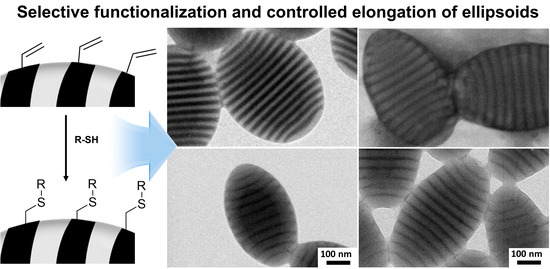
Effect of Site-Specific Functionalization on the Shape of Nonspherical Block Copolymer Particles
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Characterization
2.3. Preparation of PS34k-b-PB25k Ellipsoids via Membrane Emulsification
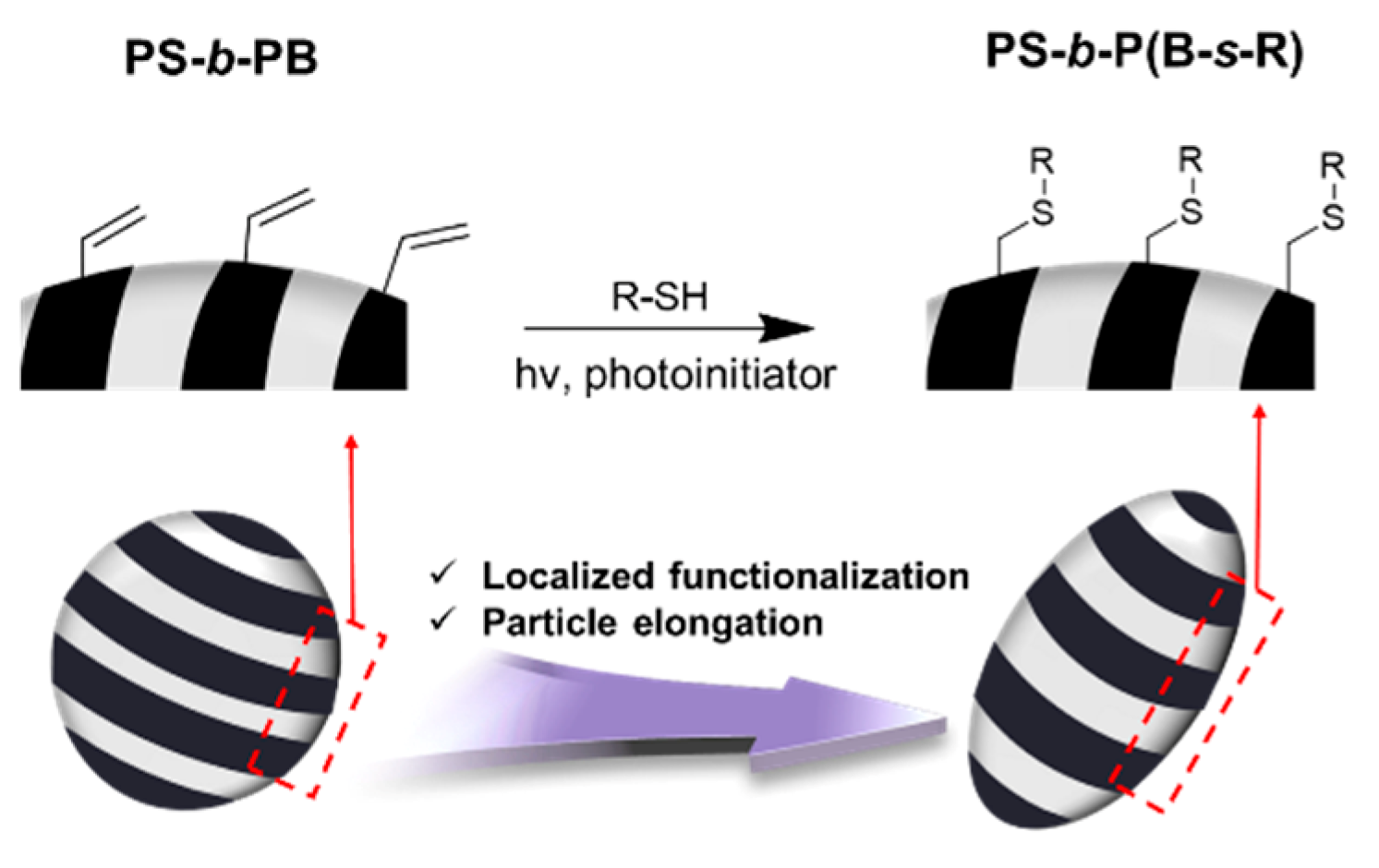
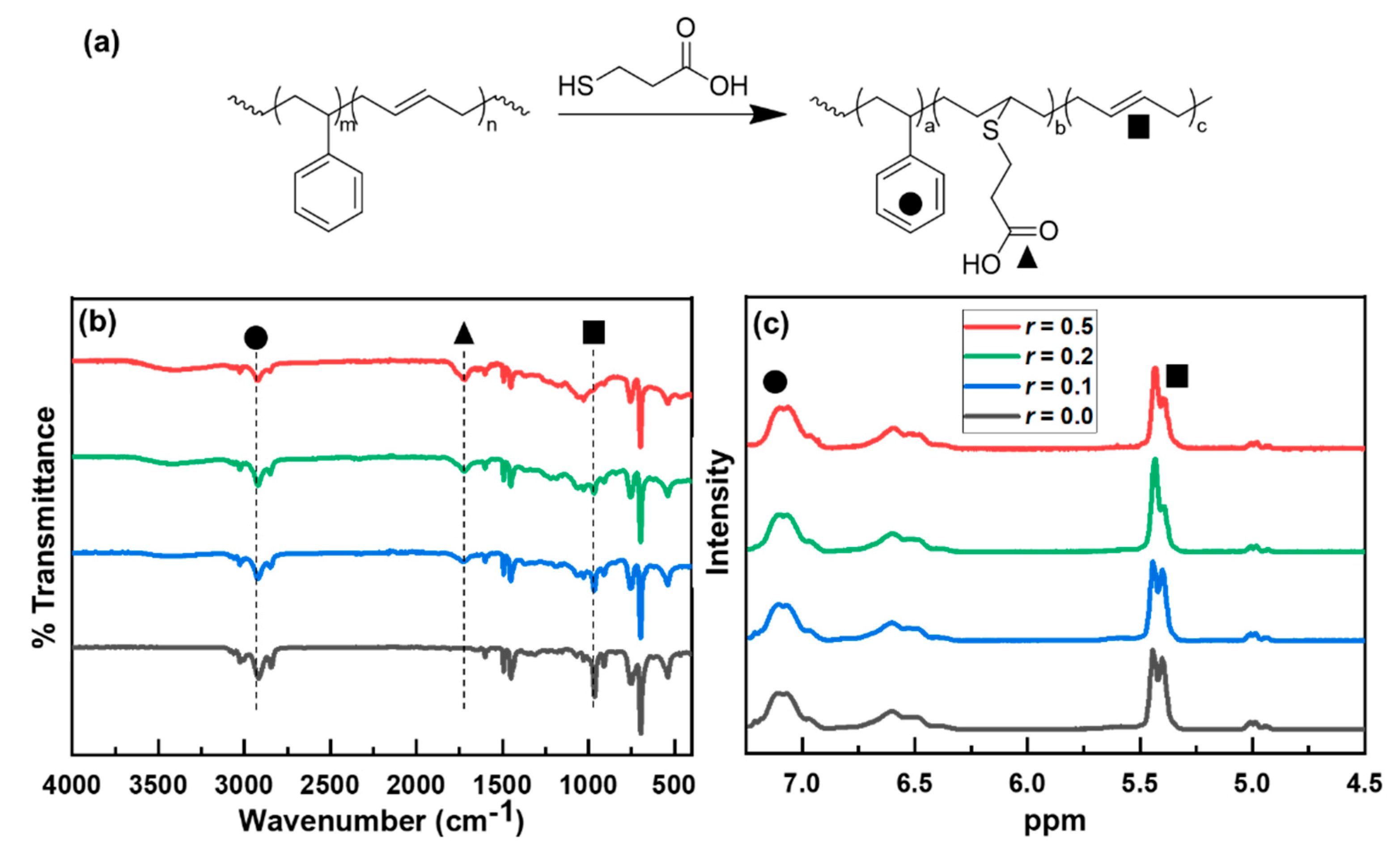
2.4. Modification of PB domain of the Ellipsoidal Particles via Thiol-Ene Photochemistry
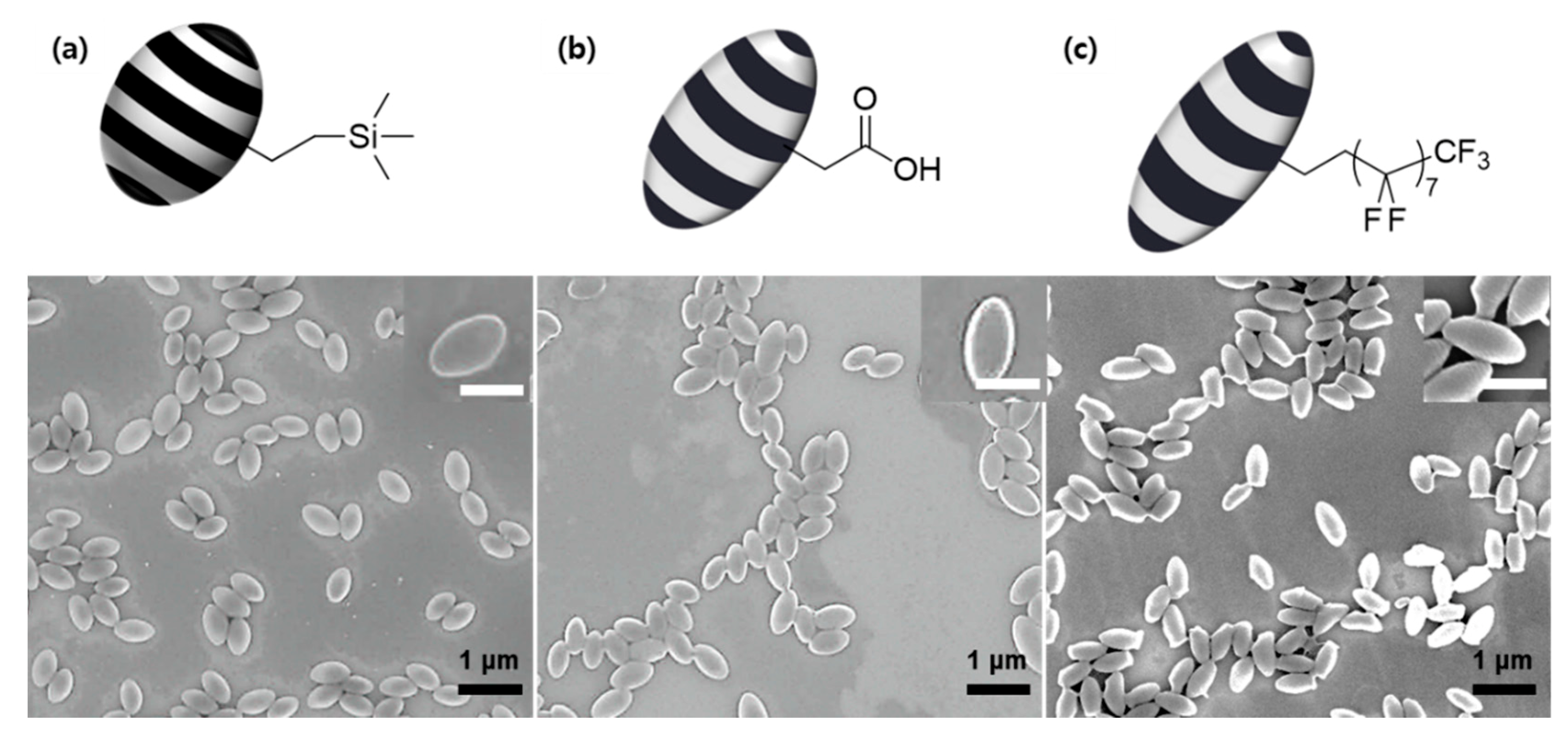
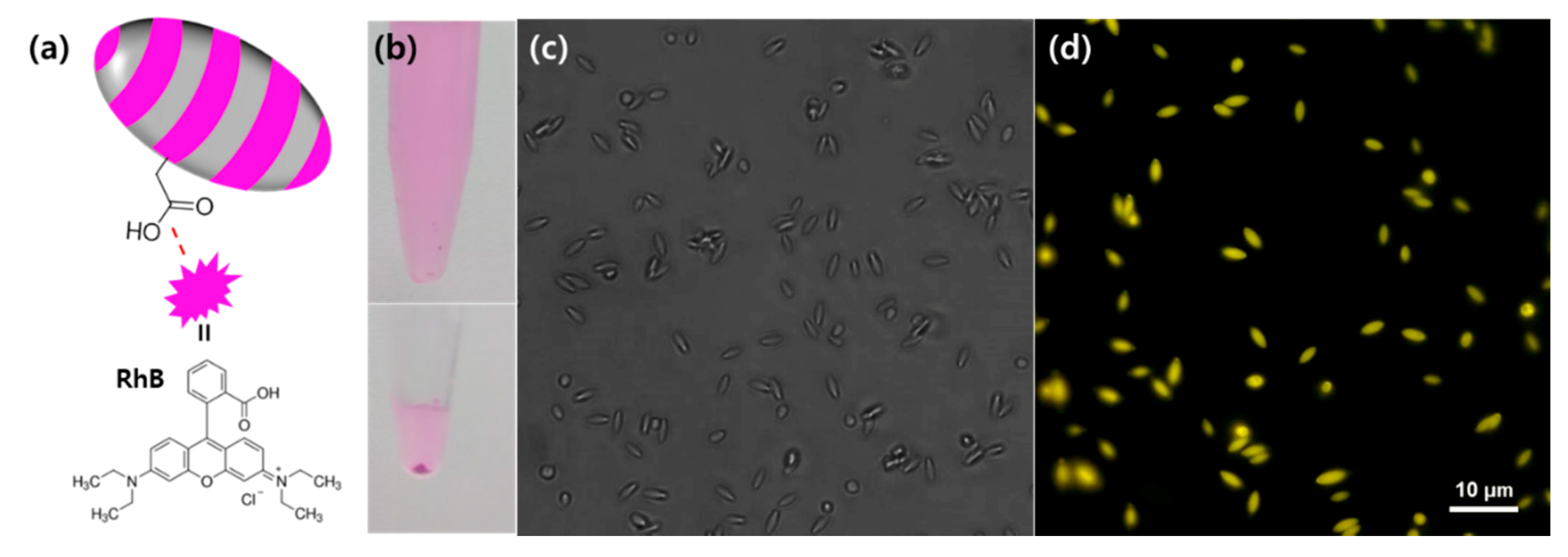
3. Results and Discussion
4. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Wang, Y.; Zheng, X.; Yi, G.R.; Sacanna, S.; Pine, D.J.; Weck, M. Three-dimensional lock and key colloids. J. Am. Chem. Soc. 2014, 136, 6866–6869. [Google Scholar] [CrossRef] [PubMed]
- Sacanna, S.; Irvine, W.T.M.; Chaikin, P.M.; Pine, D.J. Lock and key colloids. Nature 2010, 464, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.S.; Yi, G.R.; Pine, D.J. Reconfigurable Self-Assembly and Kinetic Control of Multiprogrammed DNA-Coated Particles. ACS Nano 2020, 14, 4595–4600. [Google Scholar] [CrossRef] [PubMed]
- Reisch, A.; Klymchenko, A.S. Fluorescent Polymer Nanoparticles Based on Dyes: Seeking Brighter Tools for Bioimaging. Small 2016, 12, 1968–1992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otsuka, H.; Nagasaki, Y.; Kataoka, K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv. Drug Deliv. Rev. 2012, 64, 246–255. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Yang, Y.; Urban, M.W. Stimuli-Responsive Polymeric Nanoparticles. Macromol. Rapid Commun. 2017, 38, 1700030. [Google Scholar] [CrossRef] [Green Version]
- Ratcliffe, L.P.D.; Blanazs, A.; Williams, C.N.; Brown, S.L.; Armes, S.P. RAFT polymerization of hydroxy-functional methacrylic monomers under heterogeneous conditions: Effect of varying the core-forming block. Polym. Chem. 2014, 5, 3643–3655. [Google Scholar] [CrossRef] [Green Version]
- Song, X.J.; Hu, J.; Wang, C.C. Synthesis of highly surface functionalized monodispersed poly(St/DVB/GMA) nanospheres with soap-free emulsion polymerization followed by facile “ click chemistry” with functionalized alkylthiols. Colloids Surfaces A Physicochem. Eng. Asp. 2011, 380, 250–256. [Google Scholar] [CrossRef]
- Oh, J.S.; Dang, L.N.; Yoon, S.W.; Lee, P.C.; Kim, D.O.; Kim, K.J.; Nam, J. Do Amine-functionalized polyglycidyl methacrylate microsphere as a unified template for the synthesis of gold nanoparticles and single-crystal gold plates. Macromol. Rapid Commun. 2013, 34, 1585. [Google Scholar] [CrossRef]
- Sekerak, N.M.; Hutchins, K.M.; Luo, B.; Kang, J.G.; Braun, P.V.; Chen, Q.; Moore, J.S. Size control of cross-linked carboxy-functionalized polystyrene particles: Four orders of magnitude of dimensional versatility. Eur. Polym. J. 2018, 101, 202–210. [Google Scholar] [CrossRef]
- Oh, J.S.; Wang, Y.; Pine, D.J.; Yi, G.R. High-Density PEO-b-DNA Brushes on Polymer Particles for Colloidal Superstructures. Chem. Mater. 2015, 27, 8337–8344. [Google Scholar] [CrossRef]
- Abdelrahman, A.I.; Thickett, S.C.; Liang, Y.; Ornatsky, O.; Baranov, V.; Winnik, M.A. Surface functionalization methods to enhance bioconjugation in metal-labeled polystyrene particles. Macromolecules 2011, 44, 4801–4813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamagami, T.; Kitayama, Y.; Okubo, M. Preparation of stimuli-responsive “mushroom-like” janus polymer particles as particulate surfactant by site-selective surface-initiated AGET ATRP in aqueous dispersed systems. Langmuir 2014, 30, 7823–7832. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.S.; Lee, S.; Glotzer, S.C.; Yi, G.R.; Pine, D.J. Colloidal fibers and rings by cooperative assembly. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roh, K.; Martin, D.C.; Lahann, J. Biphasic Janus particles with nanoscale anisotropy. Nat. Mater. 2005, 4, 759–763. [Google Scholar] [CrossRef]
- Bradley, L.C.; Stebe, K.J.; Lee, D. Clickable Janus Particles. J. Am. Chem. Soc. 2016, 138, 11437–11440. [Google Scholar] [CrossRef]
- Jin, Z.; Fan, H. Self-assembly of nanostructured block copolymer nanoparticles. Soft Matter 2014, 10, 9212–9219. [Google Scholar] [CrossRef]
- Wyman, I.; Njikang, G.; Liu, G. When emulsification meets self-assembly: The role of emulsification in directing block copolymer assembly. Prog. Polym. Sci. 2011, 36, 1152–1183. [Google Scholar] [CrossRef]
- Wong, C.K.; Qiang, X.; Müller, A.H.E.; Gröschel, A.H. Self-Assembly of block copolymers into internally ordered microparticles. Prog. Polym. Sci. 2020, 102, 101211. [Google Scholar] [CrossRef]
- Ku, K.H.; Shin, J.M.; Yun, H.; Yi, G.-R.; Jang, S.G.; Kim, B.J. Multidimensional Design of Anisotropic Polymer Particles from Solvent-Evaporative Emulsion. Adv. Funct. Mater. 2018, 28, 1802961. [Google Scholar] [CrossRef]
- Shin, J.J.; Kim, E.J.; Ku, K.H.; Lee, Y.J.; Hawker, C.J.; Kim, B.J. 100th Anniversary of Macromolecular Science Viewpoint: Block Copolymer Particles: Tuning Shape, Interfaces, and Morphology. ACS Macro Lett. 2020, 9, 306–317. [Google Scholar] [CrossRef]
- Chen, C.; Wylie, R.A.L.; Klinger, D.; Connal, L.A. Shape Control of Soft Nanoparticles and Their Assemblies. Chem. Mater. 2017, 29, 1918–1945. [Google Scholar] [CrossRef]
- Jang, S.G.; Audus, D.J.; Klinger, D.; Krogstad, D.V.; Kim, B.J.; Cameron, A.; Kim, S.W.; Delaney, K.T.; Hur, S.M.; Killops, K.L.; et al. Striped, ellipsoidal particles by controlled assembly of diblock copolymers. J. Am. Chem. Soc. 2013, 135, 6649–6657. [Google Scholar] [CrossRef] [PubMed]
- Klinger, D.; Wang, C.X.; Connal, L.A.; Audus, D.J.; Jang, S.G.; Kraemer, S.; Killops, K.L.; Fredrickson, G.H.; Kramer, E.J.; Hawker, C.J. A facile synthesis of dynamic, shape-changing polymer particles. Angew. Chem. Int. Ed. 2014, 53, 7018–7022. [Google Scholar] [CrossRef] [Green Version]
- Deng, R.; Liang, F.; Zhou, P.; Zhang, C.; Qu, X.; Wang, Q.; Li, J.; Zhu, J.; Yang, Z. Janus nanodisc of diblock copolymers. Adv. Mater. 2014, 26, 4469–4472. [Google Scholar] [CrossRef]
- Jeon, S.J.; Yi, G.R.; Yang, S.M. Cooperative assembly of block copolymers with deformable interfaces: Toward nanostructured particles. Adv. Mater. 2008, 20, 4103–4108. [Google Scholar] [CrossRef]
- Schmidt, B.V.K.J.; Elbert, J.; Scheid, D.; Hawker, C.J.; Klinger, D.; Gallei, M. Metallopolymer-Based Shape Anisotropic Nanoparticles. ACS Macro Lett. 2015, 4, 731–735. [Google Scholar] [CrossRef]
- Ku, K.H.; Yang, H.; Shin, J.M.; Kim, B.J. Aspect ratio effect of nanorod surfactants on the shape and internal morphology of block copolymer particles. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 188–192. [Google Scholar] [CrossRef]
- Ku, K.H.; Ryu, J.H.; Kim, J.; Yun, H.; Nam, C.; Shin, J.M.; Kim, Y.; Jang, S.G.; Lee, W.B.; Kim, B.J. Mechanistic Study on the Shape Transition of Block Copolymer Particles Driven by Length-Controlled Nanorod Surfactants. Chem. Mater. 2018, 30, 8669–8678. [Google Scholar] [CrossRef]
- Yang, H.; Ku, K.H.; Shin, J.M.; Lee, J.; Park, C.H.; Cho, H.H.; Jang, S.G.; Kim, B.J. Engineering the Shape of Block Copolymer Particles by Surface-Modulated Graphene Quantum Dots. Chem. Mater. 2016, 28, 830–837. [Google Scholar] [CrossRef]
- Shin, J.M.; Kim, Y.; Yun, H.; Yi, G.-R.; Kim, B.J. Morphological Evolution of Block Copolymer Particles: Effect of Solvent Evaporation Rate on Particle Shape and Morphology. ACS Nano 2017, 11, 2133–2142. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Motoyoshi, K.; Sugimori, H.; Jinnai, H.; Yabu, H.; Shimomura, M. Phase transition and phase transformation in block copolymer nanoparticles. Macromol. Rapid Commun. 2010, 31, 1773–1778. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.M.; Kim, M.P.; Yang, H.; Ku, K.H.; Jang, S.G.; Youm, K.H.; Yi, G.R.; Kim, B.J. Monodispserse Nanostructured Spheres of Block Copolymers and Nanoparticles via Cross-Flow Membrane Emulsification. Chem. Mater. 2015, 27, 6314–6321. [Google Scholar] [CrossRef]
- Shin, J.M.; Kim, Y.; Ku, K.H.; Lee, Y.J.; Kim, E.J.; Yi, G.-R.; Kim, B.J. Aspect Ratio-Controlled Synthesis of Uniform Colloidal Block Copolymer Ellipsoids from Evaporative Emulsions. Chem. Mater. 2018, 30, 6277–6288. [Google Scholar] [CrossRef]
- Shin, J.M.; Lee, Y.J.; Kim, M.; Ku, K.H.; Lee, J.; Kim, Y.; Yun, H.; Liao, K.; Hawker, C.J.; Kim, B.J. Development of Shape-Tuned, Monodisperse Block Copolymer Particles through Solvent-Mediated Particle Restructuring. Chem. Mater. 2019, 31, 1066–1074. [Google Scholar] [CrossRef]
- Steinhaus, A.; Chakroun, R.; Mullner, M.; Nghiem, T.L.; Hildebrandt, M.; Groschel, A.H. Confinement Assembly of ABC Triblock Terpolymers for the High-Yield Synthesis of Janus Nanorings. ACS Nano 2019, 13, 6269–6278. [Google Scholar] [CrossRef] [Green Version]
- Qiang, X.; Dai, X.; Steinhaus, A.; Gröschel, A.H. Multicompartment Microparticles with Patchy Topography through Solvent-Adsorption Annealing. ACS Macro Lett. 2019, 1654–1659. [Google Scholar] [CrossRef]
- Xu, J.; Wang, K.; Li, J.; Zhou, H.; Xie, X.; Zhu, J. ABC triblock copolymer particles with tunable shape and internal structure through 3D confined assembly. Macromolecules 2015, 48, 2628–2636. [Google Scholar] [CrossRef]
- Steinhaus, A.; Pelras, T.; Chakroun, R.; Gröschel, A.H.; Müllner, M. Self-Assembly of Diblock Molecular Polymer Brushes in the Spherical Confinement of Nanoemulsion Droplets-SI. Macromol. Rapid Commun. 2018, 39, 1–6. [Google Scholar] [CrossRef]
- Kim, E.J.; Shin, J.M.; Kim, Y.; Ku, K.H.; Yun, H.; Kim, B.J. Shape control of nanostructured cone-shaped particles by tuning the blend morphology of A-b-B diblock copolymers and C-type copolymers within emulsion droplets. Polym. Chem. 2019, 10, 2415–2423. [Google Scholar] [CrossRef]
- Deng, R.; Liu, S.; Liang, F.; Wang, K.; Zhu, J.; Yang, Z. Polymeric janus particles with hierarchical structures. Macromolecules 2014, 47, 3701–3707. [Google Scholar] [CrossRef]
- Robb, M.J.; Connal, L.A.; Lee, B.F.; Lynda, N.A.; Hawker, C.J. Functional block copolymer nanoparticles: Toward the next generation of delivery vehicles. Polym. Chem. 2012, 3, 1618–1628. [Google Scholar] [CrossRef] [PubMed]
- Varadharajan, D.; Turgut, H.; Lahann, J.; Yabu, H.; Delaittre, G. Surface-Reactive Patchy Nanoparticles and Nanodiscs Prepared by Tandem Nanoprecipitation and Internal Phase Separation. Adv. Funct. Mater. 2018, 28, 1–11. [Google Scholar] [CrossRef]
- Turgut, H.; Dingenouts, N.; Trouillet, V.; Krolla-Sidenstein, P.; Gliemann, H.; Delaittre, G. Reactive block copolymers for patterned surface immobilization with sub-30 nm spacing. Polym. Chem. 2019, 10, 1344–1356. [Google Scholar] [CrossRef] [Green Version]
- Jeon, S.J.; Yang, S.M.; Kim, B.J.; Petrie, J.D.; Jang, S.G.; Kramer, E.J.; Pine, D.J.; Yi, G.R. Hierarchically structured colloids of diblock copolymers and Au nanoparticles. Chem. Mater. 2009, 21, 3739–3741. [Google Scholar] [CrossRef]
- Yan, N.; Liu, H.; Zhu, Y.; Jiang, W.; Dong, Z. Entropy-Driven Hierarchical Nanostructures from Cooperative Self-Assembly of Gold Nanoparticles/Block Copolymers under Three-Dimensional Confinement. Macromolecules 2015, 48, 5980–5987. [Google Scholar] [CrossRef]
- Schmidt, B.V.K.J.; Wang, C.; Kraemer, S.; Connal, L.A.; Klinger, D. Highly Functional Ellipsoidal Block Copolymer Nanoparticles: A Generalized Approach to Nanostructured Chemical Ordering in Phase Separated Colloidal Particles. Polym. Chem. 2018, 9, 1638–1649. [Google Scholar] [CrossRef]
- Xu, J.; Yang, Y.; Wang, K.; Li, J.; Zhou, H.; Xie, X.; Zhu, J. Additives Induced Structural Transformation of ABC Triblock Copolymer Particles. Langmuir 2015, 31, 10975–10982. [Google Scholar] [CrossRef]
- Yan, N.; Liu, X.; Zhu, J.; Zhu, Y.; Jiang, W. Well-Ordered Inorganic Nanoparticle Arrays Directed by Block Copolymer Nanosheets. ACS Nano 2019, 13, 6638–6646. [Google Scholar] [CrossRef]
- Lowe, A.B. Thiol-ene “click” reactions and recent applications in polymer and materials synthesis: A first update. Polym. Chem. 2014, 5, 4820–4870. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Bowman, C.N. Thiol-ene click chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Lowe, A.B.; Bowman, C.N. Thiol-click chemistry: A multifaceted toolbox for small molecule and polymer synthesis. Chem. Soc. Rev. 2010, 39, 1355–1387. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, G.; Phillips, H.; Hill, J.M.; Chang, J.; Kydd, R.A. Palladium Nanoparticle Catalyst Prepared in Poly(Acrylic Acid)-lined Channels of Diblock Copolymer Microspheres. Nano Lett. 2001, 1, 683–687. [Google Scholar] [CrossRef]
- Kim, M.P.; Ku, K.H.; Kim, H.J.; Jang, S.G.; Yi, G.R.; Kim, B.J. Surface intaglio nanostructures on microspheres of gold-cored block copolymer spheres. Chem. Mater. 2013, 25, 4416–4422. [Google Scholar] [CrossRef]
- Ren, Y.; Lodge, T.P.; Hillmyer, M.A. Synthesis, characterization, and interaction strengths of difluorocarbene-modified polystyrene-polyisoprene block copolymers. Macromolecules 2000, 33, 866–876. [Google Scholar] [CrossRef]
- Ren, Y.; Lodge, T.P.; Hillmyer, M.A. Effect of selective perfluoroalkylation on the segregation strength of polystyrene-1,2-polybutadiene block copolymers. Macromolecules 2002, 35, 3889–3894. [Google Scholar] [CrossRef]
- Rodwogin, M.D.; Spanjers, C.S.; Leighton, C.; Hillmyer, M.A. Polylactide-poly(dimethylsiloxane)-polylactide triblock copolymers as multifunctional materials for nanolithographic applications. ACS Nano 2010, 4, 725–732. [Google Scholar] [CrossRef]
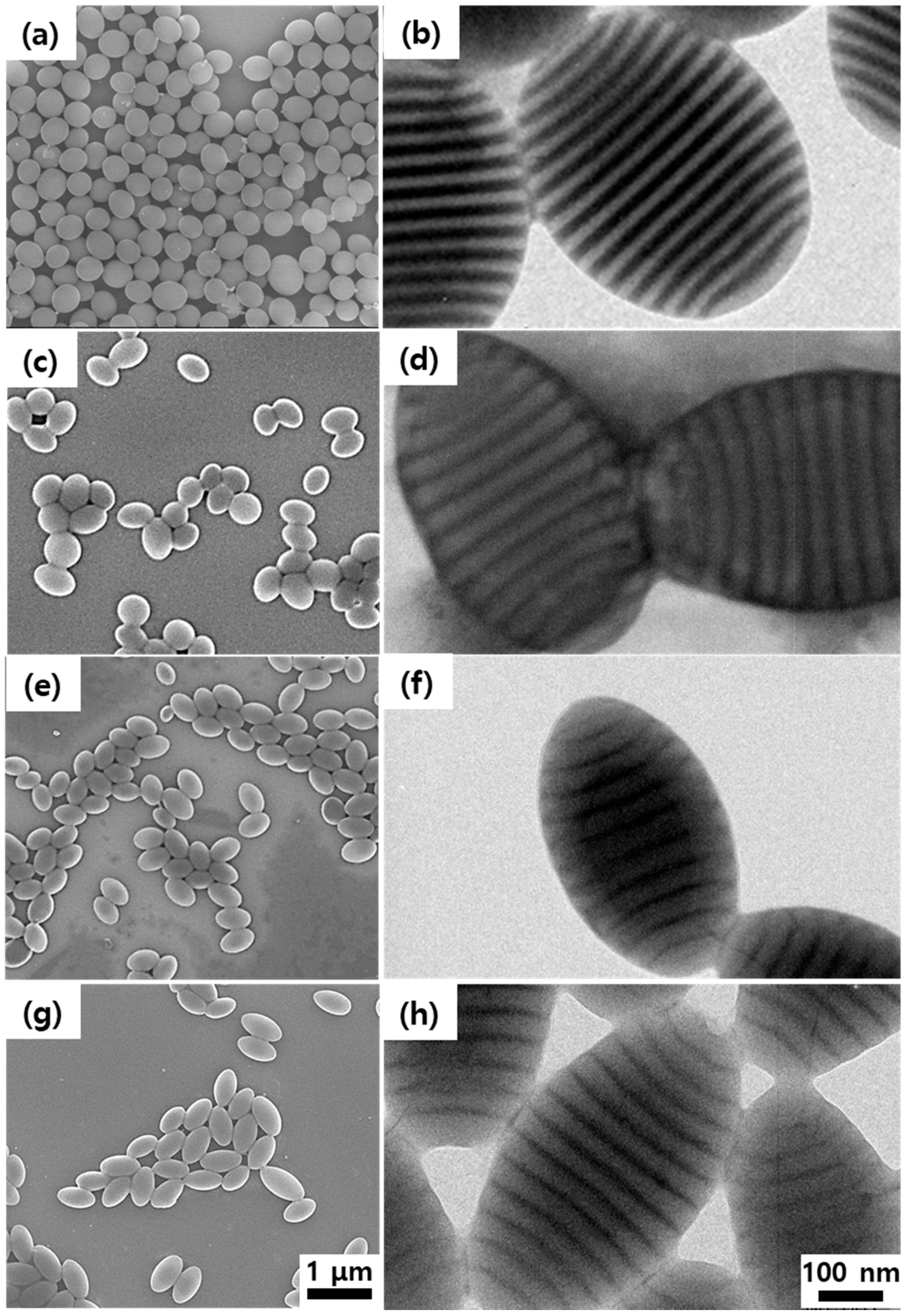

| L0 (nm) | dG (nm) | dD (nm) | L (nm) | S (nm) | AR | |
|---|---|---|---|---|---|---|
| r = 0.0 | 33.7 1.8 | 14.4 1.3 | 18.9 0.7 | 450 45 | 376 36 | 1.20 |
| r = 0.1 | 36.9 2.7 | 22.1 1.2 | 16.1 1.2 | 501 40 | 320 23 | 1.56 |
| r = 0.2 | 38.0 2.5 | 25.7 1.9 | 14.0 1.8 | 567 56 | 322 31 | 1.75 |
| r = 0.5 | 39.6 2.1 | 24.2 1.7 | 14.0 1.0 | 585 51 | 328 23 | 1.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.J. Effect of Site-Specific Functionalization on the Shape of Nonspherical Block Copolymer Particles. Polymers 2020, 12, 2804. https://doi.org/10.3390/polym12122804
Shin JJ. Effect of Site-Specific Functionalization on the Shape of Nonspherical Block Copolymer Particles. Polymers. 2020; 12(12):2804. https://doi.org/10.3390/polym12122804
Chicago/Turabian StyleShin, Jaeman J. 2020. "Effect of Site-Specific Functionalization on the Shape of Nonspherical Block Copolymer Particles" Polymers 12, no. 12: 2804. https://doi.org/10.3390/polym12122804
APA StyleShin, J. J. (2020). Effect of Site-Specific Functionalization on the Shape of Nonspherical Block Copolymer Particles. Polymers, 12(12), 2804. https://doi.org/10.3390/polym12122804