Cation Effect in the Corrosion Inhibition Properties of Coumarate Ionic Liquids and Acrylic UV-Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Monomer Synthesis
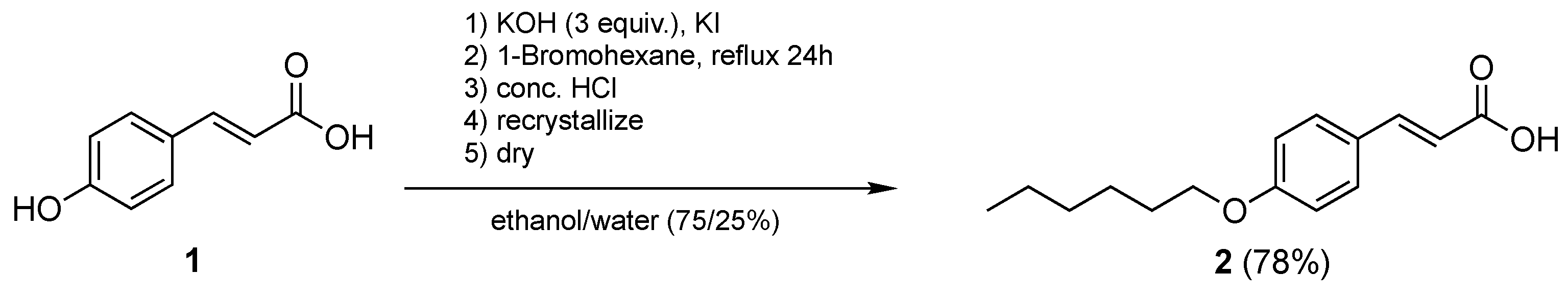
2.2.1. Synthesis of (E)-3-(4-(Hexyloxy)Phenyl)Acrylic Acid, 2
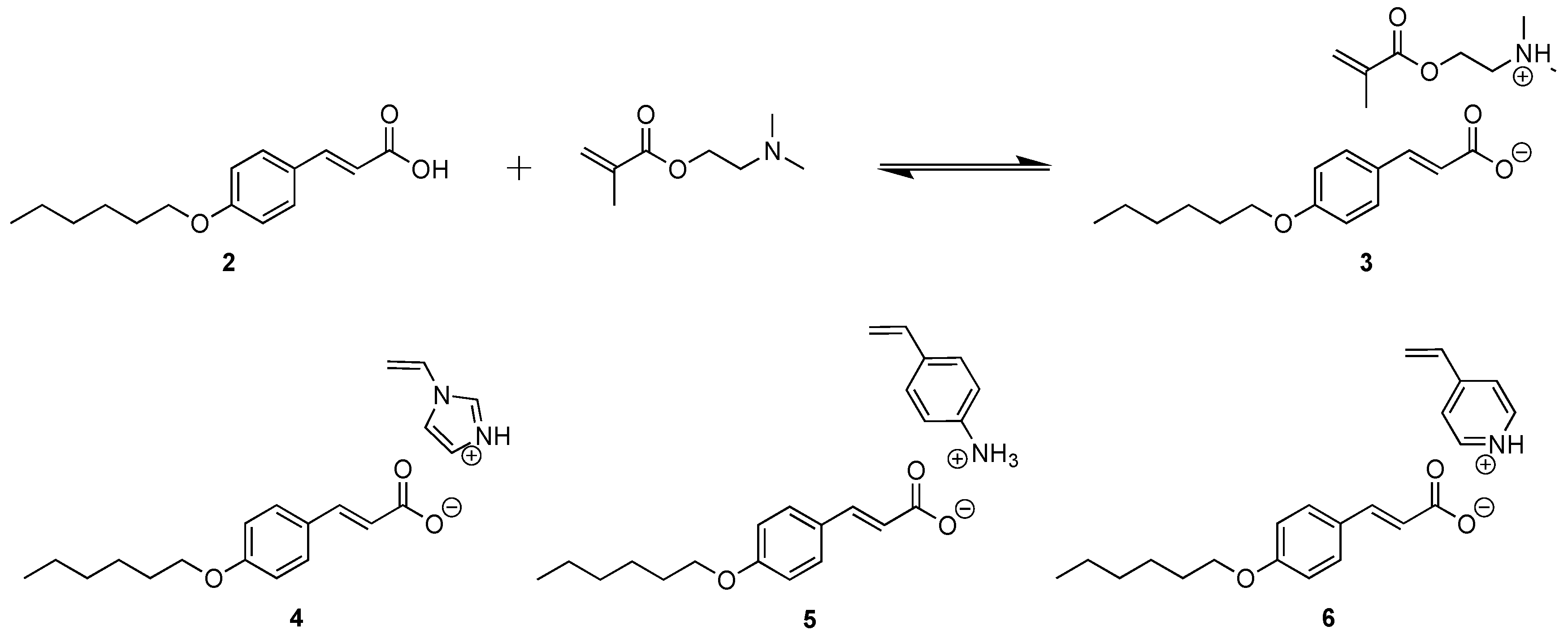
2.2.2. Synthesis of 2-(Dimethyl Ammonium)Ethyl Methacrylate p-Hexoxy Coumarate 3, [DMAEM+ HexOCou-]
2.2.3. Synthesis of 4-Vinylimidazolium p-Hexoxy Coumarate 4, [VIm+ HexOCou-]
2.2.4. Synthesis of 4-Vinylanilinium p-Hexoxy Coumarate 5, [VAn+ HexOCou-]
2.2.5. Synthesis of 4-Vinylpyridinium p-Hexoxy Coumarate 6, [VPy+ HexCou-]
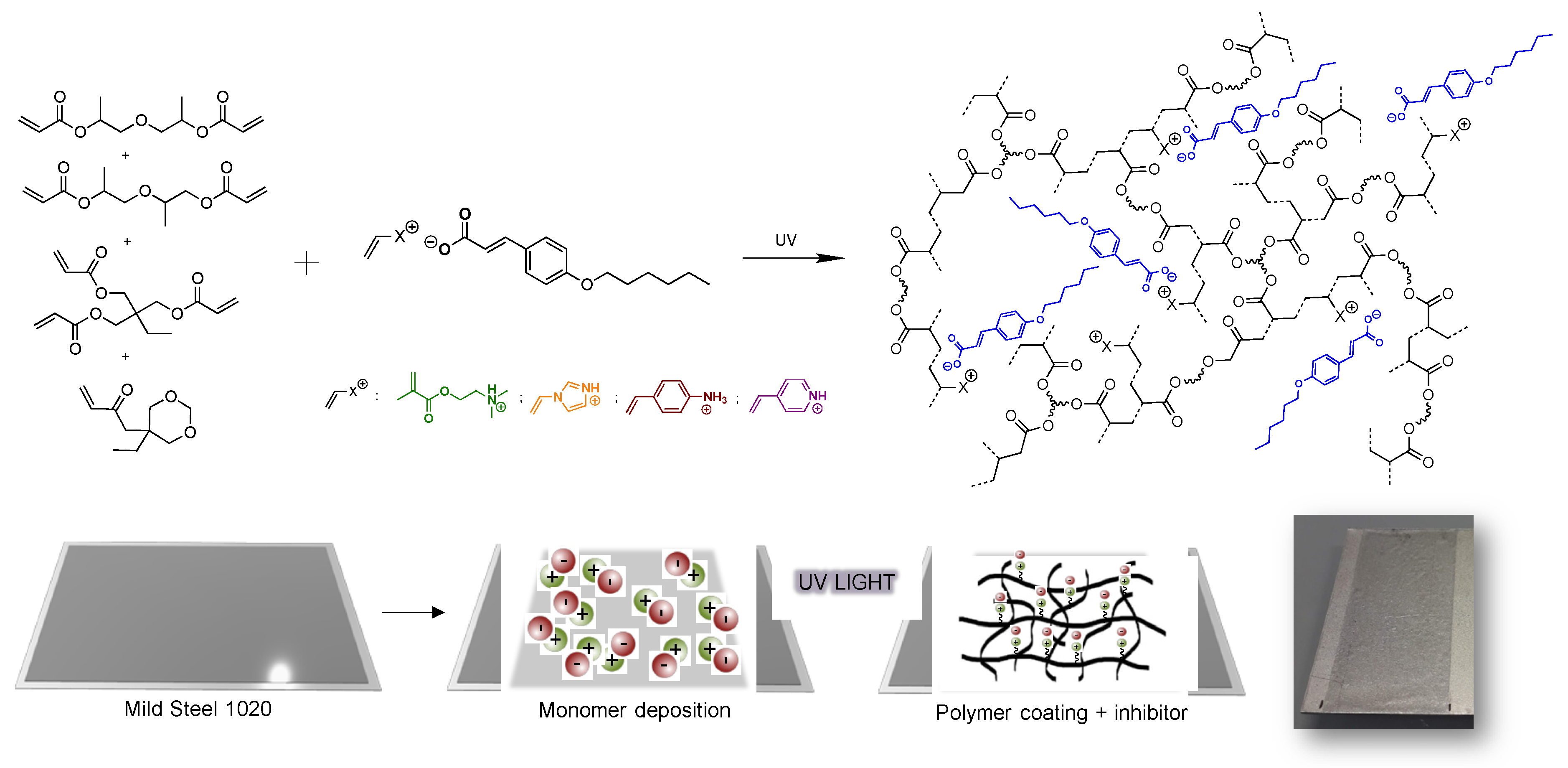
2.2.6. Preparation of the UV-Polymer Coatings
2.3. Characterization Methods
2.3.1. Nuclear Magnetic Resonance (NMR)
2.3.2. Attenuated Total Reflectance–Fourier Transport Infrared Spectroscopy (ATR-FTIR)
2.3.3. Potentiodynamic Polarization (PP) Experiments
- Corrosion current density (icorr) and corrosion potential (Ecorr)
2.3.4. Immersion Test
2.3.5. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-Ray Spectroscopy (EDS)
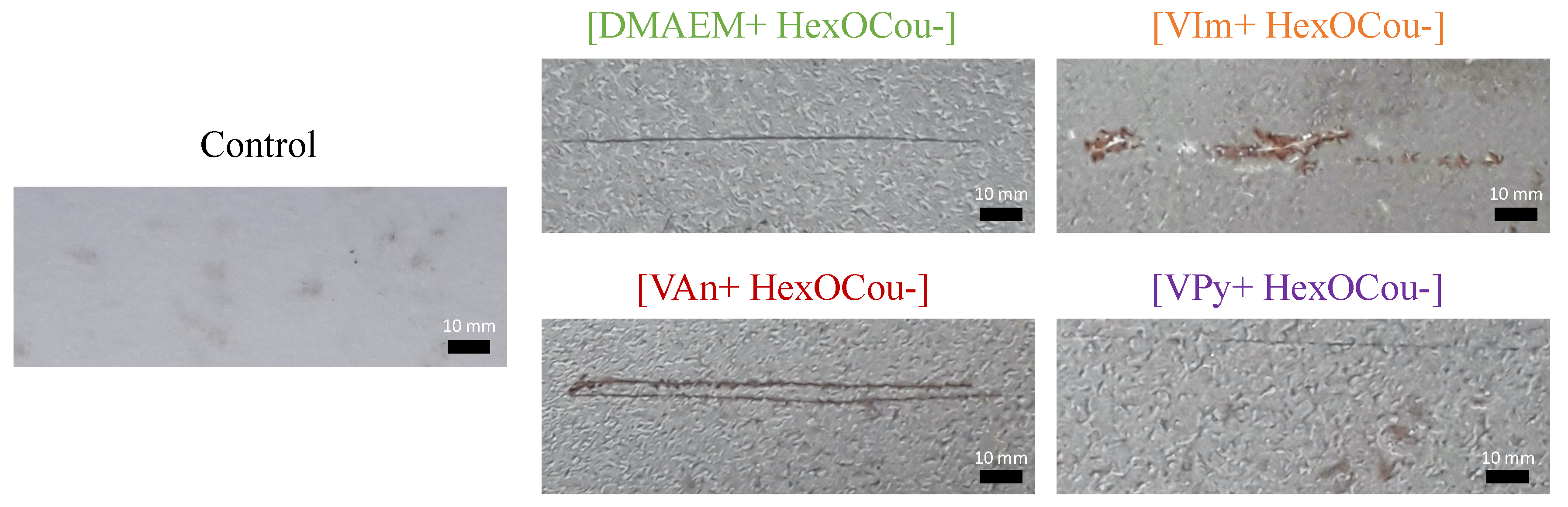
2.3.6. Scribe Test
2.3.7. Water Uptake of the Polymer Coatings
3. Results and Discussion
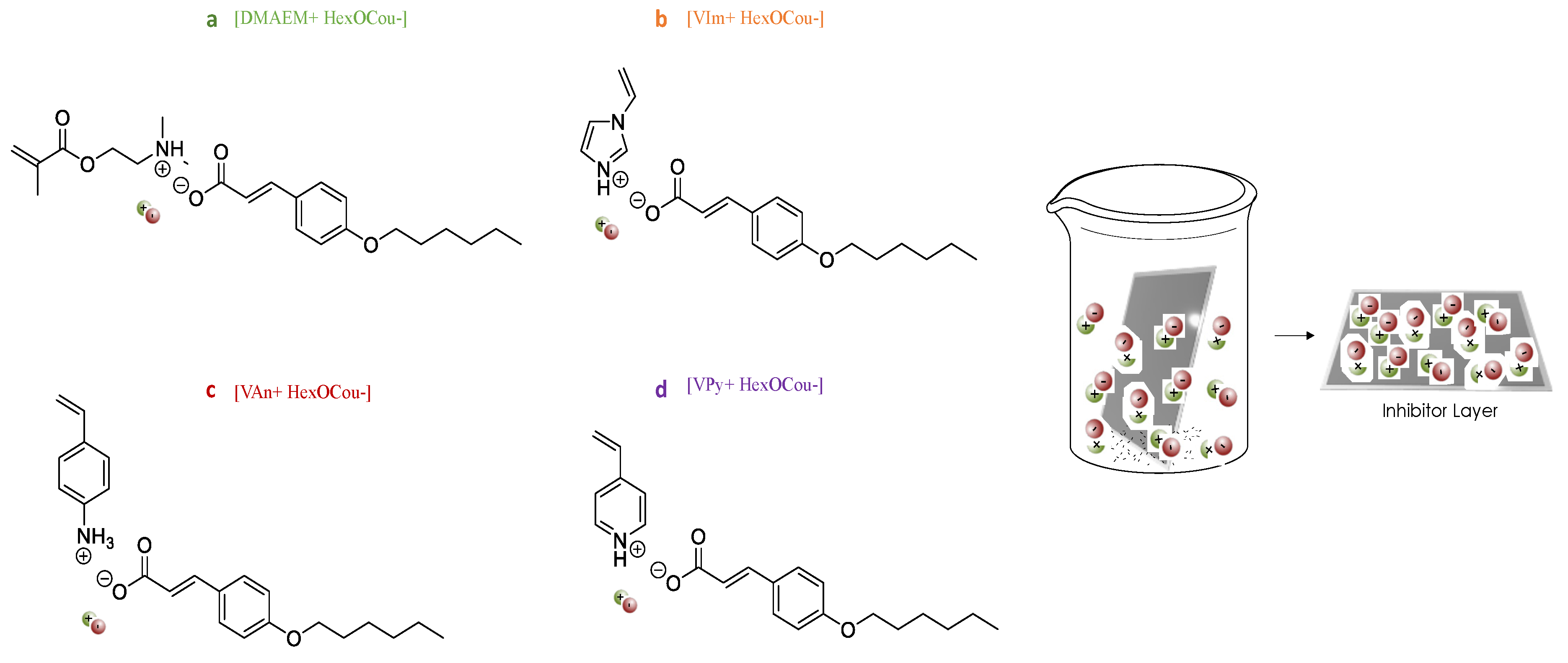
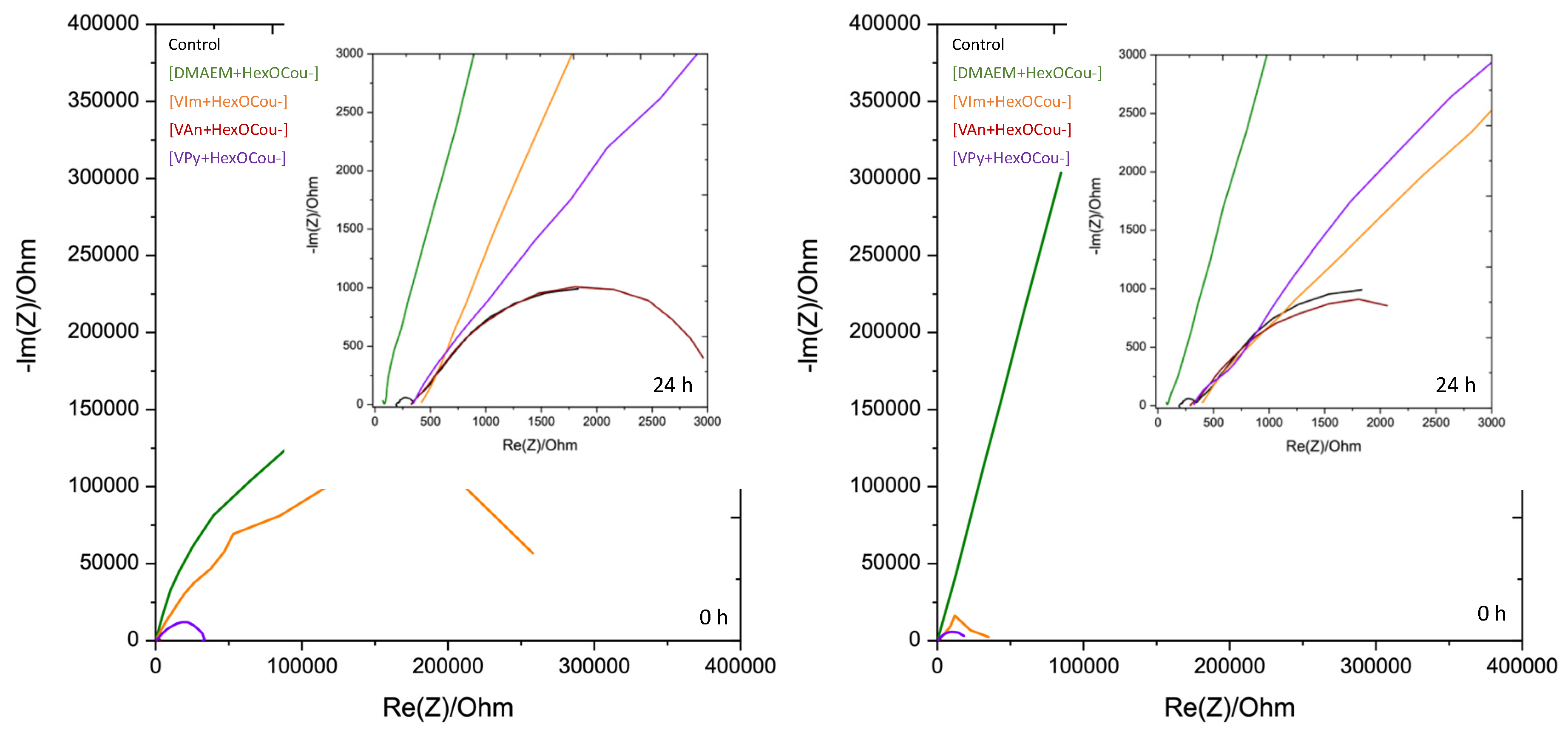
3.1. Mild Steel Corrosion Inhibition Properties of p-Hexoxy Coumarate Based Monomeric Ionic Liquids
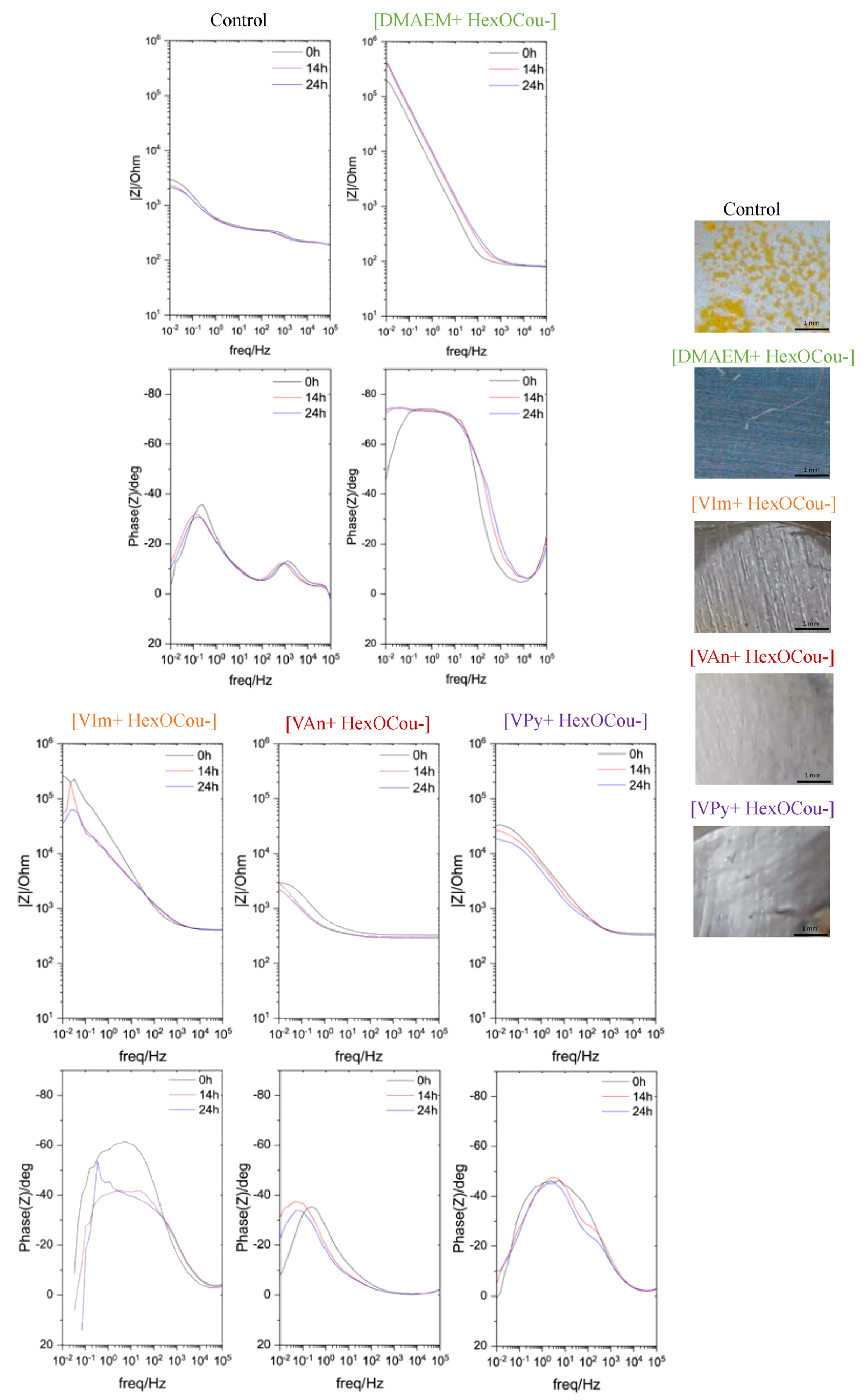
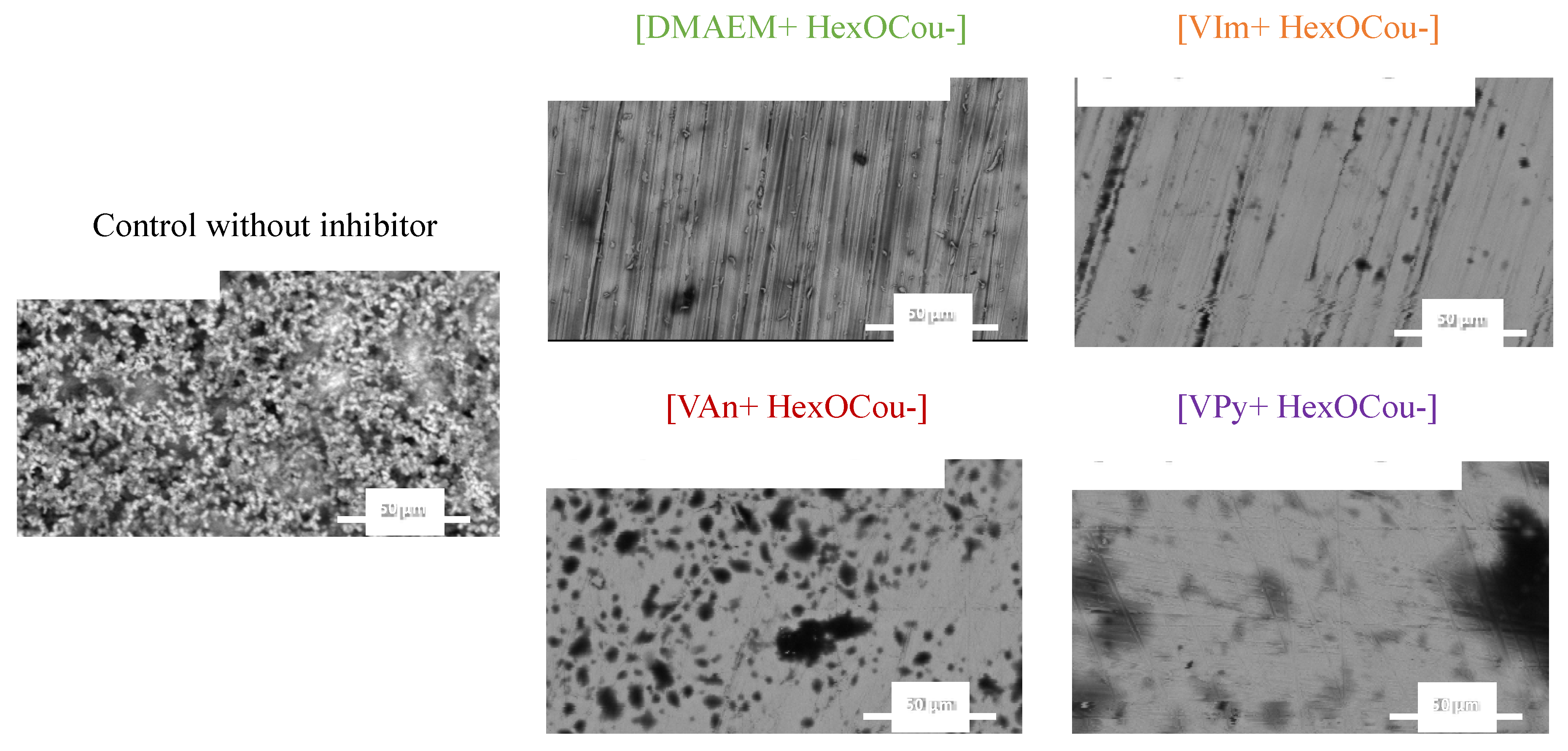
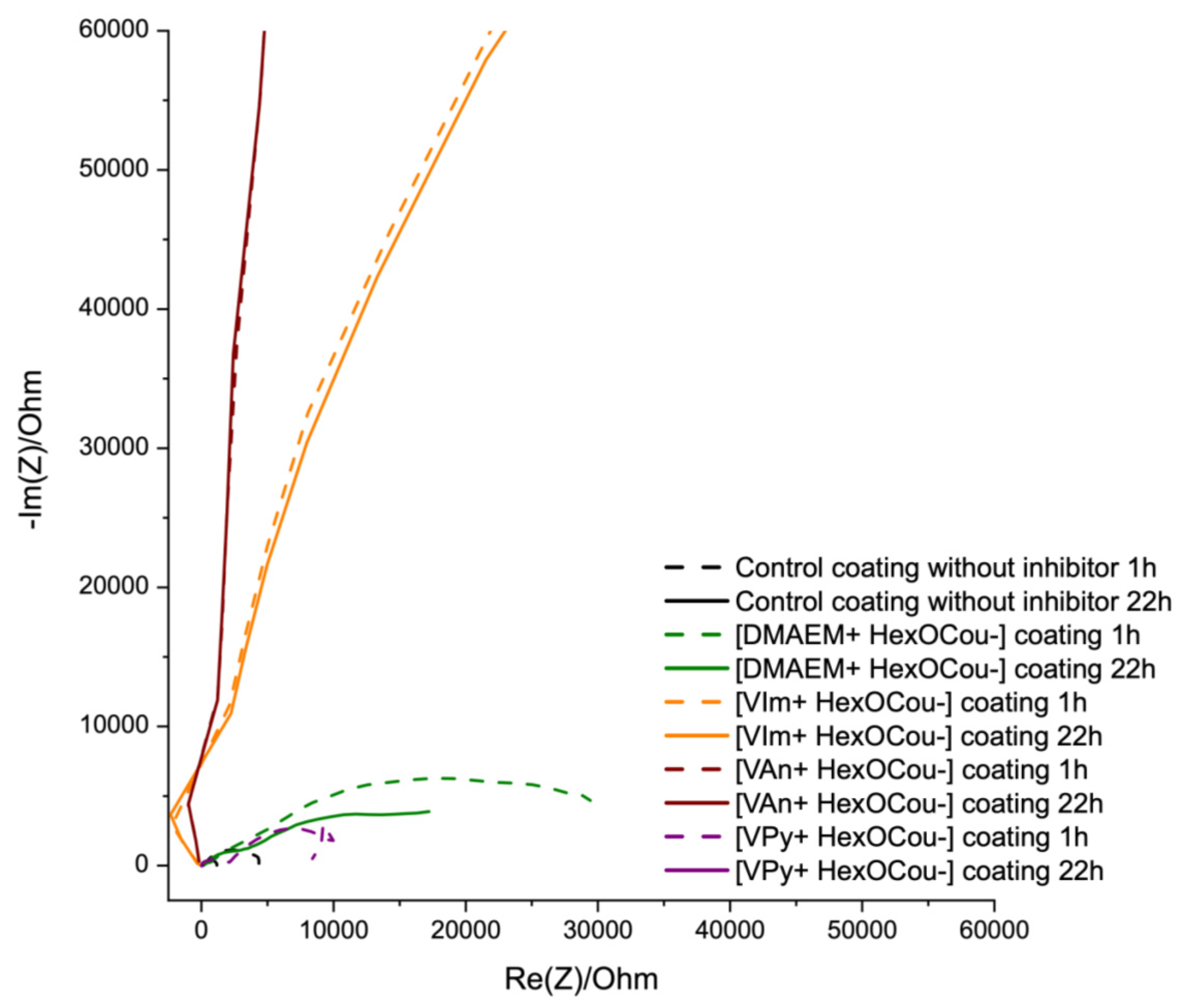
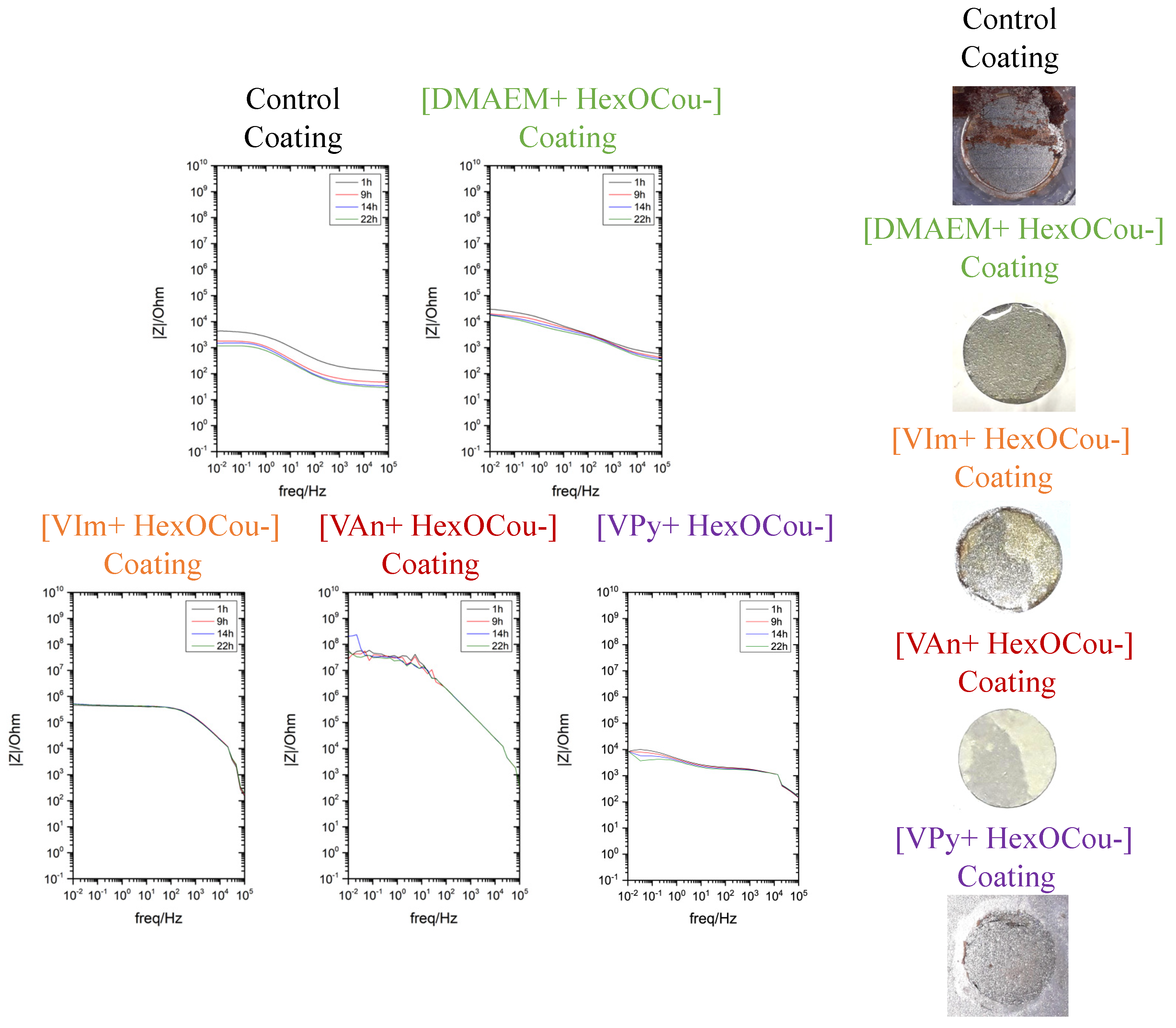
3.2. Covalent Incorporation of p-Hexoxy Coumarate Ionic Liquid Monomers into Acrylic Polymer Coatings by UV-Photopolymerization
Scribe Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Catubig, R.; Seter, M.; Neil, W.; Forsyth, M.; Hinton, B.R.W. Effects of corrosion inhibiting pigment lanthanum 4-hydroxy cinnamate on the filiform corrosion of coated steel. J. Electrochem. Soc. 2011, 158, C353. [Google Scholar] [CrossRef]
- McCafferty, E. Introduction to Corrosion Science; Springer: Berlin/Heidelberg, Germany, 2010; Volume 1, pp. 1–11. [Google Scholar]
- Sinko, J. Challenges of chromate inhibitor pigments replacement in organic coatings. Prog. Org. Coat. 2001, 42, 267–282. [Google Scholar] [CrossRef]
- Wilbur, S.; Abadin, H.; Fay, M.; Yu, D.; Tencza, B.; Ingerman, L.; Klotzbach, J.; James, S. Toxicological Profile for Chromium. Agency Toxic Subst. Dis. Reg. 2012, 1, 1–398. [Google Scholar]
- Anatas, P.T.; Williamson, T.C. Designing Chemistry for the Environment. J. Am. Chem. Soc. 1996, 118, 10945. [Google Scholar]
- Frey, M.; Harris, S.; Holmes, J.; Nation, D.; Parsons, S.; Tasker, P.; Teat, S.; Winpenny, R. Modeling Surface Engineering: Use of Polymetallic Iron Cages and Computer Graphics To Understand the Mode of Action of a Corrosion Inhibitor. Angew. Chem. Int. Ed. 1998, 37, 3245–3248. [Google Scholar] [CrossRef]
- Frey, M.; Harris, S.; Holmes, J.; Nation, D.; Parsons, S.; Tasker, P.; Winpenny, R. Elucidating the Mode of Action of a Corrosion Inhibitor for Iron. Chem. Eur. J. 2000, 6, 1407–1415. [Google Scholar] [CrossRef]
- Shawky, M.H.; Moussa, M.N.; Taha, F.I.M.; Fouda, A.S. The effect of some B-diketo compounds on the retardation of aluminium dissolution in HCl. Corros. Sci. 1981, 21, 439. [Google Scholar]
- Seter, M.; Hinton, B.; Forsyth, M. Understanding speciation of lanthanum 4-hydroxy cinnamate and its impact on the corrosion inhibition mechanism for AS1020 steel. J. Electrochem. Soc. 2012, 159, C181–C189. [Google Scholar] [CrossRef]
- Brycki, B.; Kowalczyk, I.; Szulc, A.; Kaczerewska, O.; Pakiet, M. Organic Corrosion Inhibitors. In Corrosion Inhibitors, Principles and Recent Applications; IntechOpen: Rijeka, Croatia, 2018; Volume 1, pp. 3–33. [Google Scholar]
- Chong, A.; Forsyth, M.; MacFarlane, D. Novel imidazolinium ionic liquids and organic salts. Electrochim. Acta 2015, 159, 219–226. [Google Scholar] [CrossRef]
- Tawfik, S. Ionic liquids based gemini cationic surfactants as corrosion inhibitors for carbon steel in hydrochloric acid solution. J. Mol. Liq. 2016, 216, 624–635. [Google Scholar] [CrossRef]
- Peng, Y.; Hughes, A.; Deacon, G.; Junk, P.; Hinton, B.; Forsyth, M.; Mardel, J.; Somers, A. A study of rare-earth 3-(4-methylbenzoyl)-propanoate compounds as corrosion inhibitors for AS1020 mild steel in NaCl solutions. Corros. Sci. 2018, 145, 199–211. [Google Scholar] [CrossRef]
- Mercer, A. Proceedings of the 5th European Symposium on Corrosion Inhibition; Ann. Univ.: Ferrara, Italy, 1980; Volume 2, pp. 564–581. [Google Scholar]
- Riggs, O. Corrosion Inhibitors; NACE International: Houston, TX, USA, 1973; Volume 7. [Google Scholar]
- Wilcox, G.; Gabe, D.; Warwick, M. The role of molibdates in corrosion prevention. Corros. Rev. 1986, 6, 327–365. [Google Scholar]
- Blin, F.; Koutsoukos, P.; Klepetsianis, P.; Forsyth, M. The corrosion inhibition mechanism of new rare earth cinnamate compounds—Electrochemical studies. Electrochim. Acta 2007, 52, 6212–6220. [Google Scholar] [CrossRef]
- Hosseini, M.; Hamdy Makhlouf, A.S. Industrial Applications for Intelligent Polymers and Coatings; Springer: New York, NY, USA, 2016; Volume 1, pp. 1–710. [Google Scholar]
- Sørensen, P.; Kiil, S.; Dam-Johansen, K.; Weinell, C. Anticorrosive coatings: A review. J. Coat. Technol. Res. 2009, 6, 135–176. [Google Scholar] [CrossRef]
- Yuan, J.; Mecerreyes, D.; Antonietti, M. Poly(ionic liquid)s: An update. Prog. Polym. Sci. 2013, 38, 1009–1036. [Google Scholar] [CrossRef]
- Anastro, A.; Lago, N.; Berlanga, C.; Galcerán, M.; Hilder, M.; Forsyth, M.; Mecerreyes, D. Poly(ionic liquid) iongel membranes for all solid-state rechargeable sodium-ion battery. J. Membr. Sci. 2019, 582, 435–441. [Google Scholar] [CrossRef]
- Isik, M.; Porcarelli, L.; Lago, N.; Zhu, H.; Forsyth, M.; Mecerreyes, D. Proton Conducting Membranes Based on Poly(Ionic Liquids) Having Phosphonium Counter-Cations. Macromol. Rapid Commun. 2017, 39, 1700627. [Google Scholar] [CrossRef] [PubMed]
- Isik, M.; Gracia, R.; Kollnus, L.; Tomé, L.; Marrucho, I.; Mecerreyes, D. Cholinium-Based Poly(ionic liquid)s: Synthesis, Characterization, and Application as Biocompatible Ion Gels and Cellulose Coatings. ACS Macro Lett. 2013, 2, 975–979. [Google Scholar] [CrossRef]
- Isik, M.; Fernandes, A.; Kari, V.; Paulis, M.; Mecerreyes, D. Preparation of poly(ionic liquid) nanoparticles and their novel application as flocculants for water purification. Polym. Chem. 2016, 7, 1668–1674. [Google Scholar] [CrossRef]
- Udabe, E.; Forsyth, M.; Somers, A.; Mecerreyes, D. Metal-free coumarate based ionic liquids and poly(ionic liquid)s as corrosion inhibitors. Mater. Adv. 2020, 1, 584–589. [Google Scholar] [CrossRef]
- Chong, A.L.; Mardel, J.I.; MacFarlane, D.R.; Forsyth, M.; Somers, A.E. Synergistic Corrosion Inhibition of Mild Steel in Aqueous Chloride Solutions by an Imidazolinium Carboxylate Salt. ACS Sustain. Chem. Eng. 2016, 4, 1746–1755. [Google Scholar] [CrossRef]
- Myrdal, R. Corrosion Inhibitors—State of the Art; COIN Project: Oslo, Norway, 2010. [Google Scholar]
- Schweitzer, P.A. Corrosion inhibitors. In Corrosion and Corrosion Protection Handbook; Routledge: Monticello, NY, USA, 2017; Volume 2, pp. 47–52. [Google Scholar] [CrossRef]
- Al-Mayouf, A.M.; Al-Suhybani, A.A.; Al-Ameery, A.K. Corrosion inhibition of 304SS in sulfuric acid solutions by 2-methyl benzoazole derivatives. Desalination 1998, 116, 25–33. [Google Scholar] [CrossRef]
- Gallegos-Melgar, A.; Serna, S.A.; Lázaro, I.; Gutiérrez-Castañeda, E.-J.; Mercado-Lemus, V.H.; Arcos-Gutierrez, H.; Hernández-Hernández, M.; Porcayo-Calderón, J.; Mayen, J.; Monroy, M.D.A. Potentiodynamic Polarization Performance of a Novel Composite Coating System of Al2O3/Chitosan-Sodium Alginate, Applied on an Aluminum AA6063 Alloy for Protection in a Chloride Ions Environment. Coatings 2020, 10, 45. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udabe, E.; Sommers, A.; Forsyth, M.; Mecerreyes, D. Cation Effect in the Corrosion Inhibition Properties of Coumarate Ionic Liquids and Acrylic UV-Coatings. Polymers 2020, 12, 2611. https://doi.org/10.3390/polym12112611
Udabe E, Sommers A, Forsyth M, Mecerreyes D. Cation Effect in the Corrosion Inhibition Properties of Coumarate Ionic Liquids and Acrylic UV-Coatings. Polymers. 2020; 12(11):2611. https://doi.org/10.3390/polym12112611
Chicago/Turabian StyleUdabe, Esther, Anthony Sommers, Maria Forsyth, and David Mecerreyes. 2020. "Cation Effect in the Corrosion Inhibition Properties of Coumarate Ionic Liquids and Acrylic UV-Coatings" Polymers 12, no. 11: 2611. https://doi.org/10.3390/polym12112611
APA StyleUdabe, E., Sommers, A., Forsyth, M., & Mecerreyes, D. (2020). Cation Effect in the Corrosion Inhibition Properties of Coumarate Ionic Liquids and Acrylic UV-Coatings. Polymers, 12(11), 2611. https://doi.org/10.3390/polym12112611







