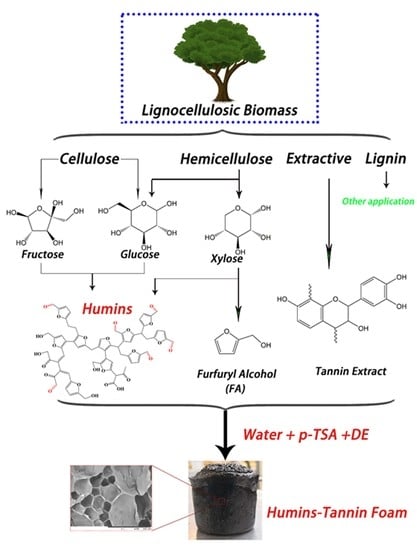
Ambient Temperature Self-Blowing Tannin-Humins Biofoams
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Humins
2.3. Preparation of Tannin-Humin Foams
- 1
- FA + glyoxal control, thus an only furanic foam without tannin [32]
- 2
- FA + glyoxal + humins: 2.5 g humins + 9 g FA + 11.4 g glyoxal + 4 g olive powder + 3.6 g p-TSA+ 1.5 DE (foaming agent); thus, a mixed furfuryl alcohol-humins. An only furanic foam.
- 3
- Tannin + FA + humins (epoxy): 12 g tannin + 5.2 g FA + 4.4 g humins + 6 g p-TSA + 1.5 g DE + 6.6 g water.
- 4
- Tannin + FA + humins: 15 g tannin + 5.2g FA + 3.7 g humins + 6 g p-TSA + 1.5 g DE + 3 g water.
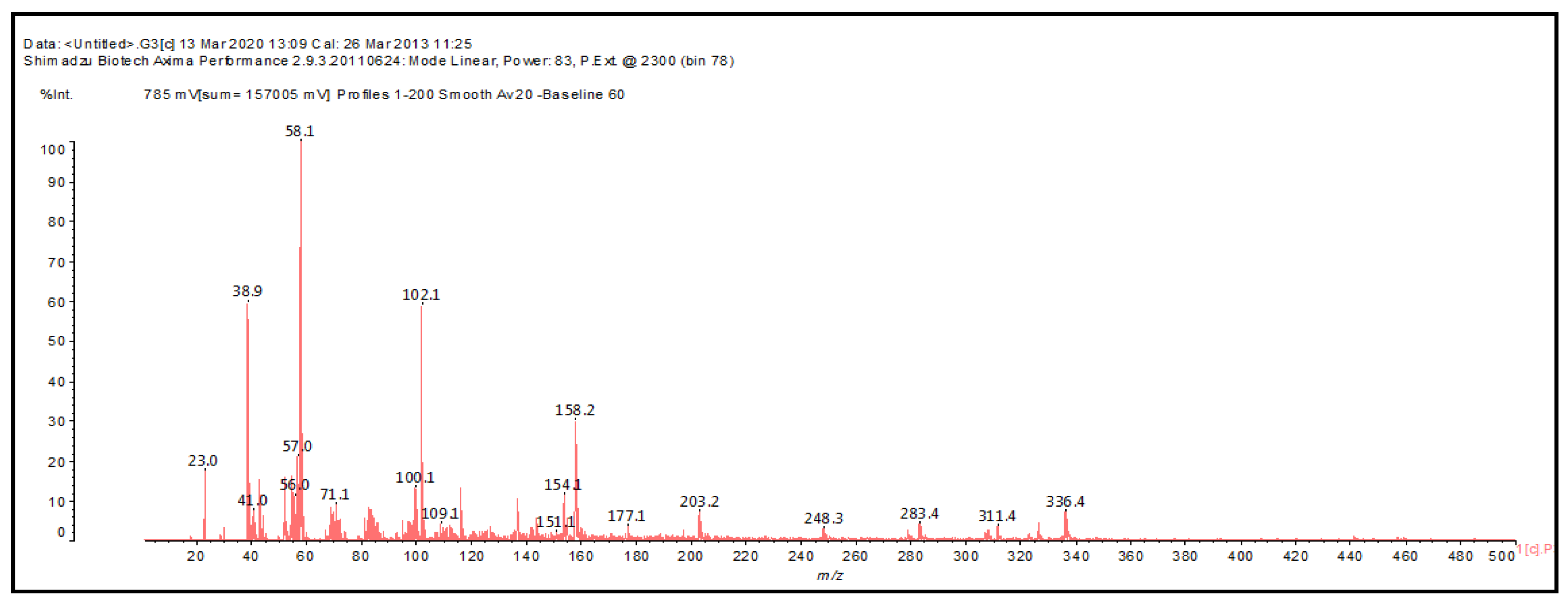
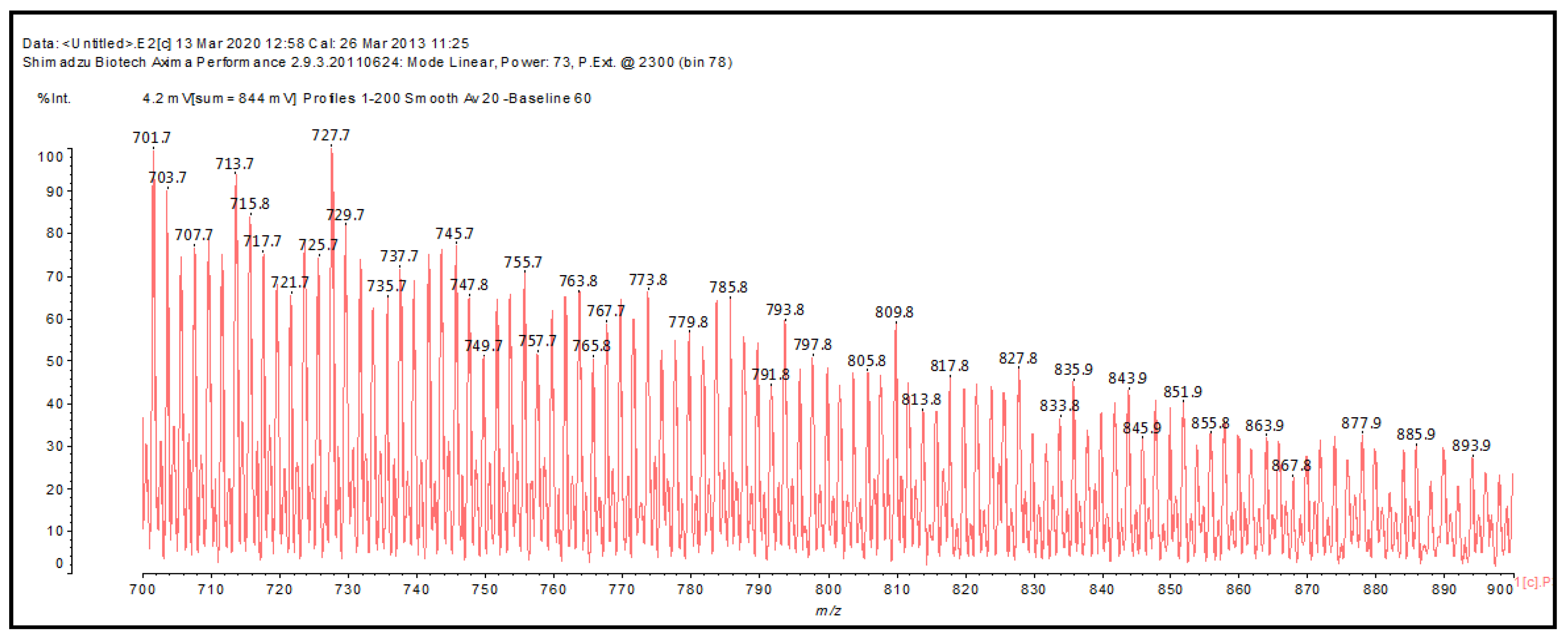
2.4. MALDI-ToF Analysis
2.5. FTIR
2.6. Apparent Density
2.7. Scanning Electron Microscopy (SEM) Analysis
2.8. Compression Strength
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pizzi, A. Tannin-based biofoams-A review. J. Renew. Mater. 2019, 7, 474–489. [Google Scholar] [CrossRef]
- Meikleham, N.; Pizzi, A. Acid and alkali-setting tannin-based rigid foams. J. Appl. Polym. Sci. 1994, 53, 1547–1556. [Google Scholar] [CrossRef]
- Tondi, G.; Zhao, W.; Pizzi, A.; Du, G.; Fierro, V.; Celzard, A. Tannin-based rigid foams: A survey of chemical and physical properties. Bioresour. Technol. 2009, 100, 5162–5169. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pizzi, A.; Cangemi, M.; Fierro, V.; Celzard, A. Flexible natural tannin-based and protein-based biosourced foams. Ind. Crops Prod. 2012, 37, 389–393. [Google Scholar] [CrossRef]
- Lacoste, C.; Pizzi, A.; Basso, M.C.; Laborie, M.-P.; Celzard, A. Pinus pinaster tannin/furanic foams: Part 2: Physical properties. Ind. Crops Prod. 2014, 61, 531–536. [Google Scholar] [CrossRef]
- Lacoste, C.; Pizzi, A.; Basso, M.C.; Laborie, M.P.; Celzard, A. Pinus pinaster tannin/furanic foams: Part 1. Formulation. Ind. Crops Prod. 2014, 52, 450–456. [Google Scholar] [CrossRef]
- Xi, X.; Pizzi, A.; Delmotte, L. Isocyanate-free Polyurethane Coatings and Adhesives from Mono- and Di-Saccharides. Polymers 2018, 10, 402. [Google Scholar] [CrossRef]
- Xi, X.; Pizzi, A.; Gerardin, C.; GDu, G. Glucose-based Non-Isocyanate Polyurethane Biofoams. J. Renew. Mater. 2019, 7, 301–312. [Google Scholar] [CrossRef]
- Xi, X.; Pizzi, A.; Gerardin, C.; Lei, H.; Chen, X.; Amirou, S. Preparation and evaluation of Glucose based Non-Isocyanate polyurethane Self-blowing Rigid Foams. Polymers 2019, 11, 1802. [Google Scholar] [CrossRef]
- Chen, X.; Xi, X.; Pizzi, A.; Fredon, E.; Zhou, X.; Li, J.; Gerardin, C.; Du, G. Preparation and Characterization of Condensed Tannin Non-Isocyanate Polyurethane (NIPU) Rigid Foams by Ambient Temperature Blowing. Polymers 2020, 12, 750. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Xi, X.; Pizzi, A.; Zhou, X.; Fredon, E.; Du, G.; Gerardin, C. Condensed Glucose-Tannin-based NIPU BioFoams of Improved Fire Retardancy. Polym. Degrad. Stabil. 2020, 175, 109121. [Google Scholar] [CrossRef]
- Lacoste, C.; Basso, M.C.; Pizzi, A.; Celzard, A.; Ella Bang, E.; Gallon, N.; Charrier, B. Pine (P. pinaster) and quebracho (Schinopsis lorentzii) tannin based foams as green acoustic absorbers. Ind. Crops Prod. 2015, 67, 70–73. [Google Scholar] [CrossRef]
- Pizzi, A.; Tondi, G.; Pasch, H.; Celzard, A. MALDI-TOF Structure determination of complex thermoset networks—Polyflavonoid tannin-furanic rigid foams. J. Appl. Polym. Sci. 2008, 110, 1451–1456. [Google Scholar] [CrossRef]
- Tondi, G.; Pizzi, A.; Pasch, H.; Celzard, A. Structure degradation, conservation and rearrangement in the carbonization of polyflavonoid tannin/furanic rigid foams—a MALDI-TOF investigation. Polym. Degrad. Stabil. 2008, 93, 968–975. [Google Scholar] [CrossRef]
- Tondi, G.; Pizzi, A.; Olives, G.R. Natural tannin-based rigid foams as insulation in wood construction. Maderas-Cienc. Tecnol. 2008, 10, 219–227. [Google Scholar] [CrossRef]
- Tondi, G.; Pizzi, A. Tannin based rigid foams: Characterisation and modification. Ind. Crops Prod. 2009, 29, 356–363. [Google Scholar] [CrossRef]
- Basso, M.C.; Pizzi, A.; Al-Marzouki, F.; Abdalla, S. Horticultural/hydroponics and floral foams from tannins. Ind. Crops Prod. 2016, 87, 177–181. [Google Scholar] [CrossRef]
- Basso, M.C.; Pizzi, A.; Celzard, A. Influence of formulation on the dynamics of preparation of tannin based foam. Ind. Crops Prod. 2013, 51, 396–400. [Google Scholar] [CrossRef]
- Basso, M.C.; Pizzi, A.; Celzard, A. Dynamic monitoring of tannin foams preparation: Surfactant effects. BioResources 2013, 8, 5807–5816. [Google Scholar] [CrossRef]
- Basso, M.C.; Lagel, M.C.; Pizzi, A.; Celzard, A.; Abdalla, S. First tools for tannin-furanic foams design. BioResources 2015, 10, 5233–5241. [Google Scholar] [CrossRef]
- Sangregorio, A.; Guigo, N.; van der Waal, J.C.; Sbirrazzuoli, N. Humins from Biorefineries as Thermoreactive Macromolecular Systems. ChemSusChem 2018, 11, 4246–4255. [Google Scholar] [CrossRef] [PubMed]
- Sangregorio, A. Valorisation of Biorefinery-Derived Humins: Towards the Development of Sustainable Thermosets and Composites. Ph.D. Thesis, Université de la Cote d’Azur, Nice, France, 2019. [Google Scholar]
- Van Zandvoort, I.; Wang, Y.; Rasrendra, C.B.; van Eck, E.R.H.; Bruijnincx, P.C.A.; Heeres, H.J.; Weckhuysen, B.M. Formation, Molecular Structure, and Morphology of Humins in Biomass Conversion: Influence of Feedstock and Processing Conditions. ChemSusChem 2013, 6, 1745–1758. [Google Scholar] [CrossRef] [PubMed]
- Van Zandvoort, I.; Koers, E.J.; Weingarth, M.; Bruijnincx, P.C.A.; Baldus, M.; Weckhuysen, B.M. Structural characterization of 13C-enriched humins and alkali-treated 13C humins by 2D solid-state NMR. Green Chem. 2015, 17, 4383–4392. [Google Scholar] [CrossRef]
- Hoang, T.M.C.; van Eck, E.R.H.; Bula, W.P.; JGardeniers, J.G.E.; Lefferts, L.; Seshan, K. Humin based by-products from biomass processing as a potential carbonaceous source for synthesis gas production. Green Chem. 2015, 17, 959–972. [Google Scholar] [CrossRef]
- Sangregorio, A.; Guigo, N.; de Jong, E.; Sbirrazzuoli, N. Kinetics and Chemorheological Analysis of Cross-Linking Reactions in Humins. Polymers 2019, 11, 1804. [Google Scholar] [CrossRef] [PubMed]
- Sangregorio, A.; Muralidhara, A.; Guigo, N.; Marlair, G.; Angelici, C.; Thygesen, L.G.; de Jong, E.; Sbirrazzuoli, N. Humins based resin for wood modification and properties improvement. Green Chem. 2020, 22, 2786–2798. [Google Scholar] [CrossRef]
- Mija, A.C.; de Jong, E.; van der Waal, J.C.; van Klink, G.P.M. Humins-Containing Foam. U.S. Patent No. 10,752,747, 25 August 2020. [Google Scholar]
- Licsandru, E.; Mija, A.C. From biorefinery by-product to bioresins. Thermosets based on humins and epoxidized linseed oil. Cell. Chem. Technol. 2019, 53, 963–969. [Google Scholar] [CrossRef]
- Cornillet, C.; Labat, G.; Gaillard, J.-M. FCBA, Analyse des Cycles de Vie (LCA), Presentation project BEMA, 16 February 2012. In Restitution du programme Bois Eco Matériaux Aquitaine (BEMA); Bois Ecomateriaux Aquitaine: Bordeaux, France, 2012; pp. 73–88. [Google Scholar]
- Arias, A.; González-García, S.; Feijoo, G.; Moreira, M.T. Tannin-based bio-adhesives for the wood panel industry as an environmentally friendly alternative to petrochemical resins. J. Ind. Ecol. 2020, in press. [Google Scholar]
- Xi, X.; Pizzi, A.; Lei, H.; Du, G.; Zhou, X.; Lin, Y. Characterization and preparation of furanic-glyoxal foams. Polymers 2020, 12, 692. [Google Scholar] [CrossRef]
- Goodner, M.D.; DeSimone, J.M.; Kiserow, D.J.; Roberts, G.W. An Equilibrium Model for Diffusion-Limited Solid-State Polycondensation. Ind. Eng. Chem. Res. 2000, 39, 2797–2806. [Google Scholar] [CrossRef]
- Aronhime, M.T.; Gillham, J.K. Time-temperature-transformation (TTT) cure diagram of thermosetting polymeric systems. In Epoxy Resins and Composites III. Advances in Polymer Science; Dušek, K., Ed.; Springer: Berlin/Heidelberg, Germany, 1986; Volume 78. [Google Scholar] [CrossRef]
- Abdullah, U.H.B.; Pizzi, A. Tannin-Furfuryl alcohol wood panel adhesives without formaldehyde. Eur. J. Wood Prod. 2013, 71, 131–132. [Google Scholar] [CrossRef]






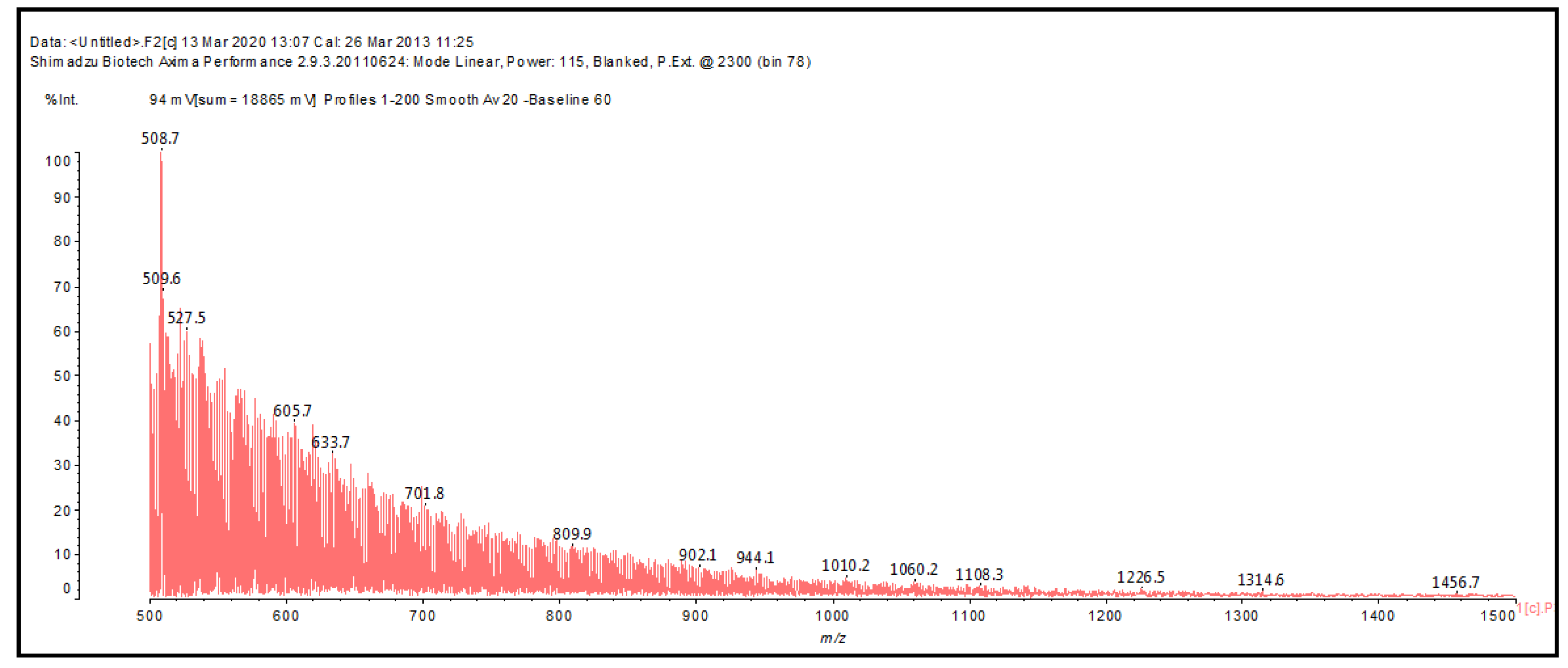
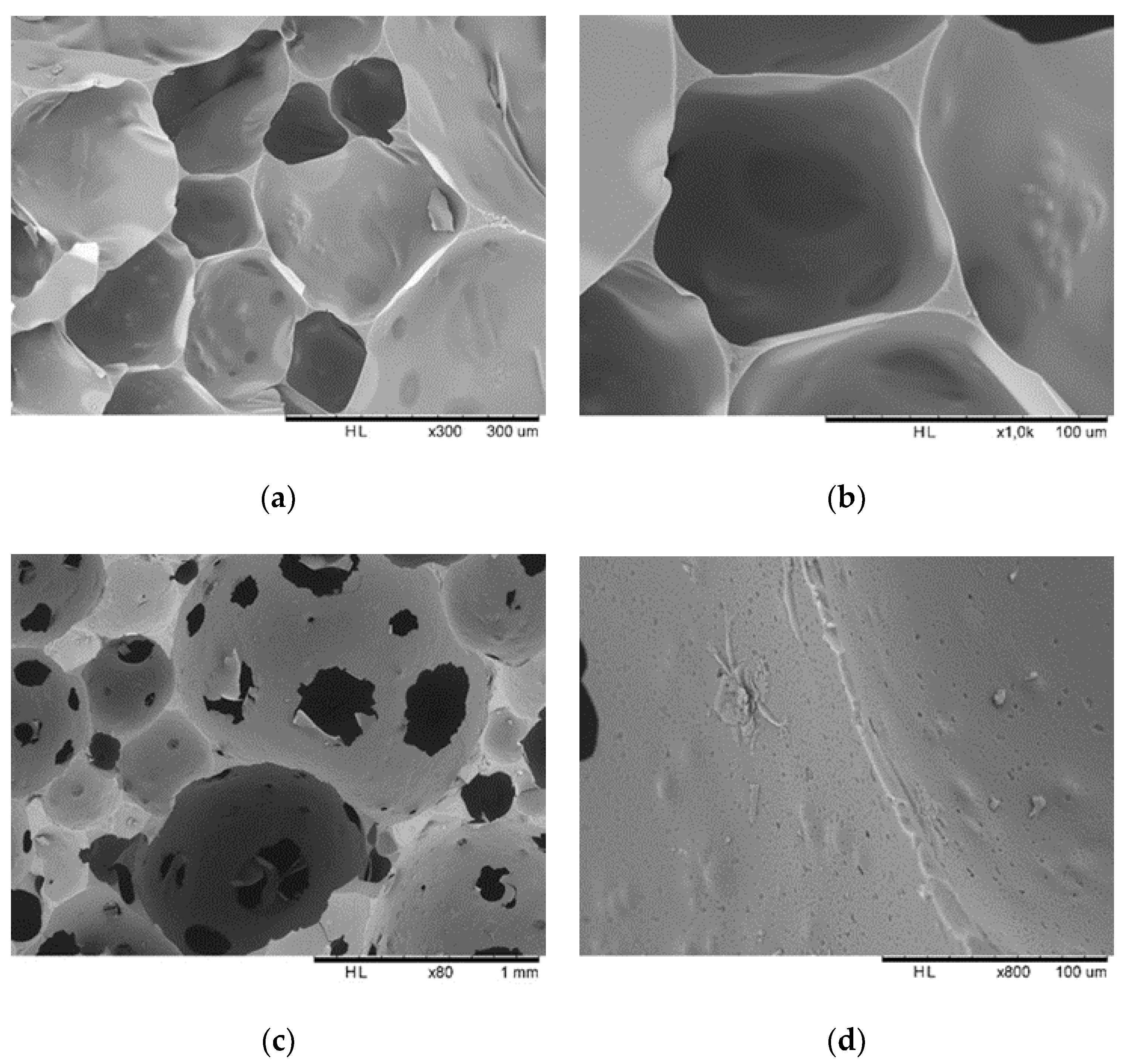
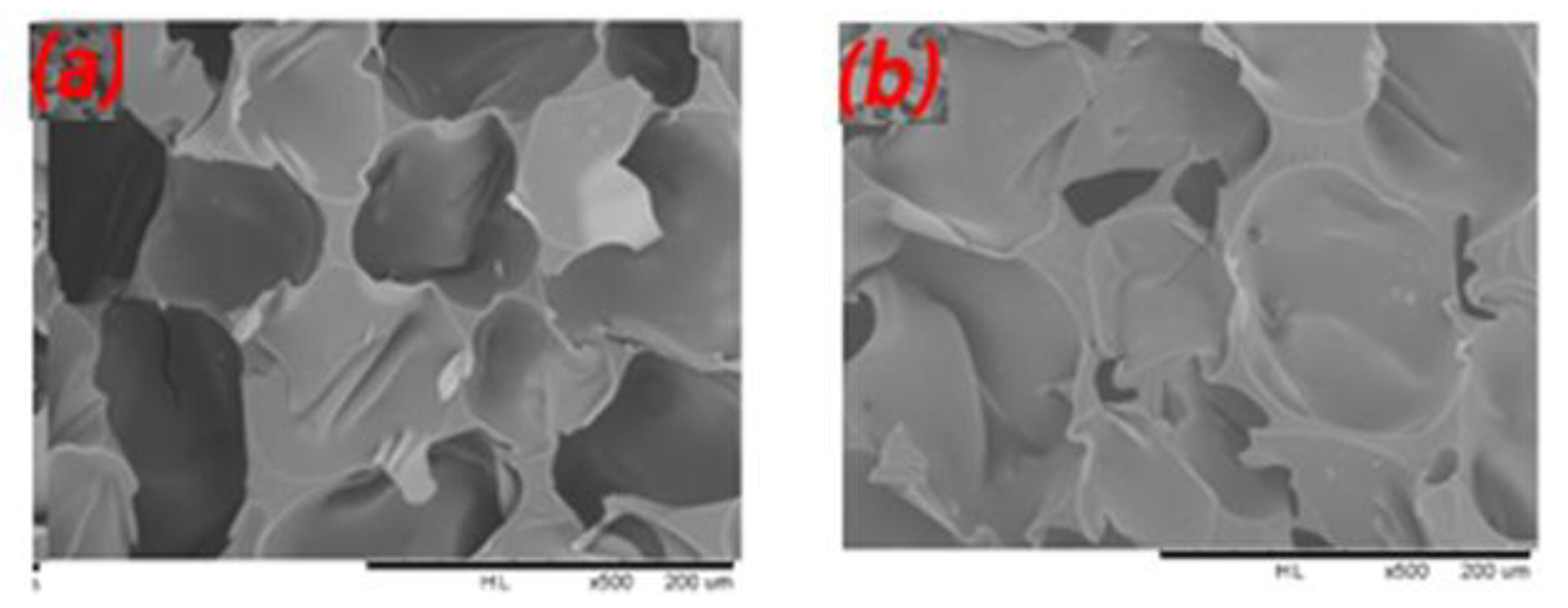
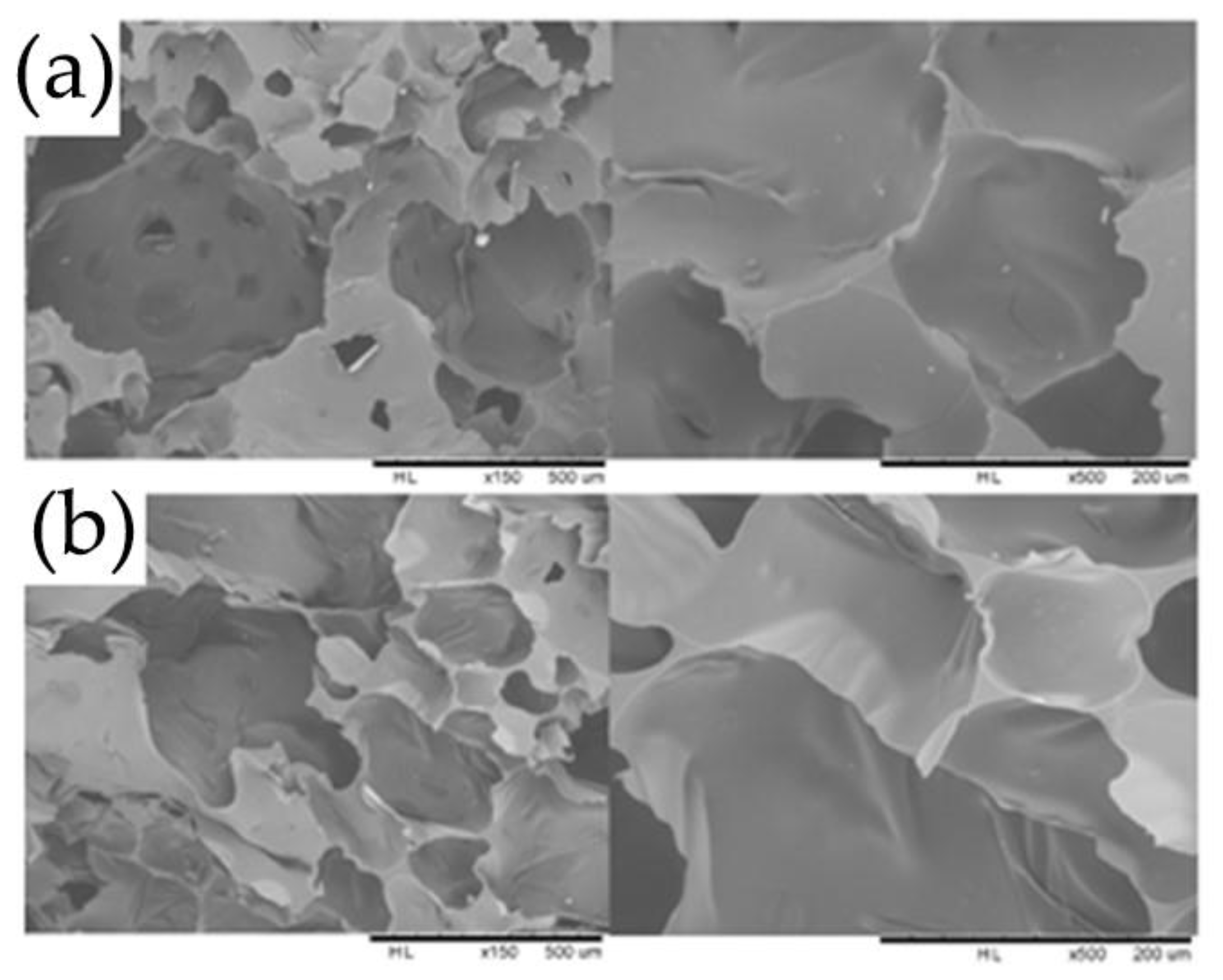
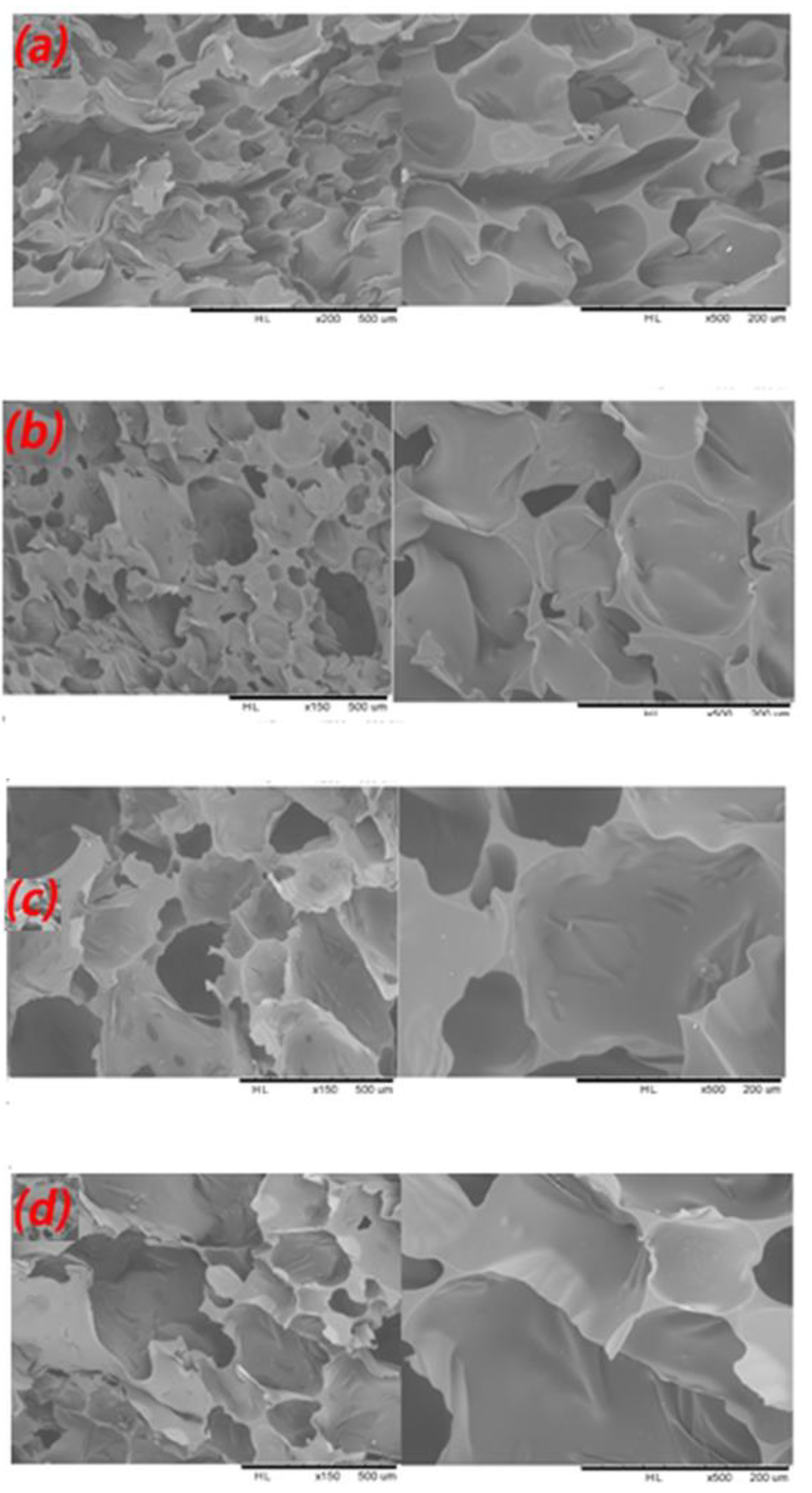
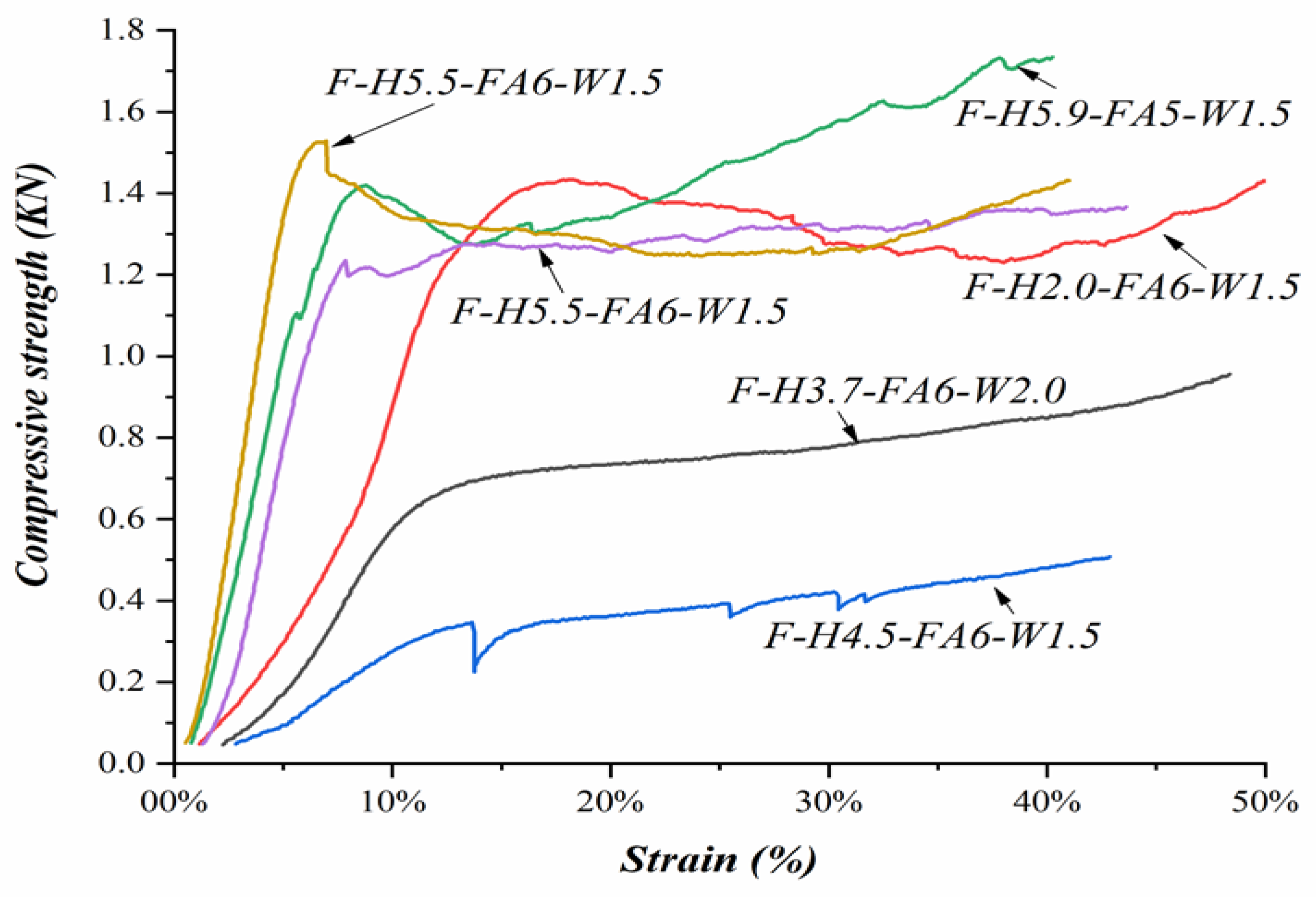
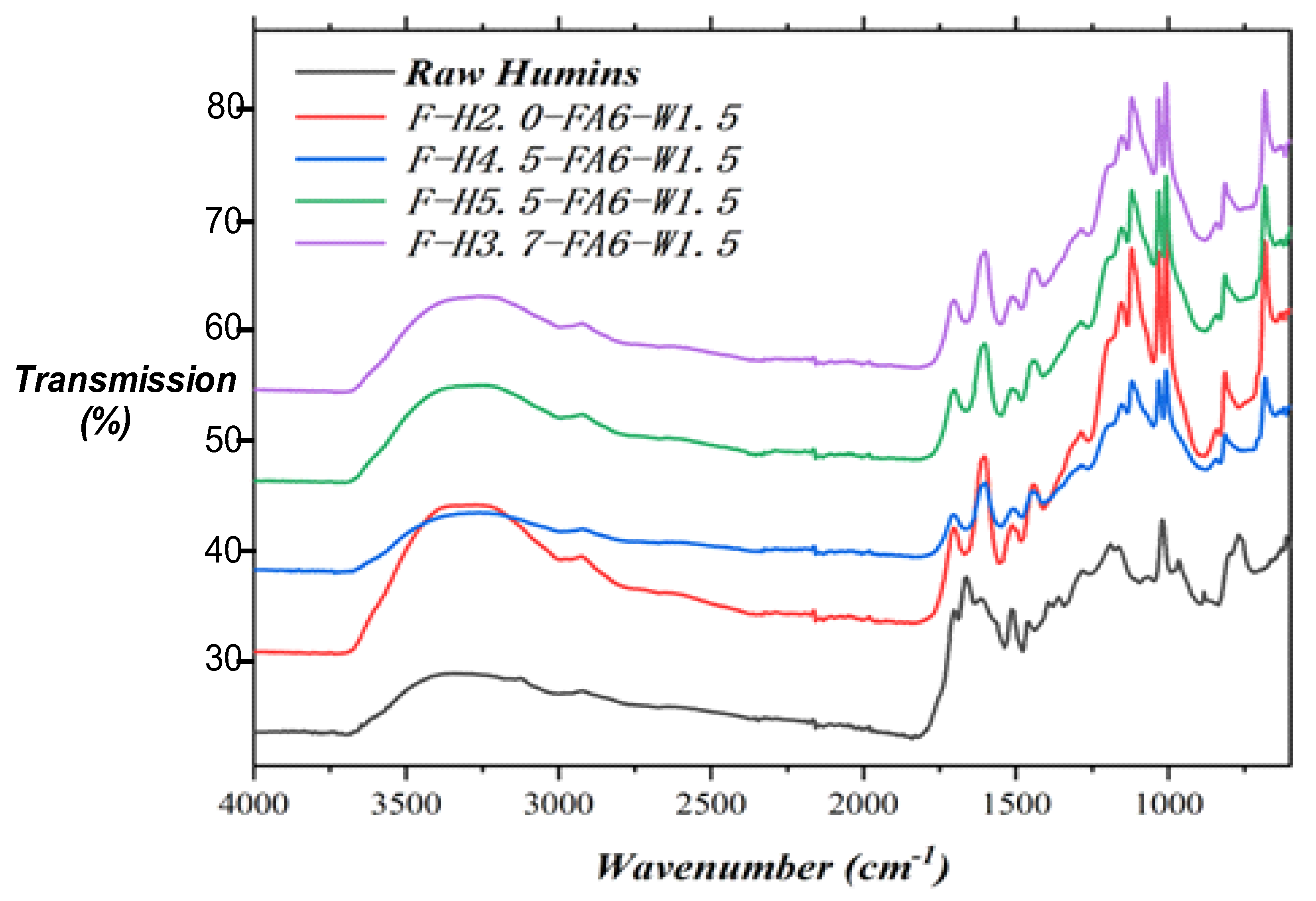



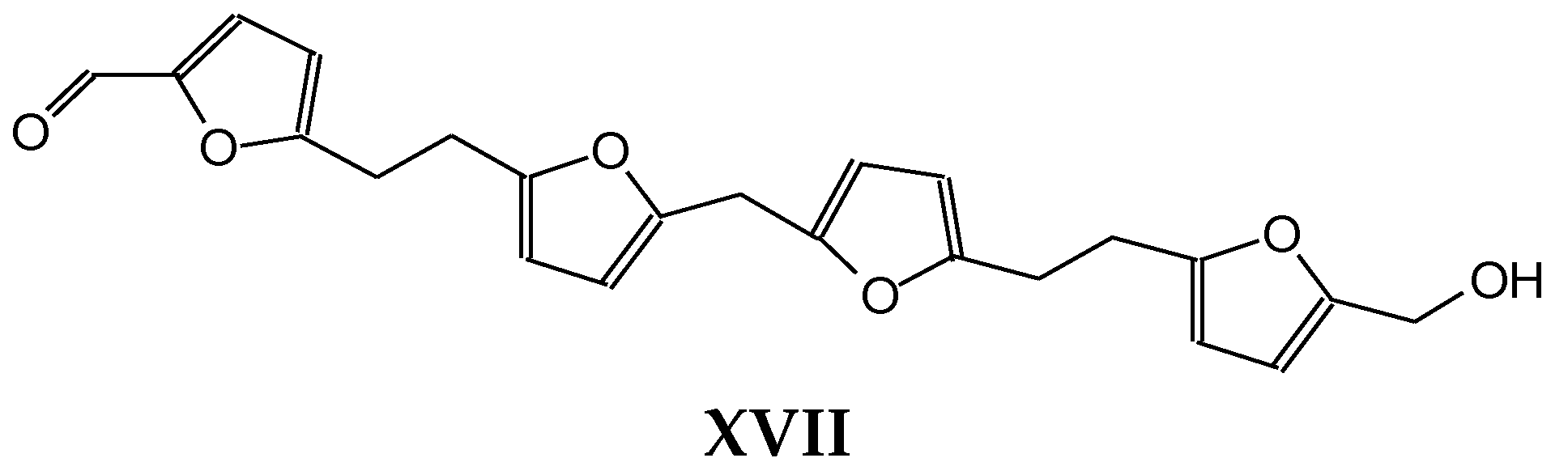
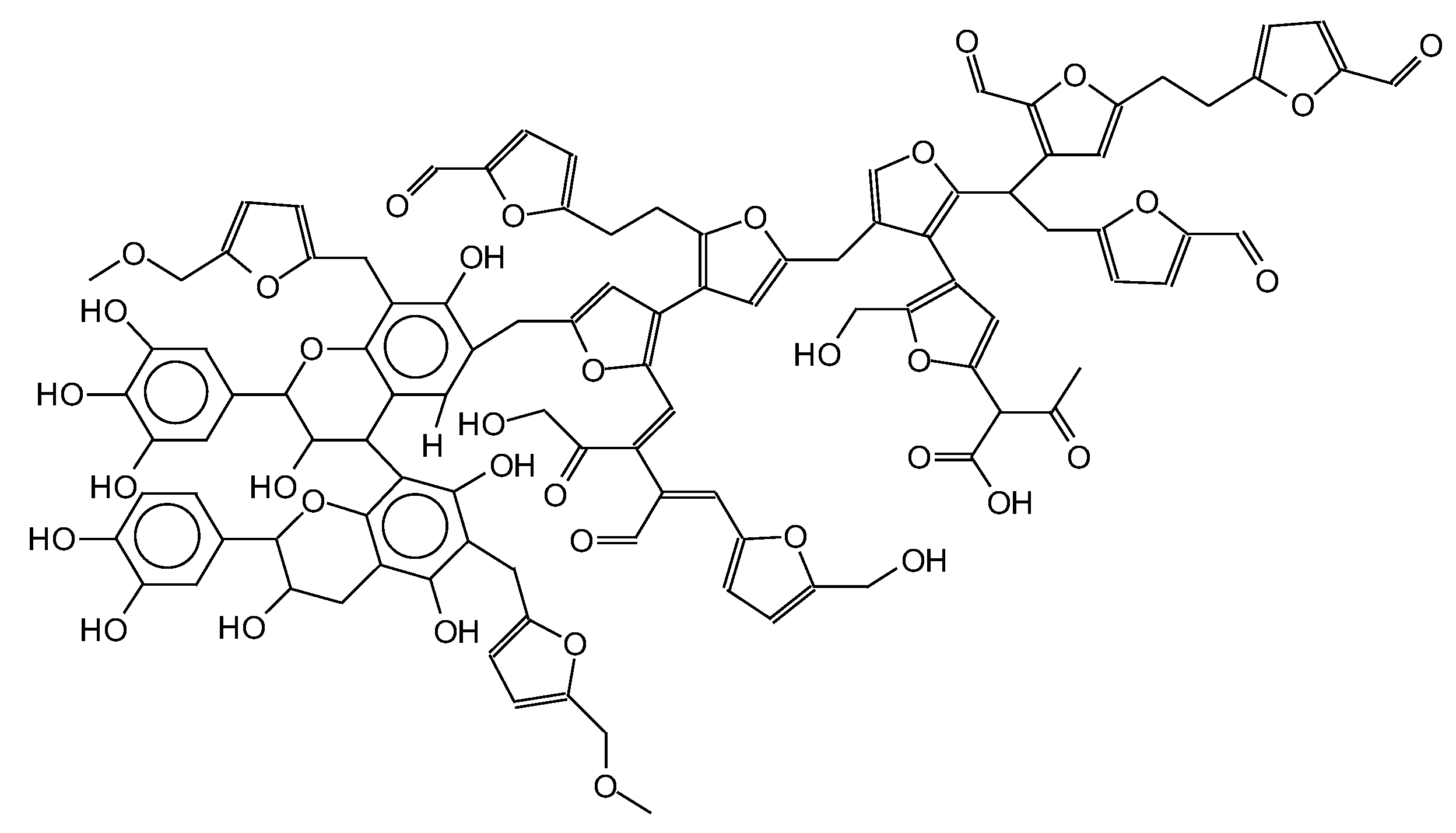
| Samples | Tannin (g) | Humins (g) | FA (g) | Water (g) | p-TSA (g) | DE (g) |
|---|---|---|---|---|---|---|
| F-H2.0-FA6-W1.5 | 15.0 | 2.0 | 6.0 | 1.5 | 6.0 | 1.5 |
| F-H3.7-FA6-W1.5 | 3.7 | 6.0 | 1.5 | |||
| F-H4.5-FA6-W1.5 | 4.5 | 6.0 | 1.5 | |||
| F-H5.5-FA6-W1.5 | 5.5 | 6.0 | 1.5 | |||
| F-H5.9-FA5-W1.5 | 5.9 | 5.0 | 1.5 | |||
| F-H3.7-FA6-W2.0 | 3.7 | 6.0 | 2.0 |
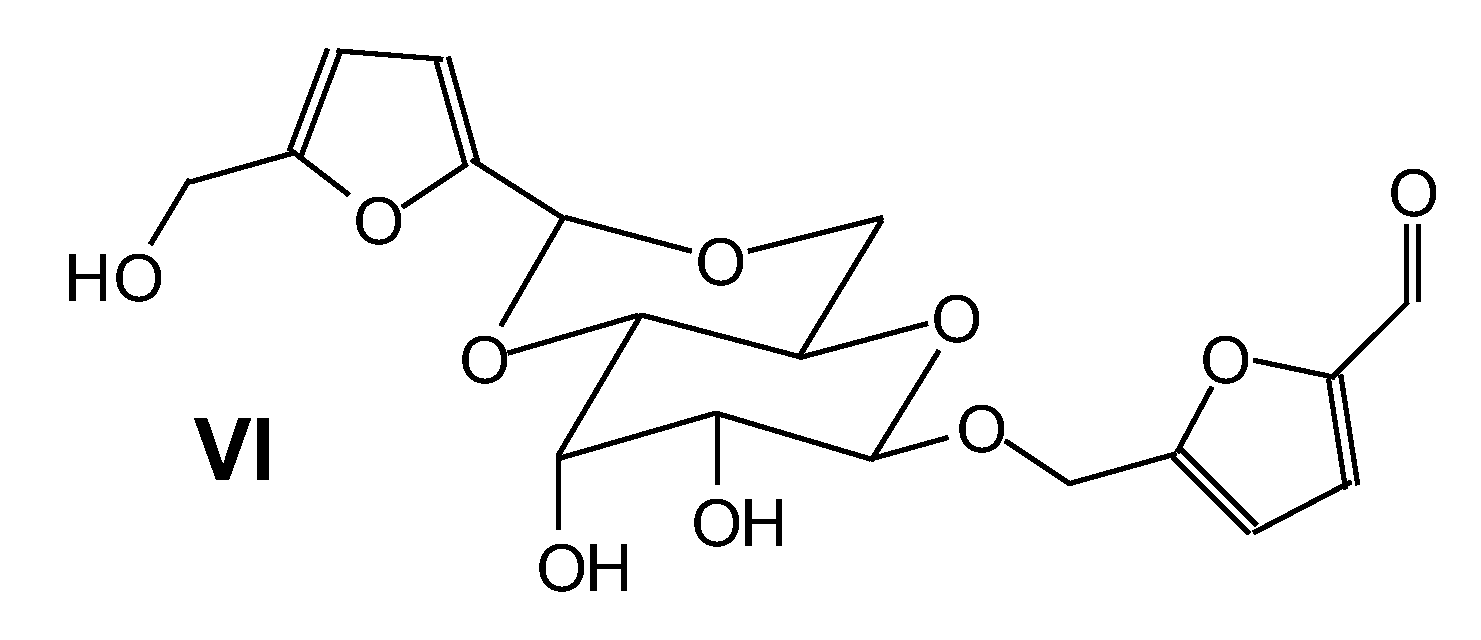
| 139 Da both theoretical and experimental, no Na+ |
 |
| 139 Da (calculated 140) no Na+ |
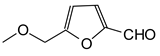 |
| 159 Da (calculated 158 Da) no Na° |
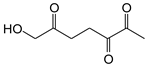 |
| 159 Da (calculated 156 Da) no Na+ |
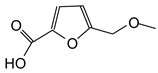 |
| 177–179 Da (Calculated 178 Da) with Na+ |
 |
| 202–203 Da (Calc 203) with Na+ |
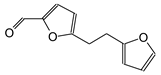 |
| 203 Da (Calc 203 Da) with Na+) [22] |
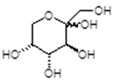 |
| 257 Da (calc 252 Da) |
 |
| 257 Da (Calc 254 Da) |
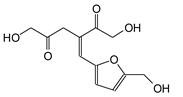 |
| 268 Da (Calculated 270 Da) no Na+, (180 °C no oxidation spectrum) |
 |
| 284 Da no Na+ (150 °C and 180° oxidized spectra) |
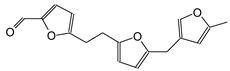 |
| 322 Da (Calculated 324 Da) no Na+ (180 °C not oxidized) [22] |
 |
| 391 Da (Calculated 389 Da) with Na+, (180 °C not oxidieed) |
 |
| 392 Da (Calculated 396 Da) (180 °C not oxidized) [22] |
 |
| 506 Da (no Na+) and 526 Da (with Na+) Calc.: 504 Da and 527 Da |
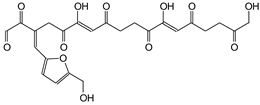 |
| 623 Da (Calculated 623 Da) with Na+ (150 °C spectrum) |
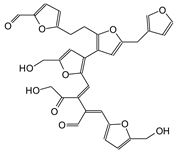 |
| 635 Da (with Na+) |
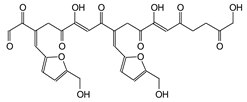 |
| 636 Da (Calculated 637 Da) with Na+, (180 °C not oxidized spectrum) |
 |
| 720 Da no Na+ |
 |
| 741 Da (calc 743 Da) with Na+ |
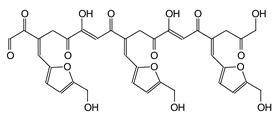 |
| 810 Da (Calculated 810 Da) without Na+ (180 °C Oxidized, 180 °C not oxidized, 150 °C) |
| 833 Da (Calculated 833 Da) with Na+ (180 °C Oxidized, 180 °C not oxidized) |
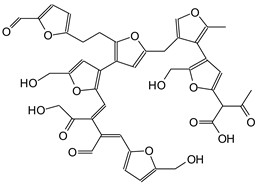 |
| 935 Da (Calculated 932 Da) without Na+ (180 °C Oxidized, 150 °C |
| 950-952-954 Da (Calculated 955 Da) with Na+ (180 °C oxidized and not oxidized) |
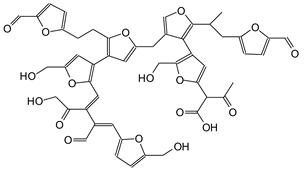 |
| 1010 Da (Calculated 1012 Da) no Na+ (180 °C not oxidized) |
| 1033 Da (Calculated 1035 Da) with Na° (180 °C oxidized) |
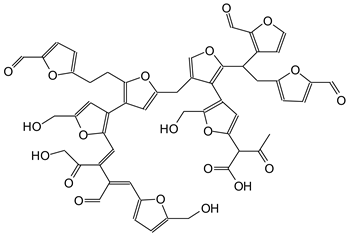 |
| 1137 Da (Calculated 1135 Da) no Na+ (180 °C oxidized) |
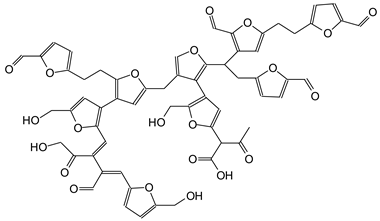 |
| 1227 Da (Calculated 1228 Da) no Na+ (180 °C not oxidized) |
| 1250 Da (Calculated 1252 Da) with Na+ (150 °C) |
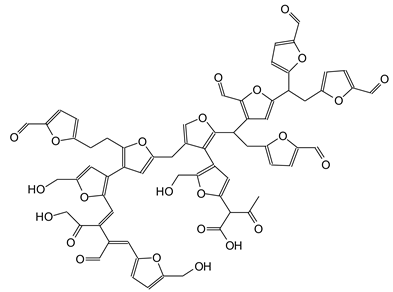 |
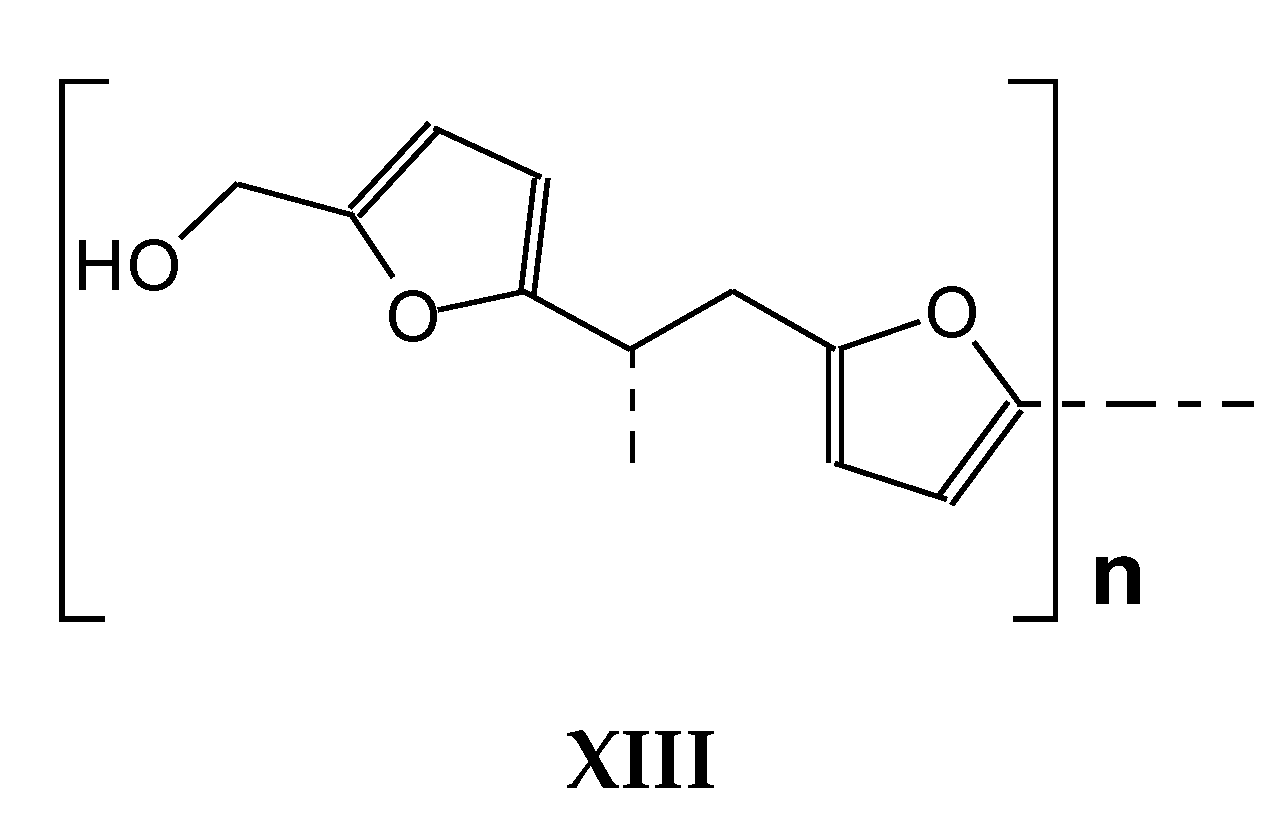
| 1351 Da With Na+ |
 |
| Samples | Density (g/cm3) | Compressive Strength (MPa) | Specific Compressive Strength (kPa/kg∙m−3) |
|---|---|---|---|
| F-H2.0-FA6-W1.5 | 0.20 | 1.59 | 7.95 |
| F-H3.7-FA6-W1.5 | 0.16 | 0.81 | 5.06 |
| F-H4.5-FA6-W1.5 | 0.11 | 0.39 | 3.54 |
| F-H5.5-FA6-W1.5 | 0.21 | 1.38 | 6.57 |
| F-H5.9-FA5-W1.5 | 0.24 | 1.58 | 6.58 |
| F-H3.7-FA6-W2.0 | 0.19 | 1.70 | 8.94 |
| 304 Da Flavonoid gallocatechin, no Na+ |
| 326–327 Da Flavonoid gallocatechin with Na+ |
 |
| 393 Da peak |
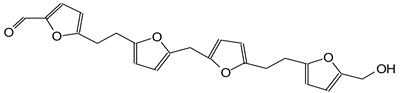 |
| 411 Da (Calculated 414 Da) no Na+ |
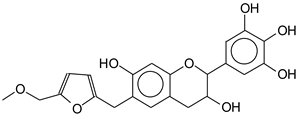 |
| 427–428 Da (Calculated 430) no Na+ reaction of a flavonoid with the humins aldehyde at 141 Da in humins only spectrum. |
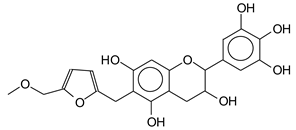 |
| 605 Da= (Calculated 605 Da) no Na+ |
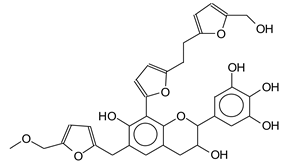 |
| 621 Da with Na+ |
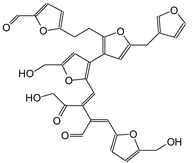 |
| 635 Da |
 |
| 731 Da (calculated 733 Da) without Na+ |
| 758 Da (calculated 756 Da)with Na+ |
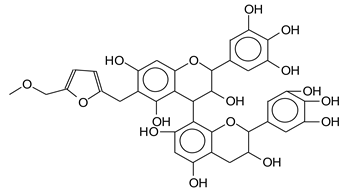 |
| 749 Da (Calculated 752 Da) no Na+, and |
| 774 Da (Calculated 775 Da) with Na+ |
 |
| 792 Da = 774 Da + 1x-OH (on A-ring of one flavonoid unit) |
| 793 Da (Calculated 794 Da) no Na+ and 815 Da with Na+ |
 |
| 783 Da = as 798 Da but based on a fisetinidin + fisetinidin dimer |
| 798.8 Da (Calculated 798.5 Da) no Na+. A delphinidin–robinetinidin dimer reacted with a humins low molecular weight species and with furfuryl alcohol: |
 |
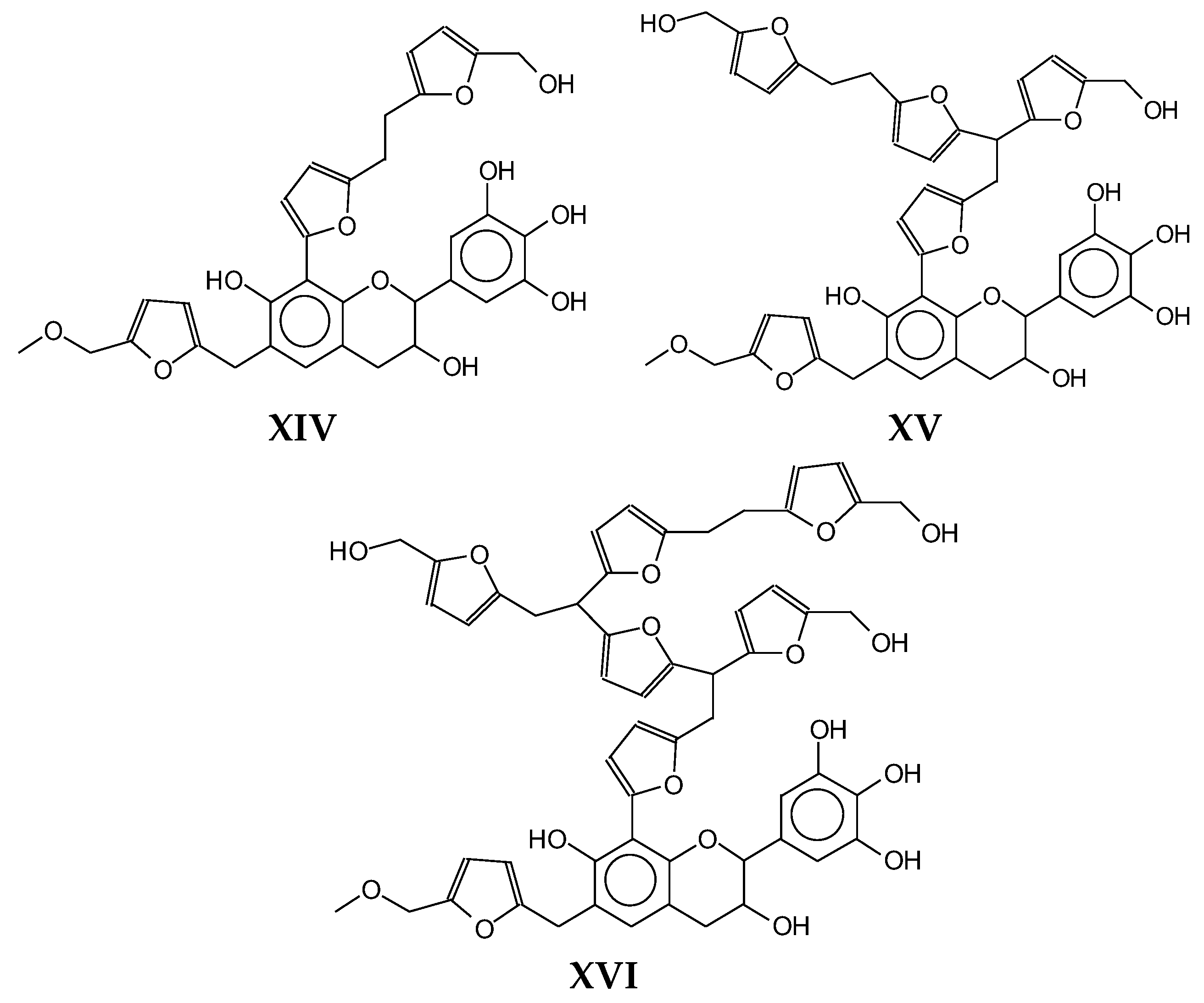
| 815 Da (calculated 915 Da) no Na+, reaction of flavonoid dimer with furfuryl alcohol and a 141 Da humins low molecular weight species |
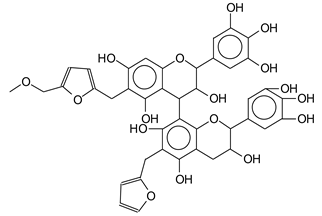 |
| 835 Da (calculated 833 Da) no Na+ |
 |
| 912 Da (Calculated 912) no Na+, small peak, reaction of a flavonoid dimer with polyfurfuryl alcohol and a humins compound |
 |
| 925 Da = (calculated 928 Da) = 912 + 1x-OH, no Na+ |
| 1008 Da (Calculated 1007 Da) with Na+. |
 |
| 1009 Da no Na+ |
| 1035 Da (Calculated 1035 Da) with Na+ in 180 °C oxidized |
 |
| 1136 Da (Calculated 1135 Da) no Na+ in 180 °C oxidized |
 |
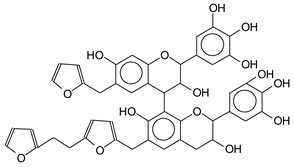
| 1535–1541 Da (Calc. 1538) with Na+ |
 |
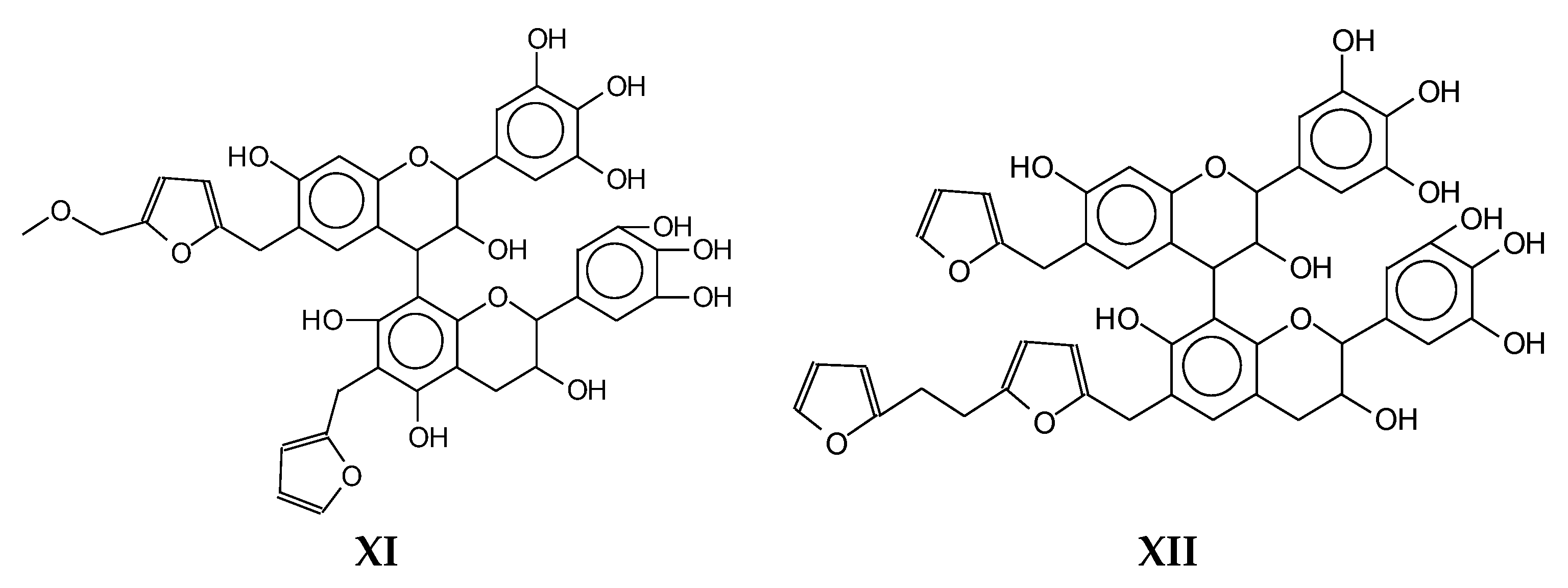
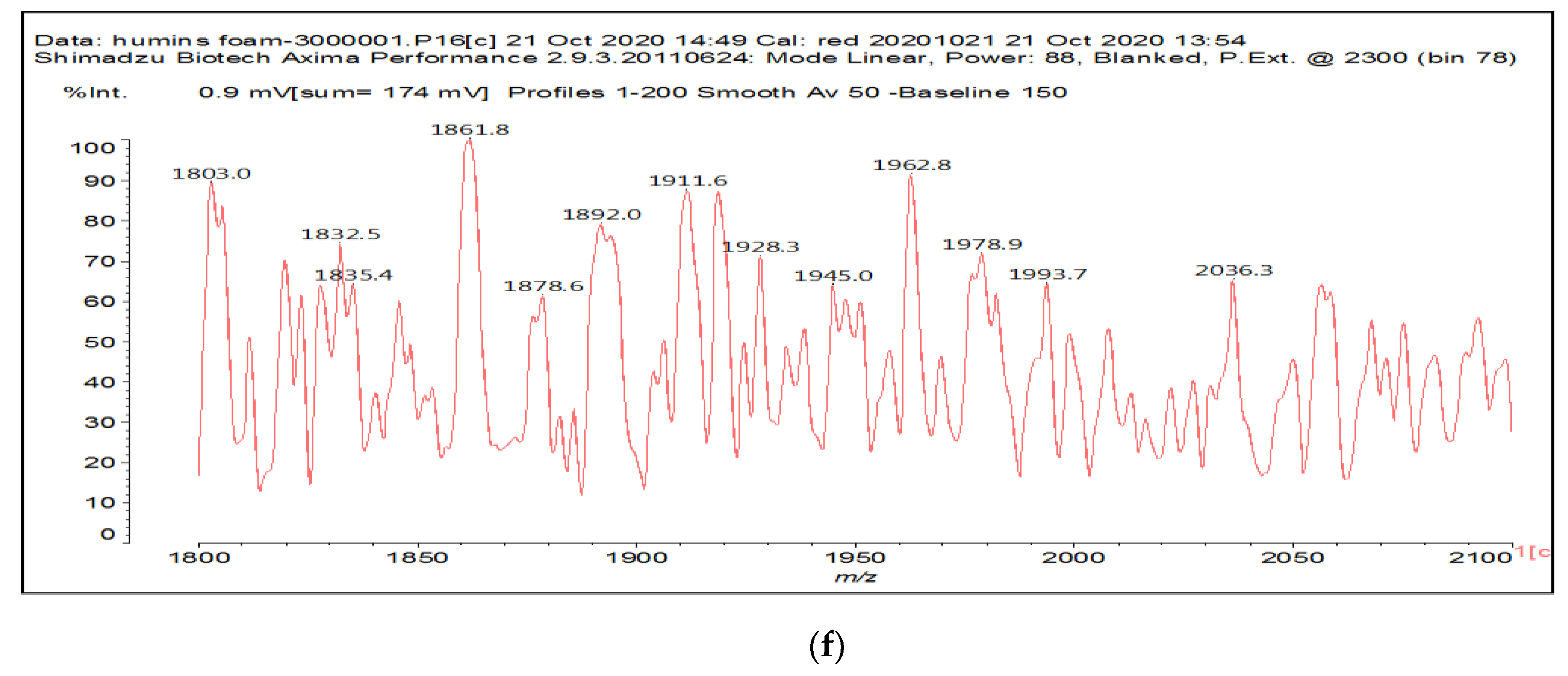
| 1803 Da (Calculated 1803) no Na+ |
 |
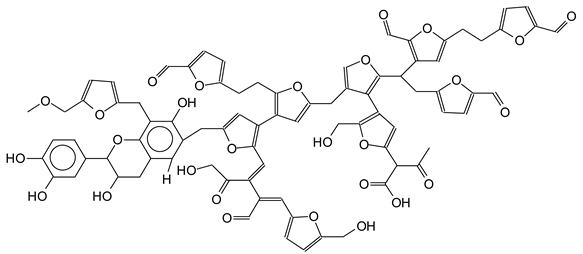
| 1819 Da (Calculated 1819 Da) no Na+ |
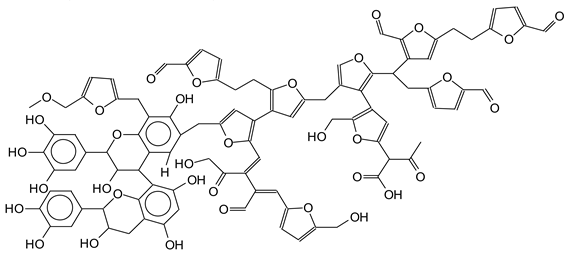 |
| 1945 Da (Calculated 1943) no Na+ |
 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Guigo, N.; Pizzi, A.; Sbirrazzuoli, N.; Li, B.; Fredon, E.; Gerardin, C. Ambient Temperature Self-Blowing Tannin-Humins Biofoams. Polymers 2020, 12, 2732. https://doi.org/10.3390/polym12112732
Chen X, Guigo N, Pizzi A, Sbirrazzuoli N, Li B, Fredon E, Gerardin C. Ambient Temperature Self-Blowing Tannin-Humins Biofoams. Polymers. 2020; 12(11):2732. https://doi.org/10.3390/polym12112732
Chicago/Turabian StyleChen, Xinyi, Nathanael Guigo, Antonio Pizzi, Nicolas Sbirrazzuoli, Bin Li, Emmanuel Fredon, and Christine Gerardin. 2020. "Ambient Temperature Self-Blowing Tannin-Humins Biofoams" Polymers 12, no. 11: 2732. https://doi.org/10.3390/polym12112732
APA StyleChen, X., Guigo, N., Pizzi, A., Sbirrazzuoli, N., Li, B., Fredon, E., & Gerardin, C. (2020). Ambient Temperature Self-Blowing Tannin-Humins Biofoams. Polymers, 12(11), 2732. https://doi.org/10.3390/polym12112732