Carbon Nanomaterials for Electro-Active Structures: A Review
Abstract
:1. Introduction
2. Carbon Nanomaterials for Electro-Active Scaffolds
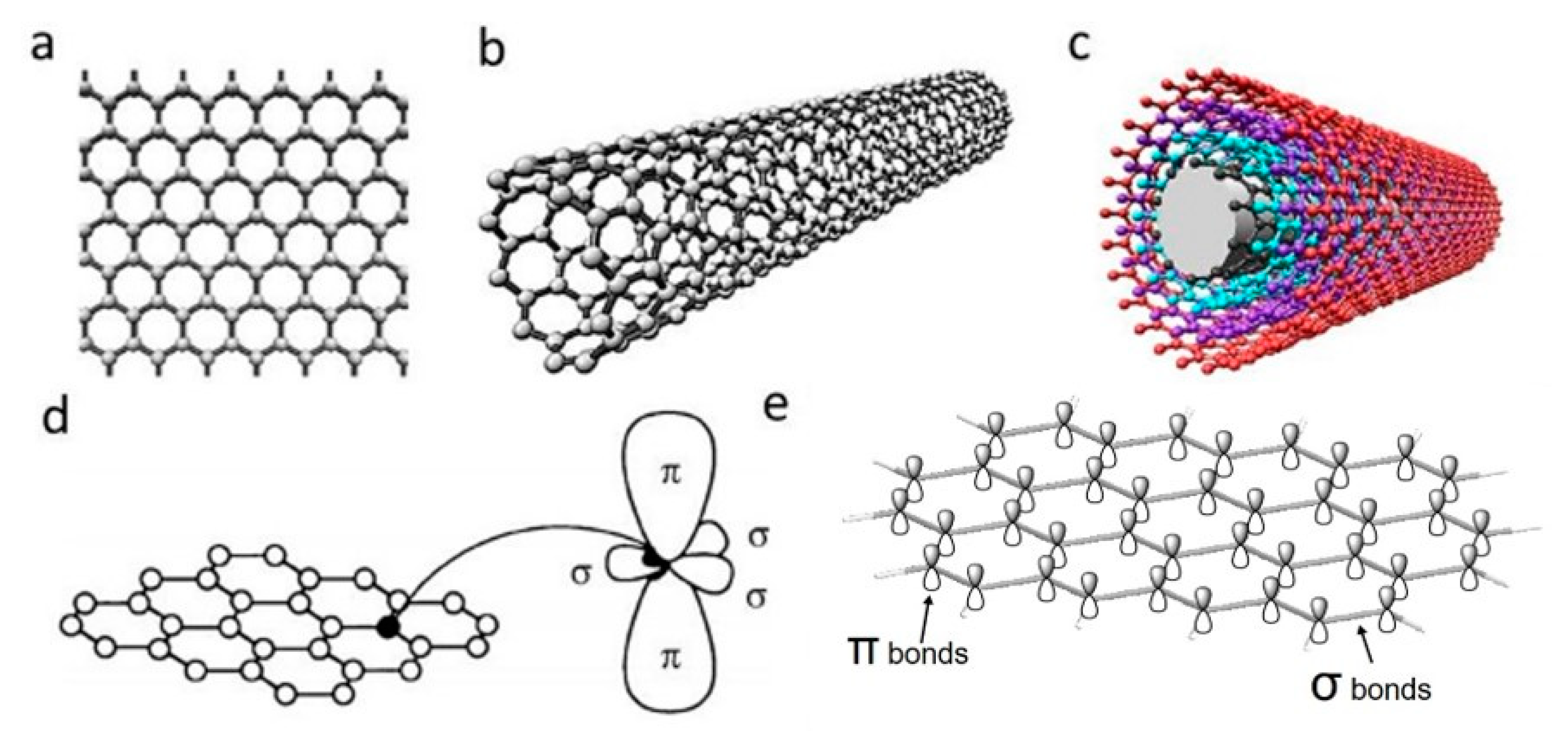
2.1. Graphene
2.1.1. Electrical Properties
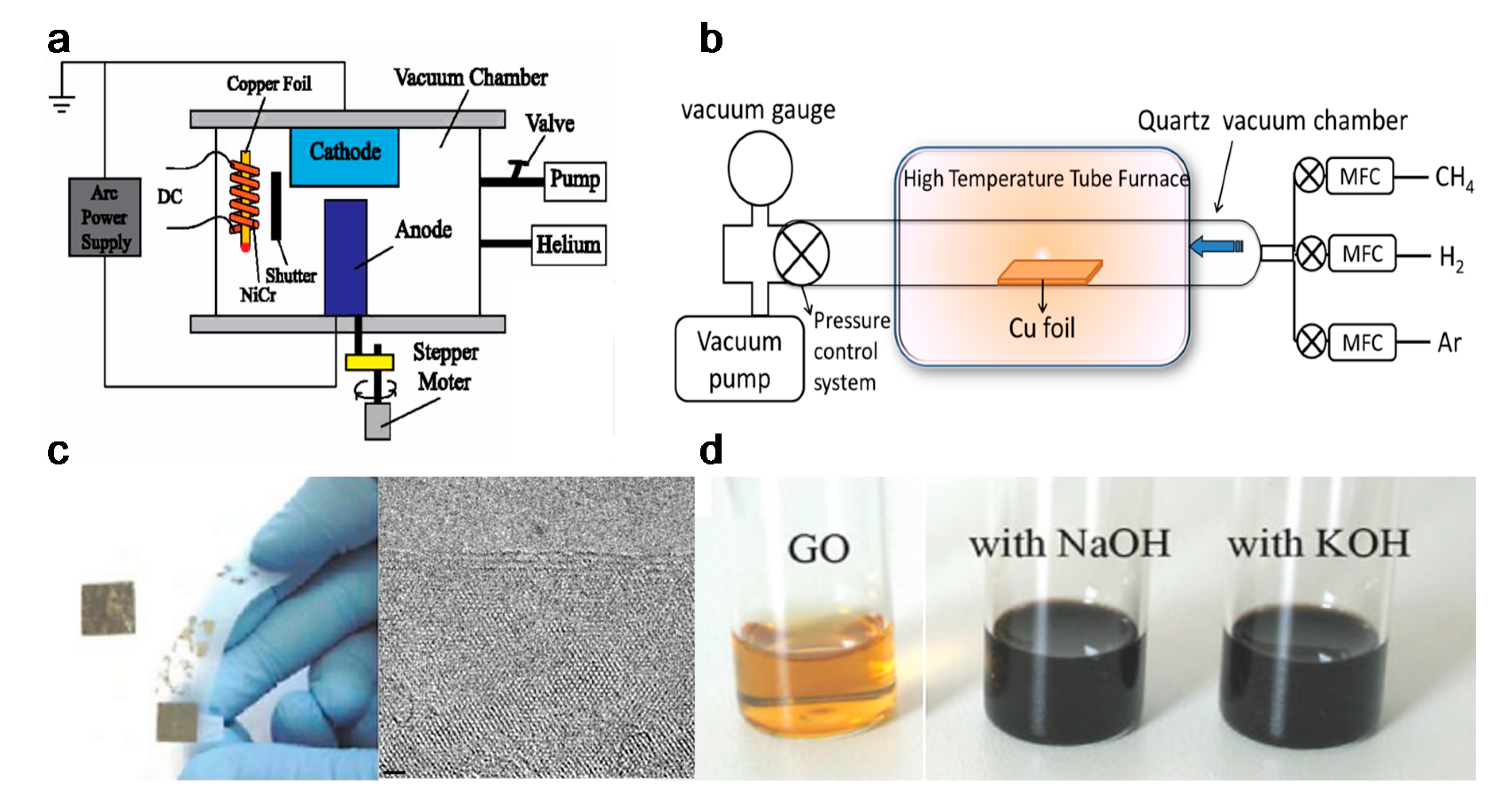
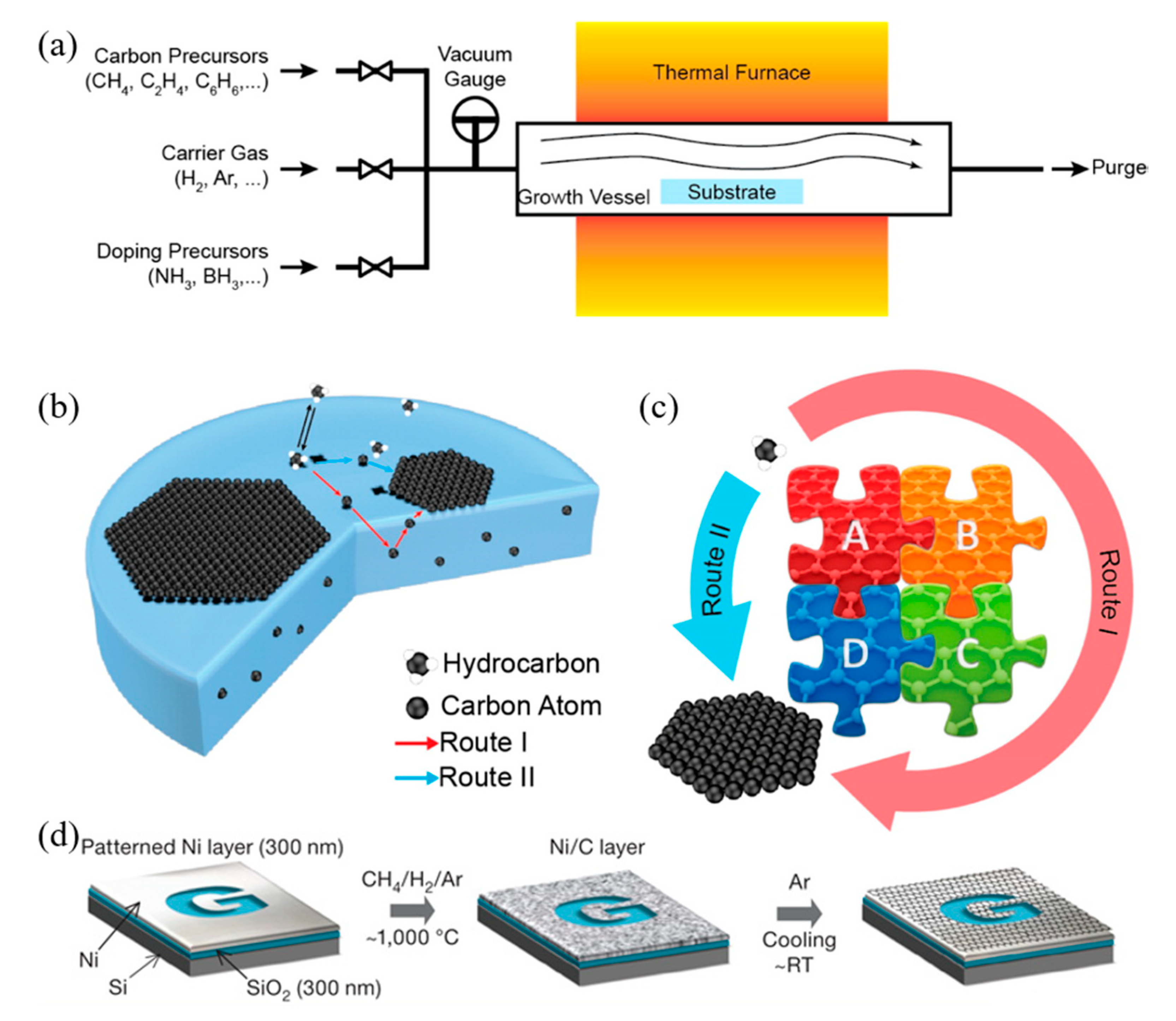
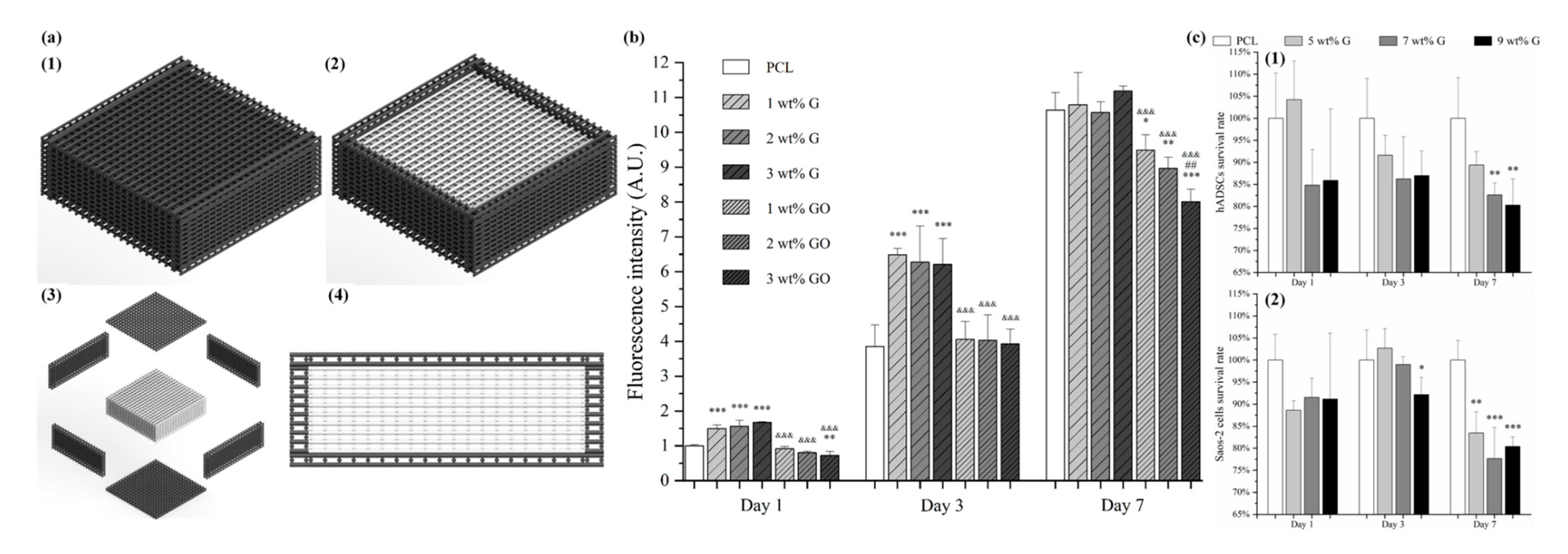
2.1.2. Materials Synthesis
- Adsorption and catalytic decomposition of precursor gas.
- Diffusion and dissolution of decomposed carbon species on the surface and into the bulk metal.
- Segregation of dissolved carbon atoms onto the metal surface.
- Surface nucleation and growth of graphene.
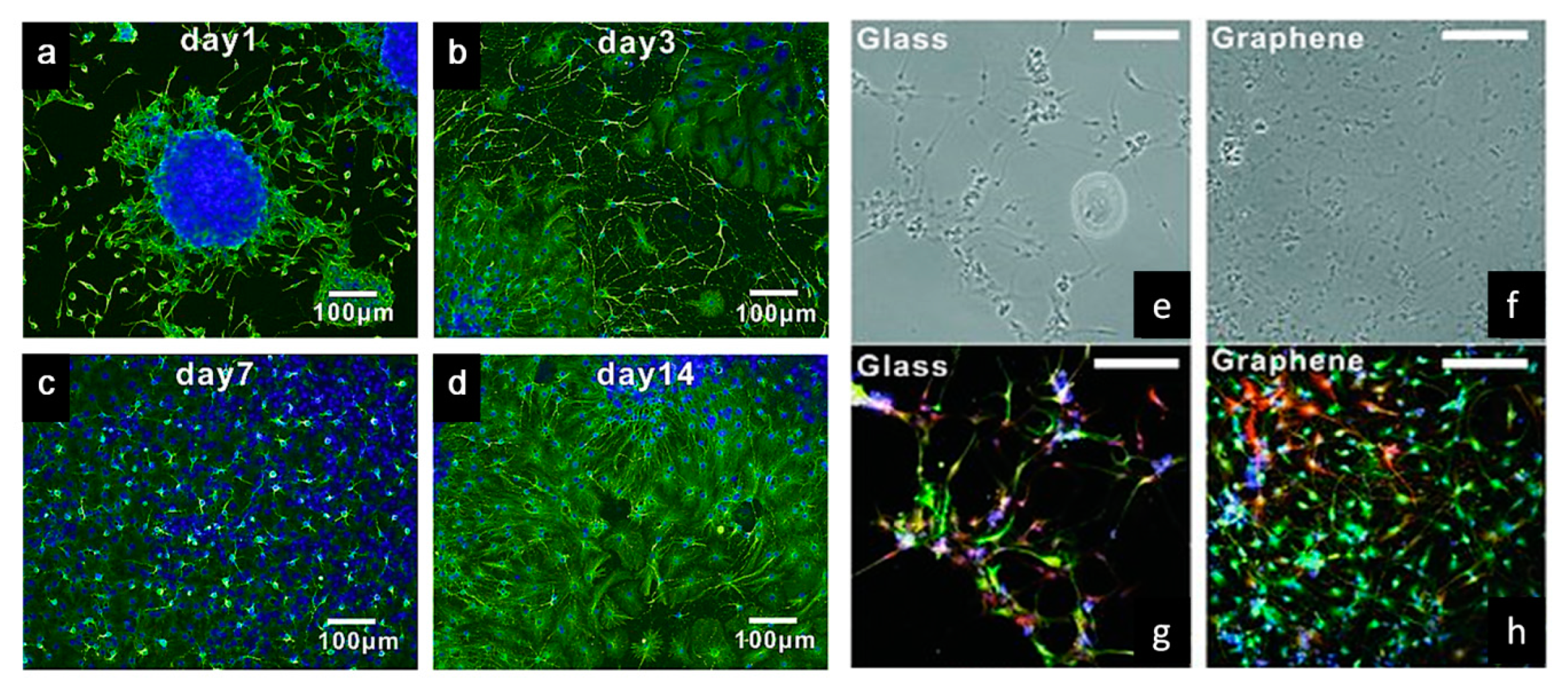
2.1.3. Tissue Engineering Applications
2.2. Carbon Nanotubes
2.2.1. Electrical Properties
2.2.2. Materials Synthesis
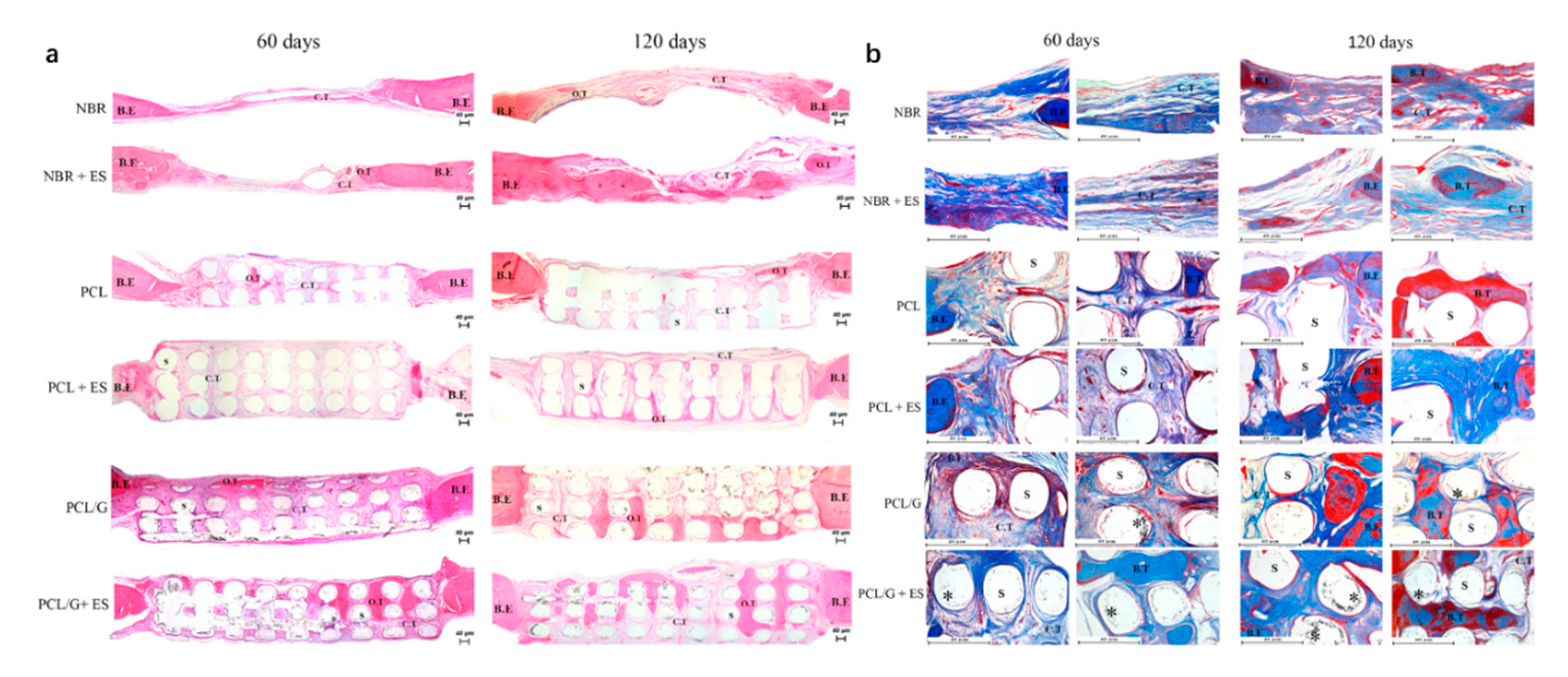
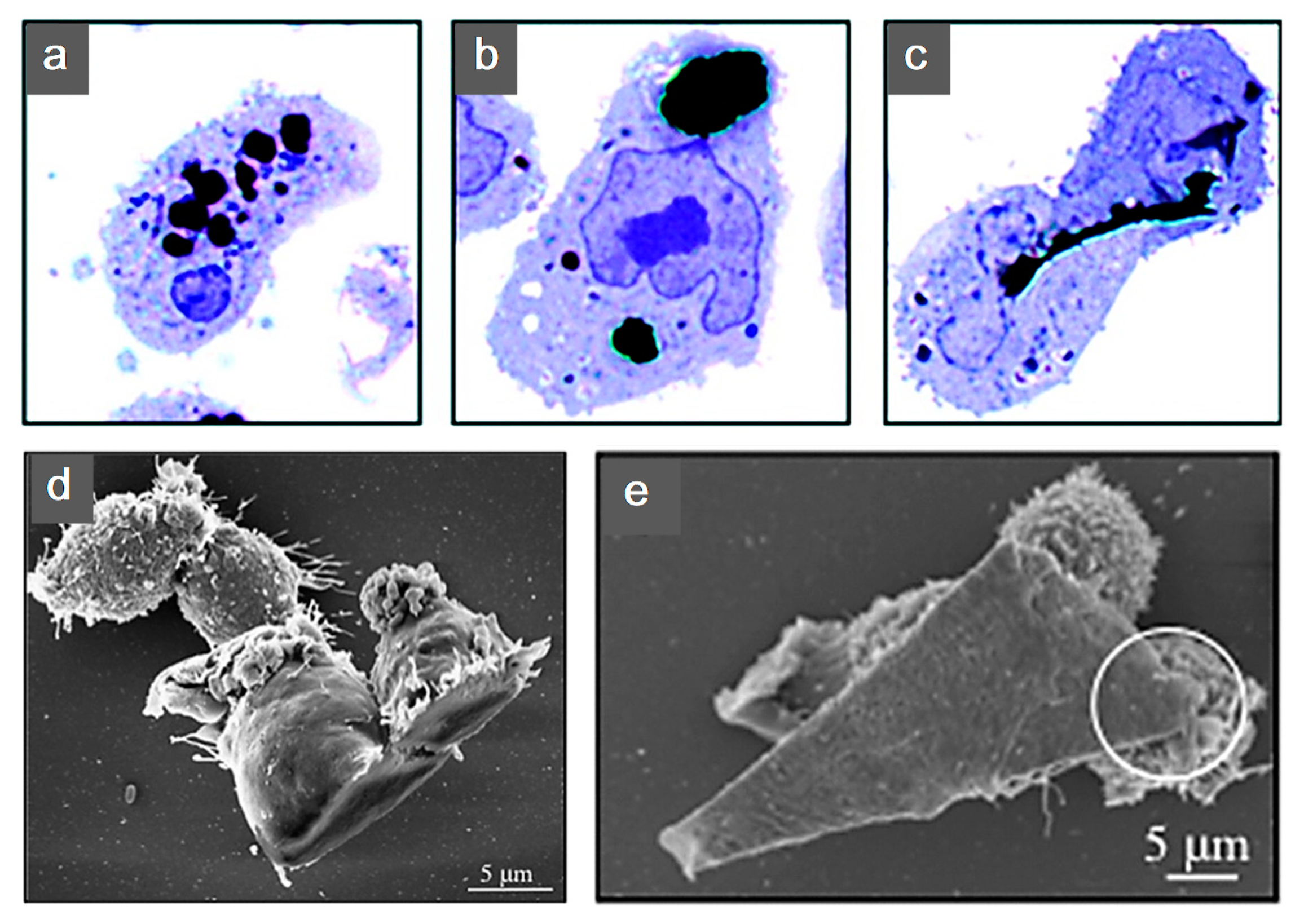
2.2.3. Tissue Engineering Applications
3. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Bártolo, P.J.; Almeida, H.A.; Rezende, R.A.; Laoui, T.; Bidanda, B. Advanced processes to fabricate scaffolds for tissue engineering. In Virtual Prototyping & Bio Manufacturing in Medical Applications; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2007; pp. 149–170. [Google Scholar]
- Bártolo, P.J.; Chua, C.K.; Almeida, H.A.; Chou, S.M.; Lim, A.S.C. Biomanufacturing for tissue engineering: Present and future trends. Virtual Phys. Prototyp. 2009, 4, 203–216. [Google Scholar] [CrossRef]
- Bártolo, P.J.; Almeida, H.A.; Laoui, T. Rapid prototyping and manufacturing for tissue engineering scaffolds. Int. J. Comput. Appl. Technol. 2009, 36, 1–9. [Google Scholar] [CrossRef]
- Bártolo, P.J.; Kruth, J.-P.; Silva, J.; Levy, G.; Malshe, A.; Rajurkar, K.; Mitsuishi, M.; Ciurana, J.; Leu, M. Biomedical production of implants by additive electro-chemical and physical processes. CIRP Ann. 2012, 61, 635–655. [Google Scholar] [CrossRef]
- Mattioli-Belmonte, M.; Giavaresi, G.; Biagini, G.; Virgili, L.; Giacomini, M.; Fini, M.; Giantomassi, F.; Natali, D.; Torricelli, P.; Giardino, R. Tailoring biomaterial compatibility: In vivo tissue response versus in vitro cell behavior. Int. J. Artif. Organs 2003, 26, 1077–1085. [Google Scholar] [CrossRef]
- Qazi, T.H.; Rai, R.; Boccaccini, A.R. Tissue engineering of electrically responsive tissues using polyaniline based polymers: A review. Biomaterials 2014, 35, 9068–9086. [Google Scholar] [CrossRef] [PubMed]
- Ko, U.H.; Park, S.; Bang, H.; Kim, M.; Shin, H.; Shin, J.H. Promotion of myogenic maturation by timely application of electric field along the topographical alignment. Tissue Eng. Part A 2018, 24, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Ganji, Y.; Li, Q.; Quabius, E.S.; Böttner, M.; Selhuber-Unkel, C.; Kasra, M. Cardiomyocyte behavior on biodegradable polyurethane/gold nanocomposite scaffolds under electrical stimulation. Mater. Sci. Eng. C 2016, 59, 10–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Bai, X.; Ding, Y.; Lee, I.-S. Electrical stimulation as a novel tool for regulating cell behavior in tissue engineering. Biomater. Res. 2019, 23, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouabhia, M.; Zhang, Z. Electrical stimulation in tissue regeneration. In Applied Biomedical Engineering; Gargiulo, G., McEwan, A., Eds.; IntechOpen: London, UK, 2011. [Google Scholar]
- Rivers, T.J.; Hudson, T.W.; Schmidt, C.E. Synthesis of a novel, biodegradable electrically conducting polymer for biomedical applications. Adv. Funct. Mater. 2002, 12, 33–37. [Google Scholar] [CrossRef]
- Staniforth, P. Electrical stimulation—Its role in growth, repair, and remodeling of the musculoskeletal system. Injury 1988, 19, 50. [Google Scholar] [CrossRef]
- Mayor, D. Book review: Electrotherapy: Evidence-Based Practice (12th Edition). Acupunct. Med. 2009, 27, 135–136. [Google Scholar] [CrossRef]
- Rangarajan, S.; Madden, L.; Bursac, N. Use of flow, electrical, and mechanical stimulation to promote engineering of striated muscles. Ann. Biomed. Eng. 2013, 42, 1391–1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Electrical stimulation: A novel tool for tissue engineering. Tissue Eng. Part B Rev. 2013, 19, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M. Electrical stimulation and angiogenesis. In Contemporary Neuroscience; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2008; pp. 495–509. [Google Scholar]
- Beugels, J.; Molin, D.G.M.; Ophelders, D.R.M.G.; Rutten, T.; Kessels, L.; Kloosterboer, N.; de Grzymala, A.A.P.; Kramer, B.W.W.; van der Hulst, R.R.W.J.; Wolfs, T.G.A.M. Electrical stimulation promotes the angiogenic potential of adipose-derived stem cells. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yonemori, K.; Matsunaga, S.; Ishidou, Y.; Maeda, S.; Yoshida, H. Early effects of electrical stimulation on osteogenesis. Bone 1996, 19, 173–180. [Google Scholar] [CrossRef]
- Srirussamee, K.; Mobini, S.; Cassidy, N.J.; Cartmell, S.H. Direct electrical stimulation enhances osteogenesis by inducing Bmp2 and Spp1 expressions from macrophages and preosteoblasts. Biotechnol. Bioeng. 2019, 116, 3421–3432. [Google Scholar] [CrossRef]
- Bassett, C.A.L.; Pawluk, R.J.; Becker, R.O. Effects of electric currents on bone in vivo. Nature 1964, 204, 652–654. [Google Scholar] [CrossRef]
- Asensio-Pinilla, E.; Udina, E.; Jaramillo, J.; Navarro, X. Electrical stimulation combined with exercise increase axonal regeneration after peripheral nerve injury. Exp. Neurol. 2009, 219, 258–265. [Google Scholar] [CrossRef]
- Ozawa, H.; Abe, E.; Shibasaki, Y.; Fukuhara, T.; Suda, T. Electric fields stimulate DNA synthesis of mouse osteoblast-like cells (MC3T3-E1) by a mechanism involving calcium ions. J. Cell. Physiol. 1989, 138, 477–483. [Google Scholar] [CrossRef]
- Tandon, N.; Marsano, A.; Maidhof, R.; Wan, L.; Park, H.; Vunjak-Novakovic, G. Optimization of electrical stimulation parameters for cardiac tissue engineering. J. Tissue Eng. Regen. Med. 2011, 5, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Park, H.; Bhalla, R.; Saigal, R.; Radisic, M.; Watson, N.; Langer, R.; Vunjak-Novakovic, G. Effects of electrical stimulation in C2C12 muscle constructs. J. Tissue Eng. Regen. Med. 2008, 2, 279–287. [Google Scholar] [CrossRef]
- Ma, X.; Ge, J.; Li, Y.; Guo, B.; Ma, P.X. Nanofibrous electroactive scaffolds from a chitosan-grafted-aniline tetramer by electrospinning for tissue engineering. RSC Adv. 2014, 4, 13652–13661. [Google Scholar] [CrossRef]
- Yu, P.; Bao, R.-Y.; Shi, X.-J.; Yangb, W.; Yang, M.-B. Self-assembled high-strength hydroxyapatite/graphene oxide/chitosan composite hydrogel for bone tissue engineering. Carbohydr. Polym. 2017, 155, 507–515. [Google Scholar] [CrossRef]
- Purohit, S.D.; Singh, H.; Bhaskar, R.; Yadav, I.; Bhushan, S.; Gupta, M.K.; Kumar, A.; Mishra, N.C. Fabrication of graphene oxide and nanohydroxyapatite reinforced gelatin–alginate nanocomposite scaffold for bone tissue regeneration. Front. Mater. 2020, 7, 1–10. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, K.M.; Han, S.S. Mechanically viscoelastic nanoreinforced hybrid hydrogels composed of polyacrylamide, sodium carboxymethylcellulose, graphene oxide, and cellulose nanocrystals. Carbohydr. Polym. 2018, 193, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Zo, S.M.; Kim, J.H.; Kim, S.-C.; Han, S.S. Enhanced physical, mechanical, and cytocompatibility behavior of polyelectrolyte complex hydrogels by reinforcing halloysite nanotubes and graphene oxide. Compos. Sci. Technol. 2019, 175, 35–45. [Google Scholar] [CrossRef]
- Bin Jo, S.; Erdenebileg, U.; Dashnyam, K.; Jin, G.-Z.; Cha, J.-R.; El-Fiqi, A.; Knowles, J.C.; Patel, K.D.; Lee, H.-H.; Lee, J.-H.; et al. Nano-graphene oxide/polyurethane nanofibers: Mechanically flexible and myogenic stimulating matrix for skeletal tissue engineering. J. Tissue Eng. 2020, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ku, S.H.; Lee, M.; Park, C.B. Carbon-based nanomaterials for tissue engineering. Adv. Heal. Mater. 2012, 2, 244–260. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Junior, J.R.P.; Nalesso, P.R.L.; Musson, D.; Cornish, J.; Mendonça, F.; Caetano, G.F.; Bártolo, P. Engineered 3D printed poly(ε-caprolactone)/graphene scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2019, 100, 759–770. [Google Scholar] [CrossRef]
- Xu, S.; Rezvanian, O.; Peters, K.; Zikry, M. The viability and limitations of percolation theory in modeling the electrical behavior of carbon nanotube–polymer composites. Nanotechnology 2013, 24, 155706. [Google Scholar] [CrossRef]
- Grady, B.P. Recent developments concerning the dispersion of carbon nanotubes in polymers. Macromol. Rapid Commun. 2010, 31, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Xu, L.; Yan, D.-X.; Li, Z.-M. Conductive polymer composites with segregated structures. Prog. Polym. Sci. 2014, 39, 1908–1933. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Bakhshandeh, B.; Reza, G.M.; Heshmatian, B.; Ganjali, M.R. A novel electroactive agarose-aniline pentamer platform as a potential candidate for neural tissue engineering. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Leong, K.-F.; Du, Z.; Chua, C.-K. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 2001, 7, 679–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeong, W.-Y.; Chua, C.K.; Leong, K.-F.; Chandrasekaran, M. Rapid prototyping in tissue engineering: Challenges and potential. Trends Biotechnol. 2004, 22, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Gao, H.; Zhu, G.; Cao, X.; Shi, X.; Wang, Y. The preparation and characterization of polycaprolactone/graphene oxide biocomposite nanofiber scaffolds and their application for directing cell behaviors. Carbon 2015, 95, 1039–1050. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; Maio, A.; Botta, L.; Rigogliuso, S.; Ghersi, G. Electrospun PCL/GO-g-PEG structures: Processing-morphology-properties relationships. Compos. Part A Appl. Sci. Manuf. 2017, 92, 97–107. [Google Scholar] [CrossRef]
- Aznar-Cervantes, S.D.; Martinez, J.G.; Bernabeu-Esclapez, A.; Lozano-Pérez, A.A.; Meseguer-Olmo, L.; Otero, T.F.; Cenis, J.L. Fabrication of electrospun silk fibroin scaffolds coated with graphene oxide and reduced graphene for applications in biomedicine. Bioelectrochemistry 2016, 108, 36–45. [Google Scholar] [CrossRef]
- Shanks, R. Biomimetic materials: A challenge for nano-scale self-assembly. Express Polym. Lett. 2014, 8, 543. [Google Scholar] [CrossRef]
- Maio, A.; Gammino, M.; Gulino, E.F.; Megna, B.; Fara, P.; Scaffaro, R. Rapid one-step fabrication of graphene oxide-decorated polycaprolactone three-dimensional templates for water treatment. ACS Appl. Polym. Mater. 2020, 2, 4993–5005. [Google Scholar] [CrossRef]
- Alenezi, H.; Çam, M.E.; Edirisinghe, M. Experimental and theoretical investigation of the fluid behavior during polymeric fiber formation with and without pressure. Appl. Phys. Rev. 2019, 6, 041401. [Google Scholar] [CrossRef]
- ASTM International. Standard Terminology for Additive Manufacturing–General Principles–Terminology; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad family of carbon nanoallotropes: Classification, chemistry, and applications of fullerenes, carbon dots, nanotubes, graphene, nanodiamonds, and combined superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef] [PubMed]
- Baptista, F.R.; Belhout, S.A.; Giordani, S.; Quinn, S.J. Recent developments in carbon nanomaterial sensors. Chem. Soc. Rev. 2015, 44, 4433–4453. [Google Scholar] [CrossRef]
- Serp, P.; Figueiredo, J.L. (Eds.) Carbon Materials for Catalysis; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Ignatova, T.; Rotkin, S.V. Discovering properties of nanocarbon materials as a pivot for device applications. Electrochem. Soc. Interface 2013, 22, 57–60. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, S.F.; Bisker, G.; Bakh, N.A.; Gibbs, S.L.; Landry, M.P.; Strano, M.S. Protein functionalized carbon nanomaterials for biomedical applications. Carbon 2015, 95, 767–779. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.R.; Jang, H.L.; Khoshakhlagh, P.; Akbari, M.; Nasajpour, A.; Zhang, Y.S.; Tamayol, A.; Khademhosseini, A. Graphene-based materials for tissue engineering. Adv. Drug Deliv. Rev. 2016, 105, 255–274. [Google Scholar] [CrossRef] [Green Version]
- Jorio, A.; Saito, R.; Dresselhaus, G.; Dresselhaus, M.S. Raman Spectroscopy in Graphene Related Systems; Wiley: Weinheim, Germany, 2011. [Google Scholar]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Pei, Z.; Lai, H.-C.; Wang, J.-Y.; Chiang, W.-H.; Chen, C.-H. High-responsivity and high-sensitivity graphene dots/a-IGZO thin-film phototransistor. IEEE Electron. Device Lett. 2015, 36, 44–46. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb carbon: A review of graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, Y.-W.; Stormer, H.L.; Kim, P. Experimental observation of the quantum Hall effect and Berry’s phase in graphene. Nature 2005, 438, 201–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kane, C.L.; Mele, E.J. Quantum spin Hall effect in graphene. Phys. Rev. Lett. 2005, 95, 226801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novoselov, K.S.; McCann, E.; Morozov, S.V.; Falko, V.I.; Katsnelson, M.I.; Geim, A.K.; Schedin, F.; Jiang, D. Unconventional quantum Hall effect and Berry’s phase of 2pi in bilayer graphene. Nat. Phys. 2006, 2, 177–180. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Jiang, Z.; Zhang, Y.; Morozov, S.V.; Stormer, H.L.; Zeitler, U.; Maan, J.C.; Boebinger, G.S.; Kim, P.; Geim, A.K. Room-temperature quantum Hall effect in graphene. Science 2007, 315, 1379. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Zhang, Y.; Stormer, H.L.; Kim, P. Quantum Hall states near the charge-neutral Dirac point in graphene. Phys. Rev. Lett. 2007, 99, 106802. [Google Scholar] [CrossRef] [Green Version]
- Morozov, S.V.; Novoselov, K.S.; Katsnelson, M.I.; Schedin, F.; Elias, D.C.; Jaszczak, J.A.; Geim, A.K. Giant intrinsic carrier mobilities in graphene and its bilayer. Phys. Rev. Lett. 2008, 100, 016602. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Skachko, I.; Barker, A.; Andrei, E.Y. Approaching ballistic transport in suspended graphene. Nat. Nanotechnol. 2008, 3, 491–495. [Google Scholar] [CrossRef] [Green Version]
- Bolotin, K.I.; Sikes, K.J.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H.L. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar] [CrossRef] [Green Version]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nanosci. Technol. 2009, 11–19. [Google Scholar] [CrossRef]
- Mao, H.Y.; Laurent, S.; Chen, W.; Akhavan, O.; Imani, M.; Ashkarran, A.A.; Mahmoudi, M. Graphene: Promises, facts, opportunities, and challenges in nanomedicine. Chem. Rev. 2013, 113, 3407–3424. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.-H.; Li, Y.-S.; Chen, G.-L.; Lin, L.-Y.; Li, T.-J.; Chuang, H.-M.; Hsieh, C.-Y.; Lo, S.-C.; Chiang, W.-H.; Ho, K.-C. Facile synthesis of boron-doped graphene nanosheets with hierarchical microstructure at atmosphere pressure for metal-free electrochemical detection of hydrogen peroxide. Electrochim. Acta 2015, 172, 52–60. [Google Scholar] [CrossRef]
- Krane, N. Preparation of graphene. Sel. Top. Phys. Phys. Nanoscale Carbon Freie Univ. Berl. 2011, 4, 1–5. [Google Scholar]
- Avouris, P.; Dimitrakopoulos, C.D. Graphene: Synthesis and applications. Mater. Today 2012, 15, 86–97. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Sood, A.K. (Eds.) Graphene: Synthesis, Properties, and Phenomena; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Manna, K.; Huang, H.-N.; Li, W.-T.; Ho, Y.-H.; Chiang, W.-H. Toward understanding the efficient exfoliation of layered materials by water-assisted cosolvent liquid-phase exfoliation. Chem. Mater. 2016, 28, 7586–7593. [Google Scholar] [CrossRef]
- Manna, K.; Hsieh, C.-Y.; Lo, S.-C.; Li, Y.-S.; Huang, H.-N.; Chiang, W.-H. Graphene and graphene-analogue nanosheets produced by efficient water-assisted liquid exfoliation of layered materials. Carbon 2016, 105, 551–555. [Google Scholar] [CrossRef]
- Subrahmanyam, K.S.; Panchakarla, L.S.; Govindaraj, A.; Rao, C.N.R. Simple method of preparing graphene flakes by an arc-discharge method. J. Phys. Chem. C 2009, 113, 4257–4259. [Google Scholar] [CrossRef]
- Fang, X.; Shashurin, A.; Keidar, M. Role of substrate temperature at graphene synthesis in an arc discharge. J. Appl. Phys. 2015, 118, 103304. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Huei, C. Synthesis and biomedical applications of graphene: Present and future trends. In Advances in Graphene Science; Aliofkhazraei, M., Ed.; IntechOpen: London, UK, 2013. [Google Scholar]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Meyer, J.C.; Geim, A.K.; Katsnelson, M.I.; Novoselov, K.S.; Booth, T.J.; Roth, S. The structure of suspended graphene sheets. Nature 2007, 446, 60–63. [Google Scholar] [CrossRef]
- Fan, X.; Peng, W.; Li, Y.; Li, X.; Wang, S.; Zhang, G.; Zhang, F. Deoxygenation of exfoliated graphite oxide under alkaline conditions: A green route to graphene preparation. Adv. Mater. 2008, 20, 4490–4493. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, J.; Wang, C.; Leng, X.; Xiao, Y.; Fu, L. Synthesis of graphene and related two-dimensional materials for bioelectronics devices. Biosens. Bioelectron. 2017, 89, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, Z.; Li, N.; Pu, Y.-Q.; Wang, B.; Zhang, T.; Tao, J. Advanced review of graphene-based nanomaterials in drug delivery systems: Synthesis, modification, toxicity and application. Mater. Sci. Eng. C 2017, 77, 1363–1375. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, B.; Bulin, C.; Li, R.; Xing, R. High-efficient synthesis of graphene oxide based on improved Hummers method. Sci. Rep. 2016, 6, 36143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schniepp, H.C.; Li, J.-L.; McAllister, M.J.; Sai, H.; Herrera-Alonso, M.; Adamson, D.H.; Prud’homme, R.K.; Car, R.; Saville, D.A.; Aksay, I.A. Functionalized single graphene sheets derived from splitting graphite oxide. J. Phys. Chem. B 2006, 110, 8535–8539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emtsev, K.V.; Bostwick, A.; Horn, K.; Jobst, J.; Kellogg, G.L.; Ley, L.; McChesney, J.L.; Ohta, T.; Reshanov, S.A.; Röhrl, J.; et al. Towards wafer-size graphene layers by atmospheric pressure graphitization of silicon carbide. Nat. Mater. 2009, 8, 203–207. [Google Scholar] [CrossRef]
- Guo, S.; Dong, S. Graphene nanosheet: Synthesis, molecular engineering, thin film, hybrids, and energy and analytical applications. Chem. Soc. Rev. 2011, 40, 2644–2672. [Google Scholar] [CrossRef]
- Li, M.; Liu, D.; Wei, D.; Song, X.; Wei, D.; Wee, A.T.S. Controllable synthesis of graphene by plasma-enhanced chemical vapor deposition and its related applications. Adv. Sci. 2016, 3, 1600003. [Google Scholar] [CrossRef]
- Deng, B.; Liu, Z.; Peng, H. Toward mass production of CVD graphene films. Adv. Mater. 2019, 31, e1800996. [Google Scholar] [CrossRef]
- Yan, K.; Fu, L.; Peng, H.; Liu, Z. Designed CVD growth of graphene via process engineering. Acc. Chem. Res. 2013, 46, 2263–2274. [Google Scholar] [CrossRef]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.-H.; Kim, P.; Choi, J.-Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Somani, P.R.; Somani, S.P.; Umeno, M. Planer nano-graphenes from camphor by CVD. Chem. Phys. Lett. 2006, 430, 56–59. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jachak, A.C.; Creighton, M.; Qiu, Y.; Kane, A.B.; Hurt, R.H. Biological interactions and safety of graphene materials. MRS Bull. 2012, 37, 1307–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Hu, M.; Zeng, T.H.; Wu, R.; Jiang, R.; Wei, J.; Wang, L.; Kong, J.; Chen, Y. Lateral dimension-dependent antibacterial activity of graphene oxide sheets. Langmuir 2012, 28, 12364–12372. [Google Scholar] [CrossRef] [PubMed]
- Karahan, H.E.; Wang, Y.; Li, W.; Liu, F.; Wang, L.; Sui, X.; Riaz, M.A.; Chen, Y. Antimicrobial graphene materials: The interplay of complex materials characteristics and competing mechanisms. Biomater. Sci. 2018, 6, 766–773. [Google Scholar] [CrossRef]
- Karahan, H.E.; Wiraja, C.; Xu, C.; Wei, J.; Wang, Y.; Wang, L.; Liu, F.; Chen, Y. Graphene materials in antimicrobial nanomedicine: Current status and future perspectives. Adv. Healthc. Mater. 2018, 7, e1701406. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Speranza, G. Functionalization of carbon nanomaterials for biomedical applications. C J. Carbon Res. 2019, 5, 72. [Google Scholar] [CrossRef] [Green Version]
- Liao, K.-H.; Lin, Y.-S.; Macosko, C.W.; Haynes, C.L. Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. ACS Appl. Mater. Interfaces 2011, 3, 2607–2615. [Google Scholar] [CrossRef]
- Syama, S.; Mohanan, P. Safety and biocompatibility of graphene: A new generation nanomaterial for biomedical application. Int. J. Biol. Macromol. 2016, 86, 546–555. [Google Scholar] [CrossRef]
- Gardin, C.; Piattelli, A.; Zavan, B. Graphene in regenerative medicine: Focus on stem cells and neuronal differentiation. Trends Biotechnol. 2016, 34, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, X.; Song, Q.; Su, R.; Zhang, Q.; Kong, T.; Liu, L.; Jin, G.; Tang, M.; Cheng, G. The promotion of neurite sprouting and outgrowth of mouse hippocampal cells in culture by graphene substrates. Biomaterials 2011, 32, 9374–9382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heo, C.; Yoo, J.; Lee, S.; Jo, A.; Jung, S.; Yoo, H.; Lee, Y.H.; Suh, M. The control of neural cell-to-cell interactions through non-contact electrical field stimulation using graphene electrodes. Biomaterials 2011, 32, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Wang, T.; Zhao, B.; Li, J.; Jin, L. Soft graphene nanofibers designed for the acceleration of nerve growth and development. Adv. Mater. 2015, 27, 6462–6468. [Google Scholar] [CrossRef]
- López, C.; Patiño, J.; García-Rama, C.; Ferrer, M.L.; Fierro, J.L.G.; Tamayo, A.; Collazos-Castro, J.E.; del Monte, F.; Gutiérrez, M.C. 3D free-standing porous scaffolds made of graphene oxide as substrates for neural cell growth. J. Mater. Chem. B 2014, 2, 5698–5706. [Google Scholar] [CrossRef]
- Tang, M.; Song, Q.; Li, N.; Jiang, Z.; Huang, R.; Cheng, G. Enhancement of electrical signaling in neural networks on graphene films. Biomaterials 2013, 34, 6402–6411. [Google Scholar] [CrossRef]
- Park, S.Y.; Park, J.; Sim, S.H.; Sung, M.G.; Kim, K.S.; Hong, B.H.; Hong, S. Enhanced differentiation of human neural stem cells into neurons on graphene. Adv. Mater. 2011, 23, H263–H267. [Google Scholar] [CrossRef]
- Jakus, A.E.; Secor, E.B.; Rutz, A.L.; Jordan, S.W.; Hersam, M.C.; Shah, R.N. Three-dimensional printing of high-content graphene scaffolds for electronic and biomedical applications. ACS Nano 2015, 9, 4636–4648. [Google Scholar] [CrossRef]
- Zhang, C.; Fan, S.; Shao, H.; Hu, X.; Zhu, B.; Zhang, Y. Graphene trapped silk scaffolds integrate high conductivity and stability. Carbon 2019, 148, 16–27. [Google Scholar] [CrossRef]
- Zhao, Y.; Gong, J.; Niu, C.; Wei, Z.; Shi, J.; Li, G.; Yang, Y.; Wang, H. A new electrospun graphene-silk fibroin composite scaffolds for guiding Schwann cells. J. Biomater. Sci. Polym. Ed. 2017, 28, 2171–2185. [Google Scholar] [CrossRef]
- Yang, Y.; Ding, X.; Zou, T.; Peng, G.; Liu, H.; Fan, Y. Preparation and characterization of electrospun graphene/silk fibroin conductive fibrous scaffolds. RSC Adv. 2017, 7, 7954–7963. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Caetano, G.F.; Chiang, W.-H.; Blaker, J.J.; Frade, M.A.C.; Bartolo, P.J.D.S. Morphological, mechanical and biological assessment of PCL/pristine graphene scaffolds for bone regeneration. Int. J. Bioprinting 2016, 2, 204–213. [Google Scholar] [CrossRef]
- Wang, W.; Caetano, G.; Ambler, W.S.; Blaker, J.J.; Frade, M.A.; Mandal, P.; Diver, C.; Bártolo, P. Enhancing the hydrophilicity and cell attachment of 3D printed PCL/graphene scaffolds for bone tissue engineering. Materials 2016, 9, 992. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.G.; Chang, W.H.; Bartolo, P.J. Design, fabrication and evaluation of pclgraphene scaffolds for bone regeneration. In Proceedings of the 2nd International Conference on Progress in Additive Manufacturing, Singapore, 16–19 May 2016; pp. 355–360. [Google Scholar]
- Caetano, G.F.; Wang, W.; Chiang, W.-H.; Cooper, G.; Diver, C.; Blaker, J.J.; Frade, M.A.; Bártolo, P. 3D-printed poly(ε-caprolactone)/graphene scaffolds activated with P1-Latex protein for bone regeneration. 3D Print. Addit. Manuf. 2018, 5, 127–137. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.C.; Lim, C.H.Y.X.; Shi, H.; Tang, L.A.L.; Wang, Y.; Lim, C.T.; Loh, K.P. Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano 2011, 5, 7334–7341. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Shahsavar, M. Graphene nanogrids for selective and fast osteogenic differentiation of human mesenchymal stem cells. Carbon 2013, 59, 200–211. [Google Scholar] [CrossRef]
- Caetano, G.; Wang, W.; Murashima, A.; Passarini, J.J.R.; Bagne, L.; Leite, M.N.; Hyppolito, M.A.; Al-Deyab, S.; El-Newehy, M.; Bártolo, P.J.; et al. Tissue constructs with human adipose-derived mesenchymal stem cells to treat bone defects in rats. Materials 2019, 12, 2268. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Wang, W.; Bártolo, P.J.D.S. Investigating the effect of carbon nanomaterials reinforcing poly(ε-caprolactone) scaffolds for bone repair applications. Int. J. Bioprinting 2020, 6, 266. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, W.; Bártolo, P. Novel poly(ε-caprolactone)/graphene scaffolds for bone cancer treatment and bone regeneration. 3D Print. Addit. Manuf. 2020, 7, 222–229. [Google Scholar] [CrossRef]
- Liao, J.; Qu, Y.; Chu, B.; Zhang, X.; Qian, Z. Biodegradable CSMA/PECA/graphene porous hybrid scaffold for cartilage tissue engineering. Sci. Rep. 2015, 5, 9879. [Google Scholar] [CrossRef]
- Hitscherich, P.; Aphale, A.; Gordan, R.; Whitaker, R.; Singh, P.; Xie, L.-H.; Patra, P.; Lee, E.J. Electroactive graphene composite scaffolds for cardiac tissue engineering. J. Biomed. Mater. Res. Part A 2018, 106, 2923–2933. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, S.; Baheiraei, N.; Mohseni, M.; Razavi, M.; Ghaderi, A.; Azizi, B.; Rabiee, N.; Karimi, M. Three-dimensional graphene foam as a conductive scaffold for cardiac tissue engineering. J. Biomater. Appl. 2019, 34, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, A.; Panchakarla, L.S.; Chandran, P.; Menon, D.; Nair, S.; Rao, C.N.R.; Koyakutty, M. Differential nano-bio interactions and toxicity effects of pristine versus functionalized graphene. Nanoscale 2011, 3, 2461–2464. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, X.; Crook, J.M.; Wallace, G.G. Electrical stimulation-induced osteogenesis of human adipose derived stem cells using a conductive graphene-cellulose scaffold. Mater. Sci. Eng. C 2020, 107, 110312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Cheng, Y.; Chen, L.; Zhu, T.; Ye, K.; Jia, C.; Wang, H.; Zhu, M.; Fan, C.; Mo, X. In vitro and in vivo studies of electroactive reduced graphene oxide-modified nanofiber scaffolds for peripheral nerve regeneration. Acta Biomater. 2019, 84, 98–113. [Google Scholar] [CrossRef]
- Yan, L.; Zhao, B.; Liu, X.; Li, X.; Zeng, C.; Shi, H.; Xu, X.; Lin, T.; Dai, L.; Liu, Y. Aligned nanofibers from polypyrrole/graphene as electrodes for regeneration of optic nerve via electrical stimulation. ACS Appl. Mater. Interfaces 2016, 8, 6834–6840. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Mattiassi, S.; Loeblein, M.; Chin, E.; Ma, D.; Coquet, P.; Viasnoff, V.; Teo, E.H.T.; Goh, E.L.K.; Yim, E.K.F. Human Rett-derived neuronal progenitor cells in 3D graphene scaffold as an in vitro platform to study the effect of electrical stimulation on neuronal differentiation. Biomed. Mater. 2018, 13, 034111. [Google Scholar] [CrossRef] [Green Version]
- Meng, S. Nerve cell differentiation using constant and programmed electrical stimulation through conductive non-functional graphene nanosheets film. Tissue Eng. Regen. Med. 2014, 11, 274–283. [Google Scholar] [CrossRef]
- Bajaj, P.; Rivera, J.A.; Marchwiany, D.; Solovyeva, V.; Bashir, R. Graphene-based patterning and differentiation of C2C12 myoblasts. Adv. Heal. Mater. 2013, 3, 995–1000. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Zhang, T.; Zhang, Q.; Chen, X.; Zhu, C.; Xu, Y.; Yang, J.; Liu, J.; Sun, D.-P. Biointerface by cell growth on graphene oxide doped bacterial cellulose/poly(3,4-ethylenedioxythiophene) nanofibers. ACS Appl. Mater. Interfaces 2016, 8, 10183–10192. [Google Scholar] [CrossRef]
- Ahadian, S.; Ramón-Azcón, J.; Chang, H.; Liang, X.; Kaji, H.; Shiku, H.; Nakajima, K.; Ramalingam, M.; Wu, H.; Matsue, T.; et al. Electrically regulated differentiation of skeletal muscle cells on ultrathin graphene-based films. RSC Adv. 2014, 4, 9534–9541. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, J.; Song, Q.; Li, N.; Zhang, Z.; Li, K.; Du, Y.; Wu, L.; Tang, M.; Liu, L.; et al. Synthesis of amphiphilic reduced graphene oxide with an enhanced charge injection capacity for electrical stimulation of neural cells. J. Mater. Chem. B 2014, 2, 4331–4337. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Pan, S.; Ma, Y.; Kong, W.; Qi, Z.; Yang, X. Effect of electrical stimulation combined with graphene-oxide-based membranes on neural stem cell proliferation and differentiation. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1867–1876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhavan, O.; Ghaderi, E.; Shirazian, S.A.; Rahighi, R. Rolled graphene oxide foams as three-dimensional scaffolds for growth of neural fibers using electrical stimulation of stem cells. Carbon 2016, 97, 71–77. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Q.; Gao, S.; Song, Q.; Huang, R.; Wang, L.; Liu, L.; Dai, J.; Tang, M.; Cheng, G. Three-dimensional graphene foam as a biocompatible and conductive scaffold for neural stem cells. Sci. Rep. 2013, 3, 1604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balikov, D.A.; Fang, B.; Chun, Y.W.; Crowder, S.W.; Prasai, D.; Lee, J.B.; Bolotin, K.I.; Sung, H.-J. Directing lineage specification of human mesenchymal stem cells by decoupling electrical stimulation and physical patterning on unmodified graphene. Nanoscale 2016, 8, 13730–13739. [Google Scholar] [CrossRef]
- Das, S.R.; Uz, M.; Ding, S.; Lentner, M.T.; Hondred, J.A.; Cargill, A.A.; Sakaguchi, D.S.; Mallapragada, S.; Das, S.R. Electrical differentiation of mesenchymal stem cells into Schwann-cell-like phenotypes using inkjet-printed graphene circuits. Adv. Healthc. Mater. 2017, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Jo, H.; Sim, M.; Kim, S.; Yang, S.; Yoo, Y.; Park, J.-H.; Yoon, T.H.; Kim, M.-G.; Lee, J.Y. Electrically conductive graphene/polyacrylamide hydrogels produced by mild chemical reduction for enhanced myoblast growth and differentiation. Acta Biomater. 2017, 48, 100–109. [Google Scholar] [CrossRef]
- Zheng, F.; Li, R.; He, Q.; Koral, K.; Tao, J.; Fan, L.; Xiang, R.; Ma, J.; Wang, N.; Yin, Y.; et al. The electrostimulation and scar inhibition effect of chitosan/oxidized hydroxyethyl cellulose/reduced graphene oxide/asiaticoside liposome based hydrogel on peripheral nerve regeneration in vitro. Mater. Sci. Eng. C 2020, 109, 110560. [Google Scholar] [CrossRef]
- Agarwal, G.; Kumar, N.; Srivastava, A. Highly elastic, electroconductive, immunomodulatory graphene crosslinked collagen cryogel for spinal cord regeneration. Mater. Sci. Eng. C 2021, 118, 111518. [Google Scholar] [CrossRef]
- Sanchez, V.C.; Jachak, A.; Hurt, R.H.; Kane, A.B. Biological interactions of graphene-family nanomaterials: An interdisciplinary review. Chem. Res. Toxicol. 2011, 25, 15–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vajtai, R. (Ed.) Springer Handbook of Nanomaterials; Springer Science and Business Media LLC.: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Ajayan, P.M.; Ebbesen, T.W. Nanometre-size tubes of carbon. Rep. Prog. Phys. 1997, 60, 1025–1062. [Google Scholar] [CrossRef]
- Grobert, N. Carbon nanotubes–becoming clean. Mater. Today 2007, 10, 28–35. [Google Scholar] [CrossRef]
- Das, R.; Shahnavaz, Z.; Ali, E.; Islam, M.M.; Hamid, S.B.A. Can we optimize arc discharge and laser ablation for well-controlled carbon nanotube synthesis? Nanoscale Res. Lett. 2016, 11, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Kazeimzadeh, F.; Malekfar, R.; Houshiar, M. The effect of graphitic target density on carbon nanotube synthesis by pulsed laser ablation method. In Proceedings of the 6th International Biennial Conference on Ultrafine Grained and Nanostructured Materials, Kish Island, Iran, 12–13 November 2017; AIP Publishing: Melville, NY, USA, 2018; p. 020018. [Google Scholar]
- Zaytseva, O.; Neumann, G. Carbon nanomaterials: Production, impact on plant development, agricultural and environmental applications. Chem. Biol. Technol. Agric. 2016, 3, 17. [Google Scholar] [CrossRef] [Green Version]
- De Volder, M.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [Green Version]
- Cha, C.; Shin, S.R.; Annabi, N.; Dokmeci, M.R.; Khademhosseini, A. Carbon-based nanomaterials: Multifunctional materials for biomedical engineering. ACS Nano 2013, 7, 2891–2897. [Google Scholar] [CrossRef]
- Chiang, W.-H.; Chen, G.-L.; Hsieh, C.-Y.; Lo, S.-C. Controllable boron doping of carbon nanotubes with tunable dopant functionalities: An effective strategy toward carbon materials with enhanced electrical properties. RSC Adv. 2015, 5, 97579–97588. [Google Scholar] [CrossRef]
- Chiang, W.-H.; Hsieh, C.-Y.; Lo, S.-C.; Chang, Y.-C.; Kawai, T.; Nonoguchi, Y. C/BCN core/shell nanotube films with improved thermoelectric properties. Carbon 2016, 109, 49–56. [Google Scholar] [CrossRef]
- Leu, Y.-A.; Yeh, M.-H.; Lin, L.-Y.; Li, T.-J.; Chang, L.-Y.; Shen, S.-Y.; Li, Y.-S.; Chen, G.-L.; Chiang, W.-H.; Lin, J.-J.; et al. Thermally stable boron-doped multiwalled carbon nanotubes as a Pt-free counter electrode for dye-sensitized solar cells. ACS Sustain. Chem. Eng. 2016, 5, 537–546. [Google Scholar] [CrossRef]
- Saito, R.; Dresselhaus, G.; Dresselhaus, M.S. Physical Properties of Carbon Nanotubes; Imperial College Press: London, UK, 1998. [Google Scholar]
- Chiang, W.-H.; Sankaran, R.M. Linking catalyst composition to chirality distributions of as-grown single-walled carbon nanotubes by tuning NixFe1−x nanoparticles. Nat. Mater. 2009, 8, 882–886. [Google Scholar] [CrossRef]
- Chiang, W.-H.; Futaba, D.N.; Yumura, M.; Hata, K. Growth control of single-walled, double-walled, and triple-walled carbon nanotube forests by a priori electrical resistance measurement of catalyst films. Carbon 2011, 49, 4368–4375. [Google Scholar] [CrossRef]
- Bhushan, B. (Ed.) Springer Handbook of Nanotechnology; Springer Science and Business Media LLC.: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Guo, T.; Nikolaev, P.; Thess, A.; Colbert, D.; Smalley, R. Catalytic growth of single-walled manotubes by laser vaporization. Chem. Phys. Lett. 1995, 243, 49–54. [Google Scholar] [CrossRef]
- Sajid, M.I.; Jamshaid, U.; Jamshaid, T.; Zafar, N.; Fessi, H.; Elaissari, A. Carbon nanotubes from synthesis to in vivo biomedical applications. Int. J. Pharm. 2016, 501, 278–299. [Google Scholar] [CrossRef] [PubMed]
- Chiang, W.-H.; Sakr, M.; Gao, X.P.A.; Sankaran, R.M. Nanoengineering NixFe1−x catalysts for gas-phase, selective synthesis of semiconducting single-walled carbon nanotubes. ACS Nano 2009, 3, 4023–4032. [Google Scholar] [CrossRef]
- Zhang, G.; Mann, D.; Zhang, L.; Javey, A.; Li, Y.; Yenilmez, E.; Wang, Q.; McVittie, J.P.; Nishi, Y.; Gibbons, J.; et al. Ultra-high-yield growth of vertical single-walled carbon nanotubes: Hidden roles of hydrogen and oxygen. Proc. Natl. Acad. Sci. USA 2005, 102, 16141–16145. [Google Scholar] [CrossRef] [Green Version]
- Bower, C.; Zhou, O.; Zhu, W.; Werder, D.J.; Jin, S. Nucleation and growth of carbon nanotubes by microwave plasma chemical vapor deposition. Appl. Phys. Lett. 2000, 77, 2767–2769. [Google Scholar] [CrossRef]
- Ho, G.; Wee, A.T.S.; Lin, J.; Tjiu, W. Synthesis of well-aligned multiwalled carbon nanotubes on Ni catalyst using radio frequency plasma-enhanced chemical vapor deposition. Thin Solid Film. 2001, 388, 73–77. [Google Scholar] [CrossRef]
- Rao, R.; Pint, C.L.; Islam, A.E.; Weatherup, R.S.; Hofmann, S.; Meshot, E.R.; Wu, F.; Zhou, C.; Dee, N.; Amama, P.B.; et al. Carbon nanotubes and related nanomaterials: Critical advances and challenges for synthesis toward mainstream commercial applications. ACS Nano 2018, 12, 11756–11784. [Google Scholar] [CrossRef] [Green Version]
- Arenal, R.; Löthman, P.; Picher, M.; Than, T.X.; Paillet, M.; Jourdain, V. Direct evidence of atomic structure conservation along ultra-long carbon nanotubes. J. Phys. Chem. C 2012, 116, 14103–14107. [Google Scholar] [CrossRef]
- An, H.; Kumamoto, A.; Takezaki, H.; Ohyama, S.; Qian, Y.; Inoue, T.; Ikuhara, Y.; Chiashi, S.; Xiang, R.; Maruyama, S. Chirality specific and spatially uniform synthesis of single-walled carbon nanotubes from a sputtered Co–W bimetallic catalyst. Nanoscale 2016, 8, 14523–14529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachilo, S.M.; Balzano, L.; Herrera, J.E.; Pompeo, F.; Resasco, D.E.; Weisman, R.B. Narrow (n,m)-distribution of single-walled carbon nanotubes grown using a solid supported catalyst. J. Am. Chem. Soc. 2003, 125, 11186–11187. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, X.; Zhang, D.; Yang, J.; Luo, D.; Xu, Z.; Wei, J.; Wang, J.-Q.; Xu, Z.; Peng, F.; et al. Chirality-specific growth of single-walled carbon nanotubes on solid alloy catalysts. Nature 2014, 510, 522–524. [Google Scholar] [CrossRef]
- Yang, F.; Wang, X.; Zhang, D.; Qi, K.; Yang, J.; Xu, Z.; Li, M.; Zhao, X.; Bai, X.; Li, Y. Growing zigzag (16,0) carbon nanotubes with structure-defined catalysts. J. Am. Chem. Soc. 2015, 137, 8688–8691. [Google Scholar] [CrossRef]
- Yang, F.; Wang, X.; Si, J.; Zhao, X.; Qi, K.; Jin, C.; Zhang, Z.; Li, M.; Zhang, D.; Yang, J.; et al. Water-assisted preparation of high-purity semiconducting (14,4) carbon nanotubes. ACS Nano 2016, 11, 186–193. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J.; Li, H.-B.; Bhola, R.; Jackson, E.A.; Scott, L.T.; Page, A.J.; Irle, S.; Morokuma, K.; Zhou, C. Nearly exclusive growth of small diameter semiconducting single-wall carbon nanotubes from organic chemistry synthetic end-cap molecules. Nano Lett. 2014, 15, 586–595. [Google Scholar] [CrossRef] [Green Version]
- Lolli, G.; Zhang, L.; Balzano, L.; Sakulchaicharoen, N.; Tan, A.Y.; Resasco, D.E. Tailoring (n,m) structure of single-walled carbon nanotubes by modifying reaction conditions and the nature of the support of CoMo catalysts. J. Phys. Chem. B 2006, 110, 2108–2115. [Google Scholar] [CrossRef]
- Zhu, Z.; Jiang, H.; Susi, T.; Nasibulin, A.G.; Kauppinen, E.I. The use of NH3 to promote the production of large-diameter single-walled carbon nanotubes with a narrow (n,m) distribution. J. Am. Chem. Soc. 2011, 133, 1224–1227. [Google Scholar] [CrossRef]
- Li, X.; Tu, X.; Zaric, S.; Welsher, K.; Seo, W.S.; Zhao, W.; Dai, H. Selective synthesis combined with chemical separation of single-walled carbon nanotubes for chirality selection. J. Am. Chem. Soc. 2007, 129, 15770–15771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Wang, X.; Li, M.; Liu, X.; Zhao, X.; Zhang, D.; Zhang, Y.; Yang, J.; Li, Y. Templated synthesis of single-walled carbon nanotubes with specific structure. Acc. Chem. Res. 2016, 49, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, M.; Misak, H.; Yang, S.Y.; Asmatulu, R. Effects of morphology, concentration and contact duration of carbon-based nanoparticles on cytotoxicity of L929 cells. In Proceedings of the ASME 2015 International Mechanical Engineering Congress and Exposition, Houston, TX, USA, 13–19 November 2015; Volume 14. [Google Scholar] [CrossRef]
- Warheit, D.B.; Laurence, B.R.; Reed, K.L.; Roach, D.H.; Reynolds, G.A.M.; Webb, T.R. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol. Sci. 2003, 77, 117–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.H.; Chhowalla, M.; Iqbal, Z.; Sesti, F. Single-walled carbon nanotubes are a new class of ion channel blockers. J. Biol. Chem. 2003, 278, 50212–50216. [Google Scholar] [CrossRef] [Green Version]
- Poland, C.A.; Duffin, R.; Kinloch, I.; Maynard, A.; Wallace, W.A.H.; Seaton, A.; Stone, V.; Brown, S.; MacNee, W.; Donaldson, K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol. 2008, 3, 423–428. [Google Scholar] [CrossRef]
- Porter, A.E.; Gass, M.; Muller, K.; Skepper, J.N.; Midgley, P.A.; Welland, M. Direct imaging of single-walled carbon nanotubes in cells. Nat. Nanotechnol. 2007, 2, 713–717. [Google Scholar] [CrossRef]
- Shin, S.R.; Jung, S.M.; Zalabany, M.; Kim, K.; Zorlutuna, P.; Kim, S.B.; Nikkhah, M.; Khabiry, M.; Azize, M.; Kong, J.; et al. Carbon-nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators. ACS Nano 2013, 7, 2369–2380. [Google Scholar] [CrossRef] [Green Version]
- Marchesan, S.; Melchionnaa, M.; Prato, M. Wire up on carbon nanostructures! How to play a winning game. ACS Nano 2015, 9, 9441–9450. [Google Scholar] [CrossRef] [Green Version]
- Shao, S.; Zhou, S.; Li, L.; Li, J.; Luo, C.; Wang, J.; Li, X.; Weng, J. Osteoblast function on electrically conductive electrospun PLA/MWCNTs nanofibers. Biomaterials 2011, 32, 2821–2833. [Google Scholar] [CrossRef] [PubMed]
- Crowder, S.W.; Liang, Y.; Rath, R.; Park, A.M.; Maltais, S.; Pintauro, P.N.; Hofmeister, W.; Lim, C.C.; Wang, X.; Sung, H.-J. Poly(ε-caprolactone)–carbon nanotube composite scaffolds for enhanced cardiac differentiation of human mesenchymal stem cells. Nanomedicine 2013, 8, 1763–1776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahadian, S.; Yamada, S.; Ramón-Azcón, J.; Estili, M.; Liang, X.; Nakajima, K.; Shiku, H.; Khademhosseini, A.; Matsue, T. Hybrid hydrogel-aligned carbon nanotube scaffolds to enhance cardiac differentiation of embryoid bodies. Acta Biomater. 2016, 31, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.M.B.; Mishra, A.; Lin, P.T.P.; Ng, S.H.; Yeong, W.Y.; Kim, S.-W.; Yoon, Y.-J. 3D printed polycaprolactone carbon nanotube composite scaffolds for cardiac tissue engineering. Macromol. Biosci. 2017, 17, 1600250. [Google Scholar] [CrossRef]
- He, J.; Xu, F.; Dong, R.; Guo, B.; Li, D. Electrohydrodynamic 3D printing of microscale poly (ε-caprolactone) scaffolds with multi-walled carbon nanotubes. Biofabrication 2017, 9, 015007. [Google Scholar] [CrossRef] [PubMed]
- Kharaziha, M.; Shin, S.R.; Nikkhah, M.; Topkaya, S.N.; Masoumi, N.; Annabi, N.; Dokmeci, M.R.; Khademhosseini, A. Tough and flexible CNT–polymeric hybrid scaffolds for engineering cardiac constructs. Biomaterials 2014, 35, 7346–7354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pok, S.; Vitale, F.; Eichmann, S.L.; Benavides, O.M.; Pasquali, M.; Jacot, J.G. Biocompatible carbon nanotube–chitosan scaffold matching the electrical conductivity of the heart. ACS Nano 2014, 8, 9822–9832. [Google Scholar] [CrossRef] [Green Version]
- Mehdikhani, M.; Ghaziof, S. Electrically conductive poly-ε-caprolactone/polyethylene glycol/multi-wall carbon nanotube nanocomposite scaffolds coated with fibrin glue for myocardial tissue engineering. Appl. Phys. A 2018, 124, 77. [Google Scholar] [CrossRef]
- Gonçalves, E.M.; Oliveira, F.J.; Silva, R.F.; Neto, M.A.; Fernandes, M.H.; Amaral, M.; Vallet-Regí, M.; Vila, M. Three-dimensional printed PCL-hydroxyapatite scaffolds filled with CNTs for bone cell growth stimulation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 1210–1219. [Google Scholar] [CrossRef]
- Dorj, B.; Won, J.-E.; Kim, J.-H.; Choi, S.-J.; Shin, U.S.; Kim, H.-W. Robocasting nanocomposite scaffolds of poly(caprolactone)/hydroxyapatite incorporating modified carbon nanotubes for hard tissue reconstruction. J. Biomed. Mater. Res. Part A 2012, 101, 1670–1681. [Google Scholar] [CrossRef]
- Wang, W.; Huang, B.; Byun, J.J.; Bártolo, P. Assessment of PCL/carbon material scaffolds for bone regeneration. J. Mech. Behav. Biomed. Mater. 2019, 93, 52–60. [Google Scholar] [CrossRef]
- Sang, L.; Liu, Y.; Hua, W.; Xu, K.; Wang, G.; Zhong, W.; Wang, L.; Xu, S.; Xing, M.; Qiu, X. Thermally sensitive conductive hydrogel using amphiphilic crosslinker self-assembled carbon nanotube to enhance neurite outgrowth and promote spinal cord regeneration. RSC Adv. 2016, 6, 26341–26351. [Google Scholar] [CrossRef]
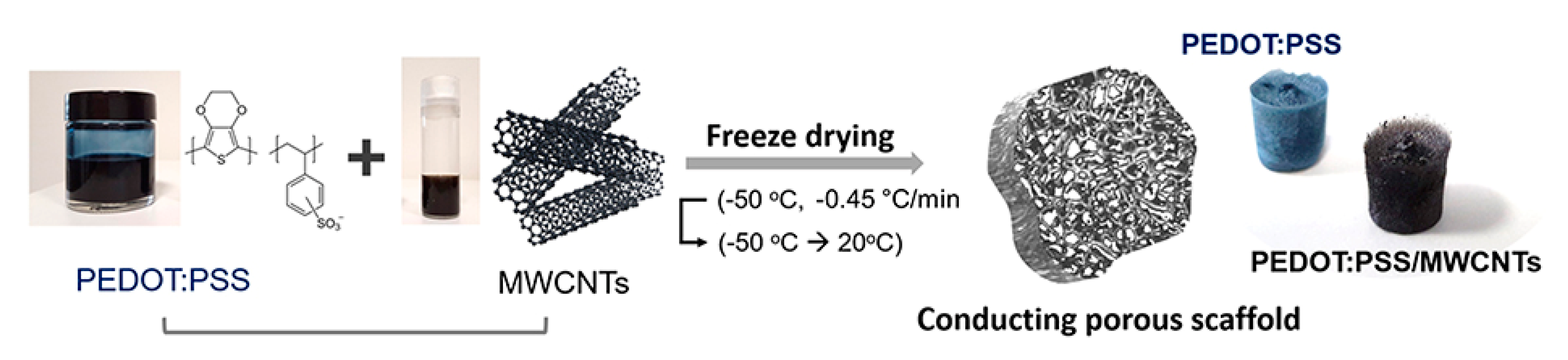
- Dominguez-Alfaro, A.; Alegret, N.; Arnaiz, B.; González-Domínguez, J.M.; Martín-Pacheco, A.; Cossío, U.; Porcarelli, L.; Bosi, S.; Vázquez, E.; Mecerreyes, D.; et al. Tailored methodology based on vapor phase polymerization to manufacture PEDOT/CNT scaffolds for tissue engineering. ACS Biomater. Sci. Eng. 2019, 6, 1269–1278. [Google Scholar] [CrossRef]
- Jayaram, A.K.; Pitsalidis, C.; Tan, E.; Moysidou, C.-M.; De Volder, M.F.L.; Kim, J.-S.; Owens, R.M. 3D hybrid scaffolds based on PEDOT:PSS/MWCNT composites. Front. Chem. 2019, 7, 363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Touri, R.; Moztarzadeh, F.; Sadeghian, Z.; Bizari, D.; Tahriri, M.; Mozafari, M. The use of carbon nanotubes to reinforce 45S5 bioglass-based scaffolds for tissue engineering applications. BioMed Res. Int. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sen, R.; Zhao, B.; Perea, D.; Itkis, M.E.; Hu, H.; Love, J.; Bekyarova, E.; Haddon, R.C. Preparation of single-walled carbon nanotube reinforced polystyrene and polyurethane nanofibers and membranes by electrospinning. Nano Lett. 2004, 4, 459–464. [Google Scholar] [CrossRef]
- Ge, J.J.; Hou, H.; Li, Q.; Graham, M.J.; Greiner, A.; Reneker, D.H.; Harris, F.W.; Cheng, S.Z.D. Assembly of well-aligned multiwalled carbon nanotubes in confined polyacrylonitrile environments: Electrospun composite nanofiber sheets. J. Am. Chem. Soc. 2004, 126, 15754–15761. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Qi, R.; Shen, M.; Cao, X.; Guo, R.; Zhang, Y.; Shi, X. Improved cellular response on multiwalled carbon nanotube-incorporated electrospun polyvinyl alcohol/chitosan nanofibrous scaffolds. Colloids Surf. B Biointerfaces 2011, 84, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Sharma, Y.; Tiwari, A.; Hattori, S.; Terada, D.; Sharma, A.K.; Ramalingam, M.; Kobayashi, H. Fabrication of conducting electrospun nanofibers scaffold for three-dimensional cells culture. Int. J. Biol. Macromol. 2012, 51, 627–631. [Google Scholar] [CrossRef]
- Meng, J.; Song, L.; Kong, H.; Zhu, G.; Wang, C.; Xu, L.; Xie, S.; Xu, H. Using single-walled carbon nanotubes nonwoven films as scaffolds to enhance long-term cell proliferation in vitro. J. Biomed. Mater. Res. Part A 2006, 79, 298–306. [Google Scholar] [CrossRef]
- Namgung, S.; Baik, K.Y.; Park, J.; Hong, S. Controlling the growth and differentiation of human mesenchymal stem cells by the arrangement of individual carbon nanotubes. ACS Nano 2011, 5, 7383–7390. [Google Scholar] [CrossRef]
- Lee, S.-J.; Zhu, W.; Nowicki, M.; Lee, G.; Heo, D.N.; Kim, J.; Zuo, Y.Y.; Zhang, L.G. 3D printing nano conductive multi-walled carbon nanotube scaffolds for nerve regeneration. J. Neural Eng. 2018, 15, 016018. [Google Scholar] [CrossRef]
- Wang, J.; Tian, L.; Chen, N.; Ramakrishna, S.; Mo, X. The cellular response of nerve cells on poly-L-lysine coated PLGA-MWCNTs aligned nanofibers under electrical stimulation. Mater. Sci. Eng. C 2018, 91, 715–726. [Google Scholar] [CrossRef]
- Ahadian, S.; Huyer, L.D.; Estili, M.; Yee, B.; Smith, N.; Xu, Z.; Sun, Y.; Radisic, M. Moldable elastomeric polyester-carbon nanotube scaffolds for cardiac tissue engineering. Acta Biomater. 2017, 52, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Mombini, S.; Mohammadnejad, J.; Bakhshandeh, B.; Narmani, A.; Nourmohammadi, J.; Vahdat, S.; Zirak, S. Chitosan-PVA-CNT nanofibers as electrically conductive scaffolds for cardiovascular tissue engineering. Int. J. Biol. Macromol. 2019, 140, 278–287. [Google Scholar] [CrossRef]
- Mooney, E.; Mackle, J.N.; Blond, D.J.-P.; O’Cearbhaill, E.; Shaw, G.; Blau, W.J.; Barry, F.P.; Barron, V.; Murphy, J.M. The electrical stimulation of carbon nanotubes to provide a cardiomimetic cue to MSCs. Biomaterials 2012, 33, 6132–6139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; George, M.N.; Park, S.; Miller, A.L., II; Gaihre, B.; Linli, L.; Waletzki, B.E.; Terzic, A.; Yaszemskiab, M.J.; Lu, L. 3D-printed scaffolds with carbon nanotubes for bone tissue engineering: Fast and homogeneous one-step functionalization. Acta Biomater. 2020, 111, 129–140. [Google Scholar] [CrossRef]
- Zhou, Z.-F.; Liu, X.; Wu, W.; Park, S.; Miller, A.L., II; Terzic, A.; Lu, L. Effective nerve cell modulation by electrical stimulation of carbon nanotube embedded conductive polymeric scaffolds. Biomater. Sci. 2018, 6, 2375–2385. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.-S.; Hwang, J.-Y.; Kim, M.S.; Lee, J.-Y.; Kim, J.-W.; Kim, H.-S.; Shin, U.S.; Knowles, J.C.; Kim, H.-W.; Hyun, J.K. Carbon-nanotube-interfaced glass fiber scaffold for regeneration of transected sciatic nerve. Acta Biomater. 2015, 13, 324–334. [Google Scholar] [CrossRef] [Green Version]
- Jin, G.; Kim, G. The effect of sinusoidal AC electric stimulation of 3D PCL/CNT and PCL/β-TCP based bio-composites on cellular activities for bone tissue regeneration. J. Mater. Chem. B 2013, 1, 1439–1452. [Google Scholar] [CrossRef]
- Fabbro, A.; Villari, A.; Laishram, J.; Scaini, D.; Toma, F.M.; Turco, A.; Prato, M.; Ballerini, L. Spinal cord explants use carbon nanotube interfaces to enhance neurite outgrowth and to fortify synaptic inputs. ACS Nano 2012, 6, 2041–2055. [Google Scholar] [CrossRef]
- Wang, L.; Hu, S.; Ullah, M.W.; Li, X.; Shi, Z.; Yang, G. Enhanced cell proliferation by electrical stimulation based on electroactive regenerated bacterial cellulose hydrogels. Carbohydr. Polym. 2020, 249, 116829. [Google Scholar] [CrossRef]
- Supronowicz, P.R.; Ajayan, P.M.; Ullmann, K.R.; Arulanandam, B.P.; Metzger, D.W.; Bizios, R. Novel current-conducting composite substrates for exposing osteoblasts to alternating current stimulation. J. Biomed. Mater. Res. 2001, 59, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Depan, D.; Misra, R.D.K. The development, characterization, and cellular response of a novel electroactive nanostructured composite for electrical stimulation of neural cells. Biomater. Sci. 2014, 2, 1727–1739. [Google Scholar] [CrossRef] [PubMed]
- Su, W.-T.; Shih, Y.-A. Nanofiber containing carbon nanotubes enhanced PC12 cell proliferation and neuritogenesis by electrical stimulation. Biomed. Mater. Eng. 2015, 26, S189–S195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.; Ben Borgens, R. Electrically controlled release of the nerve growth factor from a collagen–carbon nanotube composite for supporting neuronal growth. J. Mater. Chem. B 2013, 1, 4166–4170. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Sun, B.; Chen, C. Understanding the toxicity of carbon nanotubes. Acc. Chem. Res. 2013, 46, 702–713. [Google Scholar] [CrossRef]
- Morimoto, Y.; Hirohashi, M.; Ogami, A.; Oyabu, T.; Myojo, T.; Todoroki, M.; Yamamoto, M.; Hashiba, M.; Mizuguchi, Y.; Lee, B.W.; et al. Pulmonary toxicity of well-dispersed multi-wall carbon nanotubes following inhalation and intratracheal instillation. Nanotoxicology 2011, 6, 587–599. [Google Scholar] [CrossRef]
- Murphy, F.A.; Poland, C.A.; Duffin, R.; Al-Jamal, K.T.; Ali-Boucetta, H.; Nunes, A.; Byrne, F.; Prina-Mello, A.; Volkov, Y.; Li, S.; et al. Length-dependent retention of carbon nanotubes in the pleural space of mice initiates sustained inflammation and progressive fibrosis on the parietal pleura. Am. J. Pathol. 2011, 178, 2587–2600. [Google Scholar] [CrossRef] [Green Version]
- Kam, N.W.S.; Dai, H. Carbon nanotubes as intracellular protein transporters: Generality and biological functionality. J. Am. Chem. Soc. 2005, 127, 6021–6026. [Google Scholar] [CrossRef] [Green Version]
- Kam, N.W.S.; Liu, Z.; Dai, H. Carbon nanotubes as intracellular transporters for proteins and DNA: An investigation of the uptake mechanism and pathway. Angew. Chem. 2006, 118, 591–595. [Google Scholar] [CrossRef]
- Bussy, C.; Pinault, M.; Cambedouzou, J.; Landry, M.J.; Jegou, P.; Mayne-L’hermite, M.; Launois, P.; Boczkowski, J.; Lanone, S. Critical role of surface chemical modifications induced by length shortening on multi-walled carbon nanotubes-induced toxicity. Part. Fibre Toxicol. 2012, 9, 46. [Google Scholar] [CrossRef] [Green Version]
- Ryoo, S.-R.; Kim, Y.-K.; Kim, M.-H.; Min, D.-H. Behaviors of NIH-3T3 fibroblasts on graphene/carbon nanotubes: Proliferation, focal adhesion, and gene transfection studies. ACS Nano 2010, 4, 6587–6598. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, J.; Xu, G.; Wei, J.; Zhang, Z.; Li, X. Tuning the conductivity and inner structure of electrospun fibers to promote cardiomyocyte elongation and synchronous beating. Mater. Sci. Eng. C 2016, 69, 865–874. [Google Scholar] [CrossRef] [PubMed]


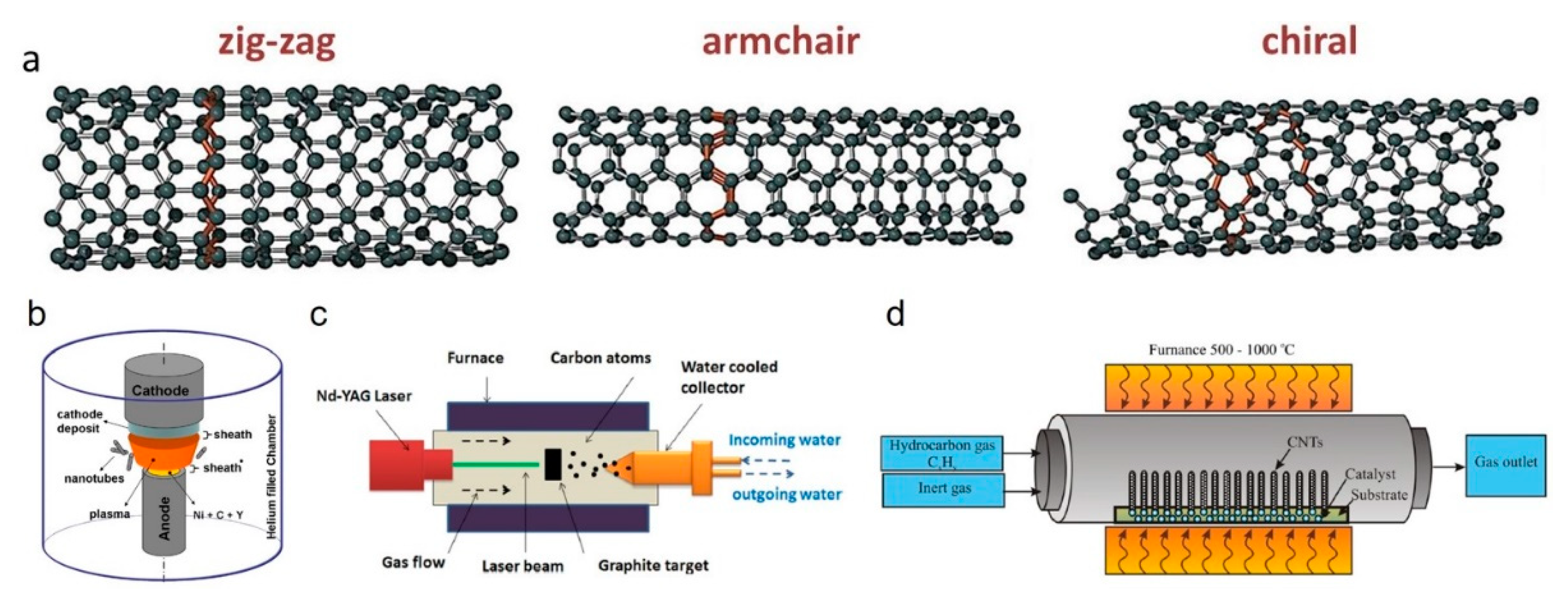
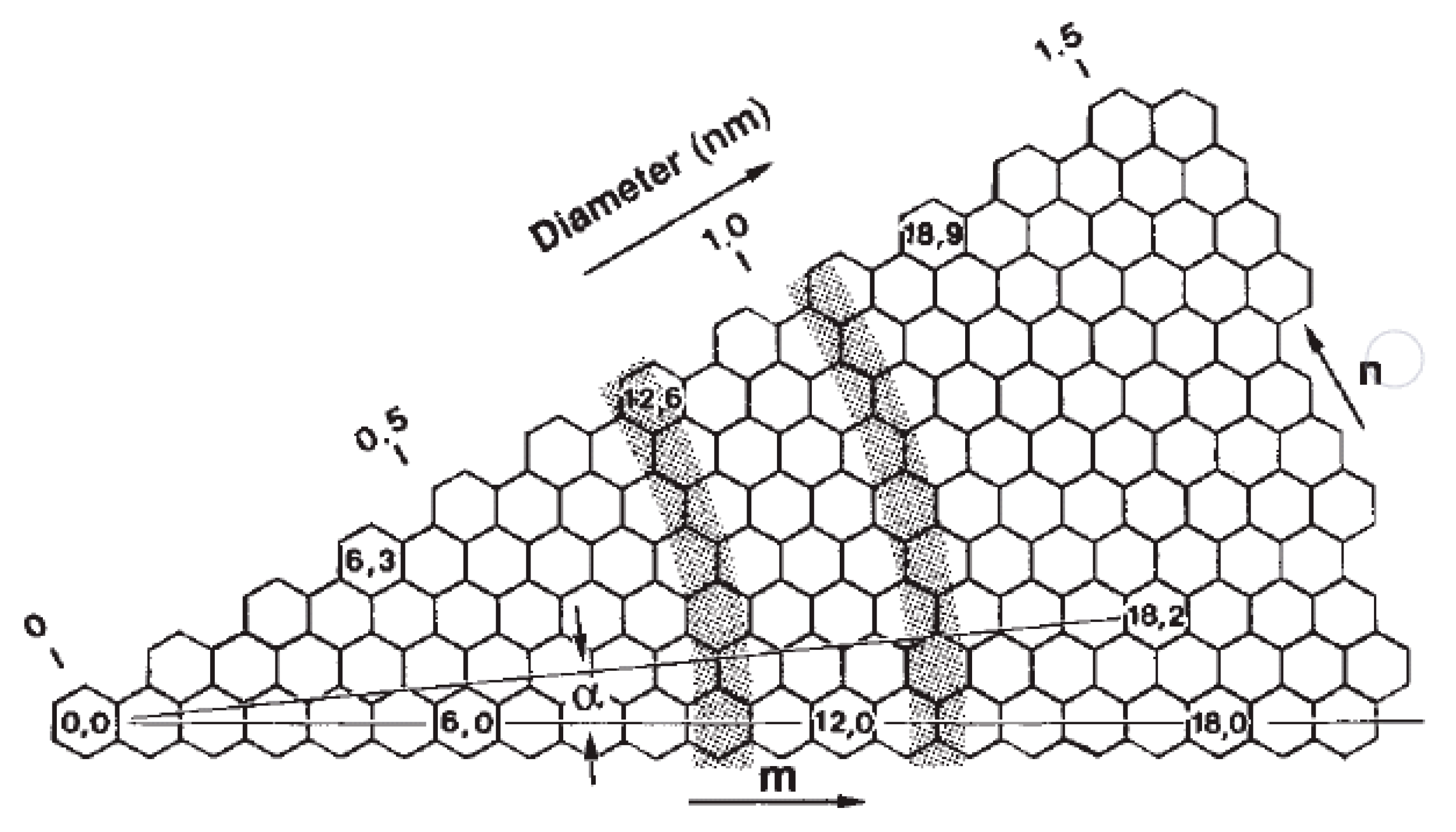
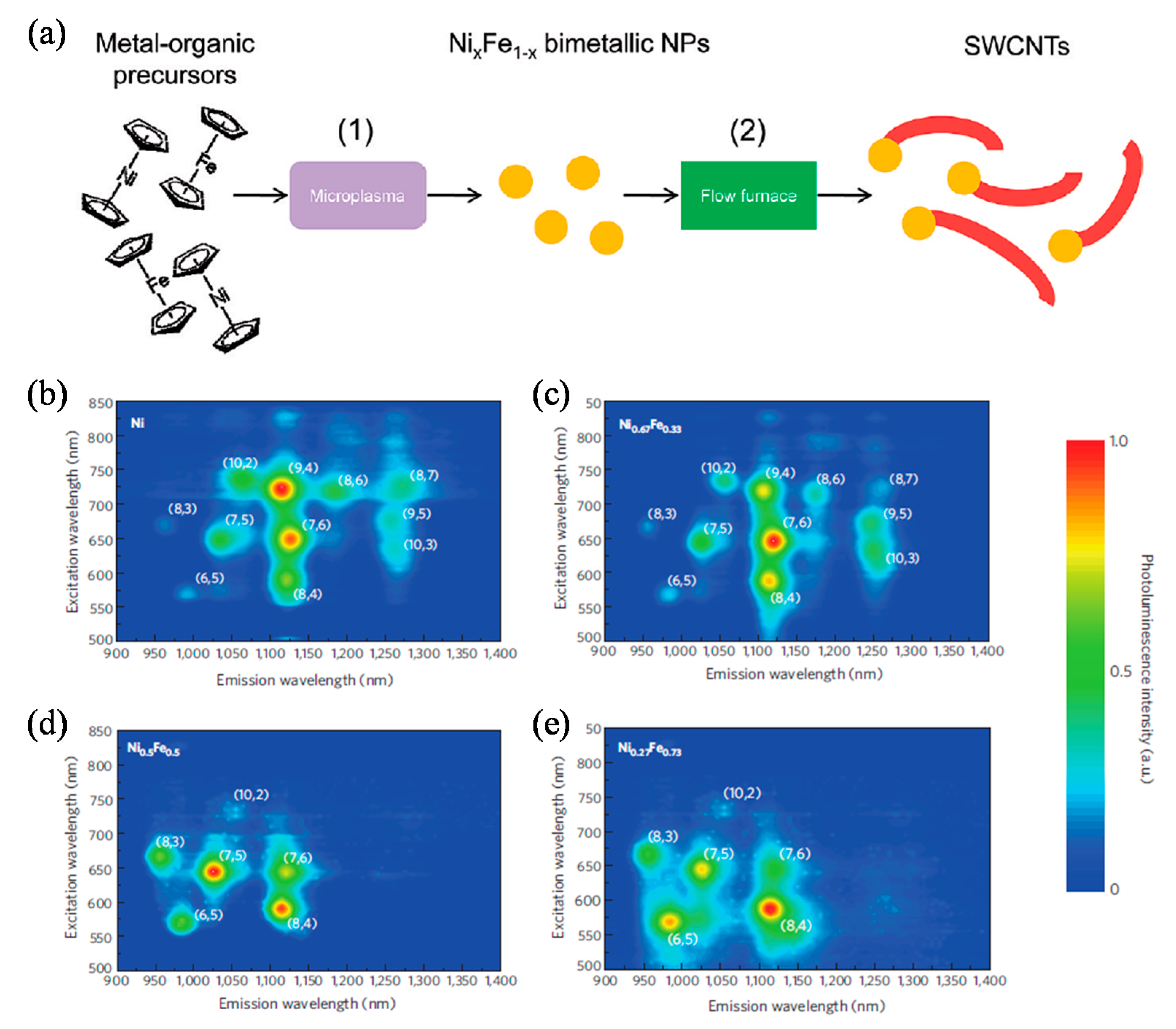
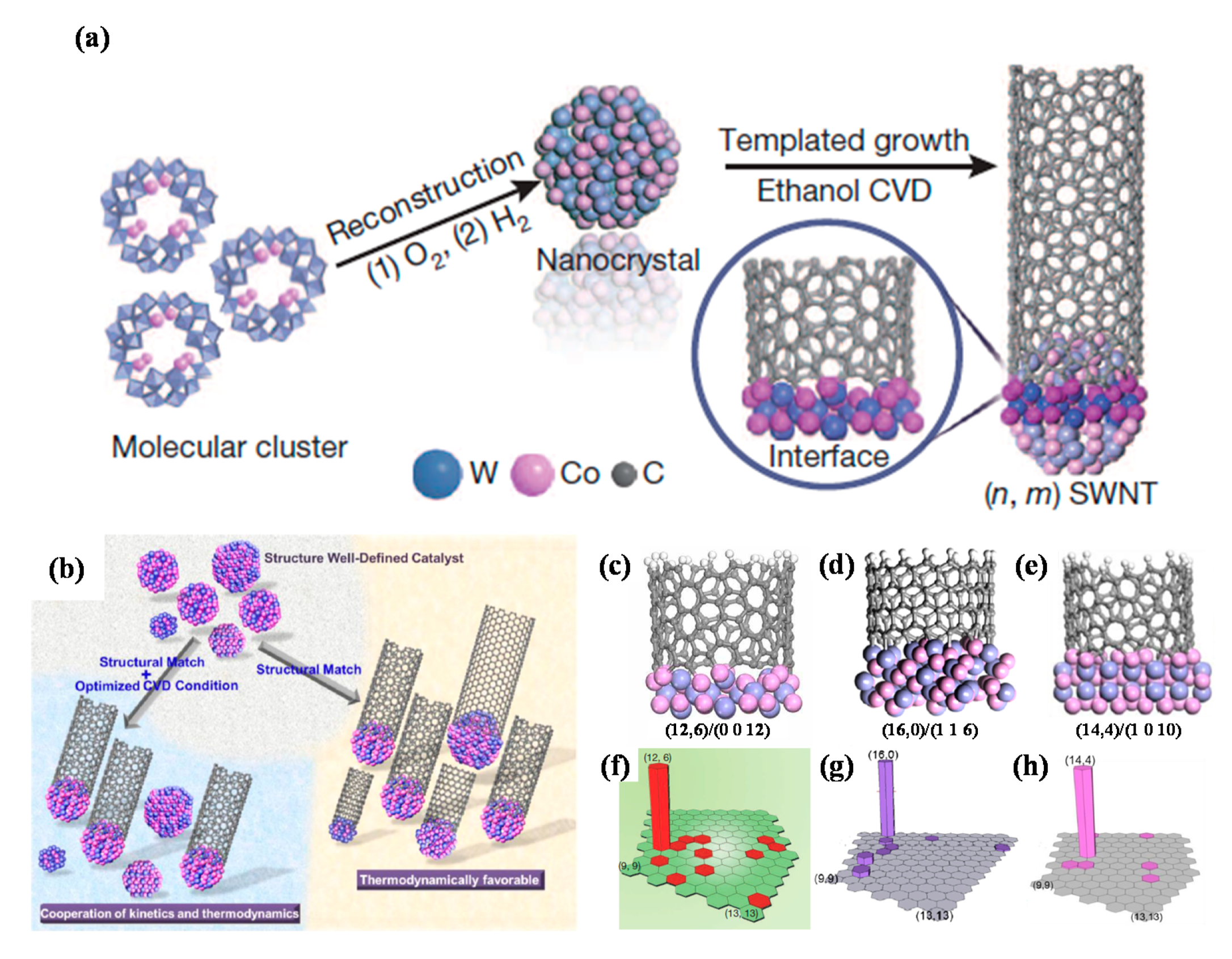
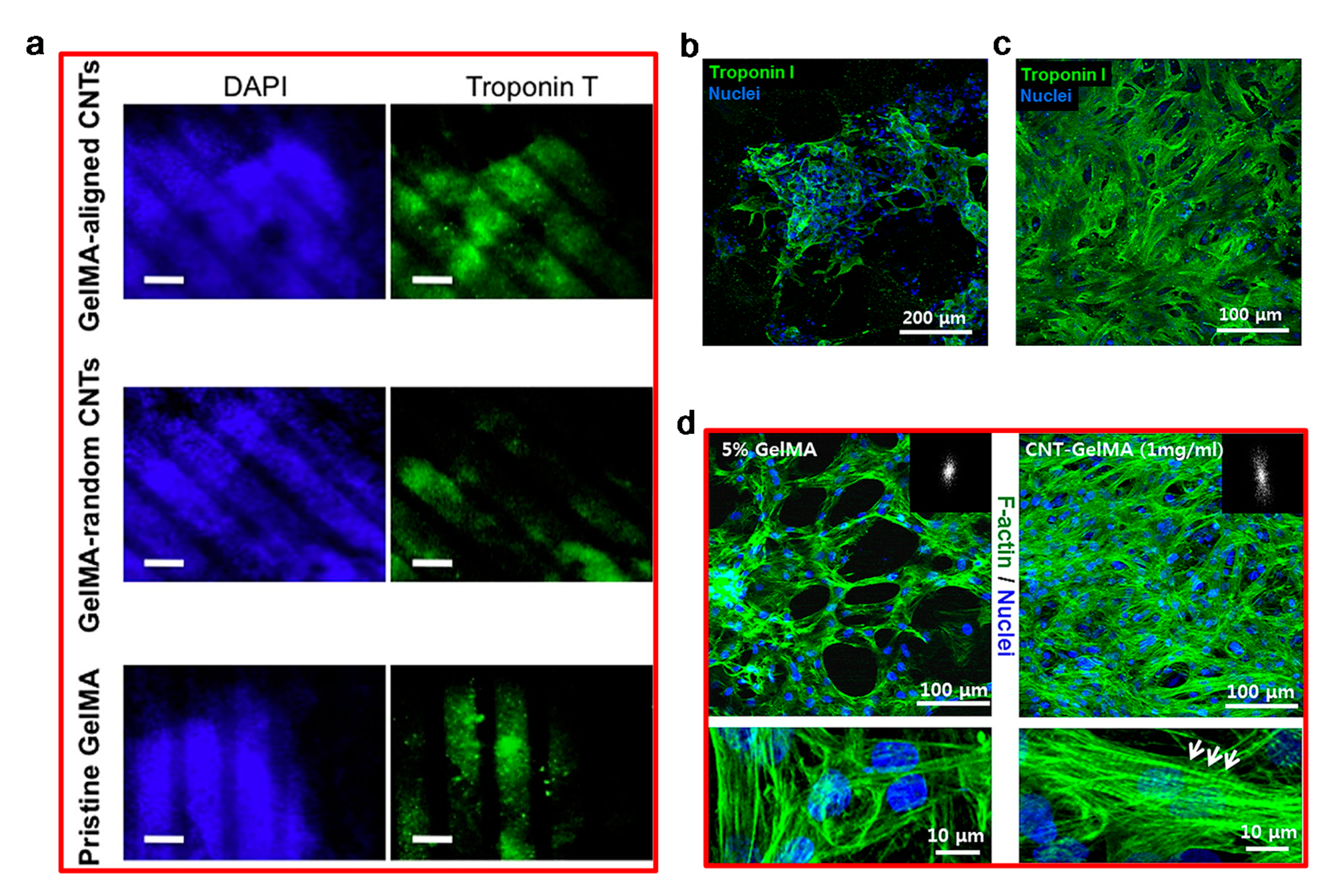
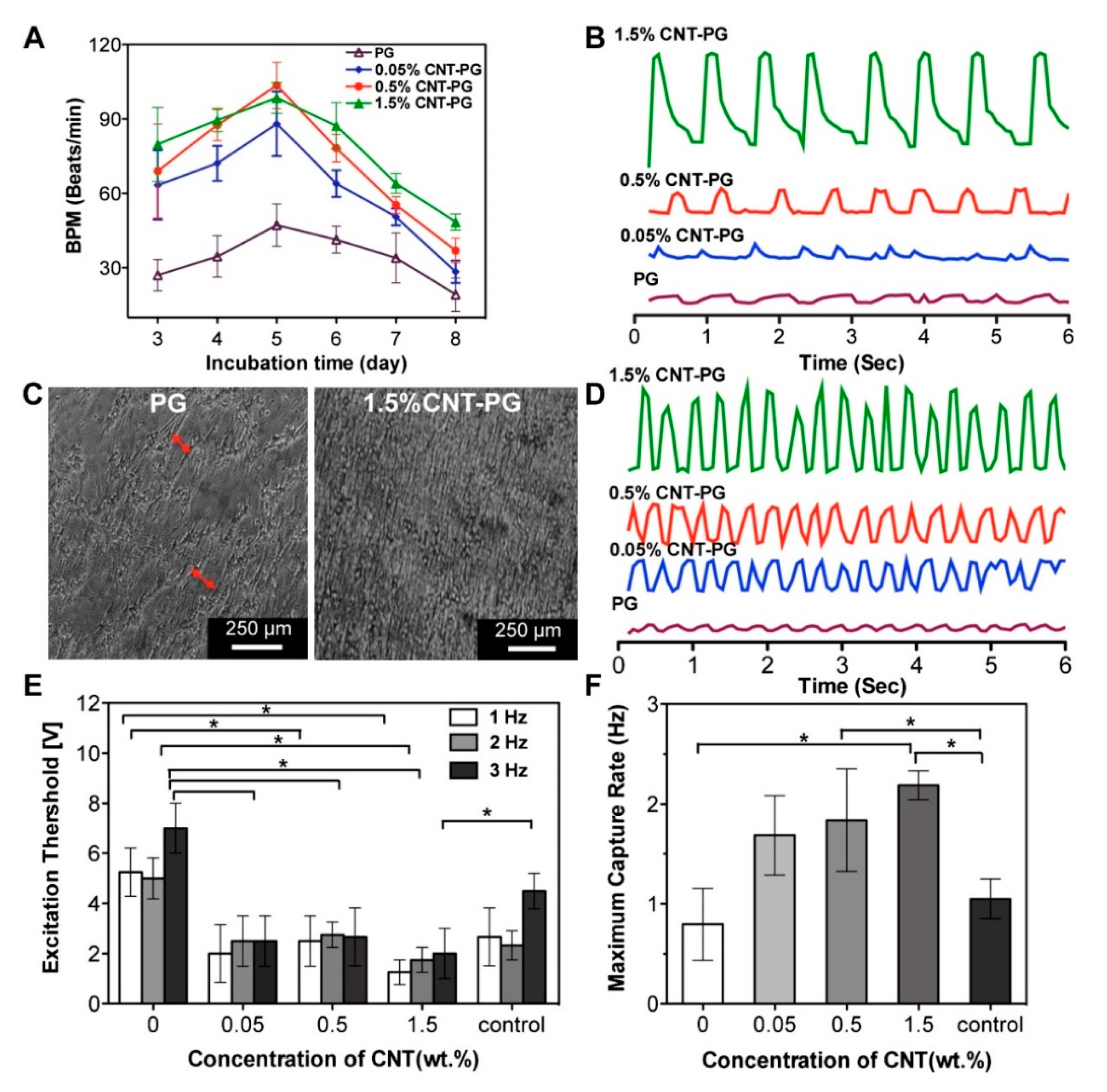
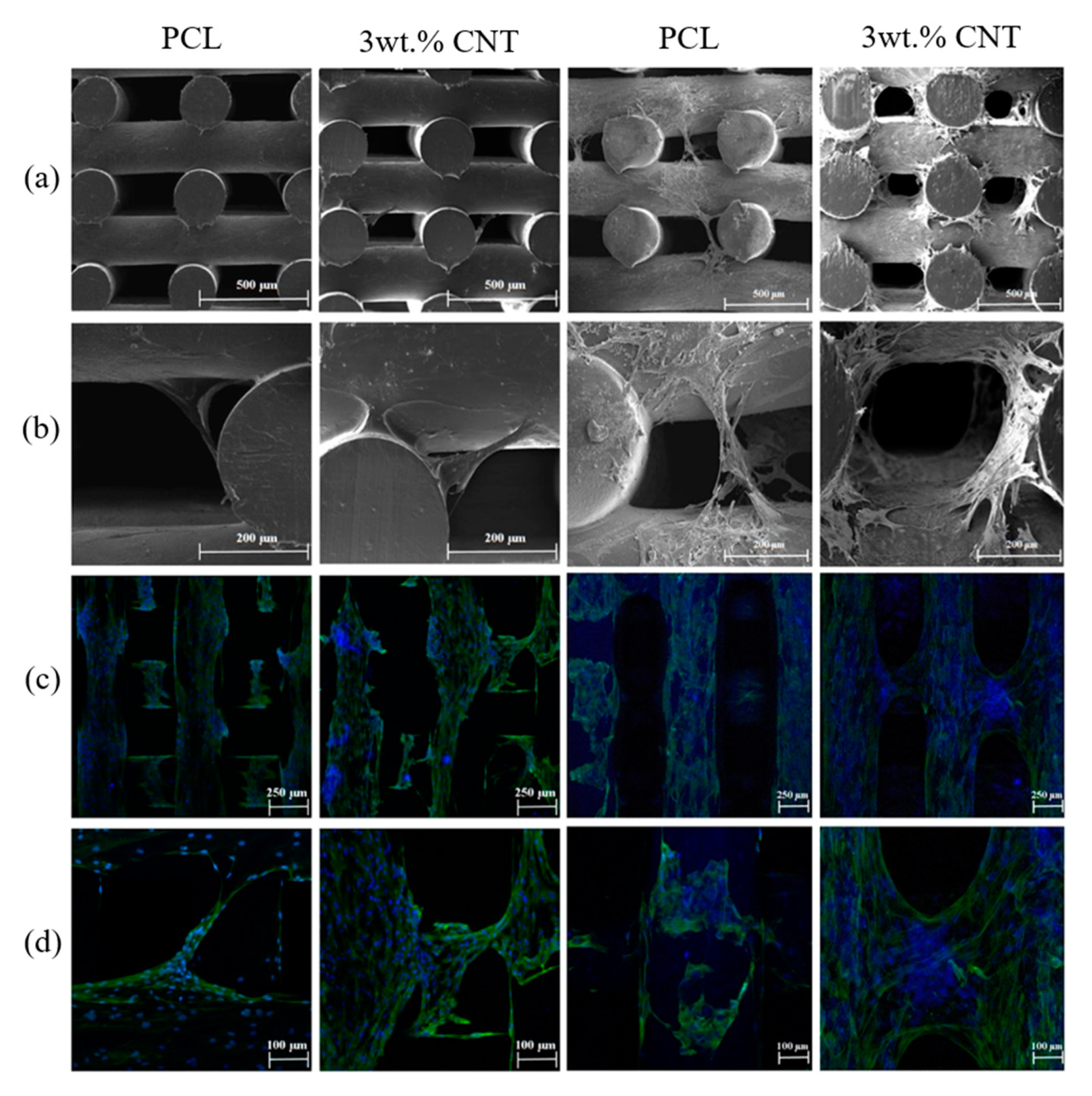
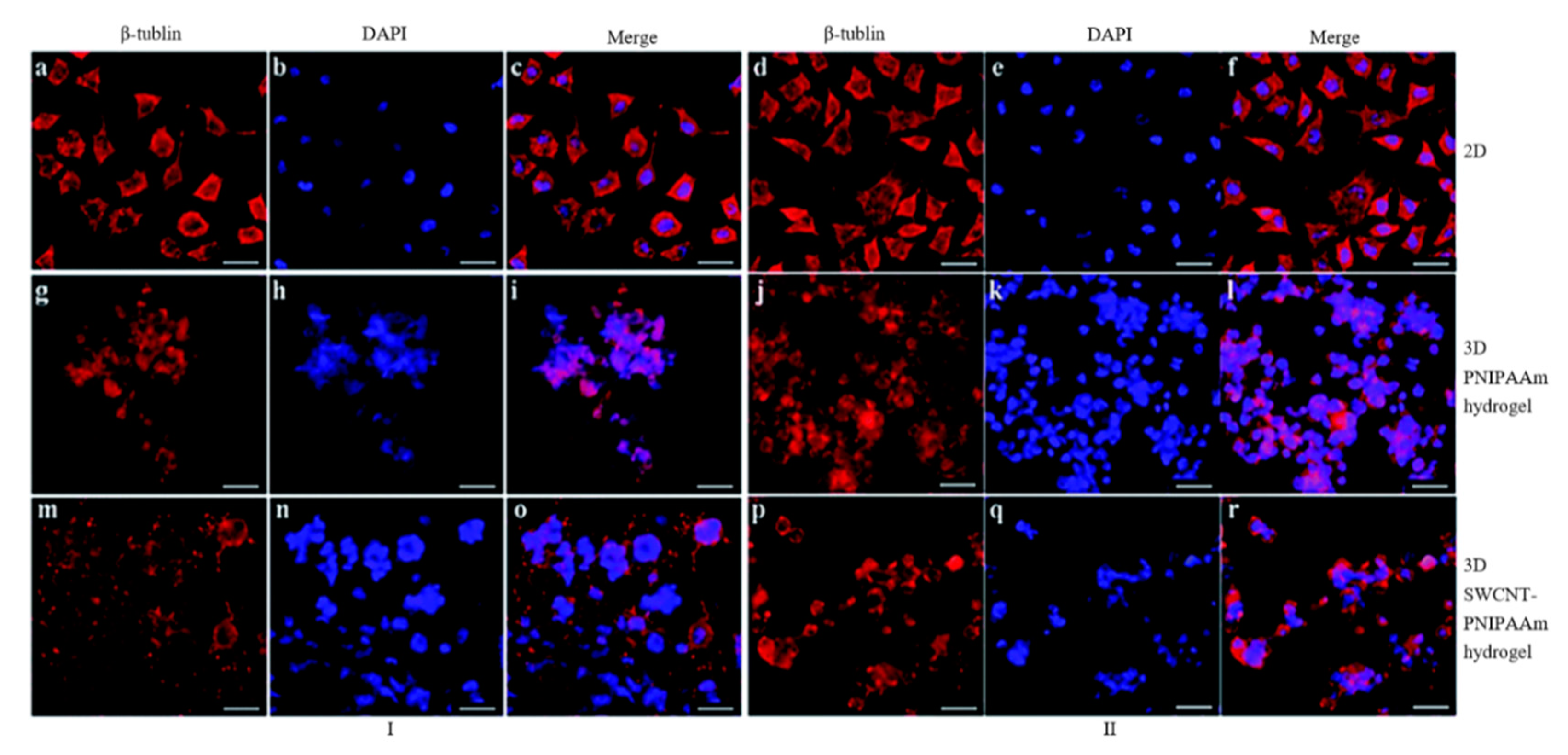
| Methods | Working Principle |
|---|---|
| Material extrusion | An additive manufacturing process in which polymers or polymer-based composites in the form of pellets or filaments are melted and selectively dispensed trough a nozzle or orifice |
| Material jetting | Polymeric droplets or bioinks (hydrogels containing cells and growth factors) are selectively deposited |
| Binder jetting | An additive manufacturing process in which a liquid binding material (e.g., colloidal system) is selectively deposited to join powder materials |
| Vat photopolymerization | An additive manufacturing process in which a liquid photopolymer is polymerized or cured (transition from liquid to solid), using a light source (laser or lamp) |
| Powder bed fusion | An additive manufacturing technique in which thermal energy from a laser or an electron beam is used to fuse in a selective way material in a powder form |
| Directed energy deposition | A technique in which focused thermal energy is used to fuse materials as the material is being deposited |
| Sheet lamination | Sheets of materials (e.g., paper, polymers, ceramics and metals) are cut and bonded together, to form a 3D object |
| Electro-Active Structures | Electrical Stimulation Settings | Cell Line | Outcome | Reference |
|---|---|---|---|---|
| Cellulose/graphene scaffold | 100 mV/mm of DC for 1 h/day | Human adipose stem cells | Increased proliferation, mineral deposition and ALP expression | Li et al., 2020 [123] |
| Reduced graphene oxide-coated ApF/poly(l-lactide-co-ε-caprolactone) scaffold | 100 mV/cm for 1 h/day | SCs and PC12 cells | Promoted SC migration, proliferation, myelin gene expression, neurotrophin secretion and induced PC12 cell differentiation | Wang et al., 2019 [124] |
| Polypyrrole/graphene nanofibrous scaffold | Forward potential varied from 0.1 to 1 V/cm while reverse potential changed from −0.1 to −1 V/cm | Retinal ganglion cells | led to 137% improvement in cell length with a significantly enhanced antiaging effect for RGCs | Yan et al., 2016 [125] |
| Graphene scaffold | Square waveform with 1 Hz and 10 μA for 30 min/day | Human Rett-derived neuronal progenitor cells | Improved cell maturation | Nguyen et al., 2018 [126] |
| Graphene membrane | Intensity of 100 mV/mm with 1 ms duration at 10 or 1 Hz | PC-12 nerve Cell | Promoted neurite extension and length growth | Meng et al., 2014 [127] |
| Graphene membrane | Pulse of 15 V, duration 50–100 ms | C2C12 Myoblasts | High degree of myogenic differentiation | Bajaj et al., 2014 [128] |
| Bacterial Cellulose/Poly(3,4-ethylenedioxythiophene) (PEDOT)/GO membrane | 0.5 V cm−1 for 1–100 ms lower than 0.6 V | PC12 neural cells | Promoted cell orientation and development of PC12 cells | Chen et al., 2016 [129] |
| Graphene-based membrane | 8 V at 1 Hz with 10 ms duration | Mouse C2C12 myoblast cells | Enhanced differentiation of skeletal muscle cells | Ahadian et al., 2014 [130] |
| Methoxy PEG/rGO membrane | 1–100 ms monophasic anodic pulses, 10 s duration, 0.6 V pulse potential | PC12 neural cells | Predominant increase in cell percentage with higher action potentials | Zhang et al., 2014 [131] |
| Poly(lactic-co-glycolic acid) (PLGA)/GO membrane | 100 mV at 20, 100 and 500 Hz for 1 h/day | Neural stem cells | Promoted proliferation, differentiation and neurite elongation in NSCs | Fu et al., 2019 [132] |
| Rolled GO foam | 100 ms cathodic voltage pulses | Human neural stem cells | More proliferation of hNSCs and their accelerated differentiation into neurons | Akhavan et al., 2016 [133] |
| Graphene-based foam | −0.2–0.8 V, 1–100 ms monophasic cathodic pulses at 10 s intervals, 20–30 μA threshold | Neural stem cell | Supported cell growth and enhanced differentiation to neurons than astrocytes | Li et al., 2013 [134] |
| Graphene-based substrate | 0.3 V at 1 Hz | Human mesenchymal stem cells | Did not create a cytotoxic environment | Balikov et al., 2016 [135] |
| Graphene-based substrate | 100 mV at 50 Hz for 10 min/day | Mesenchymal stem cells | Transdifferentiation of MSCs to SC-like phenotypes solely without the need for additional chemical growth factors | Das et al., 2017 [136] |
| Graphene/polyacrylamide hydrogel membrane | 5 V with 10 ms duration at 1 Hz for 4 h/day | Mouse C2C12 myoblast cells | Increased myogenic gene expression levels of myoblasts | Jo et al., 2017 [137] |
| CS/oxidized hydroxyethyl cellulose/rGO/asiaticoside liposome-based hydrogel membrane | 250 mV for 8 h | RSC 96 cells, PC12 cells, NIH/3 T3 cells | Promoted nerve regeneration | Zheng et al., 2019 [138] |
| Graphene crosslinked collagen cryogel membrane | 1 V for 5 min at 0.20 V/mm | BM-MSCs | Promoted proliferation of cells, aiding neural connections establishment, increase immune-modulatory secretions | Agarwal et al., 2021 [139] |
| Electro-Active Structures | Electrical Stimulation Settings | Cell Line | Outcome | Reference |
|---|---|---|---|---|
| Polylactic acid (PLA)/MWCNT scaffold | DC: 100 μA (4 h/day, 6 days) | Osteoblasts | Proliferation and elongation along the current direction | Shao et al., 2011 [183] |
| PEGDA/MWCNT scaffold | 100, 500 and 1000 µA at 100 Hz for 100 µs | Neural stem cells | Higher TUJ1 and GFAP expression | Lee et al., 2018 [204] |
| PLGA/MWCNT scaffold | 40 mV rectangular pulse for 30 min | PC12 and Schwann cells | Promoted the growth and myelination of Schwann cells | Wang et al., 2018 [205] |
| PCL/CNT scaffold | 5 V cm−1 for 5 ms duration at 1 Hz every 4 days | Human Mesenchymal Stem Cells | Rapid morphological changes and expressed cardiac genes | Crowder et al., 2013 [184] |
| 124 polymer/CNT scaffold | 2-ms pulses of 0–0.1 V at 1 Hz | Neonatal rat heart tissue | Improved tissue maturity | Ahadian et al., 2017 [206] |
| Polyvinyl acetate/Chitosan/CNT scaffold | 5 mV·cm−1 in a frequency of 1Hz for 5days at 37 °C | Undifferentiated mesenchymal stem cells | Enhanced the adherence of MSCs | Mombini et al., 2019 [207] |
| PLA/CNT nanofiber scaffold | 0.15 V/cm for 2 ms duration at 1 Hz | Mesenchymal stem cell | Increased protein expression of cardiac-associated markers | Mooney et al., 2012 [208] |
| ssDNA bound CNT scaffold | 0, 50, 100, 200, 300 and 600 mV/mm at 20 Hz | MC3T3 pre-osteoblast cells | Robust cellular filaments and strong focal adhesions sites around cell edges | Liu et al., 2020 [209] |
| Polycaprolactone fumarate/CNT scaffold | 100 mV mm−1 at 20 Hz for 2 h/day | PC-12 cell | Enhanced cell proliferation, cell migration and formation of intracellular connections | Zhou et al., 2018 [210] |
| Phosphate glass microfibers/CNT scaffold | 5 mA at 1 Hz for 1 ms duration | PC12 and DRG cells | Can support nerve regeneration | Ahn et al., 2015 [211] |
| PCL/CNT scaffold | 55 ± 8 mV cm−1 at 60 Hz for 30 min/day | Osteoblast-like cells (MG63) | Promoted bone mineralization | Jin et al., 2013 [212] |
| MWCNT scaffold | 200 μs pulses of 1–50 V at 40 s intervals | Neurons | Neurite regrowth in spinal explants is favored | Alessandra et al., 2012 [213] |
| Regenerated bacterial cellulose/polypyrrole/CNT hydrogel membrane | 10 μA for 60 min/day | Mouse embryo fibroblast | Improved cell proliferation | Wang et al., 2019 [214] |
| Poly-L-lactide/CNT substrate | AC: 10 mA (10 Hz, 6 h/day) | Osteoblasts | 46% increase in cell proliferation after 2 days | Supronowicz et al., 2002 [215] |
| PEDOT/CNT substrate | −0.9–0.5 V at scan rate of 100 mV s−1 followed by 0.30 mC cm−2 at 50 Hz | NB-39-Nu human Neuroblastoma | Higher cell proliferation and longer neurite lengths | Depan and Misra, 2014 [216] |
| PCL/CNT membrane | 750 mV, 100 Hz AC for 30 min daily for 3 to 6 days | PC12 cells | Induced neural differentiation | Su and Shih, 2015 [217] |
| Nerve growth factor/collagen/CNT membrane | 500 mV, using Ag/AgCl electrodes | PC 12 cells | Massive release of NGF consequently supporting neurite sprouting and growth | Cho and Borgens, 2013 [218] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Hou, Y.; Martinez, D.; Kurniawan, D.; Chiang, W.-H.; Bartolo, P. Carbon Nanomaterials for Electro-Active Structures: A Review. Polymers 2020, 12, 2946. https://doi.org/10.3390/polym12122946
Wang W, Hou Y, Martinez D, Kurniawan D, Chiang W-H, Bartolo P. Carbon Nanomaterials for Electro-Active Structures: A Review. Polymers. 2020; 12(12):2946. https://doi.org/10.3390/polym12122946
Chicago/Turabian StyleWang, Weiguang, Yanhao Hou, Dean Martinez, Darwin Kurniawan, Wei-Hung Chiang, and Paulo Bartolo. 2020. "Carbon Nanomaterials for Electro-Active Structures: A Review" Polymers 12, no. 12: 2946. https://doi.org/10.3390/polym12122946
APA StyleWang, W., Hou, Y., Martinez, D., Kurniawan, D., Chiang, W.-H., & Bartolo, P. (2020). Carbon Nanomaterials for Electro-Active Structures: A Review. Polymers, 12(12), 2946. https://doi.org/10.3390/polym12122946







