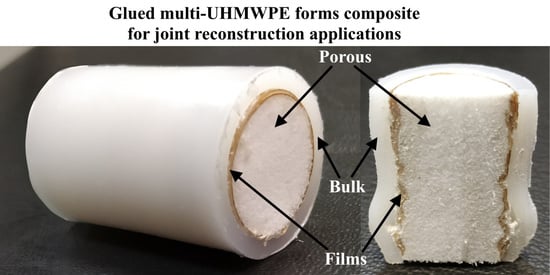
A New Approach Based on Glued Multi-Ultra High Molecular Weight Polyethylene Forms to Fabricate Bone Replacement Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Processes for Producing Cellulose Nanoparticles (CNs)
2.3. Molding, Orientation and Drawing of UHMWPE
2.4. Treatment and Modifying of the UHMWPE Films Surface
2.5. Preparing the Glues
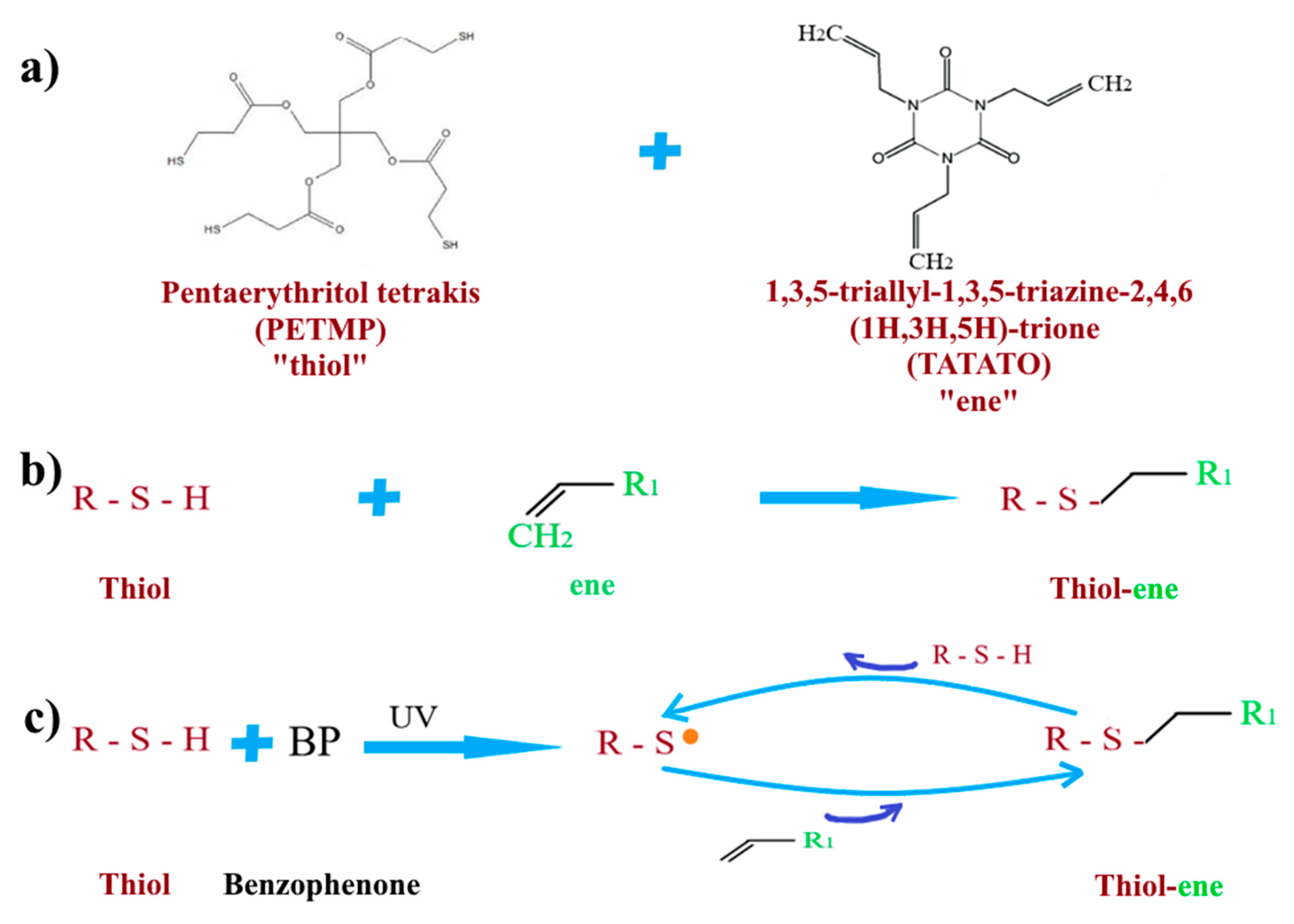
2.5.1. Glue Based on Thiol-ene Reaction
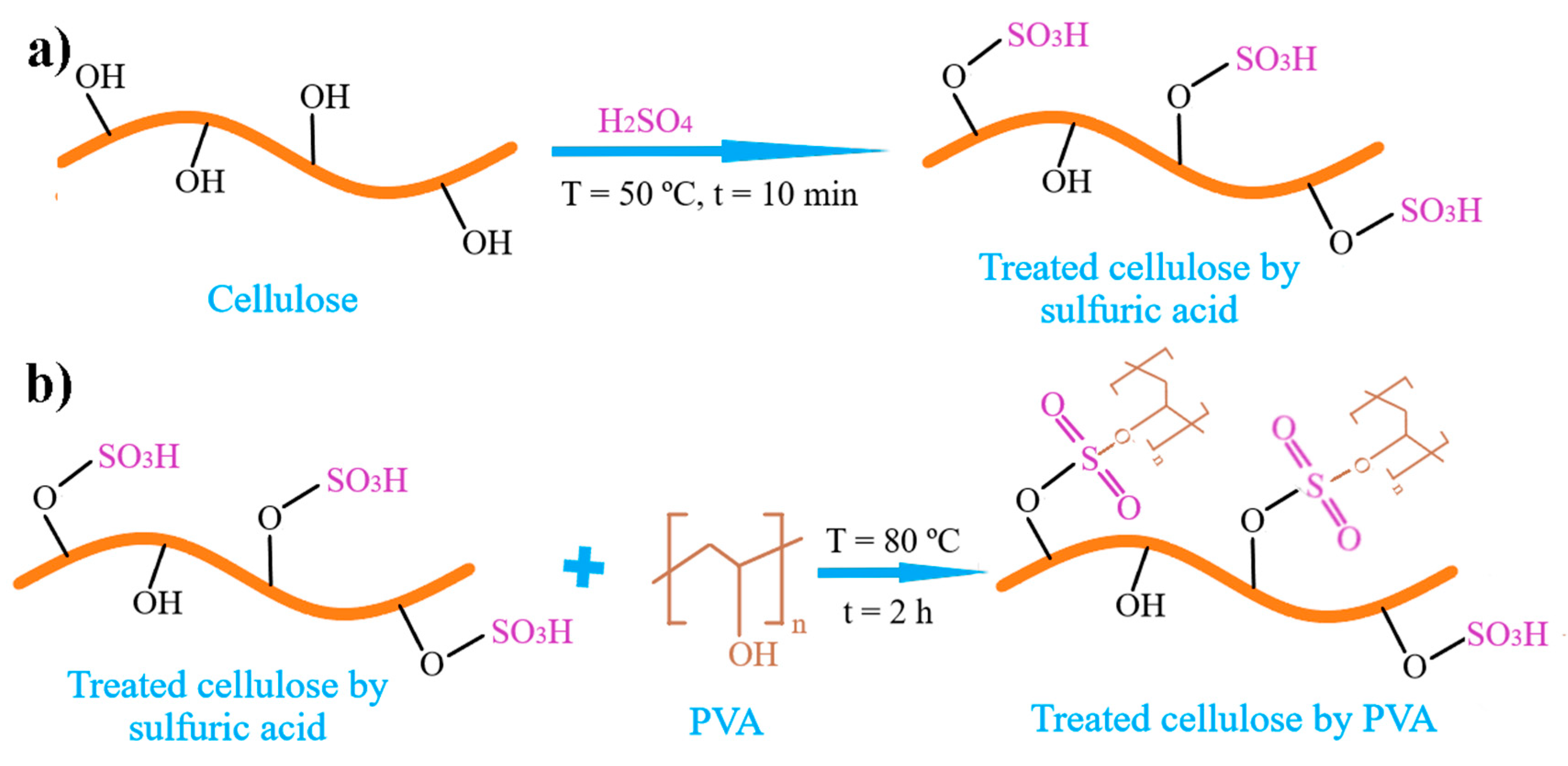
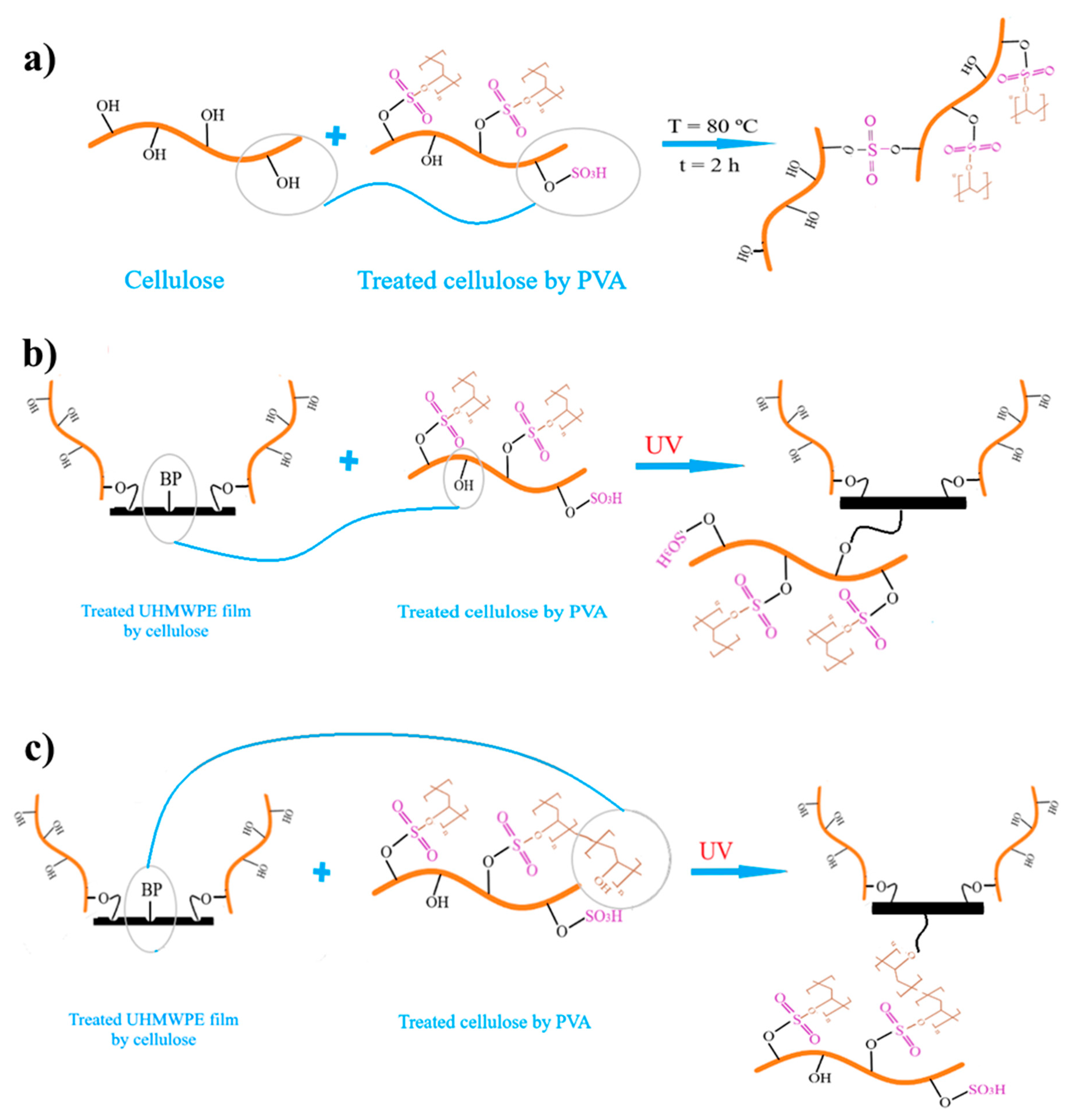
2.5.2. Glue Based on PVA/Cellulose
2.5.3. Glue Based on Phenol Formaldehyde
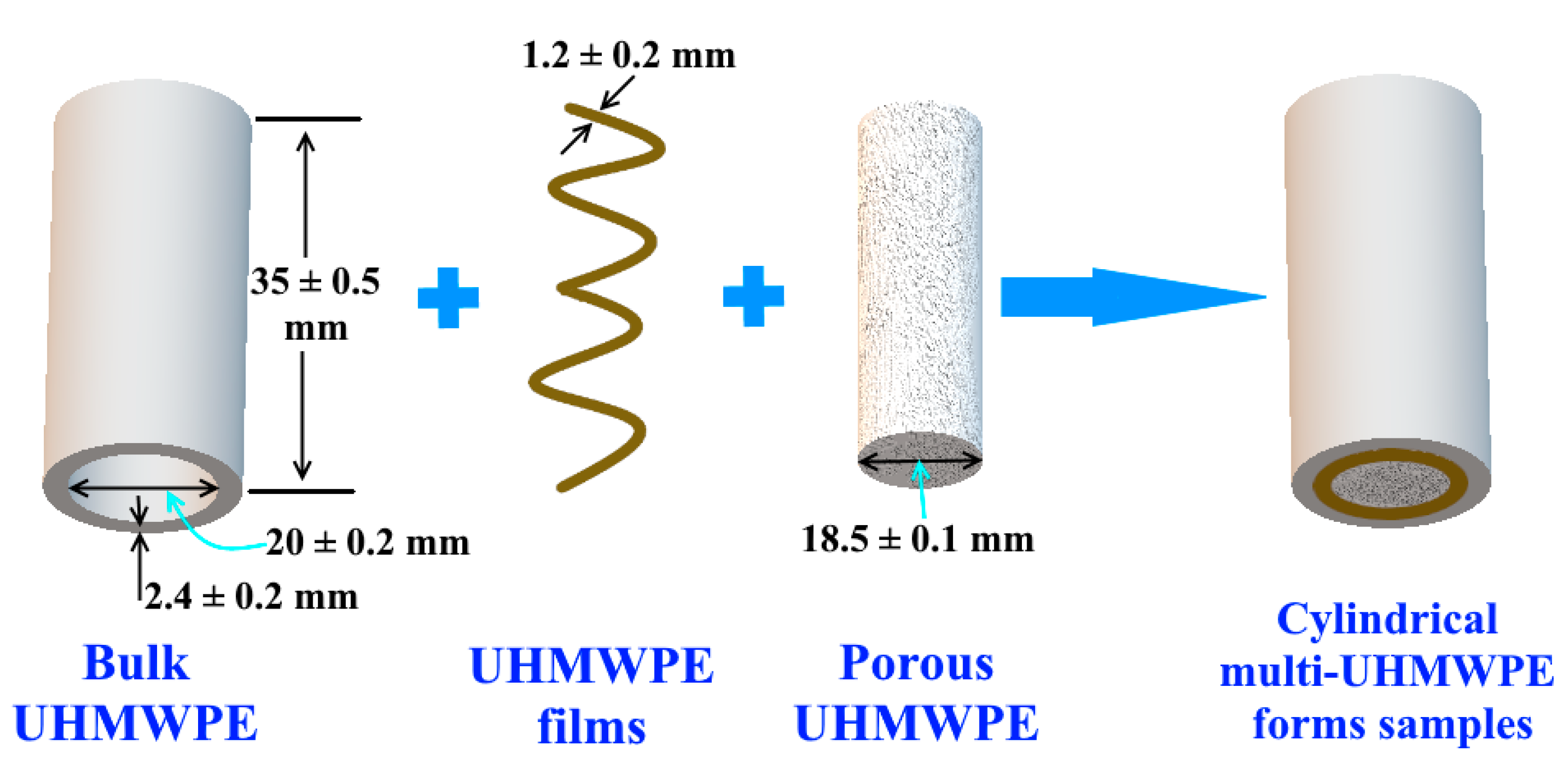
2.6. Preparing of Porous UHMWPE Cylindrical Samples
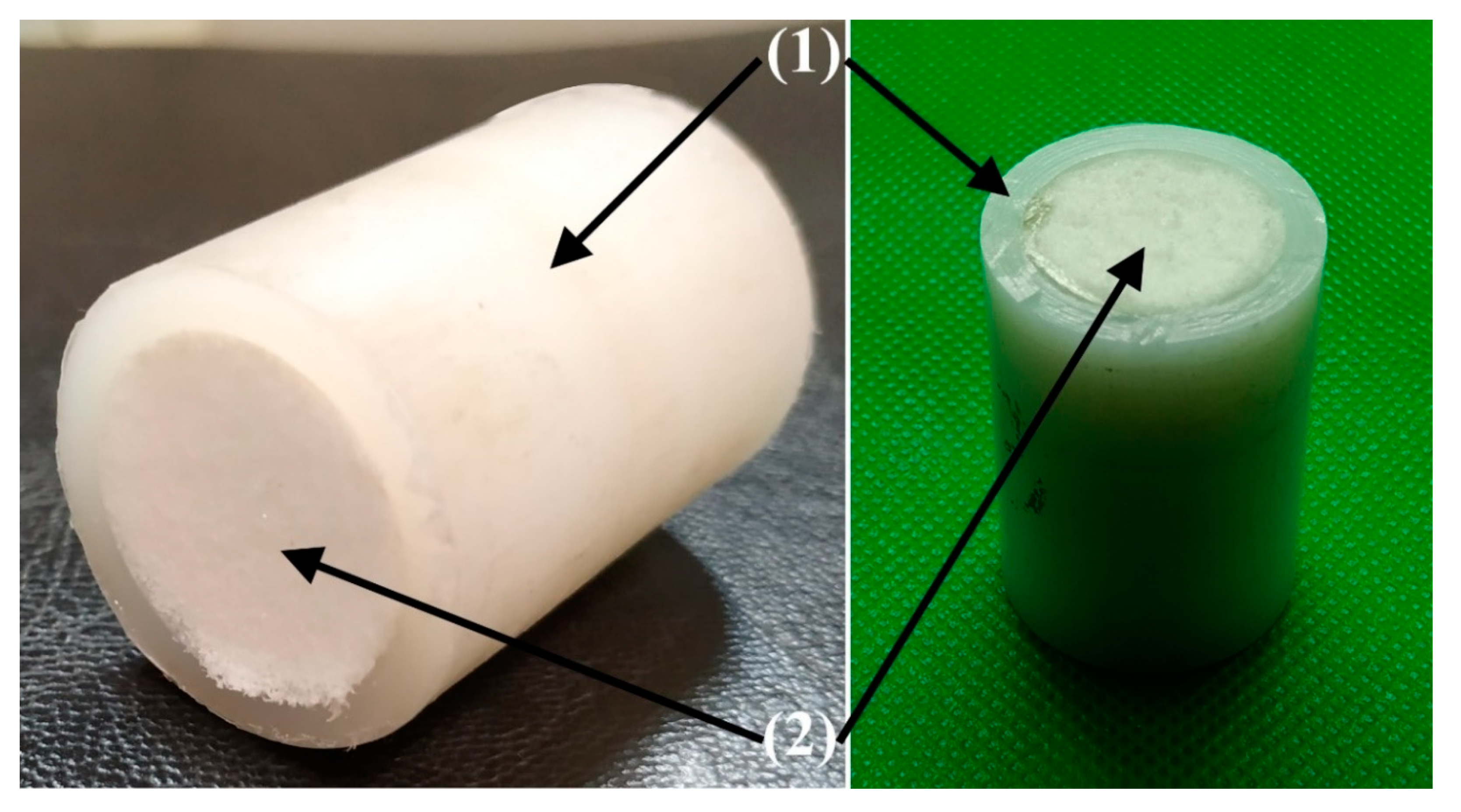
2.7. Preparing of Bulk UHMWPE Cylindrical Samples
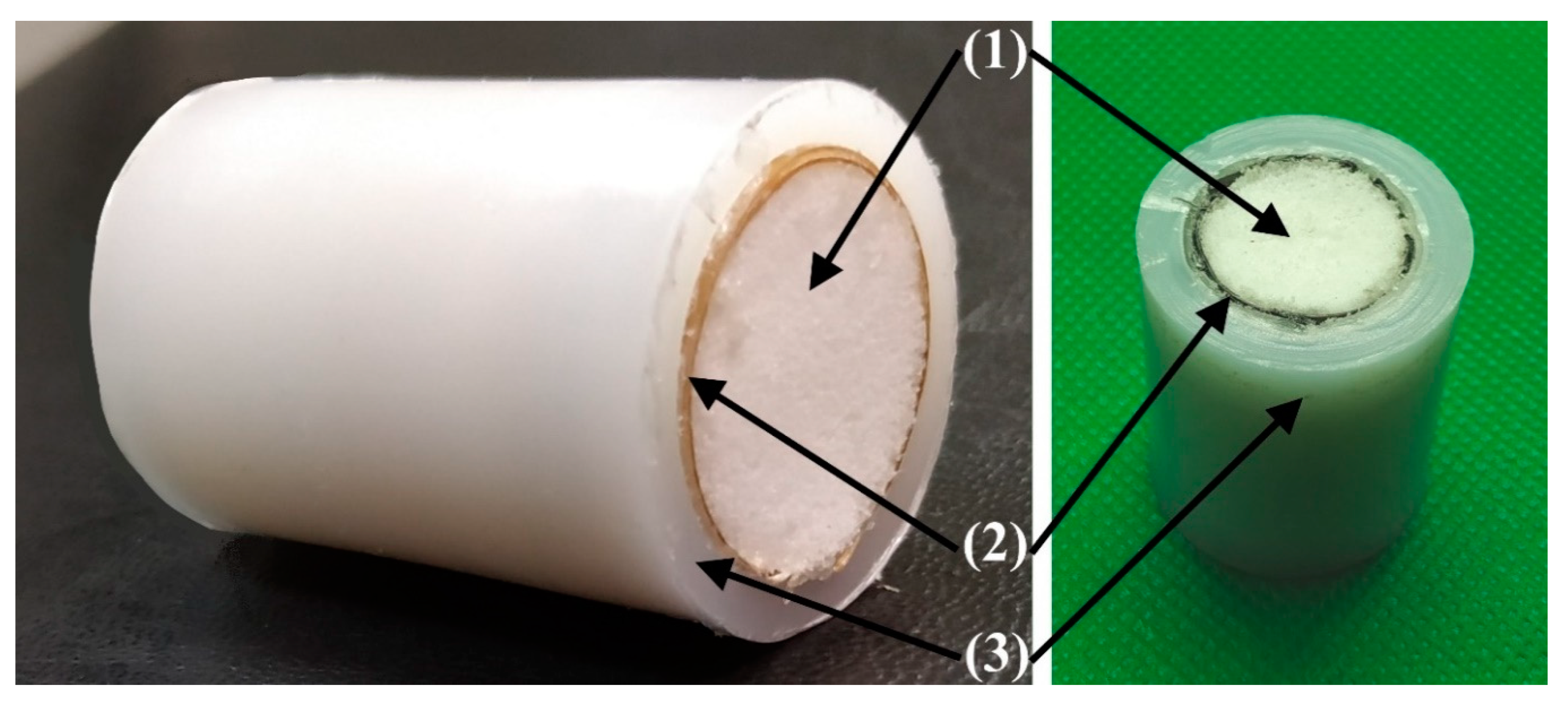
2.8. Preparing Cylindrical Multi-UHMWPE Forms Samples
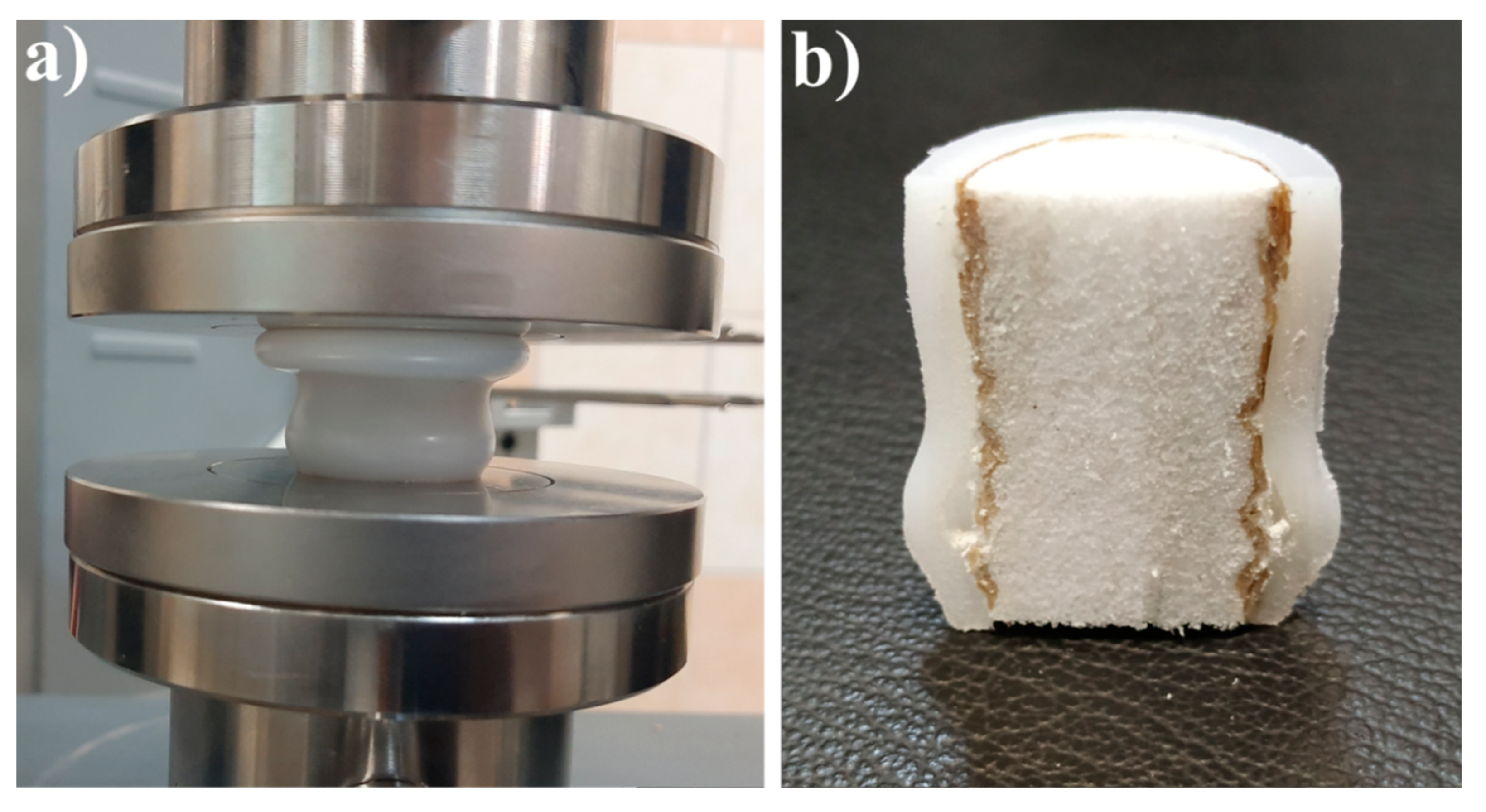
2.9. Mechanical Testing Procedures
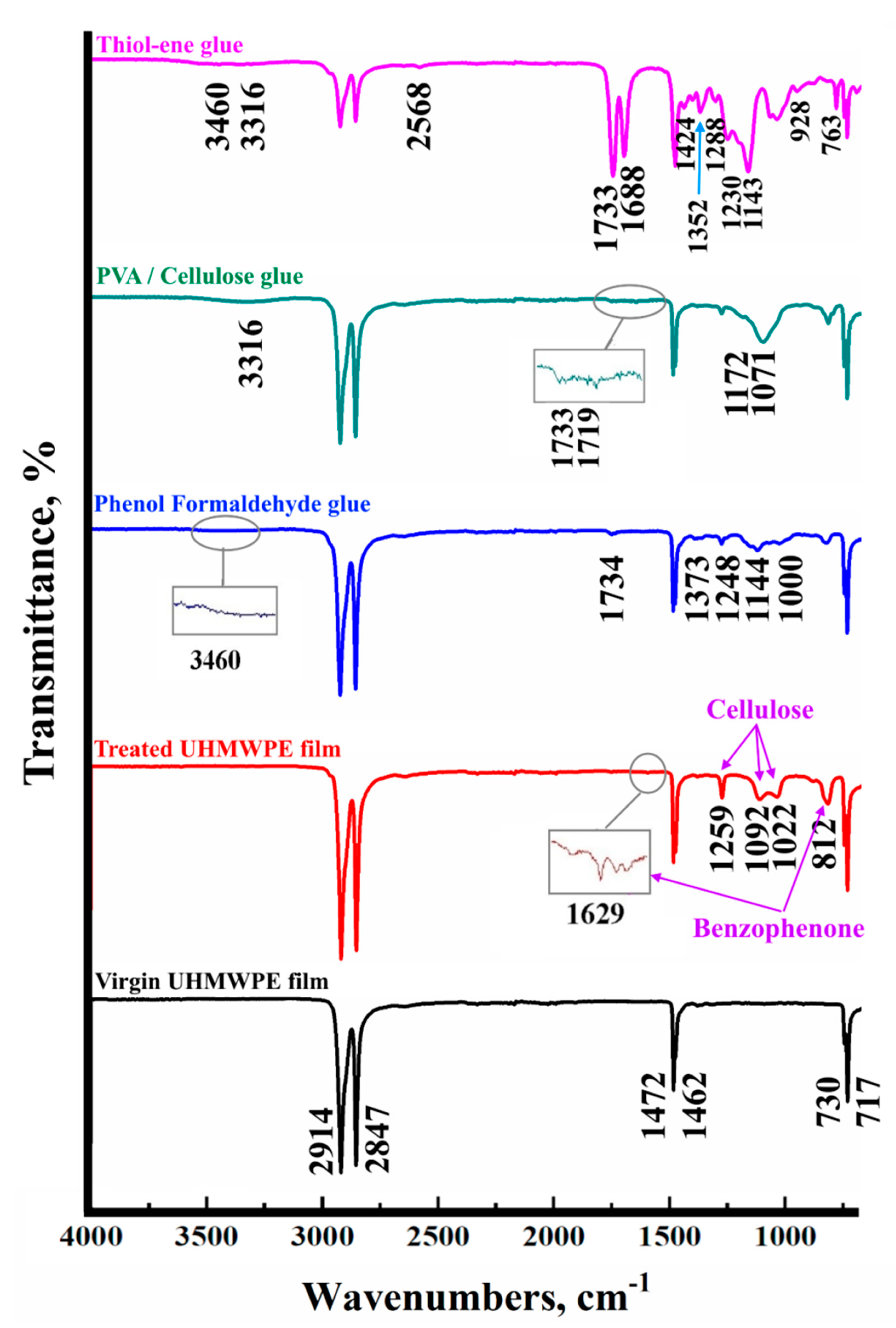
2.10. FT-IR Spectroscopy
2.11. Hemocompatibility Test
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hussain, M.; Naqvi, R.A.; Abbas, N.; Khan, S.M.; Nawaz, S.; Hussain, A.; Zahra, N.; Khalid, M.W. Ultra-High-Molecular-Weight-Polyethylene (UHMWPE) as a Promising Polymer Material for Biomedical Applications: A Concise Review. Polymers 2020, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Bracco, P.; Bellare, A.; Bistolfi, A.; Affatato, S. Ultra-High Molecular Weight Polyethylene: Influence of the Chemical, Physical and Mechanical Properties on the Wear Behavior. A Review. Materials 2017, 10, 791. [Google Scholar] [CrossRef] [PubMed]
- Graysona, W.L.; Martensa, T.P.; Enga, G.M.; Radisic, M.; Vunjak-Novakovic, G. Biomimetic approach to tissue engineering. Semin. Cell Dev. Biol. 2009, 20, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M. Recent Metallic Materials for Biomedical Applications. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2002, 33, 477–486. [Google Scholar] [CrossRef]
- Nuevo-Ordóñez, Y.; Montes-Bayón, M.; Blanco-González, E.; Paz-Aparicio, J.; Diánez Raimundez, J.; Tejerina, J.M.; Peña, M.A.; Sanz-Medel, A. Titanium release in serum of patients with different bone fixation implants and its interaction with serum biomolecules at physiological levels. Anal. Bioanal. Chem. 2011, 401, 2747–2754. [Google Scholar] [CrossRef]
- Kim, K.T.; Eo, M.Y.; Nguyen, T.T.H.; Kim, S.M. General review of titanium toxicity. Int. J. Implant Dent. 2019, 5, 10. [Google Scholar] [CrossRef]
- Dayyoub, T.; Maksimkin, A.V.; Kaloshkin, S.; Kolesnikov, E.; Chukov, D.; Dyachkova, T.P.; Gutnik, I. The Structure and Mechanical Properties of the UHMWPE Films Modified by the Mixture of Graphene Nanoplates with Polyaniline. Polymers 2019, 11, 23. [Google Scholar] [CrossRef]
- Maksimkin, A.V.; Kharitonov, A.P.; Nematulloev, S.G.; Kaloshkin, S.D.; Gorshenkov, M.V.; Chukov, D.I.; Shchetinin, I.V. Fabrication of oriented UHMWPE films using low solvent concentration. Mater. Des. 2017, 115, 133–137. [Google Scholar] [CrossRef]
- Van Vrekhem, S.; Vloebergh, K.; Asadian, M.; Vercruysse, C.; Declercq, H.; De Wilde, L.; De Geyter, N.; Morent, R.; Van Tongel, A. Improving the surface properties of an UHMWPE shoulder implant with an atmospheric pressure plasma jet. Sci. Rep. 2018, 8, 472. [Google Scholar] [CrossRef]
- Oosterom, R.; Ahmed, T.J.; Poulis, J.A.; Bersee, H.E.N. Adhesion performance of UHMWPE after different surface modification techniques. Med. Eng. Phys. 2006, 28, 323–330. [Google Scholar] [CrossRef]
- Kwon, O.H.; Nho, Y.C.; Lee, M.Y. Radiation-induced grafting of methylmethacrylate onto ultrahigh molecular weight polyethylene and its adhesive characteristics. J. Mater. Sci. Mater. Med. 2000, 11, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.R.; Feng, Y.; Chen, X.F. Graft modification of highly chlorinated polyethylene (HCPE) with methyl methacrylate by the mechanochemistry reaction. I. Synthesis and characterization. J. Appl. Polym. Sci. 2003, 89, 811–816. [Google Scholar] [CrossRef]
- Momose, K.; Takayama, S.; Hata, E.; Tomita, Y. Shift-multiplexed holographic digital data page storage in a nanoparticle-(thiol–ene) polymer composite film. Opt. Lett. 2012, 37, 2250–2252. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Kumacheva, E. Patterning surfaces with functional polymers. Nat. Mater. 2008, 7, 277–290. [Google Scholar] [CrossRef]
- DeForest, C.A.; Polizzotti, B.D.; Anseth, K.S. Sequential click reactions for synthesizing and patterning three-dimensional cell microenvironments. Nat. Mater. 2009, 8, 659–664. [Google Scholar] [CrossRef]
- Alge, D.L.; Anseth, K.S. Bioactive hydrogels: Lighting the way. Nat. Mater. 2013, 12, 950–952. [Google Scholar] [CrossRef]
- Edding, M.A.; Johnson, M.A.; Gale, B.K. Determining the optimal PDMS-PDMS bonding technique for microfluidic devices. J. Micromech. Microeng. Struct. Devices Syst. 2008, 18, 067001. [Google Scholar] [CrossRef]
- Riegger, L.; Strohmeier, O.; Faltin, B.; Zengerle, R.; Koltay, P. Adhesive bonding of microfluidic chips: Influence of process parameters. J. Micromech. Microeng. 2010, 20, 087003. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, W.; Sun, H.; Cui, C.; Zhang, L.; Jiang, Y.; Wu, Y.; Wang, Y.; Li, J.; Sumerlin, B.S.; et al. Thiol–ene click chemistry: A biocompatible way for orthogonal bioconjugation of colloidal nanoparticles. Chem. Sci. 2017, 8, 6182–6187. [Google Scholar] [CrossRef]
- Wu, Z.; Li, Y.; Zhang, L.; Zhong, Y.; Xu, H.; Mao, Z.; Wang, B.; Sui, X. Thiol–ene click reaction on cellulose sponge and its application for oil/water separation. RSC Adv. 2017, 7, 20147. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Long, K.; Zhu, X.; Yang, J. PVA Wood Adhesive Modified with Sodium Silicate Cross-linked Copolymer. In Proceedings of the 2012 International Conference on Biobase Material Science and Engineering, Changsha, China, 21–23 October 2012. [Google Scholar] [CrossRef]
- Chiellini, E.; Corti, A.; D’Antone, S.; Solaro, R. Biodegradation of poly (vinyl alcohol) based materials. Prog. Polym. Sci. 2003, 28, 963–1014. [Google Scholar] [CrossRef]
- Paradossi, G.; Cavalieri, F.; Chiessi, E.; Spagnoli, C.; Cowan, M.K. Poly (vinyl alcohol) as versatile biomaterial for potential biomedical applications. J. Mater. Sci. Mater. Med. 2003, 14, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Vrana, N.E.; Liu, Y.; McGuinness, G.B.; Cahill, P.A. Characterization of poly (vinyl alcohol)/chitosan hydrogels as vascular tissue engineering scaffolds. Macromol. Symp. 2008, 269, 106–110. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 43, 3–12. [Google Scholar] [CrossRef]
- Jorfi, M.; Foster, E.J. Recent advances in nanocellulose for biomedical applications. J. Appl. Polym. Sci. 2015, 132, 41719. [Google Scholar] [CrossRef]
- Azouz, K.B.; Ramires, E.C.; den Fonteyne, W.K.; Kissi, N.E.; Dufresne, A. Simple Method for the Melt Extrusion of a Cellulose Nanocrystal Reinforced Hydrophobic Polymer. ACS Macro Lett. 2012, 1, 236–240. [Google Scholar] [CrossRef]
- Vieira Ferreira, L.F.; Costa, A.I.; Ferreira Machado, I.; Branco, T.J.F.; Boufi, S.; Rei-Vilar, M.; Botelho do Rego, A.M. Surface Photochemistry: Benzophenone as a Probe for the Study of Modified Cellulose Fibres. Res. Lett. Phys. Chem. 2007, 1–5. [Google Scholar] [CrossRef]
- Kiziltas, A.; Rushing, T.S.; Nazari, B.; Kiziltas, E.E.; Gardner, D.J.S.; Han, Y.; Rushing, T.S. Cellulose NANOFIBER-polyethylene nanocomposites modified by polyvinyl alcohol. J. Appl. Polym. Sci. 2015, 133, 42933. [Google Scholar] [CrossRef]
- Atta-Obeng, E.; Via, B.K.; Fasina, O.; Auad, M.L.; Jiang, W. Cellulose Reinforcement of Phenol Formaldehyde: Characterization and Chemometric Elucidation. Int. J. Compos. Mater. 2013, 3, 61–68. [Google Scholar] [CrossRef]
- Adsul, M.; Soni, S.; Bhargava, S.; Bansal, V. Facile approach for the dispersion of regenerated cellulose in aqueous system in the form of nanoparticles. Biomacromolecules 2012, 13, 2890–2895. [Google Scholar] [CrossRef]
- Dayyoub, T.; Maksimkin, A.V.; Senatov, F.S.; Kaloshkin, S.D.; Zimina, A.; Kolesnikov, E.A. Treating UHMWPE surface for enhancing the adhesion properties by cellulose grafting. Int. J. Adhes. Adhes. 2020, 98, 102535. [Google Scholar] [CrossRef]
- Dayyoub, T.; Olifirov, L.K.; Chukov, D.I.; Kaloshkin, S.D.; Kolesnikov, E.; Nematulloev, S. The Structural and Mechanical Properties of the UHMWPE Films Mixed with the PE-Wax. Materials 2020, 13, 3422. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Riedl, B. Curing kinetics of phenol formaldehyde resin and wood-resin interactions in the presence of wood substrates. Wood Sci. Technol. 2004, 38, 69–81. [Google Scholar] [CrossRef]
- Maksimkin, A.V.; Kaloshkin, S.D.; Tcherdyntsev, V.V.; Chukov, D.I.; Stepashkin, A.A. Technologies for Manufacturing Ultrahigh Molecular Weight Polyethylene_Based Porous Structures for Bone Implants. Biomed. Eng. 2013, 47, 73–77. [Google Scholar] [CrossRef]
- Bayraktar, H.H.; Morgan, E.F.; Niebur, G.L.; Morris, G.E.; Wong, E.K.; Keaveny, T.M. Comparison of the elastic and yield properties of human femoral trabecular and cortical bone tissue. J. Biomech. 2004, 37, 27–35. [Google Scholar] [CrossRef]
- Maksimkin, A.V.; Senatov, F.S.; Anisimova, N.Y.; Kiselevskiy, M.V.; Zalepugin, D.Y.; Chernyshova, I.V.; Tilkunova, N.A.; Kaloshkin, S.D. Multilayer porous UHMWPE scaffolds for bone defects replacement. Mater. Sci. Eng. C 2017, 73, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.; Ni, Z.; Chen, G.; Huang, G.; Huang, H.; Zhao, Y. Mechanical and thermal properties of graphene oxide/ultrahigh molecular weight polyethylene nanocomposites. RSC Adv. 2015, 5, 63063–63072. [Google Scholar] [CrossRef]
- Stojilovic, N.N.; Dordevic, S.V.; Stojadinovic, S. Effects of clinical X-ray irradiation on UHMWPE films. Nucl. Instrum. Methods Phys. Res. B 2017, 410, 139–143. [Google Scholar] [CrossRef]
- Juchnovski, I.; Kolev, T.; Stamboliyska, B. Infrared Spectra of Benzophenone-Ketyls. Effects of Meta- and Para-Substituents on the vC[dbnd]O Frequencies. Correlation Of vC[dbnd]O of Substituted Benzophenone-ketyls With The Hueckel PC[dbnd]O Bond Order. Spectrosc. Lett. Int. J. Rapid Commun. 1993, 26, 67–78. [Google Scholar] [CrossRef]
- Agarwal, U.P.; Atalla, R.H.; Isogai, A. Nanocelluloses: Their Preparation, Properties, and Application; ACS Symposium Series: Washington, DC, USA, 2017; Volume 1251, ISBN 9780841232174. [Google Scholar] [CrossRef]
- Fan, M.; Dai, D.; Biao Huang, B. Fourier Transform Infrared Spectroscopy for Natural Fibres. In Fourier Transform—Materials Analysis, Salih Mohammed Salih; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies. Tables and Charts, 3rd ed.; John Wiley & Sons Ltd.: West Sussex, UK, 2001; ISBN 0470093072. [Google Scholar]
- Abdulkhania, A.; Marvasta, E.H.; Ashorib, A.; Hamzeha, Y.; Karimi, A.N. Preparation of cellulose/polyvinyl alcohol biocomposite films using1-n-butyl-3-methylimidazolium chloride. Int. J. Biol. Macromol. 2013, 62, 379–386. [Google Scholar] [CrossRef]
- Zioupos, P.; Currey, J.D. Changes in the Stiffness, Strength, and Toughness of Human Cortical Bone with Age. Bone 1998, 22, 57–66. [Google Scholar] [CrossRef]
- Teoh, S.H. Introduction to Biomaterials Engineering and Processing—An Overview. Biomater. Eng. Process. Ser. 2004, 1-1–1-16. [Google Scholar] [CrossRef]

| Tensile Strength, MPa | Young’s Modulus, GPa | Elongation, % |
|---|---|---|
| 1100 ± 50 | 50 ± 3 | 3.4 ± 1.0 |
| Type of Glue | Before the Treatment | After the Treatment |
|---|---|---|
| Peel Strength, Kg/cm | Peel Strength, Kg/cm | |
| Glue based on thiol-ene reaction | 0.010 ± 0.002 | 0.40 ± 0.08 |
| Glue based on PVA/Cellulose | 0.015 ± 0.002 | 0.43 ± 0.09 |
| Glue based on phenol formaldehyde | 0.015 ± 0.002 | 0.62 ± 0.10 |
| Type of Glue | Cylindrical Multi-UHMWPE Forms Samples (Bulk and Porous) | Cylindrical Multi-UHMWPE Forms Samples (Bulk, Porous and 2 Layers of Films) | ||||
|---|---|---|---|---|---|---|
| Compressive Modulus, GPa | Compressive Yield Strength, MPa | Deformation at Compressive Yield Strength, % | Compressive Modulus, GPa | Compressive Yield Strength, Mpa | Deformation at Compressive Yield Strength, % | |
| Glue based on thiol-ene reaction | 0.642 ± 0.007 | 11.9 ± 1.1 | 19.2 ± 0.6 | 0.862 ± 0.009 | 12.9 ± 1.0 | 17.7 ± 0.9 |
| Glue based on PVA/Cellulose | 0.601 ± 0.008 | 9.87 ± 0.9 | 13.8 ± 0.8 | 0.720 ± 0.010 | 12.4 ± 0.9 | 18.5 ± 0.5 |
| Glue based on phenol formaldehyde | 0.590 ± 0.006 | 10.1 ± 1.0 | 14.2 ± 0.9 | 0.592 ± 0.011 | 10.5 ± 0.7 | 15.5 ± 0.6 |
| Type of Glue | Bulk Armored Layer | Bulk Armored Layer with 2 Layers of Films | ||||
|---|---|---|---|---|---|---|
| Compressive Modulus, GPa | Compressive Yield Strength, MPa | Deformation at Yield Compressive Strength, % | Compressive Modulus, GPa | Compressive Yield Strength, MPa | Deformation at Compressive Yield Strength, % | |
| Glue based on thiol-ene reaction | 0.728 ± 0.007 | 36.5 ± 0.9 | 19.1 ± 1.1 | 0.912 ± 0.008 | 41.8 ± 0.5 | 17.2 ± 1.1 |
| Glue based on PVA/Cellulose | 0.775 ± 0.011 | 29.9 ± 1.2 | 20.0 ± 0.8 | 1.050 ± 0.006 | 37.1 ± 0.9 | 16.2 ± 0.9 |
| Glue based on phenol formaldehyde | 0.677 ± 0.009 | 30.8 ± 0.8 | 18.2 ± 0.9 | 0.693 ± 0.009 | 33.4 ± 0.8 | 13.1 ± 1.3 |
| Type of Sample | Four Layers of the UHMWPE Films | Six Layers of the UHMWPE Films | ||||
|---|---|---|---|---|---|---|
| Compressive Modulus, GPa | Compressive Yield Strength, MPa | Deformation at Compressive Yield Strength, % | Compressive Modulus, GPa | Compressive Yield Strength, MPa | Deformation at Compressive Yield Strength, % | |
| Cylindrical multi-UHMWPE forms samples (bulk, porous and films) | 0.836 ± 0.016 | 13.3 ± 0.9 | 19.5 ± 0.6 | 0.884 ± 0.011 | 13.8 ± 1.5 | 17.8 ± 0.5 |
| Cylindrical multi-UHMWPE forms samples (two armored layers (bulk and films) | 1.120 ± 0.011 | 43.7 ± 1.5 | 18.1 ± 0.6 | 1.130 ± 0.010 | 44.1 ± 1.1 | 17.8 ± 0.2 |
| UHMWPE [38,39] | Benzophenone [40] | Cellulose [41,42] | |||
|---|---|---|---|---|---|
| Wave Number, cm−1 | Functional Group | Wave Number, cm−1 | Functional Group | Wave Number, cm−1 | Functional Group |
| 717 | rocking vibration peak due to the high degree of polymerization and long molecular chain of UHMWPE | 812 | C–CO–C sym. str. | 1022 | C–O stretching group of 3,6-anhydrogalactose |
| 730 | |||||
| 1092 | |||||
| 1462 | in-plane bending vibration peak of C–H | ||||
| 1472 | 1629 | C=O stretch | 1259 | C–OH bending at C6 | |
| 2847 | sym. stretching vibration peak of C–H | ||||
| 2914 | asym. stretching vibration peak of C–H | ||||
| Phenol formaldehyde [43,44] | PVA/Cellulose [30,43] | Thiol-ene [43] | |||
| Wave number, cm−1 | Functional group | Wave number, cm−1 | Functional group | Wave number, cm−1 | Functional group |
| 1019 | alcohol C–O stretching vibrations | 1071 | stretching vibrations of the S=O group (PVA) bending vibration in cellulose/PVA | 763 | str vib –C–S |
| 928 | CH3–S (CH3 rocking vib. Sal. Compounds) | ||||
| 1144 | C–O stretch (methylol) | 1172 | =SO2 asymmetric and symmetric stretching Vibrations dialkyl sulphites, (RO)2SO | 1143 | C–O stretch (methylol) |
| 1230 | –CH2–S– (CH2 def vib) | ||||
| 1248 | C–O–C stretch | 1248 | C–O–C stretch | 1288 | |
| 1352 | sym-triazines (Ring str, at least one band) | ||||
| 1373 | OH in plane (phenolic) | 1719 | C=O stretch (overlapped with OH scissors of water) | 1389 | |
| 1424 | CH3CH2–S– (CH2 def vib) | ||||
| 1734 | C=O stretch (overlapped with OH scissors of water) | 1733 | 1688 | dialkyl thiolesters, R–CO–SR (C=O str) | |
| 2568 | str vib S–H | ||||
| 3460 | Ortho-substituted (X–C–O X=OR) | 3316 | vOH cellulose/PVA | 3316 | vOH |
| 3460 | Ortho-substituted (X–C–OX=OR) | ||||
| Sample | Hemolysis, % | LDH Release | |
|---|---|---|---|
| Incubation Time, h | |||
| 2 | 4 | ||
| Treated UHMWPE films grafted by Cellulose | 3 ± 0.1 | 5 ± 1.0 | 1805 ± 0.039 (p = 0.093) |
| Glue based on thiol-ene reaction | 3 ± 0.2 | 4 ± 1.5 | 1814 ± 0.096 (p = 0.216) |
| Glue based on PVA/Cellulose | 4 ± 0.1 | 9 ± 3.3 | 2033 ± 0.051 (p = 0.071) |
| Untreated cells (Control) | - | - | 1933 ± 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dayyoub, T.; Maksimkin, A.; Senatov, F.; Kaloshkin, S.; Anisimova, N.; Kiselevskiy, M. A New Approach Based on Glued Multi-Ultra High Molecular Weight Polyethylene Forms to Fabricate Bone Replacement Products. Polymers 2020, 12, 2545. https://doi.org/10.3390/polym12112545
Dayyoub T, Maksimkin A, Senatov F, Kaloshkin S, Anisimova N, Kiselevskiy M. A New Approach Based on Glued Multi-Ultra High Molecular Weight Polyethylene Forms to Fabricate Bone Replacement Products. Polymers. 2020; 12(11):2545. https://doi.org/10.3390/polym12112545
Chicago/Turabian StyleDayyoub, Tarek, Aleksey Maksimkin, Fedor Senatov, Sergey Kaloshkin, Natalia Anisimova, and Mikhail Kiselevskiy. 2020. "A New Approach Based on Glued Multi-Ultra High Molecular Weight Polyethylene Forms to Fabricate Bone Replacement Products" Polymers 12, no. 11: 2545. https://doi.org/10.3390/polym12112545
APA StyleDayyoub, T., Maksimkin, A., Senatov, F., Kaloshkin, S., Anisimova, N., & Kiselevskiy, M. (2020). A New Approach Based on Glued Multi-Ultra High Molecular Weight Polyethylene Forms to Fabricate Bone Replacement Products. Polymers, 12(11), 2545. https://doi.org/10.3390/polym12112545