Thermal and Structural Analysis of Epoxidized Jatropha Oil and Alkaline Treated Kenaf Fiber Reinforced Poly(Lactic Acid) Biocomposites
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Epoxidation of Jatropha Oil
2.3. Alkaline Treatment of Kenaf Fiber
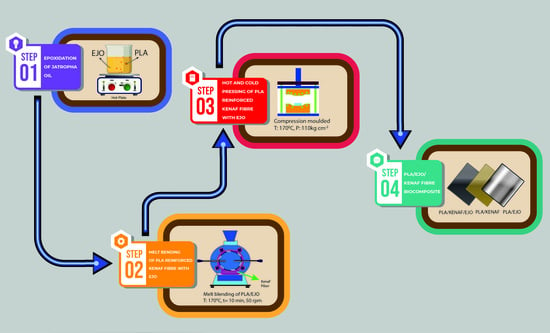
2.4. Preparation of Biocomposites
2.5. Kenaf Fiber Analysis
2.6. Biocomposites Analysis
3. Results and Discussion
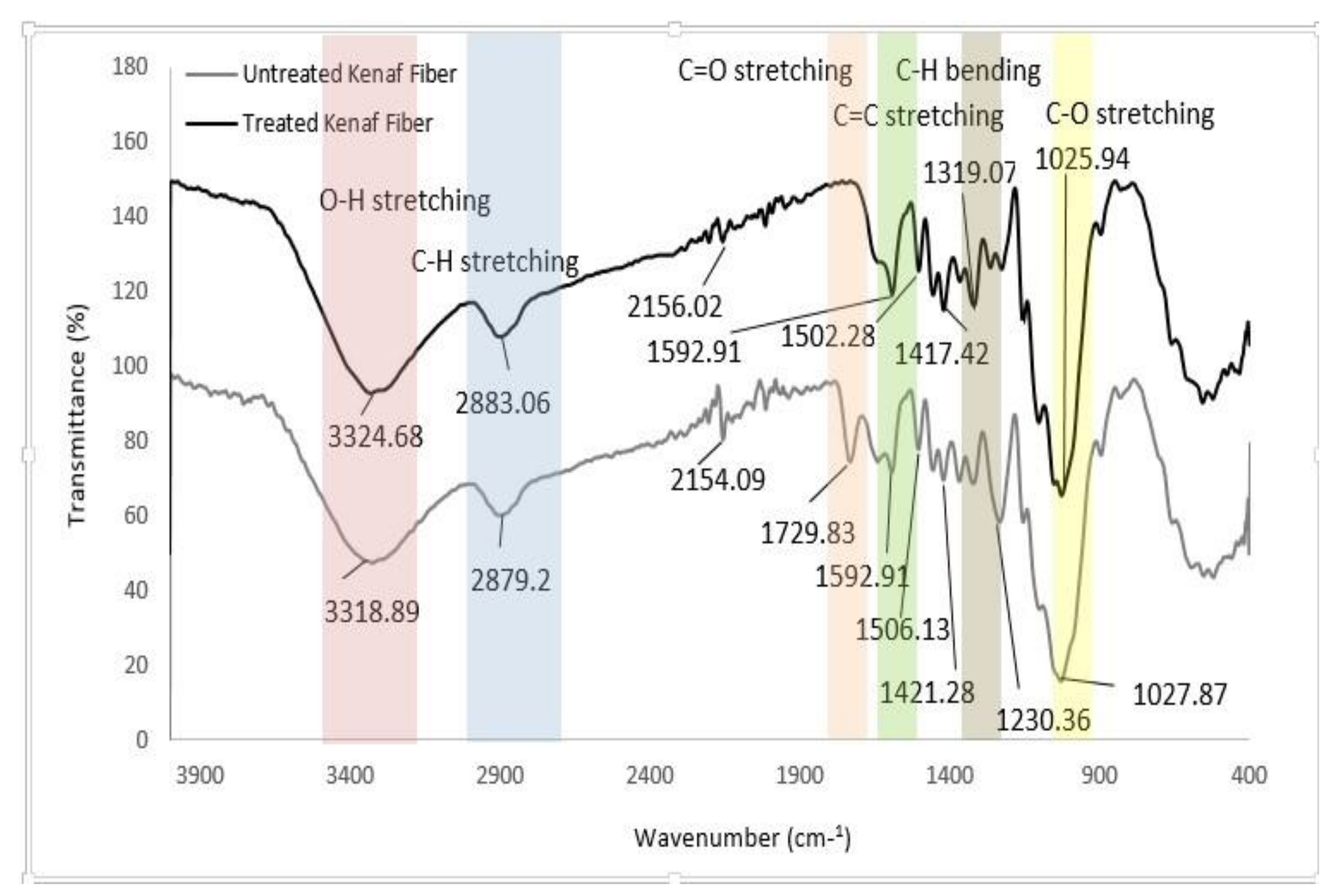
3.1. Fourier Transform Infrared (FTIR) Spectroscopy Analysis
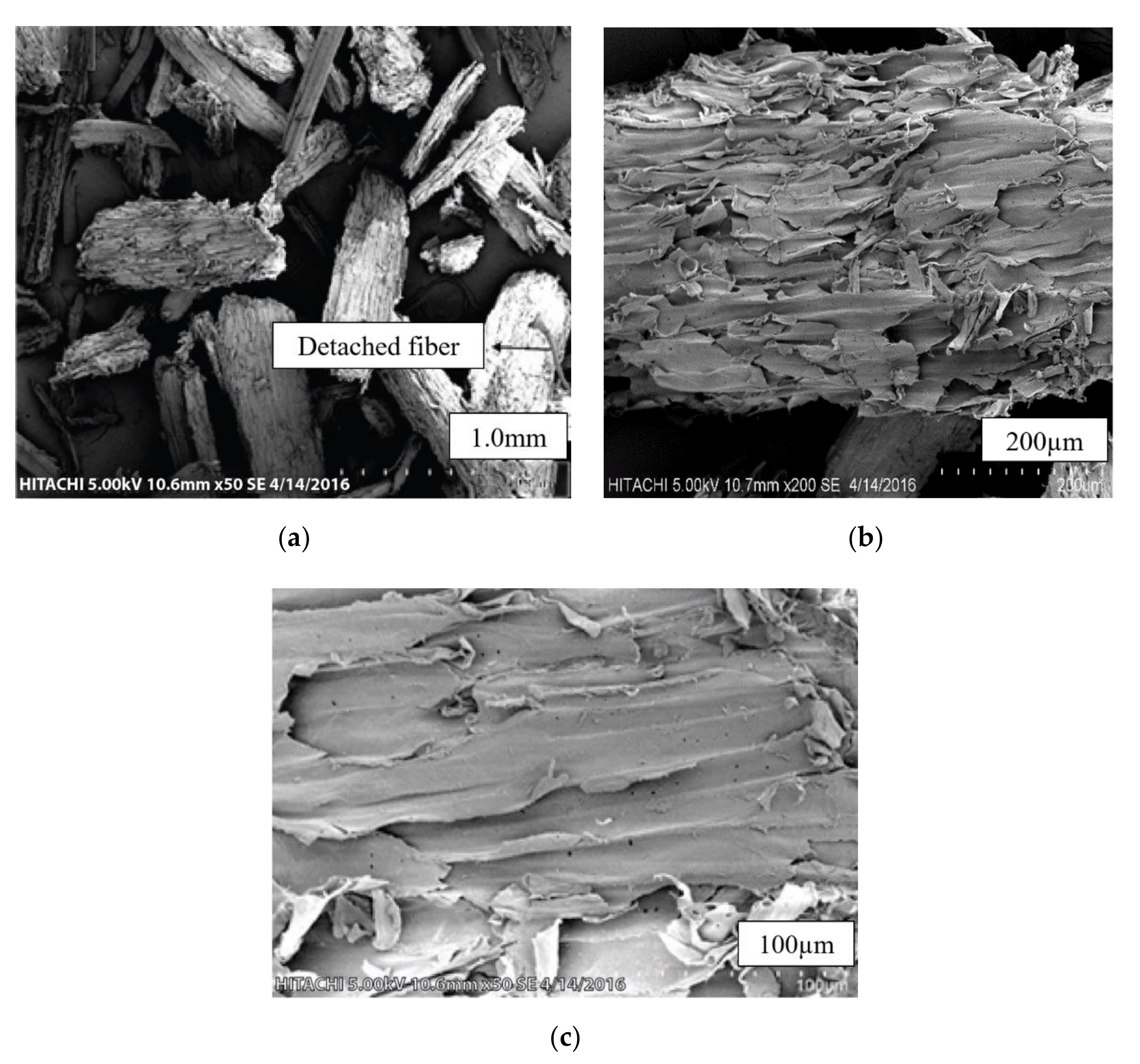
3.2. Scanning Electron Microscopy (SEM) Analysis
3.3. Chemical Composition Analysis
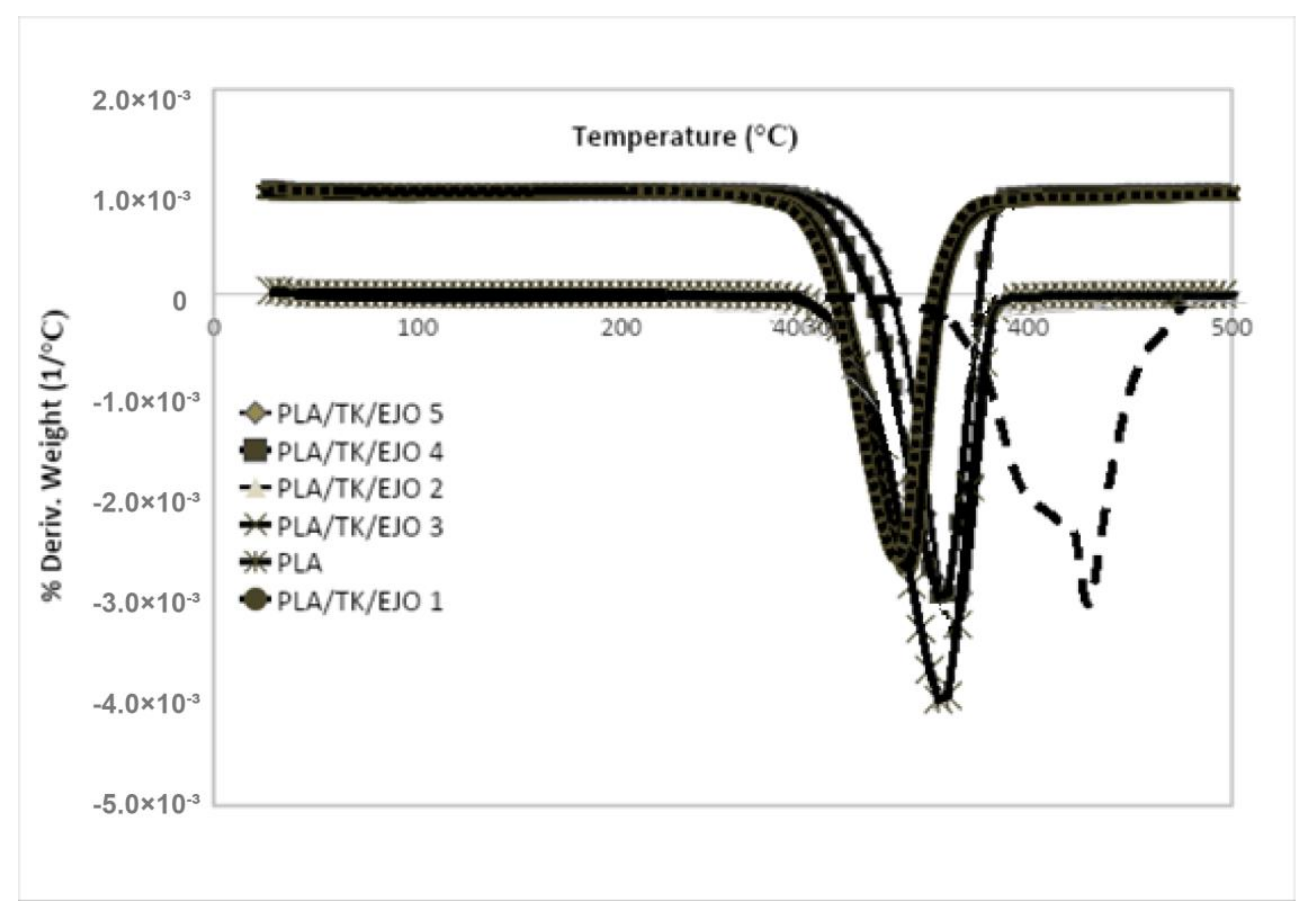
3.4. Thermogravimetric Analysis Properties
3.5. Differential Scanning Calorimetry Properties
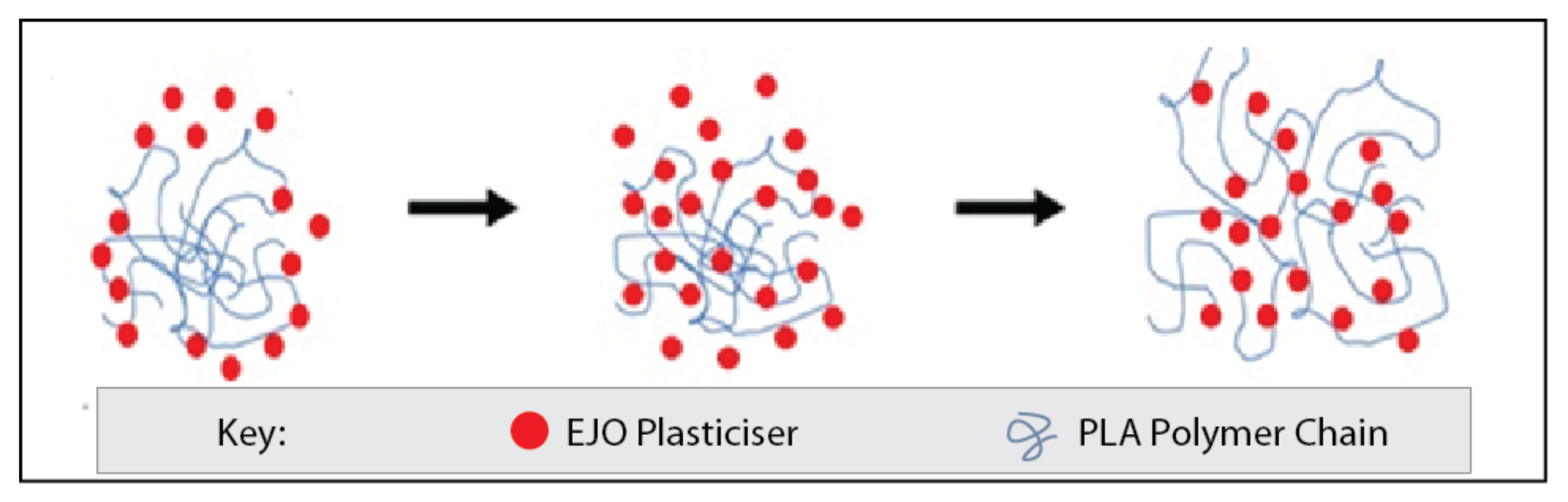
3.6. Plasticizing Effects
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stevens, E.S. Green Plastics: An Introduction to the New Science of Biodegradable Plastics; Princeton University Press: Princeton, NJ, USA, 2002. [Google Scholar]
- Ray, S.S.; Bousmina, M. Biodegradable polymers and their layered silicate nanocomposites: In greening the 21st century materials world. Prog. Mater. Sci. 2005, 50, 962–1079. [Google Scholar] [CrossRef]
- Kamarudin, S.H.; Abdullah, L.C.; Aung, M.M.; Ratnam, C.T. Mechanical and physical properties of kenaf-reinforced poly (lactic acid) plasticized with epoxidized jatropha oil. BioResources 2019, 14, 9001–9020. [Google Scholar] [CrossRef]
- Iwata, T. Biodegradable and bio-based polymers: Future prospects of eco-friendly plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef]
- Elsawy, M.A.; Kim, K.H.; Park, J.W.; Deep, A. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew. Sustain. Energy Rev. 2017, 79, 1346–1352. [Google Scholar] [CrossRef]
- Kamarudin, S.H.; Abdullah, L.C.; Aung, M.M.; Ratnam, C.T.; Jusoh, E.R. A study of mechanical and morphological properties of PLA based biocomposites prepared with EJO vegetable oil based plasticiser and kenaf fibres. Mater. Res. Express 2018, 5, 085314. [Google Scholar] [CrossRef]
- Serizawa, S.; Inoue, K.; Iji, M. Kenaf-fiber-reinforced poly (lactic acid) used for electronic products. J. Appl. Polym. Sci. 2006, 100, 618–624. [Google Scholar] [CrossRef]
- Surya, I.; Olaiya, N.G.; Rizal, S.; Zein, I.; Sri, A.; Hasan, N.A.M.; Abdul Khalil, H.P.S. Plasticizer enhancement on the miscibility and thermomechanical properties of polylactic acid-chitin-starch composites. Polymers 2020, 12, 115. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Jaszkiewicz, A.; Scherzer, D. Mechanical properties of PLA composites with man-made cellulose and abaca fibres. Compos. Part A Appl. Sci. Manuf. 2009, 40, 404–412. [Google Scholar] [CrossRef]
- Anakabe, J.; Zaldua Huici, A.M.; Eceiza, A.; Arbelaiz, A. The effect of the addition of poly (styrene-co-glycidyl methacrylate) copolymer on the properties of polylactide/poly (methyl methacrylate) blend. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Broström, J.; Boss, A.; Chronakis, I.S. Biodegradable films of partly branched poly (L-lactide)-co-poly (ε-caprolactone) copolymer: Modulation of phase morphology, plasticization properties and thermal depolymerization. Biomacromolecules 2004, 5, 1124–1134. [Google Scholar] [CrossRef]
- Dai, X.; Xiong, Z.; Na, H.; Zhu, J. How does epoxidized soybean oil improve the toughness of microcrystalline cellulose filled polylactide acid composites? Compos. Sci. Technol. 2014, 90, 9–15. [Google Scholar] [CrossRef]
- Mihai, M.; Huneault, M.A.; Favis, B.D. Rheology and extrusion foaming of chain-branched poly (lactic acid). Polym. Eng. Sci. 2010, 50, 629–642. [Google Scholar] [CrossRef]
- Balart, J.F.; Fombuena, V.; Fenollar, O.; Boronat, T.; Sánchez-Nacher, L. Processing and characterization of high environmental efficiency composites based on PLA and hazelnut shell flour (HSF) with biobased plasticizers derived from epoxidized linseed oil (ELO). Comp. Part B Eng. 2016, 86, 168–177. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhang, L.; Ma, S.; Yang, Y.; Zhang, C.; Tang, Z.; Zhu, J. Effect of castor oil enrichment layer produced by reaction on the properties of PLA/HDI-g-starch blends. Carbohydr. Polym. 2013, 94, 235–243. [Google Scholar] [CrossRef]
- Al Mulla, E.A.J.; Suhail, A.H.; Aowda, S.A. New biopolymer nanocomposites based on epoxidized soybean oil plasticized poly (lactic acid)/fatty nitrogen compounds modified clay: Preparation and characterization. Ind. Crops Prod. 2011, 33, 23–29. [Google Scholar] [CrossRef]
- Robertson, M.L.; Chang, K.; Gramlich, W.M.; Hillmyer, M.A. Toughening of polylactide with polymerized soybean oil. Macromolecules 2010, 43, 1807–1814. [Google Scholar] [CrossRef]
- Alam, A.M.; Mina, M.F.; Beg, M.D.H.; Mamun, A.A.; Bledzki, A.K.; Shubhra, Q.T.H. Thermo-mechanical and morphological properties of short natural fiber reinforced poly (lactic acid) biocomposite: Effect of fiber treatment. Fibers Polym. 2014, 15, 1303–1309. [Google Scholar] [CrossRef]
- Zhang, K.; Misra, M.; Mohanty, A.K. Toughened sustainable green composites from poly (3 hydroxybutyrate-co-3-hydroxyvalerate) based ternary blends and miscanthus biofibr. ACS Sustain. Chem. Eng. 2014, 2, 2345–2354. [Google Scholar]
- Dicker, M.P.; Duckworth, P.F.; Baker, A.B.; Francois, G.; Hazzard, M.K.; Weaver, P.M. Green composites: A review of material attributes and complementary application. Compos. Part A Appl. Sci. Manuf. 2014, 56, 280–289. [Google Scholar] [CrossRef]
- Rahman, M.M.; Netravali, A.N. Advanced green composites using liquid crystalline cellulose fibres and waxy maize starch based resin. Compos. Sci. Technol. 2018, 162, 110–116. [Google Scholar] [CrossRef]
- Bogoeva-Gaceva, G.; Avella, M.; Malinconico, M.; Buzarovska, A.; Grozdanov, A.; Gentile, G.; Errico, M.E. Natural fiber eco-composite. Polym. Comp. 2007, 28, 98–107. [Google Scholar] [CrossRef]
- Properties and Performance of Natural-Fibre Composites; Pickering, K., Ed.; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Ahmad, F.; Choi, H.S.; Park, M.K. A review: Natural fiber composites selection in view of mechanical, light weight, and economic properties. Macromol. Mater. Eng. 2015, 300, 10–24. [Google Scholar] [CrossRef]
- Davoodi, M.M.; Sapuan, S.M.; Ahmad, D.; Ali, A.; Khalina, A.; Jonoobi, M. Mechanical properties of hybrid kenaf/glass reinforced epoxy composite for passenger car bumper beam. Mater. Des. 2010, 31, 4927–4932. [Google Scholar] [CrossRef]
- Fogorasi, M.S.; Barbu, I. The potential of natural fibres for automotive sector-review. IOP Conf. Ser. Mater. Sci. Eng. 2017, 252, 012044. [Google Scholar] [CrossRef]
- Fuentes, C.A.; Tran, L.Q.N.; van Hellemont, M.; Janssens, V.; Dupont, G.; van Vuure, C.A.W.; Verpoest, I. Effect of physical adhesion on mechanical behaviour of bamboo fibre reinforced thermoplastic composites. Coll. Surf. A. 2013, 418, 7–15. [Google Scholar] [CrossRef]
- Kim, H.; Okubo, K.; Fujii, T.; Takemura, K. Influence of fiber extraction and surface modification on mechanical properties of green composites with bamboo fiber. J. Adhes. Sci. Technol. 2012, 27, 1348–1358. [Google Scholar] [CrossRef]
- Huber, T.; Müssig, J. Fibre matrix adhesion of natural fibres cotton, flax and hemp in polymeric matrices analyzed with the single fibre fragmentation test. Compos. Interfac. 2008, 15, 335–349. [Google Scholar] [CrossRef]
- Felix, J.M.; Gatenholm, P. Effect of transcrystalline morphology on interfacial adhesion in cellulose/polypropylene composites. J. Mater. Sci. 1994, 29, 3043–3049. [Google Scholar] [CrossRef]
- Graupner, N.; Rößler, J.; Ziegmann, G.; Müssig, J. Fibre/matrix adhesion of cellulose fibres in PLA, PP and MAPP: A critical review of pull-out test, microbond test and single fibre fragmentation test results. Compos. Part A 2014, 63, 133–148. [Google Scholar] [CrossRef]
- Le Duigou, A.; Davies, P.; Baley, C. Exploring durability of interfaces in flax fibre/epoxy micro-composites. Compos. Part A 2013, 48, 121–128. [Google Scholar] [CrossRef]
- Nahar, S.; Khan, R.A.; Dey, K.; Sarker, B.; Das, A.K.; Ghoshal, S. Comparative studies of mechanical and interfacial properties between jute and bamboo fiber-reinforced polypropylene-based composites. J. Thermoplast. Compos. Mater. 2012, 25, 15–32. [Google Scholar] [CrossRef]
- Park, J.M.; Quang, S.T.; Hwang, B.S.; de Vries, K.L. Interfacial evaluation of modified jute and hemp fibers/polypropylene (PP)-maleic anhydride polypropylene copolymers (PPMAPP) composites using micromechanical technique and nondestructive acoustic emission. Compos. Sci. Technol. 2006, 66, 2686–2699. [Google Scholar] [CrossRef]
- Garkhail, S.K. Composites Based on Natural Fibres and Thermoplastic Matrices. Ph.D. Thesis, Queen Mary College, University of London, London, UK, 2001. [Google Scholar]
- Van de Velde, K.; Kiekens, P. Influence of fiber surface characteristics on the flax/polypropylene interface. J. Thermoplast. Compos. Mater. 2001, 14, 244–260. [Google Scholar] [CrossRef]
- Orue, A.; Jauregi, A.; Peña-Rodriguez, C.; Labidi, J.; Eceiza, A.; Arbelaiz, A. The effect of surface modifications on sisal fiber properties and sisal/poly (lactic acid) interface adhesion. Compos. Part B Eng. 2015, 73, 132–138. [Google Scholar] [CrossRef]
- Taha, I.M.; Ziegmann, G. Potential of sisal reinforced biodegradable polylactic acid and polyvinyl alcohol composites. Key Eng. Mater. 2010, 425, 167–178. [Google Scholar] [CrossRef]
- Luo, S.; Netravali, A.N. Interfacial and mechanical properties of environment-friendly “green” composites made from pineapple fibers and poly(hydroxybutyrate-co-valerate) resin. J. Mater. Sci. 1999, 34, 3709–3719. [Google Scholar] [CrossRef]
- Awal, A.; Cescutti, G.; Ghosh, S.B.; Müssig, J. Interfacial studies of natural fibre/polypropylene composites using single fibre fragmentation test (SFFT). Compos. Part A 2011, 42, 50–56. [Google Scholar] [CrossRef]
- Pickering, K.L.; Sawpan, M.A.; Jayaraman, J.; Fernyhough, A. Influence of loading rate, alkali fibre treatment and crystallinity on fracture toughness of random short hemp fibre reinforced polylactide bio-composites. Compos. Part A 2011, 42, 1148–1156. [Google Scholar] [CrossRef]
- Anuar, H.; Zuraida, A.; Morlin, B.; Kovács, J.G. Micromechanical property investigations of poly (lactic acid) ekenaf fiber biocomposites. J. Nat. Fibers 2011, 8, 14–26. [Google Scholar] [CrossRef]
- Cho, D.; Seo, J.M.; Lee, H.S.; Cho, C.W.; Han, S.O.; Park, W.H. Property improvement of natural fiber-reinforced green composites by water treatment. Adv. Compos. Mater. 2007, 16, 299–314. [Google Scholar] [CrossRef]
- Karlsson, J.O.; Gatenholm, P.; Blachot, J.F.; Peguy, A. Improvement of adhesion between polyethylene and regenerated cellulose fibers by surface fibrillation. Polym. Compos. 1996, 17, 300–304. [Google Scholar] [CrossRef]
- Yusoff, R.B.; Takagi, H.; Nakagaito, A.N. Tensile and flexural properties of polylactic acid-based hybrid green composites reinforced by kenaf, bamboo and coir fibers. Ind. Crops Prod. 2016, 94, 562–573. [Google Scholar] [CrossRef]
- Lu, T.; Liu, S.; Jiang, M.; Xu, X.; Wang, Y.; Wang, Z.; Zhou, Z. Effects of modifications of bamboo cellulose fibers on the improved mechanical properties of cellulose reinforced poly (lactic acid) composites. Comp. Part B Eng. 2014, 62, 191–197. [Google Scholar] [CrossRef]
- Shalwan, A.; Yousif, B. In state of art: Mechanical and tribological behaviour of polymeric composites based on natural fibers. Mater. Des. 2013, 48, 14–24. [Google Scholar] [CrossRef]
- Kabir, M.; Wang, H.; Lau, K.; Cardona, F. Chemical treatments on plant-based natural fibre reinforced polymer composites: An overview. Compos Part B Eng. 2012, 43, 2883–2892. [Google Scholar] [CrossRef]
- Kalam, A.; Jumahat, A.; Salleh, Z.; Hyie, K.M. Mechanical properties and fracture toughness of alkali treated oil palm fruit bunch (OPFB) fibre/epoxy composites. Appl. Mech. Mater. 2013, 390, 521–525. [Google Scholar] [CrossRef]
- Bar, M.; Alagirusamy, R.; Das, A. Advances in Natural Fiber Reinforced Thermoplastic Composite Manufacturing: Effect of Interface and Hybrid Yarn Structure on Composite Properties. In Advances in Natural Fiber Composites; Springer: Berlin, Germany, 2018; pp. 99–117. [Google Scholar]
- Chowdhury, M.F.M.; Lavelli, A. FBK-irst: A multi-phase kernel based approach for drug-drug interaction detection and classification that exploits linguistic information. In Second Joint Conference on Lexical and Computational Semantics (*SEM), Volume 2, Proceedings of the Seventh International Workshop on Semantic Evaluation (SemEval 2013), Atlanta, Georgia, 14–15 June 2013; Association for Computational Linguistics: Atlanta, GA, USA, 2013; Volume 2, pp. 351–355. [Google Scholar]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Development and characterization of sugar palm starch and poly (lactic acid) bilayer films. Carbohydr. Polym. 2016, 146, 36–45. [Google Scholar] [CrossRef]
- Wan Jaafar, W.N.R.; Siti Norasmah, S.; Azmi, N.N.; Noor Najmi, B.; Anuar, H.; Hassan, N.A.; Abdul Razak, S.B. Thermal properties of PLA/Kenaf green nanocomposite: Effect of chemi-mechanical treatment. In Advanced Materials Research; Trans Tech Publications Ltd.: Bäch, Switzerland, 2012; Volume 76, pp. 342–344. [Google Scholar]
- Asaithambi, B.; Ganesan, G.S.; Ananda Kumar, S. Banana/sisal fibers reinforced poly (lactic acid) hybrid biocomposites; influence of chemical modification of BSF towards thermal properties. Polym. Comp. 2017, 38, 1053–1062. [Google Scholar] [CrossRef]
- Rashid, A.R.B.A.; Husin, H.B.; Alauddin, S.B.M.; Shueb, M.I.B. Performance of polylactic acid natural fiber biocomposite. Int. J. Appl. Chem. 2016, 12, 72–77. [Google Scholar]
- Mohanty, A.K.; Mubarak, A.K.; Hinrichsen, G. Surface modification of jute and its influence on performance of biodegradable jute-fabric/biopol composites. Compos. Sci. Technol. 2000, 60, 1115–1124. [Google Scholar] [CrossRef]
- Eselini, N.; Tirkes, S.; Akar, A.O.; Tayfun, U. Production and characterization of poly (lactic acid)-based biocomposites filled with basalt fiber and flax fiber hybrid. J. Elastom. Plast. 2020, 52, 701–716. [Google Scholar] [CrossRef]
- Nassiopoulos, E.; Njuguna, J. Thermo-mechanical performance of poly (lactic acid)/flax fibre-reinforced biocomposites. Mater. Des. 2015, 66, 473–485. [Google Scholar] [CrossRef]
- Ahmad, Z.; Latif, H.A.; Shaari, H.A.H.; Aiman, W.M.; Izwan, N.I.; Wahab, N.M.A. The Addition of Silane Coupling Agent in Coconut Coir Husk/PLA Biocomposite: Mechanical and Biodegradability Studies; Conference Proceedings; AIP Publishing LLC.: Melville NY, USA, 2018; Volume 2031, p. 020012. [Google Scholar]
- Rayung, M.; Ibrahim, N.A.; Zainuddin, N.; Saad, W.Z.; Razak, N.I.A.; Chieng, B.W. The effect of fiber bleaching treatment on the properties of poly (lactic acid)/oil palm empty fruit bunch fiber composites. Int. J. Mol. Sci. 2014, 15, 14728–14742. [Google Scholar] [CrossRef]
- Kaewpirom, S.; Worrarat, C. Preparation and properties of pineapple leaf fiber reinforced poly (lactic acid) green composites. Fibers Polym. 2014, 15, 1469–1477. [Google Scholar] [CrossRef]
- Anuar, H.; Zuraida, A. Thermal properties of injection moulded polylactic acid–kenaf fibre biocomposite. Malays. Polym. J. 2011, 6, 51–57. [Google Scholar]
- Saalah, S.; Abdullah, L.C.; Aung, M.M.; Salleh, M.Z.; Biak, D.R.A.; Basri, M.; Jusoh, E.R. Waterborne polyurethane dispersions synthesized from jatropha oil. Ind. Crops Prod. 2015, 64, 194–200. [Google Scholar] [CrossRef]
- Wise, L.E. Chlorite holocellulose, its fractionation and bearing on summative wood analysis and on studies on the hemicelluloses. Pap. Trade 1946, 122, 35–43. [Google Scholar]
- Sarasini, F.; Tirillò, J.; Valente, M.; Valente, T.; Cioffi, S.; Iannace, S.; Sorrentino, L. Effect of basalt fiber hybridization on the impact behavior under low impact velocity of glass/basalt woven fabric/epoxy resin composites. Comp. Part A Appl. Sci. Manuf. 2013, 47, 109–123. [Google Scholar] [CrossRef]
- Silverajah, V.S.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Hassan, H.A.; Woei, C.B. A comparative study on the mechanical, thermal and morphological characterization of poly (lactic acid)/epoxidized palm oil blend. Int. J. Mol. Sci. 2012, 13, 5878–5898. [Google Scholar] [CrossRef]
- Tee, Y.B.; Talib, R.A.; Abdan, K.; Chin, N.L.; Basha, R.K.; Yunos, K.F.M. Comparative study of chemical, mechanical, thermal, and barrier properties of poly (lactic acid) plasticized with epoxidized soybean oil and epoxidized palm oil. BioResources 2016, 11, 1518–1540. [Google Scholar] [CrossRef]
- Liu, W.; Mohanty, A.K.; Askeland, P.; Drzal, L.T.; Misra, M. Influence of fiber surface treatment on properties of Indian grass fiber reinforced soy protein based biocomposites. Polymer 2004, 45, 7589–7596. [Google Scholar] [CrossRef]
- Tserki, V.; Zafeiropoulos, N.E.; Simon, F.; Panayiotou, C. A study of the effect of acetylation and propionylation surface treatments on natural fibres. Comp. Part A Appl. Sci. Manuf. 2005, 36, 1110–1118. [Google Scholar] [CrossRef]
- Sgriccia, N.; Hawley, M.C.; Misra, M. Characterization of natural fiber surfaces and natural fiber composites. Comp. Part A Appl. Sci. Manuf. 2008, 39, 1632–1637. [Google Scholar] [CrossRef]
- Alemdar, A.; Sain, M. Biocomposites from wheat straw nanofibers: Morphology, thermal and mechanical properties. Comp. Sci. Technol. 2008, 68, 557–565. [Google Scholar] [CrossRef]
- Mwaikambo, L.Y.; Ansell, M.P. Chemical modification of hemp, sisal, jute, and kapok fibers by alkalization. J. Appl. Polym. Sci. 2002, 84, 2222–2234. [Google Scholar] [CrossRef]
- Le Troedec, M.; Sedan, D.; Peyratout, C.; Bonnet, J.P.; Smith, A.; Guinebretiere, R.; Krausz, P. Influence of various chemical treatments on the composition and structure of hemp fibres. Comp. Part A Appl. Sci. Manuf. 2008, 39, 514–522. [Google Scholar] [CrossRef]
- Jonoobi, M.; Harun, J.; Mishra, M.; Oksman, K. Chemical composition, crystallinity and thermal degradation of bleached and unbleached kenaf bast (Hibiscus cannabinus) pulp and nanofiber. BioResources 2009, 4, 626–639. [Google Scholar]
- Nacos, M.K.; Katapodis, P.; Pappas, C.; Daferera, D.; Tarantilis, P.A.; Christakopoulos, P.; Polissiou, M. Kenaf xylan–a source of biologically active acidic oligosaccharides. Carbohydr. Polym. 2006, 66, 126–134. [Google Scholar] [CrossRef]
- Abral, H.; Gafar, M.F.; Andriyanto, H.I.; Sapuan, S.M.; Ishak, M.R.E. Alkali treatment of screw pine (Pandanus Odoratissimus) fibers and its effect on unsaturated polyester composites. Polym. Plast. Technol. Eng. 2012, 51, 12–18. [Google Scholar] [CrossRef]
- Lai, C.Y.; Sapuan, S.M.; Ahmad, M.; Yahya, N.; Dahlan, K.Z.H.M. Mechanical and electrical properties of coconut coir fiber-reinforced polypropylene composites. Polym. Plast. Technol. Eng. 2005, 44, 619–632. [Google Scholar] [CrossRef]
- Jayaraman, K. Manufacturing sisal–polypropylene composites with minimum fibre degradation. Comp. Sci. Technol. 2003, 63, 367–374. [Google Scholar] [CrossRef]
- John, M.J.; Thomas, S. Biofibres and biocomposites. Carbohydr. Polym. 2008, 71, 343–364. [Google Scholar] [CrossRef]
- Godavarti, S. Thermoplastic wood fiber composites. Nat. Fibers Biopolym. Biocomp. 2005, 348–386. [Google Scholar] [CrossRef]
- Thomas, S.; Pothan, L.A.; Cherian, B.M. Advances in natural fibre reinforced polymer composites: Macro to nanoscales. Int. J. Mater. Prod. Technol. 2009, 36, 317–333. [Google Scholar] [CrossRef]
- Nevell, T.P.; Zeronian, S.H. Cellulose Cemistry and Its Applications; Ellis Horwood: New York, NY, USA, 1985; pp. 30–83. [Google Scholar]
- Yahaya, R.; Sapuan, S.M.; Jawaid, M.; Leman, Z.; Zainudin, E.S. Effect of layering sequence and chemical treatment on the mechanical properties of woven kenaf–aramid hybrid laminated composites. Mater. Des. 2015, 67, 173–179. [Google Scholar] [CrossRef]
- Mbada, N.I.; Aponbiede, O.; Ause, T.; Alabi, A. Effects of mercerization treatment on kenaf fibre (Hibiscus cannabinus L.). Int. J. Mater. Eng. 2016, 6, 8–14. [Google Scholar]
- Suardana, N.P.G.; Piao, Y.; Lim, J.K. Mechanical properties of hemp fibers and hemp/pp composites: Effects of chemical surface treatment. Mater. Phys. Mech. 2011, 11, 1–8. [Google Scholar]
- Ferreira, J.; Errajhi, O.; Richardson, M. Thermogravimetric analysis of aluminised E-glass fiber reinforced poly (lactic acid) composites. Polym. Test 2006, 25, 1091–1094. [Google Scholar] [CrossRef][Green Version]
- Saba, N.; Paridah, M.; Abdan, K.; Ibrahim, N. Dynamic mechanical properties of oil palm nano filler/kenaf/epoxy hybrid nanocomposites. Constr. Build Mater. 2016, 124, 133–138. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, Y.; Zhang, M.; Zhou, W. Phase behavior and its viscoelastic response of polylactide/poly (ε-caprolactone) blend. Eur. Polym. J. 2008, 44, 2171–2183. [Google Scholar] [CrossRef]
- Ray, D.; Sarkar, B.K.; Bose, N.R. Impact fatigue behaviour of vinylester resin matrix composites reinforced with alkali treated jute fibres. Comp. Part A Appl. Sci. Manuf. 2002, 33, 233–241. [Google Scholar] [CrossRef]
- El-Shekeil, Y.A.; Sapuan, S.M.; Khalina, A.; Zainudin, E.S.; Al-Shuja’a, O.M. Effect of alkali treatment on mechanical and thermal properties of Kenaf fiber-reinforced thermoplastic polyurethane composite. J. Therm. Anal. Calorim. 2012, 109, 1435–1443. [Google Scholar] [CrossRef]
- Kalam, A.; Berhan, M.N.; Ismail, H. Physical and mechanical characterizations of oil palm fruit bunch fiber filled polypropylene composites. J. Reinf. Plast. Comp. 2010, 29, 3173–3184. [Google Scholar] [CrossRef]
- Arbelaiz, A.; Fernandez, B.; Ramos, J.A.; Retegi, A.; Llano-Ponte, R.; Mondragon, I. Mechanical properties of short flax fibre bundle/polypropylene composites: Influence of matrix/fibre modification, fibre content, water uptake and recycling. Comp. Sci. Technol. 2005, 65, 1582–1592. [Google Scholar] [CrossRef]
- Sreekala, M.S.; Kumaran, M.G.; Thomas, S. Oil palm fibers: Morphology, chemical composition, surface modification, and mechanical properties. J. Appl. Polym. Sci. 1997, 66, 821–835. [Google Scholar] [CrossRef]
- Nomura, S.; Kugo, Y.; Erata, T. 13 C NMR and XRD studies on the enhancement of cellulose II crystallinity with low concentration NaOH post-treatments. Cellulose 2020, 27, 1–11. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; Rayón, E.; López, J.; Kenny, J.M. Multifunctional PLA–PHB/cellulose nanocrystal films: Processing, structural and thermal properties. Carbohydr. Polym. 2014, 107, 16–24. [Google Scholar] [CrossRef]
- Jia, Z.; Tan, J.; Han, C.; Yang, Y.; Dong, L. Poly (ethylene glycol-co-propylene glycol) as a macromolecular plasticizing agent for polylactide: Thermomechanical properties and aging. J. Appl. Polym. Sci. 2009, 114, 1105–1117. [Google Scholar] [CrossRef]
- Burgos, N.; Martino, V.P.; Jiménez, A. Characterization and ageing study of poly (lactic acid) films plasticized with oligomeric lactic acid. Polym. Degrad. Stab. 2013, 98, 651–658. [Google Scholar] [CrossRef]
- Maizatul, N.; Norazowa, I.; Yunus, W.M.Z.W.; Khalina, A.; Khalisanni, K. FTIR and TGA analysis of biodegradable poly (lactic acid)/treated kenaf bast fibre: Effect of plasticizers. Pertan. J. Sci. Technol. 2013, 21, 151–160. [Google Scholar]
- Cao, X.; Mohamed, A.; Gordon, S.H.; Willett, J.L.; Sessa, D.J. DSC study of biodegradable poly (lactic acid) and poly (hydroxy ester ether) blends. Thermochim. Acta 2003, 406, 115–127. [Google Scholar] [CrossRef]
- Fischer, E.W.; Sterzel, H.J.; Wegner, G.K.Z.Z. Investigation of the structure of solution grown crystals of lactide copolymers by means of chemical reactions. Kolloid Z. Z. Polym. 1973, 251, 980–990. [Google Scholar] [CrossRef]
- Han, S.O.; Karevan, M.; Sim, I.N.; Bhuiyan, M.A.; Jang, Y.H.; Ghaffar, J.; Kalaitzidou, K. Understanding the reinforcing mechanisms in kenaf fiber/PLA and kenaf fiber/PP composites: A comparative study. Int. J. Polym. Sci. 2012, 2012. [Google Scholar] [CrossRef]
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Perspective on polylactic acid (PLA) based sustainable materials for durable applications: Focus on toughness and heat resistance. ACS Sustain. Chem. Eng. 2016, 4, 2899–2916. [Google Scholar] [CrossRef]
- Cheung, H.Y.; Ho, M.P.; Lau, K.T.; Cardona, F.; Hui, D. Natural fibre-reinforced composites for bioengineering and environmental engineering applications. Comp. Part B Eng. 2009, 40, 655–663. [Google Scholar] [CrossRef]
- Huda, M.S.; Drzal, L.T.; Mohanty, A.K.; Misra, M. Effect of fiber surface-treatments on the properties of laminated biocomposites from poly (lactic acid) (PLA) and kenaf fibers. Comp. Sci. Technol. 2008, 68, 424–432. [Google Scholar] [CrossRef]
- Aji, I.S.; Sapuan, S.M.; Zainudin, E.S.; Abdan, K. Kenaf fibres as reinforcement for polymeric composites: A review. Int. J. Mech. Mater. Eng. 2009, 4, 239–248. [Google Scholar]
- Dehbari, N.; Moazeni, N.; Rahman, W.A. Effects of Kenaf core on properties of poly (lactic acid) bio-composite. Polym. Comp. 2014, 35, 1220–1227. [Google Scholar] [CrossRef]
- Dobreva, T.; Pereña, J.M.; Pérez, E.; Benavente, R.; García, M. Crystallization behavior of poly (L-lactic acid)-based ecocomposites prepared with kenaf fiber and rice straw. Polym. Comp. 2010, 31, 974–984. [Google Scholar] [CrossRef]
- Suryanegara, L.; Nakagaito, A.N.; Yano, H. The effect of crystallization of PLA on the thermal and mechanical properties of microfibrillated cellulose-reinforced PLA composites. Comp. Sci. Technol. 2009, 69, 1187–1192. [Google Scholar] [CrossRef]
- Ten, E.; Jiang, L. Wolcott, M.P. Crystallization kinetics of poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/cellulosenanowhiskers composites. Carbohydr. Polym. 2012, 90, 541–550. [Google Scholar] [CrossRef]
- Baiardo, M.; Frisoni, G.; Scandola, M.; Rimelen, M.; Lips, D.; Ruffieux, K.; Wintermantel, E. Thermal and mechanical properties of plasticized poly (L-lactic acid). J. Appl. Polym. Sci. 2003, 90, 1731–1738. [Google Scholar] [CrossRef]
- Kulinski, B.; Piorkowska, E. Crystallization, structure and properties of plasticized poly (L-lactide). Polymer 2005, 46, 10290–10300. [Google Scholar] [CrossRef]
- Ali, F.; Chang, Y.W.; Kang, S.C.; Yoon, J.Y. Thermal, mechanical and rheological properties of poly (lactic acid)/epoxidized soybean oil blends. Polym. Bull. 2009, 62, 91–98. [Google Scholar] [CrossRef]
- Wasanasuk, K.; Tashiro, K. Crystal structure and disorder in Poly (l-lactic acid) δ form (α′ form) and the phase transition mechanism to the ordered α form. Polymer 2011, 52, 6097–6109. [Google Scholar] [CrossRef]





| Properties | PLA 2003D | American Society for Testing and Materilals (ASTM) |
|---|---|---|
| Specific gravity, g/cm3 | 1.24 | D792 |
| Notched Izod Impact, J/m | 16.0 | D256 |
| Melting point, °C | 145–160 | D3418 |
| Glass transition temperature, °C | 55.0–66.0 | D3418 |
| Deflection temperature at 0.46 MPa (66 psi), °C | 55.0 | E2092 |
| D-lactide % | 4.0–4.5 |
| PLA Compositions (wt.%) | Kenaf Fiber Compositions (wt.%) | Epoxidized Jatropha Oil Compositions (wt.%) |
|---|---|---|
| 69 | 30 | 1 |
| 68 | 30 | 2 |
| 67 | 30 | 3 |
| 66 | 30 | 4 |
| 65 | 30 | 5 |
| Bond Type | Hemp | Sisal | Jute | Kapok | This Work |
|---|---|---|---|---|---|
| –OH stretching | 3448 | 3447.2 | 3447.9 | 3419.7 | 3318.89, 3324.68 |
| –CH–vibration | 2920.5 | 2942.2 | 2918.8 | 2918.1 | 2879.2, 2883.06 |
| –C=O–stretching | 1736.5 | 1737.2 | 1741.1 | 1729.83 | |
| –C=C–stretching | 1645 | 1653.9 | 1653.8 | 1596.1 | 1592.91 |
| –CH–bending | 1384.1 | 1384.1, 1259.9 | 1384.1, 1255.6 | 1383.6, 1245.5 | 1319.07, 1230.36 |
| –C–C–stretching | 1000–1162 | 1000–1162 | 1000–1162 | 1000–1162 | 1156–1031 |
| –CH–stretching | 897.9 | 897 | |||
| –OH | 668.9 | 668.9 | 668.5 | 600 |
| Chemical Constituents | Compositions (%) | |
|---|---|---|
| Untreated Fiber | Treated Fiber | |
| Cellulose | 50.80 | 55.82 |
| Hemicellulose | 20.23 | 14.91 |
| Lignin | 16.75 | 13.84 |
| Sample | Onset Degradation Temperature, Tonset (°C) | Final Degradation Temperature, Tfinal (°C) | Rapid Decomposition Temperature, Tmax (°C) | Residue (%) |
|---|---|---|---|---|
| PLA | 315.53 | 377.83 | 357.85 | 2.19 |
| PLA/TK/EJO 1 | 290.98 | 371.17 | 322.15 | 3.24 |
| PLA/TK/EJO 2 | 292.20 | 377.50 | 324.37 | 4.79 |
| PLA/TK/EJO 3 | 293.65 | 378.17 | 324.53 | 4.36 |
| PLA/TK/EJO 4 | 294.64 | 378.33 | 335.13 | 3.27 |
| PLA/TK/EJO 5 | 295.99 | 382.17 | 336.94 | 2.91 |
| Sample | Tg (°C) | Tc (°C) | Tm1 (°C) | Tm2 (°C) | ΔHc (J/g) | Xc (%) |
|---|---|---|---|---|---|---|
| PLA | 68.64 | 107.75 | 159.40 | 163.94 | 3.40 | 3.63 |
| PLA/TK/EJO 1 | 66.49 | 99.95 | 147.06 | 157.16 | 16.1 | 17.3 |
| PLA/TK/EJO 2 | 63.96 | 99.64 | 147.67 | 156.29 | 15.7 | 16.9 |
| PLA/TK/EJO 3 | 63.56 | 99.12 | 147.48 | 155.18 | 14.8 | 15.9 |
| PLA/TK/EJO 4 | 62.96 | 99.10 | 147.37 | 155.83 | 14.7 | 15.8 |
| PLA/TK/EJO 5 | 62.48 | 99.08 | 146.02 | 155.12 | 11.4 | 12.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamarudin, S.H.; Abdullah, L.C.; Aung, M.M.; Ratnam, C.T. Thermal and Structural Analysis of Epoxidized Jatropha Oil and Alkaline Treated Kenaf Fiber Reinforced Poly(Lactic Acid) Biocomposites. Polymers 2020, 12, 2604. https://doi.org/10.3390/polym12112604
Kamarudin SH, Abdullah LC, Aung MM, Ratnam CT. Thermal and Structural Analysis of Epoxidized Jatropha Oil and Alkaline Treated Kenaf Fiber Reinforced Poly(Lactic Acid) Biocomposites. Polymers. 2020; 12(11):2604. https://doi.org/10.3390/polym12112604
Chicago/Turabian StyleKamarudin, Siti Hasnah, Luqman Chuah Abdullah, Min Min Aung, and Chantara Thevy Ratnam. 2020. "Thermal and Structural Analysis of Epoxidized Jatropha Oil and Alkaline Treated Kenaf Fiber Reinforced Poly(Lactic Acid) Biocomposites" Polymers 12, no. 11: 2604. https://doi.org/10.3390/polym12112604
APA StyleKamarudin, S. H., Abdullah, L. C., Aung, M. M., & Ratnam, C. T. (2020). Thermal and Structural Analysis of Epoxidized Jatropha Oil and Alkaline Treated Kenaf Fiber Reinforced Poly(Lactic Acid) Biocomposites. Polymers, 12(11), 2604. https://doi.org/10.3390/polym12112604