Formation and Stability of Smooth Thin Films with Soft Microgels Made of Poly(N-Isopropylacrylamide) and Poly(Acrylic Acid)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PNIPAm Microgel Synthesis
2.3. IPN Microgel Synthesis
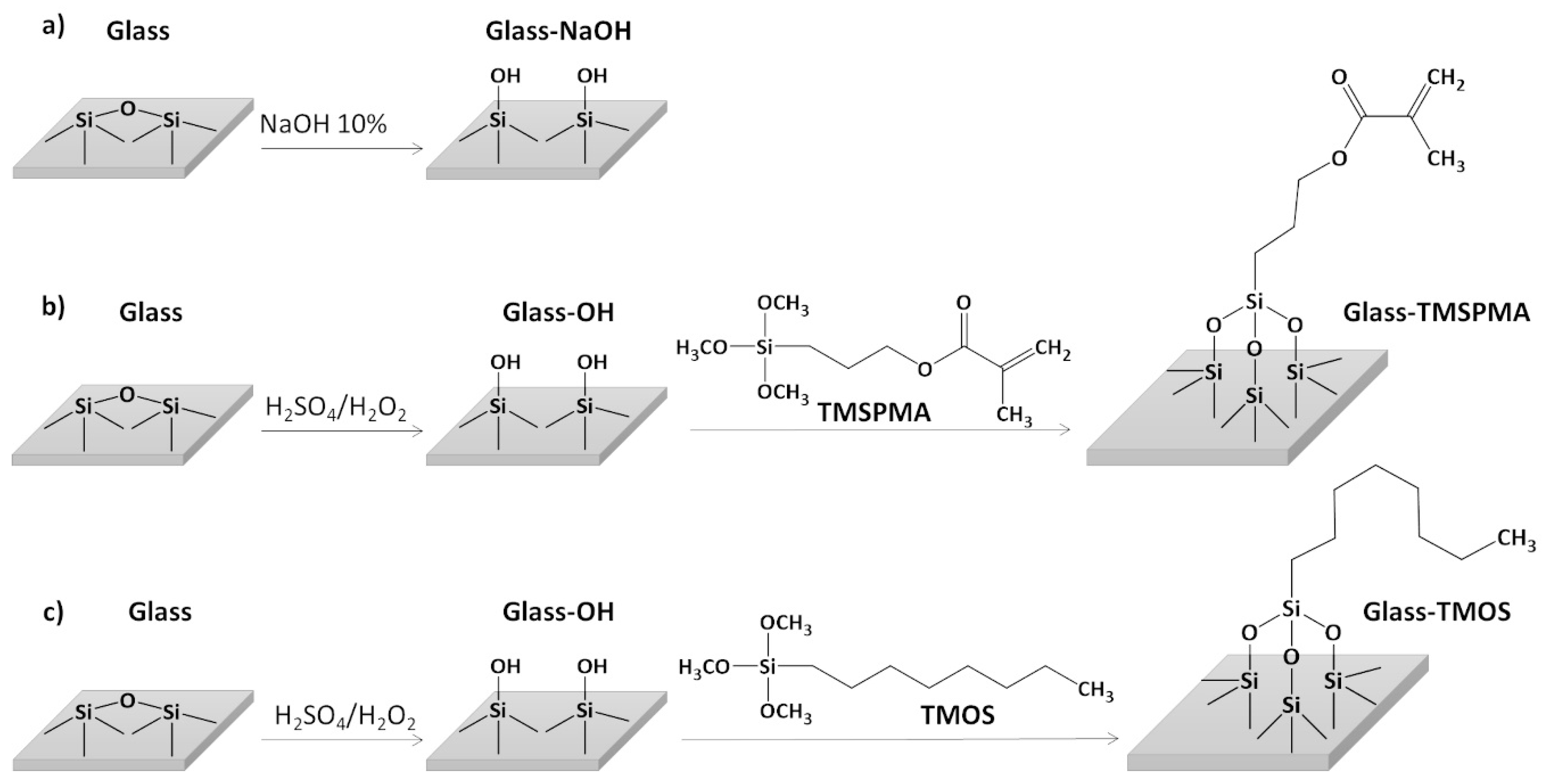
2.4. Glass Surface Functionalization
2.5. Film Preparation
2.6. Characterization Methods
3. Results and Discussion
3.1. Microgels: Preparation and Characterization
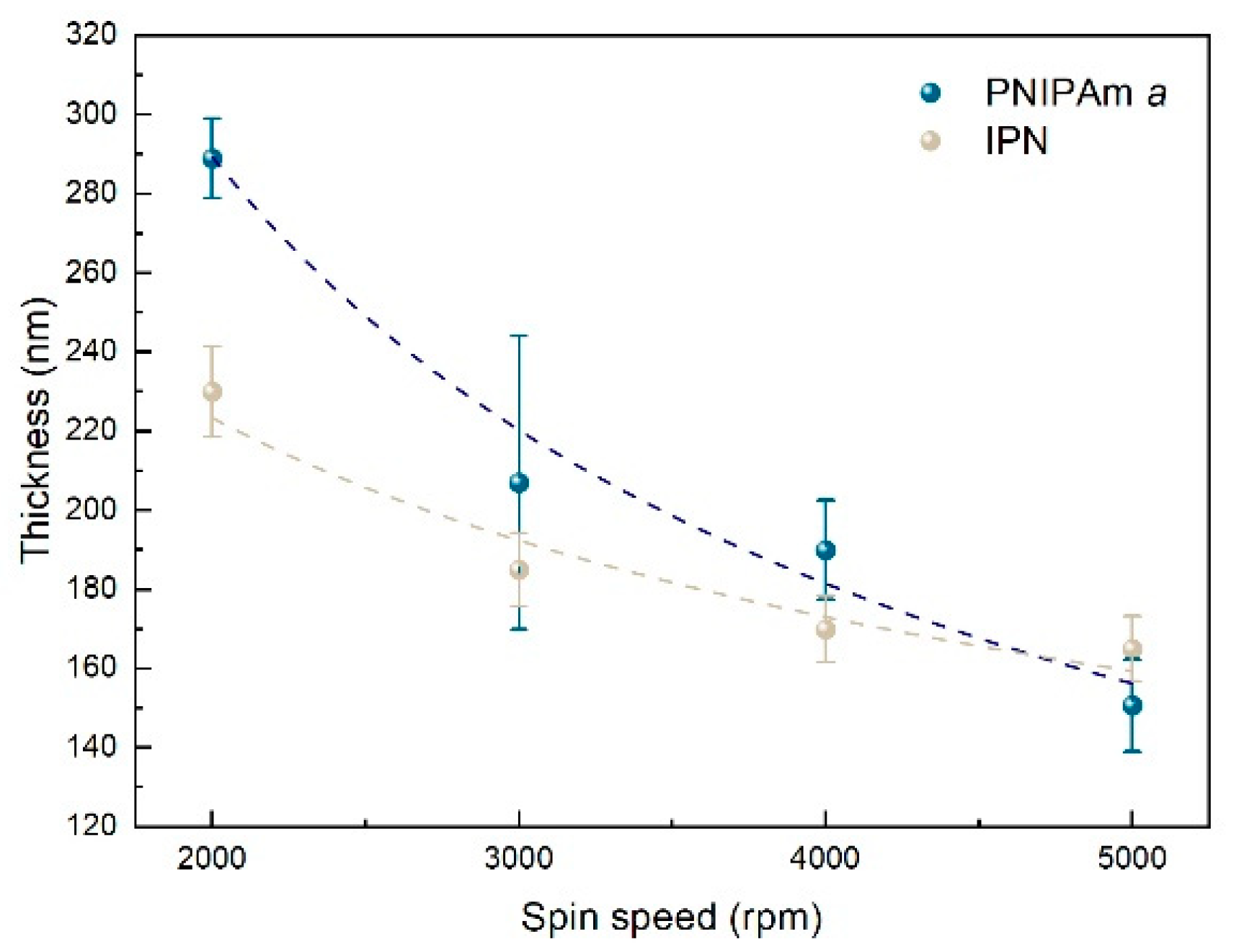
3.2. Film Characterization
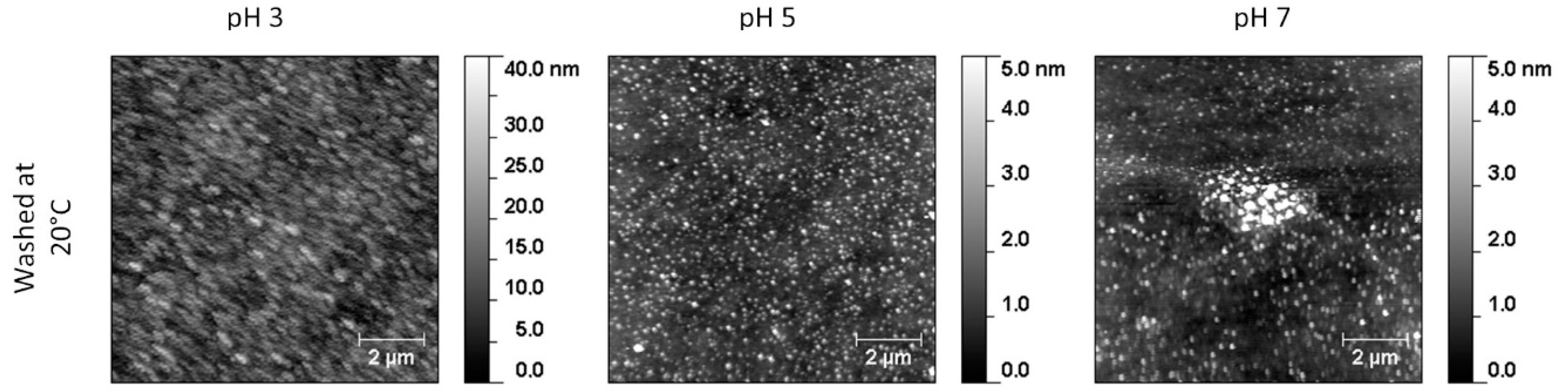
3.3. Film Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gil, E.S.; Hudson, S.M. Stimuli-reponsive polymers and their bioconjugates. Prog. Polym. Sci. 2004, 29, 1173–1222. [Google Scholar] [CrossRef]
- Wei, M.; Gao, Y.; Li, X.; Serpe, M.J. Stimuli-responsive polymers and their applications. Polym. Chem. 2017, 8, 127–143. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Soppimath, K.S.; Aminabhavi, T.M.; Dave, A.M.; Kumbar, S.G.; Rudzinski, W.E. Stimulus-Responsive “Smart” Hydrogels as Novel Drug Delivery Systems. Drug Dev. Ind. Pharm. 2002, 28, 957–974. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Zhang, Y. PNIPAM microgels for biomedical applications: From dispersed particles to 3D assemblies. Soft Matter 2011, 7, 6375. [Google Scholar] [CrossRef]
- Ferreira, N.N.; Ferreira, L.M.B.; Cardoso, V.M.O.; Boni, F.I.; Souza, A.L.R.; Gremião, M.P.D. Recent advances in smart hydrogels for biomedical applications: From self-assembly to functional approaches. Eur. Polym. J. 2018, 99, 117–133. [Google Scholar] [CrossRef]
- Migliorini, L.; Santaniello, T.; Yan, Y.; Lenardi, C.; Milani, P. Low-voltage electrically driven homeostatic hydrogel-based actuators for underwater soft robotics. Sensors Actuators B Chem. 2016, 228, 758–766. [Google Scholar] [CrossRef]
- Ruan, C.; Zeng, K.; Grimes, C.A. A mass-sensitive pH sensor based on a stimuli-responsive polymer. Anal. Chim. Acta 2003, 497, 123–131. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Vogler, S.; Jamshidi, A.; Valley, J.; Pei, S.N.; Pautot, S.; Wu, M.C. Thermo-sensitive microgels as in-situ sensor for temperature measurement in optoelectronic tweezers. Proc. IEEE Int. Conf. Micro Electro Mech. Syst. 2010, 1123–1126. [Google Scholar] [CrossRef]
- Yoshida, M.; Langer, R.; Lendlein, A.; Lahann, J. From Advanced Biomedical Coatings to Multi-Functionalized Biomaterials. J. Macromol. Sci. Part C Polym. Rev. 2006, 46, 347–375. [Google Scholar] [CrossRef]
- Wood, K.C.; Zacharia, N.S.; Schmidt, D.J.; Wrightman, S.N.; Andaya, B.J.; Hammond, P.T. Electroactive controlled release thin films. Proc. Natl. Acad. Sci. USA 2008, 105, 2280–2285. [Google Scholar] [CrossRef] [PubMed]
- Tokarev, I.; Motornov, M.; Minko, S. Molecular-engineered stimuli-responsive thin polymer film: A platform for the development of integrated multifunctional intelligent materials. J. Mater. Chem. 2009, 19, 6932. [Google Scholar] [CrossRef]
- Tokarev, I.; Minko, S. Stimuli-responsive hydrogel thin films. Soft Matter 2009, 5, 511–524. [Google Scholar] [CrossRef]
- Mendes, P.M. Stimuli-responsive surfaces for bio-applications. Chem. Soc. Rev. 2008, 37, 2512. [Google Scholar] [CrossRef]
- Senaratne, W.; Andruzzi, L.; Ober, C.K. Self-Assembled Monolayers and Polymer Brushes in Biotechnology: Current Applications and Future Perspectives. Biomacromolecules 2005, 6, 2427–2448. [Google Scholar] [CrossRef]
- Liu, Z.; Calvert, P. Multilayer Hydrogels as Muscle-Like Actuators. Adv. Mater. 2000, 12, 288–291. [Google Scholar] [CrossRef]
- Islam, M.; Ahiabu, A.; Li, X.; Serpe, M. Poly (N-isopropylacrylamide) Microgel-Based Optical Devices for Sensing and Biosensing. Sensors 2014, 14, 8984–8995. [Google Scholar] [CrossRef]
- Sigolaeva, L.V.; Gladyr, S.Y.; Gelissen, A.P.H.; Mergel, O.; Pergushov, D.V.; Kurochkin, I.N.; Plamper, F.A.; Richtering, W. Dual-Stimuli-Sensitive Microgels as a Tool for Stimulated Spongelike Adsorption of Biomaterials for Biosensor Applications. Biomacromolecules 2014, 15, 3735–3745. [Google Scholar] [CrossRef]
- Sigolaeva, L.V.; Mergel, O.; Evtushenko, E.G.; Gladyr, S.Y.; Gelissen, A.P.H.; Pergushov, D.V.; Kurochkin, I.N.; Plamper, F.A.; Richtering, W. Engineering Systems with Spatially Separated Enzymes via Dual-Stimuli-Sensitive Properties of Microgels. Langmuir 2015, 31, 13029–13039. [Google Scholar] [CrossRef]
- Nagase, K.; Kobayashi, J.; Okano, T. Temperature-responsive intelligent interfaces for biomolecular separation and cell sheet engineering. J. R. Soc. Interface 2009, 6, S293–S309. [Google Scholar] [CrossRef]
- Tang, Z.; Akiyama, Y.; Okano, T. Temperature-Responsive Polymer Modified Surface for Cell Sheet Engineering. Polymers 2012, 4, 1478–1498. [Google Scholar] [CrossRef]
- Schmidt, S.; Zeiser, M.; Hellweg, T.; Duschl, C.; Fery, A.; Möhwald, H. Adhesion and mechanical properties of PNIPAM microgel films and their potential use as switchable cell culture substrates. Adv. Funct. Mater. 2010, 20, 3235–3243. [Google Scholar] [CrossRef]
- Nash, M.E.; Carroll, W.M.; Nikoloskya, N.; Yang, R.; Connell, C.O.; Gorelov, A.V.; Dockery, P.; Liptrot, C.; Lyng, F.M.; Garcia, A.; et al. Straightforward, One-Step Fabrication of Ultrathin Thermoresponsive Films from Commercially Available pNIPAm for Cell Culture and Recovery. ACS Appl. Mater. Interfaces 2011, 3, 1980–1990. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.A.; Love, S.A.; Lucero, A.E.; Haynes, C.L.; Canavan, H.E. Effect of Polymer Deposition Method on Thermoresponsive Polymer Films and Resulting Cellular Behavior. Langmuir 2012, 28, 2281–2287. [Google Scholar] [CrossRef]
- Morra, M.; Cassinelli, C. Thermal Recovery of Cells Cultured on Poly(N-Isopropylacrylamide) Surface-Grafted Polystyrene Dishes. In Surface Modification of Polymeric Biomaterials; Springer: Boston, MA, USA, 1996; pp. 175–181. [Google Scholar]
- Pan, Y.V.; Wesley, R.A.; Luginbuhl, R.; Denton, D.D.; Ratner, B.D. Plasma Polymerized N-Isopropylacrylamide: Synthesis and Characterization of a Smart Thermally Responsive Coating. Biomacromolecules 2001, 2, 32–36. [Google Scholar] [CrossRef]
- Takei, Y.G.; Aoki, T.; Sanui, K.; Ogata, N.; Okano, T.; Sakurai, Y. Temperature-responsive bioconjugates. 1. Synthesis of temperature-responsive oligomers with reactive end groups and their coupling to biomolecules. Bioconjug. Chem. 1993, 4, 42–46. [Google Scholar] [CrossRef]
- Kanazawa, H.; Yamamoto, K.; Matsushima, Y.; Takai, N.; Kikuchi, A.; Sakurai, Y.; Okano, T. Temperature-Responsive Chromatography Using Poly(N-isopropylacrylamide)-Modified Silica. Anal. Chem. 1996, 68, 100–105. [Google Scholar] [CrossRef]
- Yakushiji, T.; Sakai, K.; Kikuchi, A.; Aoyagi, T.; Sakurai, Y.; Okano, T. Graft Architectural Effects on Thermoresponsive Wettability Changes of Poly(N-isopropylacrylamide)-Modified Surfaces. Langmuir 1998, 14, 4657–4662. [Google Scholar] [CrossRef]
- Kidoaki, S.; Ohya, S.; Nakayama, Y.; Matsuda, T. Thermoresponsive Structural Change of a Poly(N-isopropylacrylamide) Graft Layer Measured with an Atomic Force Microscope. Langmuir 2001, 17, 2402–2407. [Google Scholar] [CrossRef]
- Tang, Z.; Okano, T. Recent development of temperature-responsive surfaces and their application for cell sheet engineering. Regen. Biomater. 2014, 1, 91–102. [Google Scholar] [CrossRef]
- Nyström, L.; Malmsten, M. Surface-bound microgels—From physicochemical properties to biomedical applications. Adv. Colloid Interface Sci. 2016, 238, 88–104. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, K.; Wegener, T.; He, J.; Zeiser, M.; Bookhold, J.; Dewald, I.; Godino, N.; Jaeger, M.; Hellweg, T.; Fery, A.; et al. Patterned Thermoresponsive Microgel Coatings for Noninvasive Processing of Adherent Cells. Biomacromolecules 2016, 17, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; He, X.; Cao, M.; Wang, X.; Sun, Y.; He, H.; Xu, H.; Lu, J.R. Self-Assembled Two-Dimensional Thermoresponsive Microgel Arrays for Cell Growth/Detachment Control. Biomacromolecules 2014, 15, 4021–4031. [Google Scholar] [CrossRef] [PubMed]
- Sigolaeva, L.; Pergushov, D.; Oelmann, M.; Schwarz, S.; Brugnoni, M.; Kurochkin, I.; Plamper, F.; Fery, A.; Richtering, W. Surface Functionalization by Stimuli-Sensitive Microgels for Effective Enzyme Uptake and Rational Design of Biosensor Setups. Polymers 2018, 10, 791. [Google Scholar] [CrossRef]
- Fu, X.; Hosta-Rigau, L.; Chandrawati, R.; Cui, J. Multi-Stimuli-Responsive Polymer Particles, Films, and Hydrogels for Drug Delivery. Chem 2018, 4, 2084–2107. [Google Scholar] [CrossRef]
- Sun, H.; Kabb, C.P.; Sims, M.B.; Sumerlin, B.S. Architecture-transformable polymers: Reshaping the future of stimuli-responsive polymers. Prog. Polym. Sci. 2019, 89, 61–75. [Google Scholar] [CrossRef]
- Sanzari, I.; Buratti, E.; Huang, R.; Tusan, C.G.; Dinelli, F.; Evans, N.D.; Prodromakis, T.; Bertoldo, M. Poly(N-isopropylacrylamide) based thin microgel films for use in cell culture applications. Sci. Rep. 2020, 10, 6126. [Google Scholar] [CrossRef]
- Pelton, R.H.; Chibante, P. Preparation of aqueous latices with N-isopropylacrylamide. Colloids Surf. 1986, 20, 247–256. [Google Scholar] [CrossRef]
- Xia, X.; Hu, Z. Synthesis and Light Scattering Study of Microgels with Interpenetrating Polymer Networks. Langmuir 2004, 20, 2094–2098. [Google Scholar] [CrossRef]
- Micali, N.; Bertoldo, M.; Buratti, E.; Nigro, V.; Angelini, R.; Villari, V. Interpenetrating Polymer Network Microgels in Water: Effect of Composition on the Structural Properties and Electrosteric Interactions. ChemPhysChem 2018, 19, 1–9. [Google Scholar] [CrossRef]
- Nigro, V.; Ripanti, F.; Angelini, R.; Sarra, A.; Bertoldo, M.; Buratti, E.; Postorino, P.; Ruzicka, B. Molecular mechanisms driving the microgels behaviour: A Raman spectroscopy and dynamic light scattering study. J. Mol. Liq. 2019, 284, 718–724. [Google Scholar] [CrossRef]
- Xiong, W.; Gao, X.; Zhao, Y.; Xu, H.; Yang, X. The dual temperature/pH-sensitive multiphase behavior of poly(N-isopropylacrylamide-co-acrylic acid) microgels for potential application in in situ gelling system. Colloids Surf. B Biointerfaces 2011, 84, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Carpi, F.; Smela, E. Biomedical Applications of Electroactive Polymer Actuators; Carpi, F., Smela, E., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2009; ISBN 9780470744697. [Google Scholar]
- Asaka, K.; Okuzaki, H. Soft Actuators; Asaka, K., Okuzaki, H., Eds.; Springer: Tokyo, Japan, 2014; ISBN 978-4-431-54766-2. [Google Scholar]
- Nigro, V.; Angelini, R.; Rosi, B.; Bertoldo, M.; Buratti, E.; Casciardi, S.; Sennato, S.; Ruzicka, B. Study of network composition in interpenetrating polymer networks of poly(N isopropylacrylamide) microgels: The role of poly(acrylic acid). J. Colloid Interface Sci. 2019, 545, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Wang, C.; Yan, J.; Zhang, L.; Li, L.; Zha, L. pH/temperature dual stimuli-responsive microcapsules with interpenetrating polymer network structure. Colloid Polym. Sci. 2010, 288, 1723–1729. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, G.; Zou, L.; Tang, L.; Marquez, M.; Hu, Z. Viscoelastic behavior and in vivo release study of microgel dispersions with inverse thermoreversible gelation. Biomacromolecules 2008, 9, 142–148. [Google Scholar] [CrossRef]
- Liu, X.; Guo, H.; Zha, L. Study of pH/temperature dual stimuli-responsive nanogels with interpenetrating polymer network structure. Polym. Int. 2012, 61, 1144–1150. [Google Scholar] [CrossRef]
- Nigro, V.; Ruzicka, B.; Ruta, B.; Zontone, F.; Bertoldo, M.; Buratti, E.; Angelini, R. Relaxation Dynamics, Softness, and Fragility of Microgels with Interpenetrated Polymer Networks. Macromolecules 2020, 53, 1596–1603. [Google Scholar] [CrossRef]
- Burmistrova, A.; von Klitzing, R. Control of number density and swelling/shrinking behavior of P(NIPAM–AAc) particles at solid surfaces. J. Mater. Chem. 2010, 20, 3502. [Google Scholar] [CrossRef]
- Cutright, C.; Brotherton, Z.; Alexander, L.; Harris, J.; Shi, K.; Khan, S.; Genzer, J.; Menegatti, S. Packing density, homogeneity, and regularity: Quantitative correlations between topology and thermoresponsive morphology of PNIPAM-co-PAA microgel coatings. Appl. Surf. Sci. 2020, 508, 145129. [Google Scholar] [CrossRef]
- Seeber, M.; Zdyrko, B.; Burtovvy, R.; Andrukh, T.; Tsai, C.C.; Owens, J.R.; Kornev, K.G.; Luzinov, I. Surface grafting of thermoresponsive microgel nanoparticles. Soft Matter 2011, 7, 9962–9971. [Google Scholar] [CrossRef]
- Tsuji, S.; Kawaguchi, H. Self-Assembly of Poly( N-isopropylacrylamide)-Carrying Microspheres into Two-Dimensional Colloidal Arrays. Langmuir 2005, 21, 2434–2437. [Google Scholar] [CrossRef]
- Serpe, M.J.; Lyon, L.A. Optical and Acoustic Studies of pH-Dependent Swelling in Microgel Thin Films. Chem. Mater. 2004, 16, 4373–4380. [Google Scholar] [CrossRef]
- Nolan, C.M.; Serpe, M.J.; Lyon, L.A. Thermally Modulated Insulin Release from Microgel Thin Films. Biomacromolecules 2004, 5, 1940–1946. [Google Scholar] [CrossRef] [PubMed]
- Serpe, M.J.; Jones, C.D.; Lyon, L.A. Layer-by-Layer Deposition of Thermoresponsive Microgel Thin Films. Langmuir 2003, 19, 8759–8764. [Google Scholar] [CrossRef]
- Schmidt, S.; Hellweg, T.; Von Klitzing, R. Packing density control in P(NIPAM-Co-AAc) microgel monolayers: Effect of surface charge, pH, and preparation technique. Langmuir 2008, 24, 12595–12602. [Google Scholar] [CrossRef]
- Schmidt, S.; Motschmann, H.; Hellweg, T.; von Klitzing, R. Thermoresponsive surfaces by spin-coating of PNIPAM-co-PAA microgels: A combined AFM and ellipsometry study. Polymer 2008, 49, 749–756. [Google Scholar] [CrossRef]
- Nerapusri, V.; Keddie, J.L.; Vincent, B.; Bushnak, I.A. Swelling and Deswelling of Adsorbed Microgel Monolayers Triggered by Changes in Temperature, pH, and Electrolyte Concentration. Langmuir 2006, 22, 5036–5041. [Google Scholar] [CrossRef]
- Sechi, A.; Freitas, J.M.G.; Wünnemann, P.; Töpel, A.; Paschoalin, R.T.; Ullmann, S.; Schröder, R.; Aydin, G.; Rütten, S.; Böker, A.; et al. Surface-Grafted Nanogel Arrays Direct Cell Adhesion and Motility. Adv. Mater. Interfaces 2016, 3, 1–13. [Google Scholar] [CrossRef]
- Xia, Y.; Tang, Y.; He, X.; Pan, F.; Li, Z.; Xu, H.; Lu, J.R. Patterned Thermoresponsive Microgel Surfaces to Control Cell Detachment. Biomacromolecules 2016, 17, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Bluestein, B.M.; Reed, J.A.; Canavan, H.E. Effect of substrate storage conditions on the stability of “Smart” films used for mammalian cell applications. Appl. Surf. Sci. 2017, 392, 950–959. [Google Scholar] [CrossRef]
- Tang, D.; Zeng, Z.; Xia, Y.; Chen, B.; Gao, S.; Cao, M.; Wang, S.; Li, D. The effects of thermoresponsive microgel density on cell adhesion, proliferation, and detachment. J. Appl. Polym. Sci. 2020, 137, 1–7. [Google Scholar] [CrossRef]
- Kunz, S.; Pawlik, M.; Schärtl, W.; Seiffert, S. Polymer- vs. colloidal-type viscoelastic mechanics of microgel pastes. Colloid Polym. Sci. 2018, 296, 1341–1352. [Google Scholar] [CrossRef]
- Sanzari, I.; Humphrey, E.J.; Dinelli, F.; Terracciano, C.M.; Prodromakis, T. Effect of patterned polyacrylamide hydrogel on morphology and orientation of cultured NRVMs. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Scotti, A.; Bochenek, S.; Brugnoni, M.; Fernandez-Rodriguez, M.A.; Schulte, M.F.; Houston, J.E.; Gelissen, A.P.H.; Potemkin, I.I.; Isa, L.; Richtering, W. Exploring the colloid-to-polymer transition for ultra-low crosslinked microgels from three to two dimensions. Nat. Commun. 2019, 10, 1418. [Google Scholar] [CrossRef]
- Daoud, M.; Cotton, J.P.; Farnoux, B.; Jannink, G.; Sarma, G.; Benoit, H.; Duplessix, C.; Picot, C.; de Gennes, P.G. Solutions of Flexible Polymers. Neutron Experiments and Interpretation. Macromolecules 1975, 8, 804–818. [Google Scholar] [CrossRef]
- Whitelam, S.; Tamblyn, I.; Haxton, T.K.; Wieland, M.B.; Champness, N.R.; Garrahan, J.P.; Beton, P.H. Common physical framework explains phase behavior and dynamics of atomic, molecular, and polymeric network formers. Phys. Rev. X 2014, 4, 1–12. [Google Scholar] [CrossRef]
- Conley, G.M.; Aebischer, P.; Nöjd, S.; Schurtenberger, P.; Scheffold, F. Jamming and overpacking fuzzy microgels: Deformation, interpenetration, and compression. Sci. Adv. 2017, 3, 1–8. [Google Scholar] [CrossRef]
- Schulte, M.F.; Scotti, A.; Brugnoni, M.; Bochenek, S.; Mourran, A.; Richtering, W. Tuning the Structure and Properties of Ultra-Low Cross-Linked Temperature-Sensitive Microgels at Interfaces via the Adsorption Pathway. Langmuir 2019, 35, 14769–14781. [Google Scholar] [CrossRef]
- Kyrey, T.; Witte, J.; Pipich, V.; Feoktystov, A.; Koutsioubas, A.; Vezhlev, E.; Frielinghaus, H.; von Klitzing, R.; Wellert, S.; Holderer, O. Influence of the cross-linker content on adsorbed functionalised microgel coatings. Polymer 2019, 169, 29–35. [Google Scholar] [CrossRef]
- Danglad-Flores, J.; Eftekhari, K.; Skirtach, A.G.; Riegler, H. Controlled Deposition of Nanosize and Microsize Particles by Spin-Casting. Langmuir 2019, 35, 3404–3412. [Google Scholar] [CrossRef]
- Emslie, A.G.; Bonner, F.T.; Peck, L.G. Flow of a Viscous Liquid on a Rotating Disk. J. Appl. Phys. 1958, 29, 858–862. [Google Scholar] [CrossRef]
- Nitta, S.V.; Jain, A.; Wayner, P.C.; Gill, W.N.; Plawsky, J.L. Effect of sol rheology on the uniformity of spin-on silica xerogel films. J. Appl. Phys. 1999, 86, 5870–5878. [Google Scholar] [CrossRef]
- Peeters, T.; Remoortere, B.V. Parameters of the spin coating process. J. Appl. Polym. Sci. 2008, 12, 234–239. [Google Scholar]
- Tyona, M.D. A comprehensive study of spin coating as a thin film deposition technique and spin coating equipment. Adv. Mater. Res. 2013, 2, 181–193. [Google Scholar] [CrossRef]
- Hau, W.L.W.; Trau, D.W.; Sucher, N.J.; Wong, M.; Zohar, Y. Surface-chemistry technology for microfluidics. J. Micromech. Microeng. 2003, 13, 272–278. [Google Scholar] [CrossRef]
- Han, Y.; Mayer, D.; Offenhäusser, A.; Ingebrandt, S. Surface activation of thin silicon oxides by wet cleaning and silanization. Thin Solid Films 2006, 510, 175–180. [Google Scholar] [CrossRef]
- Gilcreest, V.P.; Carroll, W.M.; Rochev, Y.A.; Blute, I.; Dawson, K.A.; Gorelov, A.V. Thermoresponsive Poly( N-isopropylacrylamide) Copolymers: Contact Angles and Surface Energies of Polymer Films. Langmuir 2004, 20, 10138–10145. [Google Scholar] [CrossRef]
- Microgel Suspensions: Fundamentals and Applications; Fernandez-Nieves, A., Wyss, H.M., Mattsson, J., Weitz, D.A., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; ISBN 9783527632992. [Google Scholar]
- Swift, T.; Swanson, L.; Geoghegan, M.; Rimmer, S. The pH-responsive behaviour of poly(acrylic acid) in aqueous solution is dependent on molar mass. Soft Matter 2016, 12, 2542–2549. [Google Scholar] [CrossRef]
- Franco, S.; Ruzicka, B.; Buratti, E.; Nigro, V.; Zoratto, N.; Matricardi, P.; Zaccarelli, E.; Angelini, R. Volume fraction determination of microgel composed of interpenetrating polymer networks of PNIPAM and polyacrylic acid. J. Phys. Cond. Matt. 2020. Submitted. [Google Scholar]
- Franco, S.; Buratti, E.; Ruzicka, B.; Angelini, R. Unpublished data.
- Scotti, A.; Brugnoni, M.; Lopez, C.G.; Bochenek, S.; Crassous, J.J.; Richtering, W. Flow properties reveal the particle-to-polymer transition of ultra-low crosslinked microgels. Soft Matter 2020, 16, 668–678. [Google Scholar] [CrossRef]
- Varga, I.; Kardos, A.; Borsos, A.; Gilányi, T. Effect of internal charge distribution on the electrophoretic mobility of poly(N-isopropylacrylamide) based core-shell microgel particles. J. Mol. Liq. 2020, 302, 111979. [Google Scholar] [CrossRef]






| Diameter (nm) at 20 °C | Swelling Ratio D20 °C/D40 °C | BIS* (wt%) | PAAc* (wt%) | |
|---|---|---|---|---|
| PNIPAm a | 91 ± 2 | 2.1 | 1.4% | 0% |
| PNIPAm b | 558 ± 24 | 2.2 | 1.3% | 0% |
| IPN | 254 ± 12 | 1.3 | 4.9% | 19.2% |
| Substrate | Coating Stability | |||||||
|---|---|---|---|---|---|---|---|---|
| PNIPAm a Wash 20 °C | PNIPAm a Wash 50 °C | PNIPAm b Wash 20 °C | PNIPAm b Wash 50 °C | IPN wash 20 °C | IPN Wash 50 °C | |||
| glass-NaOH | Yes | No | Yes | No | pH3 Yes | pH5 No | pH7 No | No |
| glass-TMSPMA | No | Yes | Yes | Yes | No | Yes | ||
| glass-TMOS | No | Yes | Yes | Yes | No | Yes | ||
| Surface | Contact Angle |
|---|---|
| Glass | 47.3 ± 0.7 |
| Glass treated with Piranha | <10° |
| glass-NaOH | 34.5 ± 1.9 |
| glass-TMSPMA | 70.2 ± 4.1 |
| glass-TMOS | 100.6 ± 0.9 |
| Qsurface × 1020 (C/nm2) 25 °C | Qsurface × 1020 (C/nm2) 38 °C | |
|---|---|---|
| PNIPAm a | −0.74 | −2.1 |
| PNIPAm b | −0.18 | −0.36 |
| IPN at pH 5.5 | −13 | −24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buratti, E.; Sanzari, I.; Dinelli, F.; Prodromakis, T.; Bertoldo, M. Formation and Stability of Smooth Thin Films with Soft Microgels Made of Poly(N-Isopropylacrylamide) and Poly(Acrylic Acid). Polymers 2020, 12, 2638. https://doi.org/10.3390/polym12112638
Buratti E, Sanzari I, Dinelli F, Prodromakis T, Bertoldo M. Formation and Stability of Smooth Thin Films with Soft Microgels Made of Poly(N-Isopropylacrylamide) and Poly(Acrylic Acid). Polymers. 2020; 12(11):2638. https://doi.org/10.3390/polym12112638
Chicago/Turabian StyleBuratti, Elena, Ilaria Sanzari, Franco Dinelli, Themistoklis Prodromakis, and Monica Bertoldo. 2020. "Formation and Stability of Smooth Thin Films with Soft Microgels Made of Poly(N-Isopropylacrylamide) and Poly(Acrylic Acid)" Polymers 12, no. 11: 2638. https://doi.org/10.3390/polym12112638
APA StyleBuratti, E., Sanzari, I., Dinelli, F., Prodromakis, T., & Bertoldo, M. (2020). Formation and Stability of Smooth Thin Films with Soft Microgels Made of Poly(N-Isopropylacrylamide) and Poly(Acrylic Acid). Polymers, 12(11), 2638. https://doi.org/10.3390/polym12112638









