Processing Conditions of a Medical Grade Poly(Methyl Methacrylate) with the Arburg Plastic Freeforming Additive Manufacturing Process
Abstract
1. Introduction
2. Materials and Methods
3. Results
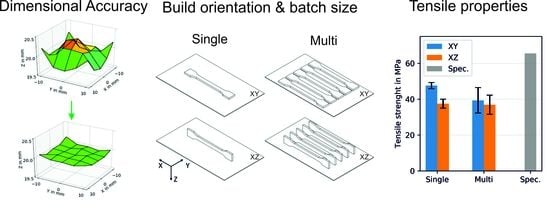
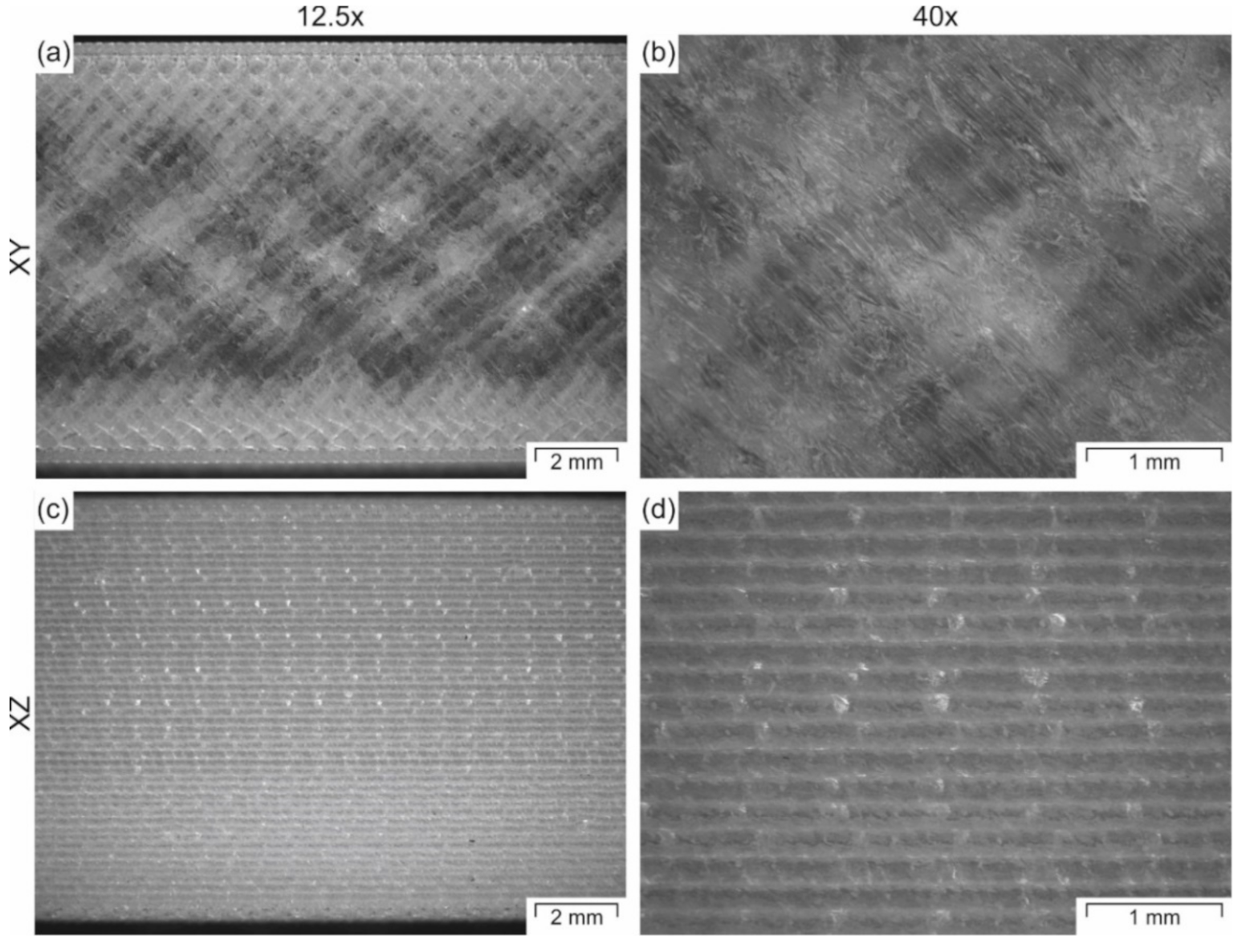
3.1. Optimized Processing Parameters
3.2. Tensile Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zolfagharian, A.; Mahmud, M.A.P.; Gharaie, S.; Bodaghi, M.; Kouzani, A.Z.; Kaynak, A. 3D/4D-printed bending-type soft pneumatic actuators: Fabrication, modelling, and control. Virtual Phys. Prototyp. 2020, 15, 373–402. [Google Scholar] [CrossRef]
- Culmone, C.; Smit, G.; Breedveld, P. Additive manufacturing of medical instruments: A state-of-the-art review. Addit. Manuf. 2019, 27, 461–473. [Google Scholar] [CrossRef]
- Hirsch, A.; Hecker, F.; Moritzer, E. Process Parameter Optimization to Improve the Mechanical Properties of Arburg Plastic Freeformed Components. In Proceedings of the 30th Annual International Solid Freeform Fabrication Symposium, Austin, TX, USA, 12–14 August 2019; pp. 705–714. [Google Scholar]
- Gaub, H. Customization of mass-produced parts by combining injection molding and additive manufacturing with Industry 4.0 technologies. Reinf. Plast. 2016, 6, 401–404. [Google Scholar] [CrossRef]
- Neff, M.; Pawelczyk, L. Resilient Hard-soft Combinations with Plastic Freeforming. ATZ Prod. Worldw. 2019, 6, 36–39. [Google Scholar] [CrossRef]
- Welsh, N.R.; Malcolm, R.K.; Devlin, B.; Boyd, P. Dapivirine-releasing vaginal rings produced by plastic freeforming additive manufacturing. Int. J. Pharm. 2019, 572, 118725. [Google Scholar] [CrossRef] [PubMed]
- Kaut, F.; Cepus, V.; Grellmann, W.; Lach, R. Struktur-Eigenschafts-Beziehungen additiv gefertigter thermo-plastischer Polymere am Beispiel der ARBURGFreeformer-Technologie. In Rapid.Tech + FabCon 3.D—International Trade Show & Conference for Additive Manufacturing, Proceedings of the 15th Rapid. Tech Conference, Erfurt, Germany, 5–7 June 2018; Kynast, M., Eichmann, M., Witt, G., Eds.; Hanser: München, Germany, 2018; pp. 217–235. ISBN 978-3-446-45811-6. [Google Scholar]
- Roehm America LLC. CYROLITE MD H12 Polymer. Available online: https://www.cyrolite.com/en/cyrolite-md (accessed on 19 August 2020).
- Petersmann, S.; Spoerk, M.; van de Steene, W.; Üçal, M.; Wiener, J.; Pinter, G.; Arbeiter, F. Mechanical properties of polymeric implant materials produced by extrusion-based additive manufacturing. J. Mech. Behav. Biomed. Mater. 2020, 104, 103611. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Michiardi, A.; Castaño, O.; Planell, J.A. Biomaterials in orthopaedics. J. R. Soc. Interf. 2008, 5, 1137–1158. [Google Scholar] [CrossRef]
- Ratner, B.D. Biomaterials Science. An Introduction to Materials in Medicine, 2nd ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2004; ISBN 0125824637. [Google Scholar]
- Ridwan-Pramana, A.; Marcián, P.; Borák, L.; Narra, N.; Forouzanfar, T.; Wolff, J. Structural and mechanical implications of PMMA implant shape and interface geometry in cranioplasty—A finite element study. J. Cranio-Maxillofac. Surg. 2016, 44, 34–44. [Google Scholar] [CrossRef]
- Kriegel, R.J. Kalottenplastik für große Schädeldefekte mit PMMA (Polymethylmethacrylat) oder Tutoplast-Prozessierten Autogenen Knochentransplantaten. Ph.D. Thesis, Rheinischen Friedrich-Wilhelms-Universität, Bonn, Germany, October 2006. [Google Scholar]
- Marchac, D.; Greensmith, A. Long-term experience with methylmethacrylate cranioplasty in craniofacial surgery. J. Plast. Reconstr. Aesthet. Surg. 2008, 61, 744–752. [Google Scholar] [CrossRef]
- Fernandes da Silva, A.L.; Borba, A.M.; Simão, N.R.; Pedro, F.L.M.; Borges, A.H.; Miloro, M. Customized Polymethyl Methacrylate Implants for the Reconstruction of Craniofacial Osseous Defects. Hindawi 2014, 358569, 8. [Google Scholar] [CrossRef]
- Moser, M.; Schmid, R.; Schindel, R.; Hildebrandt, G. Patient-specific polymethylmethacrylate prostheses for secondary reconstruction of large calvarial defects: A retrospective feasibility study of a new intraoperative moulding device for cranioplasty. J. Cranio-Maxillofac. Surg. 2017, 45, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Hieu, L.C.; Bohez, E.; Vander Sloten, J.; Oris, P.; Phien, H.N.; Vatcharaporn, E.; Binh, P.H. Design and manufacturing of cranioplasty implants by 3-axis CNC milling. Technol. Health Care 2002, 10, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Chiarini, L.; Figurelli, S.; Pollastri, G.; Torcia, E.; Ferrari, F.; Albanese, M.; Nocini, P.F. Cranioplasty using acrylic material: A new technical procedure. J. Cranio-Maxillofac. Surg. 2004, 32, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Fiaschi, P.; Pavanello, M.; Imperato, A.; Dallolio, V.; Accogli, A.; Capra, V.; Consales, A.; Cama, A.; Piatelli, G. Surgical results of cranioplasty with a polymethylmethacrylate customized cranial implant in pediatric patients: A single-center experience. J. Neurosurg. 2016, 17, 705–710. [Google Scholar] [CrossRef]
- Rotaru, H.; Baciut, M.; Stan, H.; Bran, S.; Chezan, H.; Iosif, A.; Tomescu, M.; Kim, S.-G.; Rotaru, A.; Baciut, G. Silicone rubber mould cast polyethylmethacrylate-hydroxyapatite plate used for repairing a large skull defect. J. Cranio-Maxillofac. Surg. 2006, 34, 242–246. [Google Scholar] [CrossRef]
- Kim, B.-J.; Hong, K.-S.; Park, K.-J.; Park, D.-H.; Chung, Y.-G.; Kang, S.-H. Customized Cranioplasty Implants Using Three-Dimensional Printers and Polymethyl-Methacrylate Casting. J. Korean Neurosurg. Soc. 2012, 52, 541–546. [Google Scholar] [CrossRef]
- Morales-Gómez, J.A.; Garcia-Estrada, E.; Leos-Bortoni, J.E.; Delgado-Brito, M.; Flores-Huerta, L.E.; de La Cruz-Arriaga, A.A.; Torres-Díaz, L.J.; de León, Á.R.M.-P. Cranioplasty with a low-cost customized polymethylmethacrylate implant using a desktop 3D printer. J. Neurosurg. 2018, 1–7. [Google Scholar] [CrossRef]
- Tan, E.T.W.; Ling, J.M.; Dinesh, S.K. The feasibility of producing patient-specific acrylic cranioplasty implants with a low-cost 3D printer. J. Neurosurg. 2016, 124, 1531–1537. [Google Scholar] [CrossRef]
- Verius, M.; Marreiros, F.; Heuze, Y.; Unterhofer, C.; Recheis, W. A Novel Approach for Implant Design of Large Cranial Defects using PMMA and Rapid Prototyping Techniques. Available online: https://epos.myesr.org/poster/esr/ecr2011/C-0950 (accessed on 25 August 2020).
- De La Peña, A.; De La Peña-Brambila, J.; Pérez-De la Torre, J.; Ochoa, M.; Gallardo, G.J. Low-cost customized cranioplasty using a 3D digital printing model: A case report. J. 3D Print. Med. 2018, 4. [Google Scholar] [CrossRef]
- Petersman, S.; Spoerk, M.; Huber, P.; Lang, M.; Pinter, G.; Arbeiter, F. Impact Optimization of 3D-Printed Poly(methyl methacrylate) for Cranial Implants. Macromol. Mater. Eng. 2019, 304. [Google Scholar] [CrossRef]
- Medical University of Graz. CAMed—Clinical Additive Manufacturing for Medical Applications. Available online: https://www.medunigraz.at/en/camed/ (accessed on 22 July 2020).
- Chacón, J.M.; Caminero, M.A.; García-Plaza, E.; Núñez, P.J. Additive manufacturing of PLA structures using fused deposition modelling: Effect of process parameters on mechanical properties and their optimal selection. Mater. Des. 2017, 124, 143–157. [Google Scholar] [CrossRef]
- Zaldivar, R.J.; Witkin, D.B.; McLouth, T.; Patel, D.N.; Schmitt, K.; Nokes, J.P. Influence of processing and orientation print effects on the mechanical and thermal behavior of 3D-Printed ULTEM® 9085 Material. Addit. Manuf. 2017, 13, 71–80. [Google Scholar] [CrossRef]
- Lin, X.; Coates, P.; Hebda, M.; Wang, R.; Lu, Y.; Zhang, L. Experimental analysis of the tensile property of FFF-printed elastomers. Polym. Test. 2020, 90, 106687. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Liu, C.-Y. The influence of forced-air cooling on a 3D printed PLA part manufactured by fused filament fabrication. Addit. Manuf. 2019, 25, 196–203. [Google Scholar] [CrossRef]










| Parameter | Testing Method | Typical Value |
|---|---|---|
| Tensile Strength | ASTM D 638 | 65.5 MPa |
| Tensile Modulus | ASTM D 638 | 3.2 GPa |
| Tensile Elongation at Break | ASTM D 638 | 4–6% |
| Vicat Softening Point 1.8 MPa | ASTM D1525 | 105 °C |
| Melt Flow Rate 230 °C & 3.8 kg | ASTM D1238 | 7.0 g/10 min |
| Specific Gravity | ASTM D 792 | 1.19 |
| Parameter | PMMA Initial Values | PMMA Final Values | Support Material Values |
|---|---|---|---|
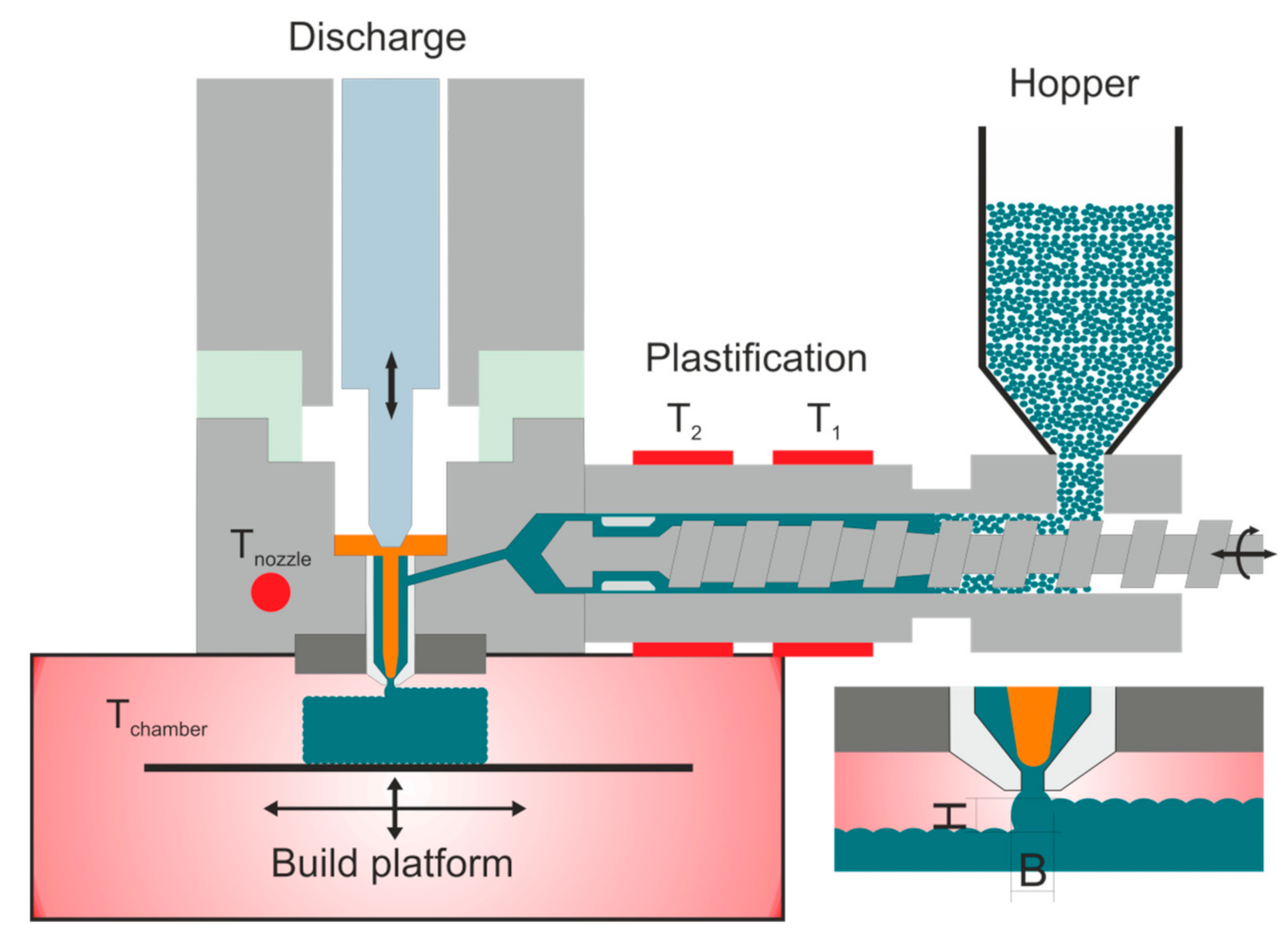
| Temperature zone 1 (T1) in °C | 195 | 200 | 140 |
| Temperature zone 2 (T2) in °C | 225 | 230 | 180 |
| Nozzle temperature (Tnozzle) in °C | 240 | 245 | 200 |
| Chamber temperature (Tchamber) in °C | 100 | 100 | 100 |
| Dosing stroke in mm | 8 | 8 | 6 |
| Backpressure in bar | 30 | 40 | 50 |
| Screw speed in m/s | 4 | 4 | 8 |
| Discharge rate in % | 70 | 67 | 100 |
| Droplet overlap in % | 50 | 25 | 40 |
| Drop aspect ratio (DAR) in - | 1.26 | 1.29 | 1.65 |
| Layer height in mm | 0.2 | 0.2 | 0.2 |
| Scale factor X-direction in - | 1.000 | 1.015 | 1.000 |
| Scale factor Y-direction in - | 1.000 | 1.015 | 1.000 |
| Scale factor Z-direction in - | 1.000 | 1.000 | 1.000 |
| Build Orientation | * Processing Conditions | Total Single Batches | Total Single Specimens | Total Multiple Batches | Total Multiple Specimens |
|---|---|---|---|---|---|
| XY | PMMA final | 15 | 15 | 3 | 15 |
| XZ | PMMA final | 15 | 15 | 3 | 15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hentschel, L.; Kynast, F.; Petersmann, S.; Holzer, C.; Gonzalez-Gutierrez, J. Processing Conditions of a Medical Grade Poly(Methyl Methacrylate) with the Arburg Plastic Freeforming Additive Manufacturing Process. Polymers 2020, 12, 2677. https://doi.org/10.3390/polym12112677
Hentschel L, Kynast F, Petersmann S, Holzer C, Gonzalez-Gutierrez J. Processing Conditions of a Medical Grade Poly(Methyl Methacrylate) with the Arburg Plastic Freeforming Additive Manufacturing Process. Polymers. 2020; 12(11):2677. https://doi.org/10.3390/polym12112677
Chicago/Turabian StyleHentschel, Lukas, Frank Kynast, Sandra Petersmann, Clemens Holzer, and Joamin Gonzalez-Gutierrez. 2020. "Processing Conditions of a Medical Grade Poly(Methyl Methacrylate) with the Arburg Plastic Freeforming Additive Manufacturing Process" Polymers 12, no. 11: 2677. https://doi.org/10.3390/polym12112677
APA StyleHentschel, L., Kynast, F., Petersmann, S., Holzer, C., & Gonzalez-Gutierrez, J. (2020). Processing Conditions of a Medical Grade Poly(Methyl Methacrylate) with the Arburg Plastic Freeforming Additive Manufacturing Process. Polymers, 12(11), 2677. https://doi.org/10.3390/polym12112677