Quantitative Analysis of Solubility Parameters and Surface Properties of Larch Bark Proanthocyanidins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.1.1. Isolation of Larch Bark Polymeric Proanthocyanidins
2.1.2. Preparation of Larch Bark Oligomeric Proanthocyanidins
2.2. Determination of Average Degree of Polymerization of Larch Bark Proanthocyanidins
2.3. Inverse Gas Chromatography Was Used to Calculate Solubility Parameters
2.3.1. Determination of Retention Time
2.3.2. Characteristic Retention Volume of Probe Solvent
2.3.3. Thermodynamic Parameters of Probe Molecules
2.3.4. Flory–Huggins Interaction Parameter
2.3.5. Solubility parameter
2.4. Molecular Dynamics Simulation Was Used to Calculate Solubility Parameters
2.4.1. Model Establishment and Optimization
2.4.2. Calculation of Solubility Parameters
2.5. Calculation of Surface Properties of Larch Bark Proanthocyanidins
2.5.1. Dispersive Surface Energy
2.5.2. Specific Surface Energy and Total Surface Energy
2.5.3. Surface Acidity and Alkalinity
3. Results and Discussion
3.1. Average Degree of Polymerization of Proanthocyanidins
3.2. Solubility Parameters of Larch Bark Proanthocyanidins
3.2.1. Retention Volumes of Probe Solvents (by IGC)
3.2.2. Thermodynamic Parameters of Probe Solvents (by IGC)
3.2.3. Flory–Huggins Interaction Parameter and Infinite Dilution Activity Coefficient (by IGC)
3.2.4. Solubility Parameter (by IGC)
3.3. Molecular Dynamics Calculation
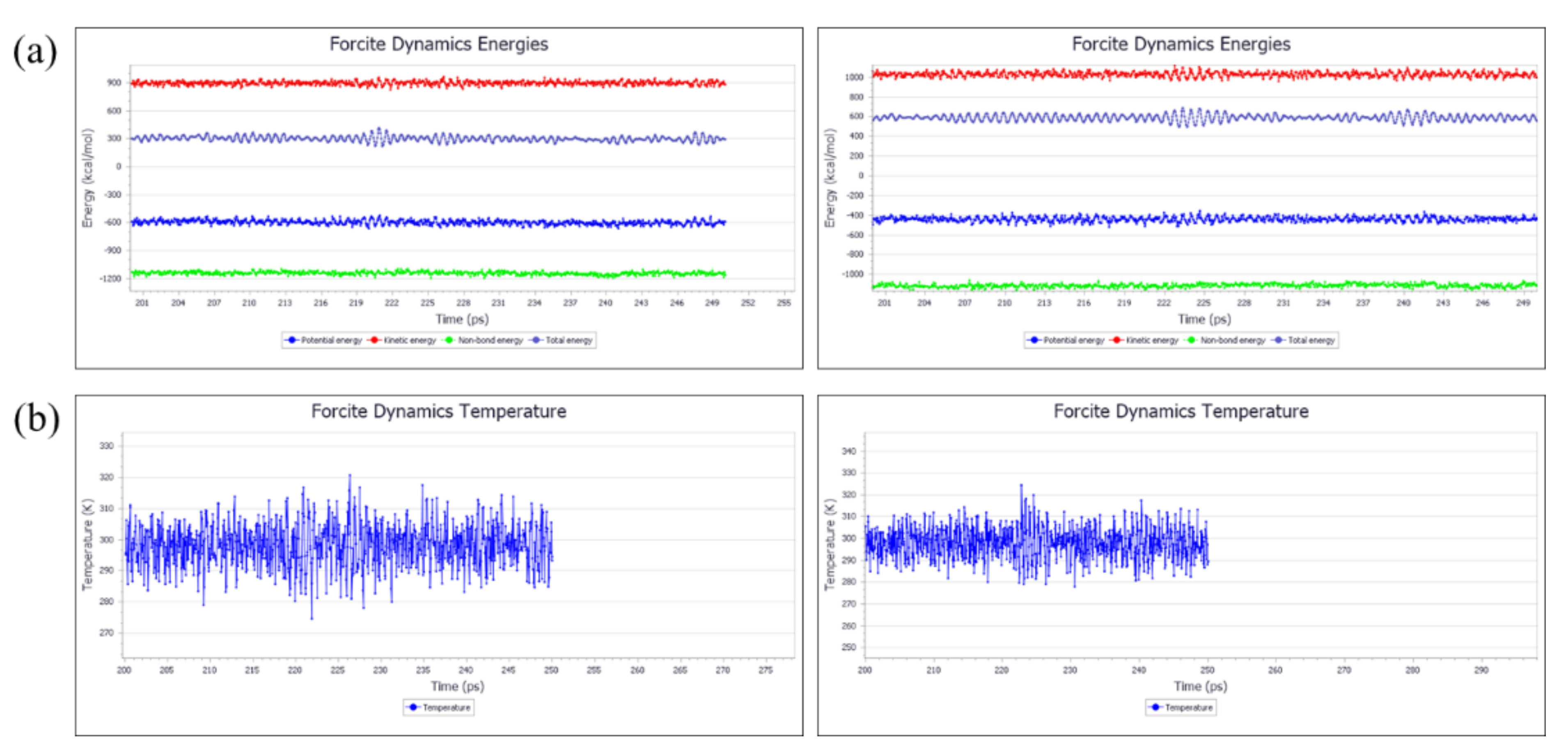
3.3.1. Molecular Modeling and Optimization
3.3.2. Calculation of Solubility Parameters (by MD)
3.4. Comparison of Solubility Parameters of Larch Bark Polymeric Proanthocyanidins and Oligomeric Proanthocyanidins Obtained by Experimental Inverse Gas Chromatography and Molecular Dynamics Simulations
3.5. Determination of Surface Properties of Larch Bark Proanthocyanidins by Inverse Gas Chromatography
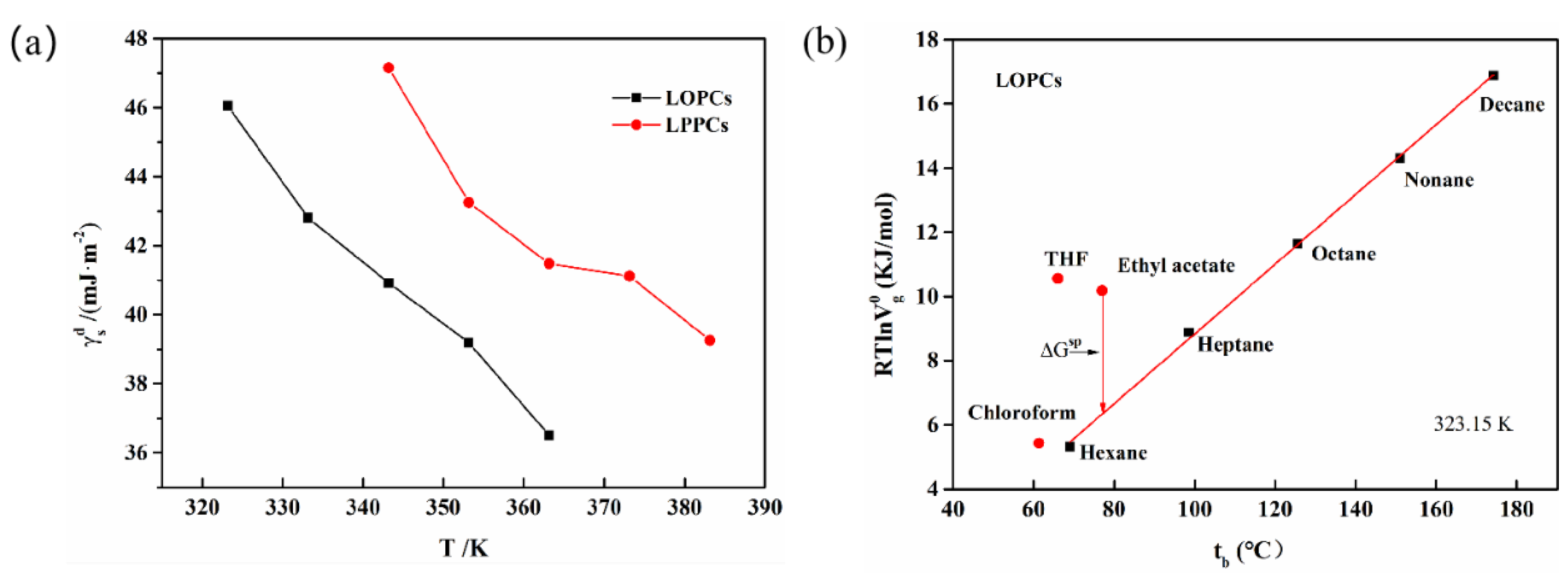
3.5.1. Dispersive Component and Specific Component
3.5.2. Surface Acidity and Alkalinity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Probe Solvent | Number of Carbon | Molecular Mass (g/mol) | Boiling Point (℃) |
|---|---|---|---|
| n-hexane | 6 | 86.18 | 69 |
| n-heptane | 7 | 100.2 | 98.5 |
| n-octane | 8 | 114.23 | 125.6 |
| n-nonane | 9 | 128.26 | 151 |
| n-decane | 10 | 142.29 | 174.2 |
| cyclopentane | 5 | 70.14 | 49.3 |
| cyclohexane | 6 | 84.16 | 80.7 |
| dichloromethane | 1 | 84.93 | 39.8 |
| trichloromethane | 1 | 119.38 | 61.3 |
| trichloroethylene | 2 | 131.39 | 87.1 |
| benzene | 6 | 78.11 | 80 |
| toluene | 7 | 92.14 | 110.6 |
| p-xylene | 8 | 106.167 | 138.37 |
| o-xylene | 8 | 106.16 | 144.4 |
| ethanol | 2 | 46.07 | 78 |
| 1-propanol | 3 | 60.1 | 97.1 |
| acetone | 2 | 58.08 | 56.53 |
| methyl ethyl ketone | 3 | 72.11 | 79.6 |
| ethyl acetate | 4 | 88.11 | 77 |
| tetrahydrofuran | 4 | 72.11 | 66 |
| Probe Solvent | LPPCs | LOPCs | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 323.15 K | 333.15 K | 343.15 K | 353.15 K | 363.15 K | 373.15 K | 383.15 K | 323.15 K | 333.15 K | 343.15 K | 353.15 K | 363.15 K | |
| n-hexane | 5.78 | 3.78 | 2.06 | 1.88 | 1.75 | 1.07 | 0.86 | 7.23 | 4.96 | 3.40 | 2.71 | 2.29 |
| n-heptane | 19.56 | 12.69 | 8.61 | 5.63 | 5.24 | 3.71 | 2.66 | 27.26 | 16.58 | 10.81 | 7.55 | 4.66 |
| n-octane | 54.12 | 35.25 | 22.47 | 14.84 | 11.94 | 7.58 | 5.64 | 76.34 | 42.98 | 26.13 | 17.33 | 11.34 |
| n-nonane | 141.08 | 87.16 | 51.4 | 34.14 | 27.19 | 16.44 | 11.74 | 205.31 | 109.09 | 62.88 | 41.43 | 26.16 |
| n-decane | 367.61 | 215.97 | 122.19 | 75.08 | 58.37 | 32.55 | 22.47 | 536.47 | 266.87 | 145.58 | 91.39 | 56.16 |
| cyclopentane | 0.71 | 0.48 | 0.37 | 0.27 | 0.19 | 0.16 | 0.31 | 1.77 | 1.47 | 1.20 | 0.97 | 0.55 |
| cyclohexane | 6.99 | 4.55 | 3.18 | 2.06 | 1.94 | 1.56 | 1.72 | 9.43 | 6.63 | 4.81 | 3.09 | 2.01 |
| dichloromethane | 2.64 | 2.13 | 1.21 | 0.89 | 0.49 | 0.33 | 0.54 | 3.66 | 2.52 | 1.70 | 1.06 | 0.82 |
| trichloromethane | 6.99 | 5.42 | 3.46 | 2.5 | 1.94 | 1.15 | 0.94 | 7.54 | 5.47 | 4.00 | 2.41 | 2.20 |
| trichloroethylene | 13.89 | 9.78 | 6.35 | 4.29 | 4.08 | 2.63 | 2.35 | 19.3 | 11.68 | 7.61 | 5.02 | 4.30 |
| benzene | 24.12 | 14.04 | 8.37 | 6.14 | 4.65 | 2.88 | 2.43 | 13.74 | 11.74 | 7.49 | 5.68 | 4.12 |
| toluene | 45.41 | 24.79 | 16.19 | 11.22 | 9.01 | 5.67 | 4.38 | 41.99 | 27.88 | 18.37 | 12.04 | 8.60 |
| p-xylene | 121.83 | 75.83 | 42.79 | 28.85 | 21.3 | 12.33 | 10.02 | 121.99 | 75.66 | 47.71 | 30.14 | 20.21 |
| o-xylene | 184.66 | 87.45 | 49.86 | 35.26 | 25.47 | 14.71 | 13.31 | 153.57 | 90.23 | 56.3 | 36.69 | 24.24 |
| ethanol | 78.45 | 42.27 | 27.84 | 14.84 | 11.14 | 5.18 | 3.91 | 103.10 | 71.89 | 31.06 | 16.08 | 11.07 |
| 1-propanol | 69.12 | 28.41 | 19.31 | 10.36 | 5.91 | 3.21 | 2.04 | 66.08 | 42.39 | 27.78 | 9.73 | 7.41 |
| acetone | 27.77 | 22.95 | 11.63 | 8.19 | 4.74 | 2.96 | 1.33 | 35.36 | 18.84 | 14.04 | 6.93 | 5.31 |
| methyl ethyl ketone | 43.18 | 33.03 | 20.37 | 10.33 | 7.94 | 4.69 | 2.58 | 67.51 | 46.89 | 23.99 | 13.10 | 8.05 |
| ethyl acetate | 34.87 | 27.99 | 26.51 | 17.99 | 6.68 | 3.45 | 2.51 | 44.2 | 28.05 | 20.51 | 11.65 | 7.04 |
| tetrahydrofuran | 41.45 | 28.09 | 20.65 | 11.13 | 6.56 | 3.86 | 3.04 | 51.04 | 33.71 | 23.10 | 11.94 | 7.77 |
| Probe Solvent | LPPCs | LOPCs | ||||
|---|---|---|---|---|---|---|
| n-hexane | −31.01 | -4.94 | 26.07 | −28.51 | −3.23 | 25.29 |
| n-heptane | −32.99 | −3.31 | 29.68 | −42.13 | −2.12 | 40.01 |
| n-octane | −38.65 | −2.21 | 36.43 | −46.15 | −1.35 | 44.8 |
| n-nonane | −42.12 | −1.47 | 40.65 | −49.75 | −0.83 | 48.92 |
| n-decane | −47.54 | −0.99 | 46.56 | −54.62 | −0.51 | 54.11 |
| cyclopentane | −29.81 | −5.31 | 24.5 | −26.79 | −3.53 | 23.26 |
| cyclohexane | −30.26 | −3.62 | 26.63 | −37.49 | −2.37 | 35.11 |
| dichloromethane | −43.24 | −4.70 | 38.53 | −37.59 | −1.89 | 35.7 |
| trichloromethane | −35.62 | −3.36 | 32.26 | −32.12 | −1.43 | 30.69 |
| trichloroethylene | −31.05 | −2.28 | 28.77 | −37.72 | −1.08 | 36.64 |
| benzene | −39.34 | −3.34 | 35.99 | −30.53 | −2.14 | 28.4 |
| toluene | −40.95 | −2.27 | 38.68 | −39.15 | −1.4 | 37.75 |
| p-xylene | −43.61 | −1.53 | 42.07 | −44.07 | −0.89 | 43.18 |
| o-xylene | −44.9 | −1.36 | 43.54 | −44.85 | −0.77 | 44.09 |
| ethanol | −51.89 | −2.26 | 49.64 | −58.15 | −1.33 | 56.82 |
| 1-propanol | −59.35 | −1.81 | 57.54 | −56.94 | −1.00 | 55.93 |
| acetone | −44.57 | −3.63 | 40.94 | −46.82 | −2.35 | 44.48 |
| methyl ethyl ketone | −48.73 | −2.9 | 45.84 | −53.85 | −1.85 | 52.00 |
| ethyl acetate | −48.95 | −3.35 | 45.59 | −44.28 | −2.13 | 42.16 |
| tetrahydrofuran | −47.56 | −11.26 | 36.31 | −46.72 | −7.68 | 39.05 |
| Probe Solvent | LPPCs | LOPCs | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 323.1 K | 333.15 K | 343.15 K | 353.15 K | 363.15 K | 373.15 K | 383.15 K | 323.15 K | 333.15 K | 343.15 K | 353.15 K | 363.15 K | |
| n-hexane | 3.47 | 3.56 | 3.85 | 3.66 | 3.46 | 3.7 | 3.69 | 3.24 | 73.28 | 3.35 | 3.29 | 3.19 |
| n-heptane | 3.13 | 3.18 | 3.2 | 3.29 | 3.05 | 3.11 | 3.17 | 2.8 | 2.91 | 2.98 | 3 | 3.17 |
| n-octane | 3.01 | 3.00 | 3.04 | 3.07 | 2.93 | 3.06 | 3.05 | 2.67 | 2.8 | 2.89 | 2.92 | 2.99 |
| n-nonane | 2.69 | 2.94 | 3.01 | 2.99 | 2.82 | 2.96 | 2.95 | 2.58 | 2.72 | 2.81 | 2.8 | 2.86 |
| n-decane | 2.92 | 2.89 | 2.95 | 2.96 | 2.77 | 2.95 | 2.94 | 2.54 | 2.68 | 2.78 | 2.77 | 2.81 |
| cyclopentane | 5.13 | 5.21 | 5.18 | 5.25 | 5.33 | 5.27 | 4.41 | 4.21 | 4.09 | 4.01 | 3.97 | 4.29 |
| cyclohexane | 3.69 | 3.77 | 3.8 | 3.93 | 3.71 | 3.66 | 3.32 | 3.39 | 3.39 | 3.39 | 3.53 | 3.67 |
| dichloromethane | 3.29 | 3.19 | 3.46 | 3.5 | 3.86 | 4.02 | 3.3 | 2.96 | 3.02 | 3.12 | 3.33 | 3.33 |
| trichloromethane | 2.7 | 2.63 | 2.77 | 2.81 | 2.8 | 3.07 | 3.04 | 2.63 | 2.62 | 2.62 | 2.85 | 2.67 |
| trichloroethylene | 2.78 | 2.77 | 2.85 | 2.93 | 2.68 | 2.84 | 2.69 | 2.46 | 2.59 | 2.67 | 2.77 | 2.63 |
| benzene | 2.53 | 2.71 | 2.89 | 2.89 | 2.88 | 3.09 | 3.01 | 3.09 | 2.89 | 3 | 2.97 | 3 |
| toluene | 2.77 | 2.97 | 3.02 | 3.04 | 2.93 | 3.09 | 3.06 | 2.85 | 2.86 | 2.9 | 2.97 | 2.98 |
| p-xylene | 2.71 | 2.72 | 2.87 | 2.87 | 2.81 | 3.01 | 2.9 | 2.71 | 2.73 | 2.76 | 2.83 | 2.86 |
| o-xylene | 2.53 | 2.81 | 2.94 | 2.88 | 2.83 | 3.03 | 2.8 | 2.72 | 2.78 | 2.82 | 2.84 | 2.88 |
| ethanol | 2.07 | 2.22 | 2.21 | 2.44 | 2.36 | 2.78 | 2.73 | 1.79 | 1.69 | 2.1 | 2.36 | 2.36 |
| 1-propanol | 2.84 | 3.21 | 3.12 | 3.3 | 3.45 | 3.68 | 3.77 | 2.88 | 2.81 | 2.76 | 3.36 | 3.22 |
| acetone | 1.87 | 1.72 | 2.09 | 2.15 | 2.42 | 2.65 | 3.21 | 1.63 | 1.92 | 1.9 | 2.32 | 2.31 |
| methyl ethyl ketone | 2.03 | 1.93 | 2.06 | 2.42 | 2.38 | 2.63 | 2.97 | 1.59 | 13.58 | 1.9 | 2.18 | 2.37 |
| ethyl acetate | 1.98 | 1.82 | 1.53 | 1.6 | 2.29 | 2.67 | 2.73 | 1.74 | 1.82 | 1.79 | 2.03 | 2.23 |
| tetrahydrofuran | 0.08 | 0.2 | 0.26 | 0.65 | 0.97 | 1.31 | 1.38 | −0.13 | 0.01 | 0.15 | 0.58 | 0.8 |
| Probe Solvent | LPPCs | LOPCs | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 323.15 K | 333.15 K | 343.15 K | 353.15 K | 363.15 K | 373.15 K | 383.15 K | 323.15 K | 333.15 K | 343.15 K | 353.15 K | 363.15 K | |
| n-hexane | 87.26 | 95.32 | 127.97 | 105.2 | 86.29 | 109.85 | 108.39 | 69.7 | 72.53 | 77.44 | 72.84 | 66.03 |
| n-heptane | 62.42 | 65.19 | 66.93 | 73.26 | 57.62 | 61 | 64.89 | 44.78 | 49.88 | 53.32 | 54.63 | 64.78 |
| n-octane | 55.19 | 54.42 | 56.65 | 58.65 | 51.15 | 57.97 | 57.35 | 39.13 | 44.63 | 48.72 | 50.21 | 53.87 |
| n-nonane | 52.35 | 51.5 | 55.1 | 54.13 | 45.72 | 52.27 | 51.86 | 35.97 | 41.15 | 45.05 | 44.61 | 47.52 |
| n-decane | 50.19 | 49.06 | 51.96 | 52.63 | 43.6 | 51.9 | 51.33 | 34.39 | 39.71 | 43.62 | 43.24 | 45.31 |
| cyclopentane | 458.92 | 495.39 | 483.87 | 518.33 | 560.58 | 527.76 | 224.26 | 183.44 | 162.88 | 150.44 | 144 | 198.4 |
| cyclohexane | 108.99 | 117.7 | 121.69 | 138.74 | 110.72 | 105.84 | 75.09 | 80.83 | 80.84 | 80.4 | 92.35 | 106.87 |
| dichloromethane | 72.66 | 65.84 | 86.61 | 90 | 129.18 | 151.36 | 73.64 | 52.3 | 55.56 | 61.77 | 75.77 | 76.2 |
| trichloromethane | 40.53 | 37.57 | 43.41 | 45.09 | 44.56 | 58.74 | 57.07 | 37.57 | 37.25 | 37.46 | 46.76 | 39.43 |
| trichloroethylene | 44.01 | 43.19 | 47.19 | 50.71 | 39.56 | 46.39 | 40 | 31.66 | 36.18 | 39.38 | 43.35 | 37.53 |
| benzene | 34.01 | 40.72 | 48.88 | 48.82 | 48.33 | 59.65 | 55 | 59.71 | 48.72 | 54.67 | 52.79 | 54.58 |
| toluene | 43.55 | 53.11 | 55.77 | 56.65 | 50.88 | 59.54 | 57.9 | 47.09 | 47.24 | 49.16 | 52.8 | 53.3 |
| p-xylene | 40.71 | 41.45 | 48.1 | 48.13 | 45.15 | 55.37 | 49.44 | 40.66 | 41.55 | 43.15 | 46.07 | 47.58 |
| o-xylene | 34.24 | 45.25 | 51.37 | 48.47 | 46.03 | 56.02 | 44.55 | 41.18 | 43.86 | 45.5 | 46.58 | 48.36 |
| ethanol | 21.47 | 25.11 | 24.83 | 31.27 | 28.72 | 43.66 | 41.78 | 16.34 | 14.77 | 22.26 | 28.85 | 28.9 |
| 1-propanol | 46.36 | 67.4 | 61.55 | 73.67 | 85.6 | 107.48 | 118.39 | 48.49 | 45.17 | 42.79 | 78.48 | 68.24 |
| acetone | 17.61 | 15.2 | 21.95 | 23.3 | 30.71 | 38.29 | 67.35 | 13.83 | 18.52 | 18.18 | 27.53 | 27.47 |
| methyl ethyl ketone | 20.77 | 18.67 | 21.39 | 30.56 | 29.47 | 37.79 | 52.84 | 13.28 | 13.15 | 18.16 | 24.11 | 29.07 |
| ethyl acetate | 19.61 | 16.8 | 12.54 | 13.4 | 26.75 | 39.21 | 41.72 | 15.47 | 16.76 | 16.21 | 20.68 | 25.38 |
| tetrahydrofuran | 2.93 | 3.31 | 3.52 | 5.21 | 7.18 | 10.08 | 10.76 | 2.38 | 2.76 | 3.15 | 4.86 | 6.06 |
| Larch Bark Proanthocyanidins | 323.15 K | 333.15 K | 343.15 K | 353.15 K | 363.15 K | 373.15 K | 383.15 K | 298.15 K |
|---|---|---|---|---|---|---|---|---|
| LPPCs | 19.16 | 18.86 | 18.66 | 18.17 | 17.57 | 16.94 | 16.52 | 20.50 |
| LOPCs | 20.74 | 20.58 | 20.06 | 19.17 | 19.02 | - | - | 22.09 |
| Proanthocyanidins | |||
|---|---|---|---|
| trimer | 16.24 | 15.03 | 22.35 |
| heptamer | 14.26 | 14.51 | 20.57 |
| T/K | |||||
|---|---|---|---|---|---|
| LPPCs | |||||
| 343.15 | 47.15 | 2.32 | 10.66 | 9.95 | 57.10 |
| 353.15 | 43.26 | 0.75 | 8.76 | 5.14 | 48.39 |
| 363.15 | 41.47 | 1.92 | 5.00 | 6.19 | 47.66 |
| 373.15 | 41.12 | 2.24 | 5.12 | 6.77 | 47.90 |
| 383.15 | 39.26 | 1.60 | 7.17 | 6.77 | 46.03 |
| LOPCs | |||||
| 323.15 | 46.06 | 2.20 | 0.04 | 0.56 | 46.65 |
| 333.15 | 42.80 | 1.71 | 1.47 | 3.17 | 45.97 |
| 343.15 | 40.92 | 1.07 | 2.88 | 3.51 | 44.44 |
| 353.15 | 39.19 | 0.01 | 1.02 | 0.18 | 39.37 |
| 363.15 | 36.50 | 0.01 | 2.01 | 0.28 | 36.78 |
| LPPCs | |||||
|---|---|---|---|---|---|
| Probe solvent | 343.15 K | 353.15 K | 363.15 K | 373.15 K | 383.15 K |
| Chloroform | 1.88 | 1.52 | 0.93 | 0.56 | 0.67 |
| THF | 6.48 | 5.42 | 4.14 | 3.86 | 3.88 |
| Ethyl acetate | 6.00 | 5.70 | 3.10 | 2.43 | 2.23 |
| LOPCs | |||||
| Probe solvent | 323.15 K | 333.15 K | 343.15 K | 353.15 K | 363.15 K |
| Chloroform | 0.80 | 0.96 | 1.11 | 0.38 | 0.88 |
| THF | 5.43 | 5.51 | 5.64 | 4.62 | 4.26 |
| Ethyl acetate | 3.85 | 3.86 | 4.19 | 3.48 | 2.94 |
References
- Yang, T.; Dong, M.; Cui, J.; Gan, L.; Han, S. Exploring the formaldehyde reactivity of tannins with different molecular weight distributions: Bayberry tannins and larch tannins. Holzforschung 2020, 74, 673–682. [Google Scholar] [CrossRef]
- Wagner, K.; Roth, C.; Willfor, S.; Musso, M.; Petutschnigg, A.; Oostingh, G.J.; Schnabel, T. Identification of Antimicrobial Compounds in Different Hydrophilic Larch Bark Extracts. BioResources 2019, 14, 5807–5815. [Google Scholar]
- Vamanu, E.; Gatea, F.; Pelinescu, D.R. Bioavailability and Bioactivities of Polyphenols Eco Extracts from Coffee Grounds after In Vitro Digestion. Foods 2020, 9, 1281. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Mitsunaga, T. Tyrosinase inhibitory activity of proanthocyanidins from woody plants. J. Wood Sci. 2003, 49, 461–465. [Google Scholar] [CrossRef]
- Yang, F.; Yao, J.; Chen, X.; Shao, Z. Preparation and antimicrobial properties of pva/tannin blend films. Acta Polym. Sin. 2012, 125–130. [Google Scholar] [CrossRef]
- Su, L.; Xing, Z.; Wang, D.; Xu, G.; Ren, S.; Fang, G. Mechanical Properties Research and Structural Characterization of Alkali Lignin/Poly(vinyl alcohol) Reaction Films. BioResources 2013, 8, 3532–3543. [Google Scholar] [CrossRef]
- Anwer, M.A.S.; Naguib, H.E.; Celzard, A.; Fierro, V. Comparison of the thermal, dynamic mechanical and morphological properties of PLA-Lignin & PLA-Tannin particulate green composites. Compos. Part B-Eng. 2015, 82, 92–99. [Google Scholar]
- Grigsby, W.J.; Bridson, J.H.; Lomas, C.; Elliot, J.A. Esterification of Condensed Tannins and Their Impact on the Properties of Poly(Lactic Acid). Polymers 2013, 5, 344–360. [Google Scholar] [CrossRef]
- Ashrafi, A.; Jokar, M.; Mohammadi Nafchi, A. Preparation and characterization of biocomposite film based on chitosan and kombucha tea as active food packaging. Int. J. Biol. Macromol. 2018, 108, 444–454. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L. Developing a bio-based packaging film from soya by-products incorporated with valonea tannin. J. Clean. Prod. 2017, 143, 624–633. [Google Scholar] [CrossRef]
- Zhai, Y.; Wang, J.; Wang, H.; Song, T.; Hu, W.; Li, S. Preparation and Characterization of Antioxidative and UV-Protective Larch Bark Tannin/PVA Composite Membranes. Molecules 2018, 23, 2073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voelkel, A. Inverse gas chromatography in characterization of surface. Chemom. Intell. Lab. Syst. 2004, 72, 205–207. [Google Scholar] [CrossRef]
- Klein, G.L.; Pierre, G.; Bellon-Fontaine, M.N.; Graber, M. Inverse Gas Chromatography with Film Cell Unit: An Attractive Alternative Method to Characterize Surface Properties of Thin Films. J. Chromatogr. Sci. 2015, 53, 1233–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domingue, J.; Burnett, D.; Thielmann, F. Using inverse gas chromatography (IGC) to investigate process-related changes in surface and bulk properties of pharmaceutical materials. Am. Lab. 2003, 35, 32. [Google Scholar]
- Balard, H.; Maafa, D.; Santini, A.; Donnet, J.B. Study by inverse gas chromatography of the surface properties of milled graphites. J. Chromatogr. A 2008, 1198, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Bilgic, C. Determination of the surface properties of kaolinite by inverse gas chromatography. Water Sci. Technol. 2018, 136, 319–328. [Google Scholar] [CrossRef]
- Chow, A.H.L.; Tong, H.H.Y.; Shekunov, B.Y.; York, P. Use of inverse gas chromatography (IGC) to determine the surface energy and surface area of powdered materials. Pharm. Res. 2004, 21, 1718–1719. [Google Scholar]
- Zhao, S.; Lin, X.; Song, J.; Shi, B. Surface characterization of ashtree wood meat by inverse gas chromatography. Chin. Sci. Bull. 2007, 52, 1178–1181. [Google Scholar] [CrossRef]
- Mohammadkazemi, F.; Aguiar, R.; Cordeiro, N. Improvement of bagasse fiber-cement composites by addition of bacterial nanocellulose: An inverse gas chromatography study. Cellulose 2017, 24, 1803–1814. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Yang, X. Surface Wettability Characterization of Synthetic Fibers by Inverse Gas Chromatography. Chem. Ind. Eng. 2017, 34, 27–32. [Google Scholar]
- Adamska, K.; Sandomierski, M.; Buchwald, Z.; Voelkel, A. Inverse gas chromatography in the examination of surface properties of experimental dental composites. Polym. Test. 2020, 90, 106697. [Google Scholar] [CrossRef]
- Farshchi, N.; Abbasian, A.; Larijani, K. Is Inverse Gas Chromatography (IGC) a Convenient Method to Determine Compatibility of Rubber Materials? Chromatographia 2019, 82, 1709–1719. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Q.; Ma, K. Solubility Parameter of Ionic Liquids: A Comparative Study of Inverse Gas Chromatography and Hansen Solubility Sphere. Acs Sustain. Chem. Eng. 2019, 7, 10544–10551. [Google Scholar] [CrossRef]
- Um, H.J.; Lee, K.; Cho, Y.S.; Kim, Y.S.; Kim, T.; Park, C.R. Experimental consideration of the Hansen solubility parameters of as-produced multi-walled carbon nanotubes by inverse gas chromatography. Phys. Chem. Chem. Phys. 2014, 16, 17466–17472. [Google Scholar]
- Adamska, K.; Voelkel, A.; Berlinska, A. The solubility parameter for biomedical polymers-Application of inverse gas chromatography. J. Pharm. Biomed. Anal. 2016, 127, 202–206. [Google Scholar] [CrossRef]
- Farshchi, N.; Abbasian, A.; Larijani, K. Inverse Gas Chromatography (IGC) study of vegetable oils solvency via solubility parameter. Chim. Oggi-Chem. Today 2017, 35, 40–46. [Google Scholar]
- Engstrom, M.T.; Palijarvi, M.; Fryganas, C.; Grabber, J.H.; Mueller-Harvey, I.; Salminen, J.P. Rapid Qualitative and Quantitative Analyses of Proanthocyanidin Oligomers and Polymers by UPLC-MS/MS. J. Agric. Food Chem. 2014, 62, 3390–3399. [Google Scholar] [CrossRef]
- Binder, K.; Horbach, J.; Kob, W.; Paul, W.; Varnik, F. Molecular dynamics simulations. J. Phys. Condens. Matter 2004, 16, 429–453. [Google Scholar] [CrossRef]
- Hansson, T.; Oostenbrink, C.; van Gunsteren, W. Molecular dynamics simulations. Curr. Opin. Struct. Biol. 2002, 12, 190–196. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, Y.; Chai, W.; Wang, T.; Zhang, Y. Effects of composition ratio on the properties of poly(vinyl alcohol)/poly (acrylic acid) blend membrane: A molecular dynamics simulation study. Mater. Des. 2016, 89, 848–855. [Google Scholar] [CrossRef]
- Gupta, J.; Nunes, C.; Vyas, S.; Jonnalagadda, S. Prediction of Solubility Parameters and Miscibility of Pharmaceutical Compounds by Molecular Dynamics Simulations. J. Phys. Chem. B 2011, 115, 2014–2023. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Zhou, X.; Xing, Z. Computing solubility parameters of phosphorous flame retardants by molecular dynamics and correlating their interactions with poly(ethylene terephthalate). Text. Res. J. 2019, 89, 195–203. [Google Scholar] [CrossRef]
- Zhu, H.; Li, P.; Ren, S.; Tan, W.; Fang, G. Low-Cost Ru/C-Catalyzed Depolymerization of the Polymeric Proanthocyanidin-Rich Fraction from Bark To Produce Oligomeric Proanthocyanidins with Antioxidant Activity. Acs Omega 2019, 4, 16471–16480. [Google Scholar] [CrossRef] [PubMed]
- Bindon, K.A.; Kennedy, J.A. Ripening-Induced Changes in Grape Skin Proanthocyanidins Modify Their Interaction with Cell Walls. J. Agric. Food Chem. 2011, 59, 2696–2707. [Google Scholar] [CrossRef] [PubMed]
- Janini, G.M.; Muschik, G.M.; Schroer, J.A.; Zielinski, W.L., Jr. Gas-liquid chromatographic evaluation and gas-chromatography/mass spectrometric application of new high-temperature liquid crystal stationary phases for polycyclic aromatic hydrocarbon separations. Anal. Chem. 1976, 48, 1879–1883. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; López, J.; Ferrándiz, S.; Peltzer, M.A. Characterization of PLA-limonene blends for food packaging applications. Polym. Test. 2013, 32, 760–768. [Google Scholar] [CrossRef]
- Huang, J.C. Probe dependency of flory–huggins interaction parameters between solvents: Cases of hydrocarbons and isosteric derivatives. J. Appl. Polym. Sci. 2013, 127, 5000–5006. [Google Scholar] [CrossRef]
- Jan-Chan, H. Probe dependency of polymer-plasticizer and polymer-polymer interaction parameters in inverse gas chromatography. J. Appl. Polym. Sci. 2007, 106, 4110–4116. [Google Scholar]
- Jiang, G.Q.; Zhang, Z.R.; Li, L.L.; Du, F.G.; Pang, J.Y. Analysis of Purified Oligomeric Proanthocyanidins from Larix gmelinii Bark and the Study of Physiological Activity of the Purified Product. BioResources 2016, 11, 1690–1706. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.M.; Zhou, T.T.; Bu, H.Y.; Zhang, L.P.; Sun, S.Q.; Ma, F. Infrared Spectroscopic Analysis of the Production Process of Larch Bark Proanthocyanidins. Spectrosc. Spectr. Anal. 2018, 38, 62–67. [Google Scholar]
- Yousefi Seyf, J.; Haghtalab, A. A junction between molecular dynamics simulation and local composition models for computation of solid-liquid equilibrium-A pharmaceutical solubility application. Fluid Phase Equilibria 2017, 437, 83–95. [Google Scholar] [CrossRef]
- Basivi, P.K.; Pasupuleti, V.R.; Seella, R.; Tukiakula, M.R.; Kalluru, S.R.; Park, S.J. Inverse Gas Chromatography Study on London Dispersive Surface Free Energy and Electron Acceptor-Donor of Fluconazole Drug. J. Chem. Eng. Data 2017, 62, 2090–2094. [Google Scholar] [CrossRef]
- Kazayawoko, M.; Balatinecz, J.J.; Romansky, M. Thermodynamics of Adsorption of n-Alkanes on Maleated Wood Fibers by Inverse Gas Chromatography. J. Colloid Interface Sci. 1997, 190, 408–415. [Google Scholar] [CrossRef]
- Gamelas, J.A.F. The surface properties of cellulose and lignocellulosic materials assessed by inverse gas chromatography: A review. Cellulose 2013, 20, 2675–2693. [Google Scholar] [CrossRef] [Green Version]
- Gutmann, V. Empirical parameters for donor and acceptor properties of solvents. Electrochim. Acta 1976, 21, 661–670. [Google Scholar] [CrossRef]
- Siboni, S.; Della Volpe, C. On the definition of scales in van Oss-Chaudhury-Good acid-base theory. Match-Commun. Math. Comput. Chem. 2006, 56, 291–316. [Google Scholar]
- Morra, M. Acid-base properties of adhesive dental polymers. Dent. Mater. Off. Publ. Acad. Dent. Mater. 1993, 9, 375–378. [Google Scholar] [CrossRef]
- Finke, H.L.; Scott, D.W.; Gross, M.E.; Messerly, J.F.; Waddington, G. Cycloheptane, Cycloöctane and 1,3,5-Cycloheptatriene. Low Temperature Thermal Properties, Vapor Pressure and Derived Chemical Thermodynamic Properties. J. Am. Chem. Soc. 1956, 78, 5469–5476. [Google Scholar] [CrossRef]
- Zou, Q.C.; Tang, Q.Q.; Wu, L.M. Comparison of methods characterizing the surface properties of polymer by inverse gas chromatography. Chin. J. Anal. Chem. 2007, 35, 1469–1474. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Song, J.; Du, L.; Ma, Y.; Ren, S.; Ren, J.; Li, S. Quantitative Analysis of Solubility Parameters and Surface Properties of Larch Bark Proanthocyanidins. Polymers 2020, 12, 2800. https://doi.org/10.3390/polym12122800
Chen S, Song J, Du L, Ma Y, Ren S, Ren J, Li S. Quantitative Analysis of Solubility Parameters and Surface Properties of Larch Bark Proanthocyanidins. Polymers. 2020; 12(12):2800. https://doi.org/10.3390/polym12122800
Chicago/Turabian StyleChen, Siqi, Jie Song, Liuping Du, Yanli Ma, Shixue Ren, Junxue Ren, and Shujun Li. 2020. "Quantitative Analysis of Solubility Parameters and Surface Properties of Larch Bark Proanthocyanidins" Polymers 12, no. 12: 2800. https://doi.org/10.3390/polym12122800
APA StyleChen, S., Song, J., Du, L., Ma, Y., Ren, S., Ren, J., & Li, S. (2020). Quantitative Analysis of Solubility Parameters and Surface Properties of Larch Bark Proanthocyanidins. Polymers, 12(12), 2800. https://doi.org/10.3390/polym12122800








