCC/n-HA | Freeze-drying | - | - | - | - | [83] |
| 1/0.2/0.25/0 | 0.29 | 0.37 | |||||||
| 1/0.2/0/2/0.25 | 0.2 | 0.46 | |||||||
| 1/0.2/0.15/0.5 | 0.19 | 0.92 | |||||||
| 1/0.2/0.1/0.75 | 0.37 | 1.2 | |||||||
| 1/0.20/0.05/1 | 0.25 | 0.37 | |||||||
| 1/0.2/0/1.25 | 0.33 | 0.4 | |||||||
| Commercialized MCC | PVA | - | PVA | Fused deposition modelling (FDM) | 11.19 | 2.88 | - | - | [47] |
| PVA/NCC-2 | 11.69 | 4.25 | |||||||
| PVA/NCC-5 | 19.32 | 4.98 | |||||||
| PVA/NCC-10 | 15.46 | 5.71 | |||||||
| Commercialized MCC | PCL | - | PCL: MCC (1:0) | Fused deposition modelling (FDM) | - | - | - | 25 | [73] |
| PCL: MCC (49:1) | 32 | ||||||||
| PCL: MCC (19:1) | 29 | ||||||||
| PCL: MCC (9:1) | 7 | ||||||||
| Softwood sulfite pulp | PCL | Surface oxidation NCC | PCL | Micro-extrusion | 10.4 ± 0.9 | 194.3 ± 12.1 | - | - | [72] |
| PCL/NCC-1 | 13.4 ± 1.5 | 275.2 ± 14.4 | |||||||
| PCL/NCC-2 | 15.3 ± 1.0 | 299.9 ± 15.2 | |||||||
| PCL/NCC-3 | 16.3 ± 1.4 | 353.1 ± 20.9 | |||||||
| PCL/NCC-5 | 16.6 ± 0.3 | 373.8 ± 18.6 | |||||||
| PCL/NCC-10 | 18.2 ± 0.3 | 492.5 ± 44.1 | |||||||
| Wood pulp | - | NCC coated with HAP | NCC | Freeze-drying | - | - | - | 80.6 ± 1.4 KPa | [84] |
| NCC/HAP at pH 7.4 | 119.6 ± 2.7 KPa | ||||||||
| NCC/HAP at pH 8.5 | 227.6 ± 2.7 KPa | ||||||||
| NCC/HAP | 92.5 ± 2.3 KPa | ||||||||
| Cotton | Chitosan/alginate/HAP | Dicationic crosslinking using CaCl2 | SC | Freeze-drying | - | - | 0.35 | - | [53] |
| SC/HA | 0.38 | ||||||||
| SC/HA/NCC-0.5 | 0.48 | ||||||||
| SC/HA/NCC-1.0 | 0.54 | ||||||||
| SC/HA/NCC-2.0 | 0.51 | ||||||||
| Not stated | Silk fibroin (SF) | Incorporated with n-HA | SF | Freeze-drying | - | - | 92.1 ± 7.3 KPa | 175.2 ± 10.65 KPa | [85] |
| SF/NCC | 100.8 ± 13.5 KPa | 200 ± 12.3 KPa | |||||||
| SF/n-HA | 140.1 ± 11.4 KPa | 428.3 ± 14.4 KPa | |||||||
| SF/n-HA/NCC | 200.7 ± 15.3 KPa | 617.5 ± 25.2 KPa | |||||||
| Cotton | PLLA | Incorporated with HA pretreatment of particles using a coupling agent | PLLA/HA/MCC | Freeze-drying | - | - | 0.5–2.3 | 8–47 | [81] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murizan, N.I.S.; Mustafa, N.S.; Ngadiman, N.H.A.; Mohd Yusof, N.; Idris, A. Review on Nanocrystalline Cellulose in Bone Tissue Engineering Applications. Polymers 2020, 12, 2818. https://doi.org/10.3390/polym12122818
Murizan NIS, Mustafa NS, Ngadiman NHA, Mohd Yusof N, Idris A. Review on Nanocrystalline Cellulose in Bone Tissue Engineering Applications. Polymers. 2020; 12(12):2818. https://doi.org/10.3390/polym12122818
Chicago/Turabian StyleMurizan, Nur Ilyana Sahira, Nur Syahirah Mustafa, Nor Hasrul Akhmal Ngadiman, Noordin Mohd Yusof, and Ani Idris. 2020. "Review on Nanocrystalline Cellulose in Bone Tissue Engineering Applications" Polymers 12, no. 12: 2818. https://doi.org/10.3390/polym12122818
APA StyleMurizan, N. I. S., Mustafa, N. S., Ngadiman, N. H. A., Mohd Yusof, N., & Idris, A. (2020). Review on Nanocrystalline Cellulose in Bone Tissue Engineering Applications. Polymers, 12(12), 2818. https://doi.org/10.3390/polym12122818





