The Viable Fabrication of Gas Separation Membrane Used by Reclaimed Rubber from Waste Tires
Abstract
1. Introduction
Research Significance
2. Experiment
2.1. Materials
2.2. Synthesis of Rubber-Derived TR Membrane
2.3. Characterization of Collected Rubber Samples and Fabricated TR Membranes
2.4. Permeation Test
3. Results and Discussion
3.1. Analysis of As-Received Rubber Sample
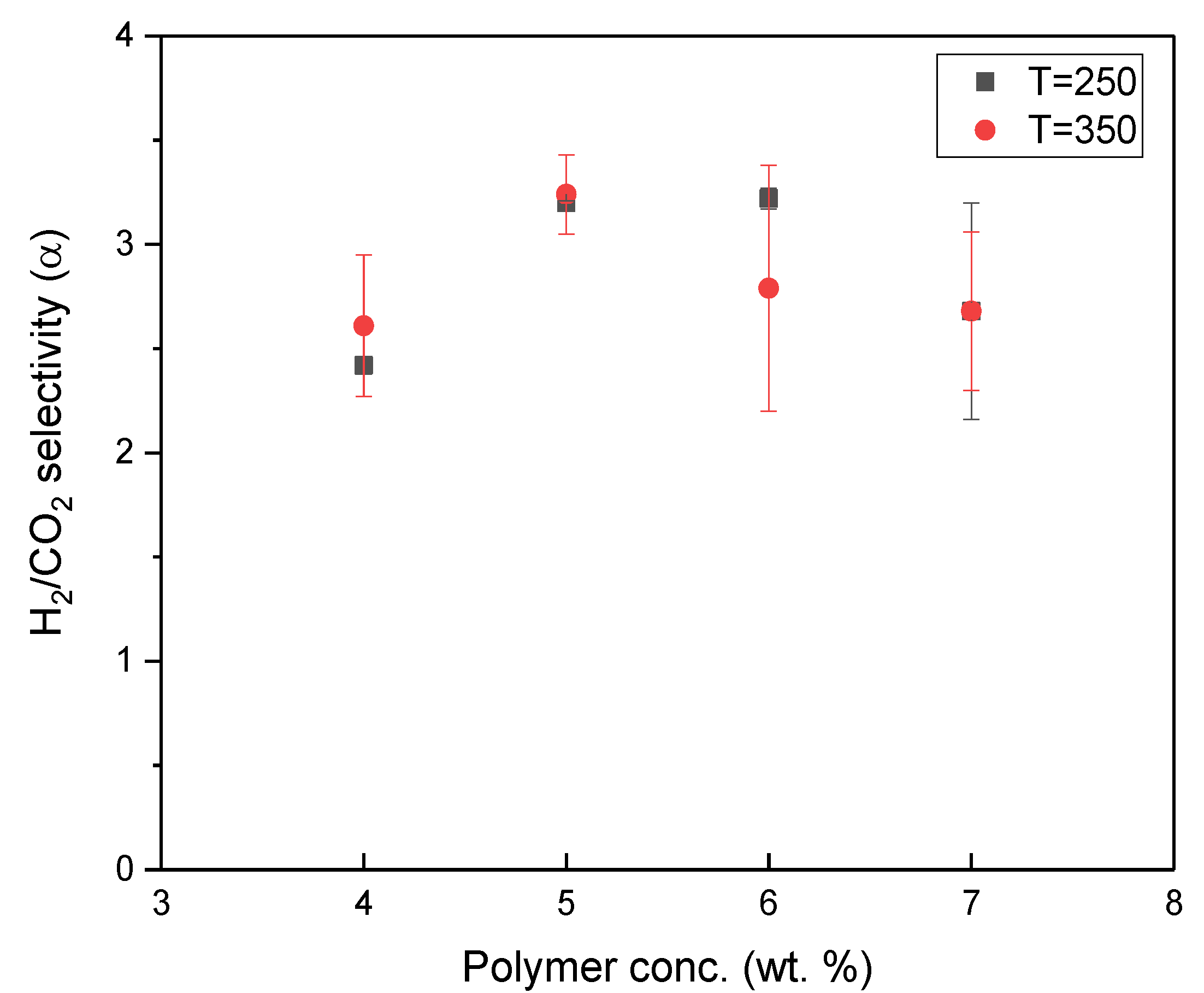
3.2. Effect of Reclaimed Rubber Precursor Concentrations on Gas Separation Performance
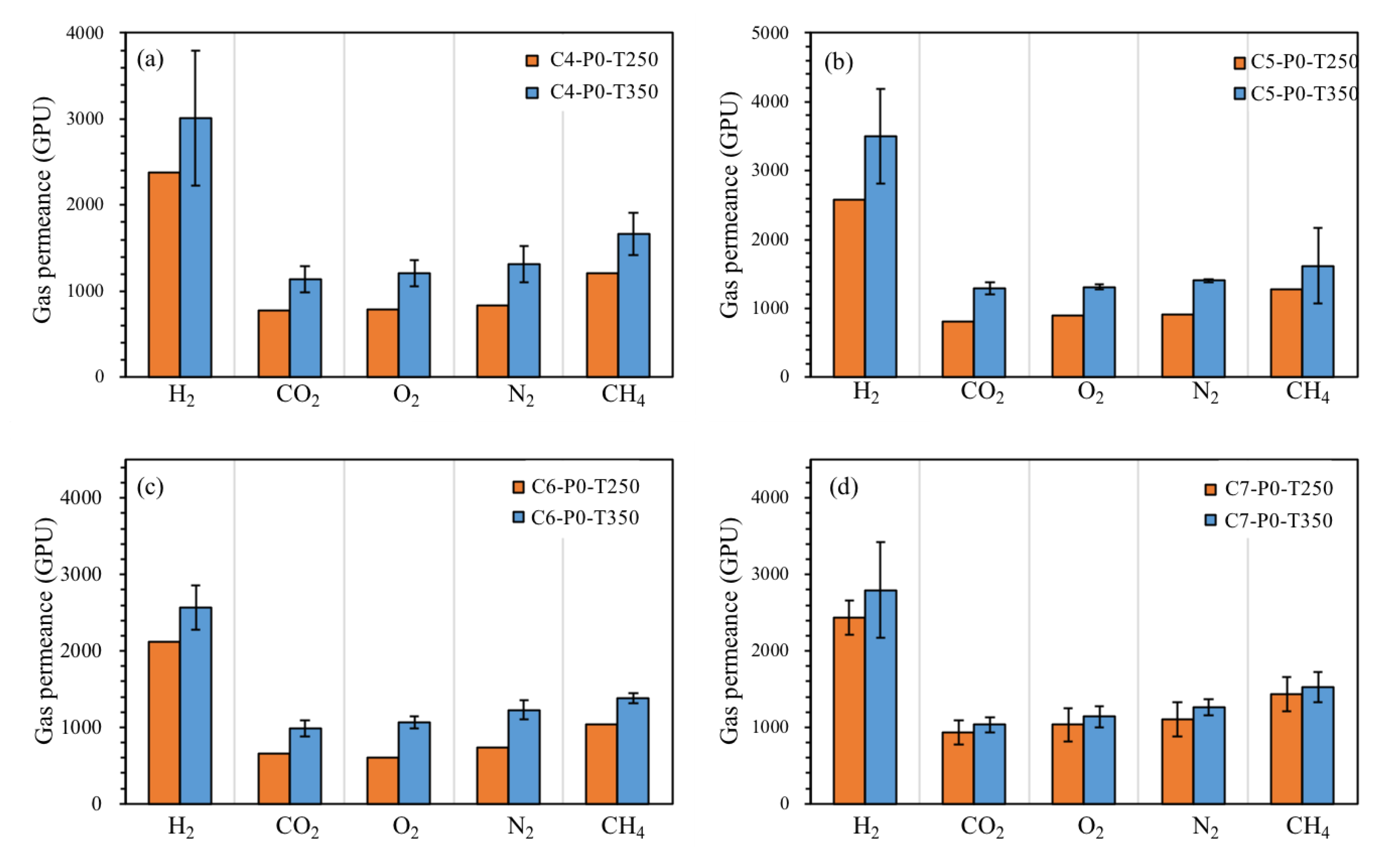
3.3. Effect of Heating Temperature on Gas Separation Performance
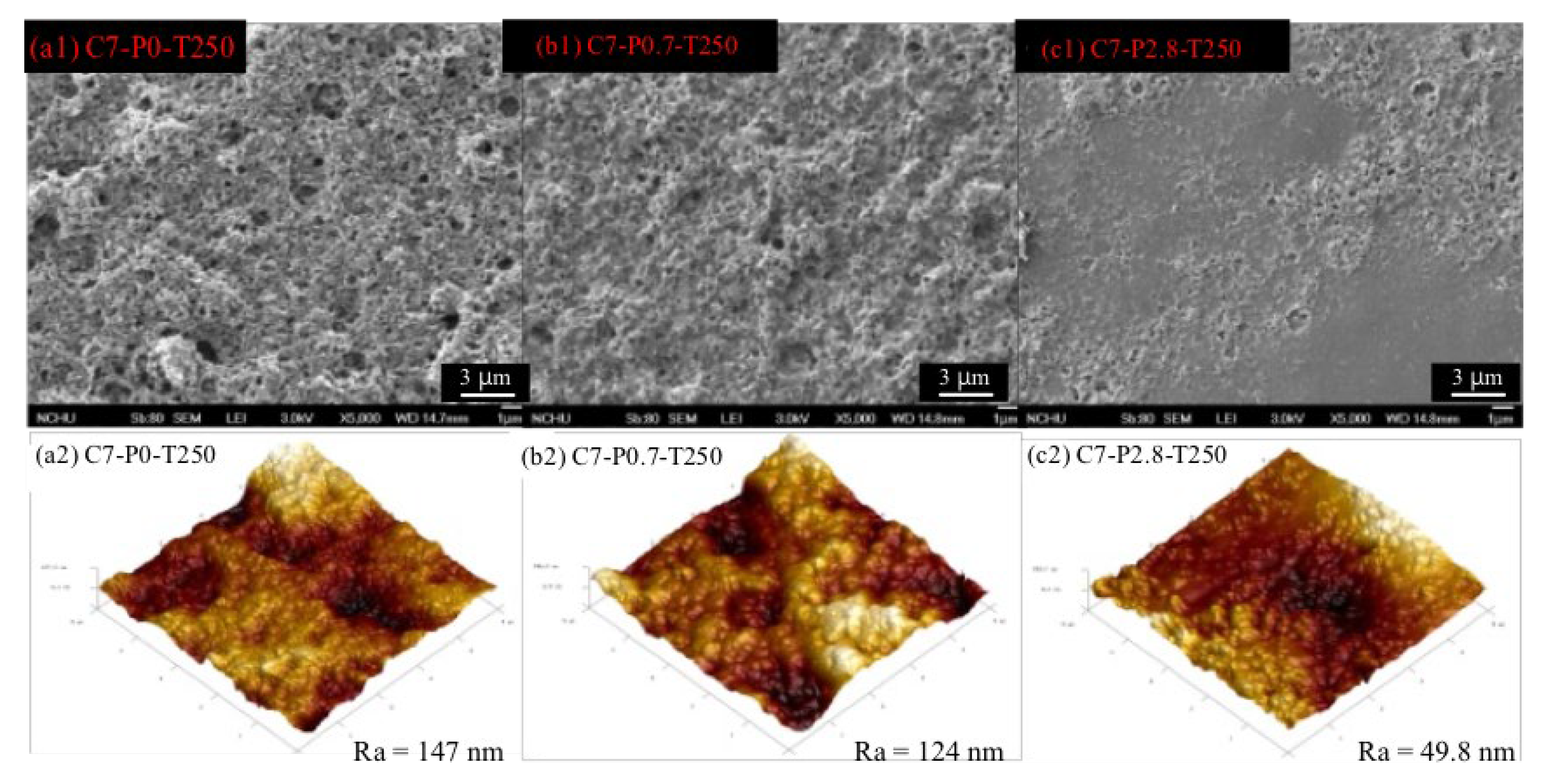
3.4. Effect of PPO Additive Content on Gas Separation Performance
3.5. Comparison of Rubber-Derived TR Membrane with Other Published Literature
4. Conclusions
- (a)
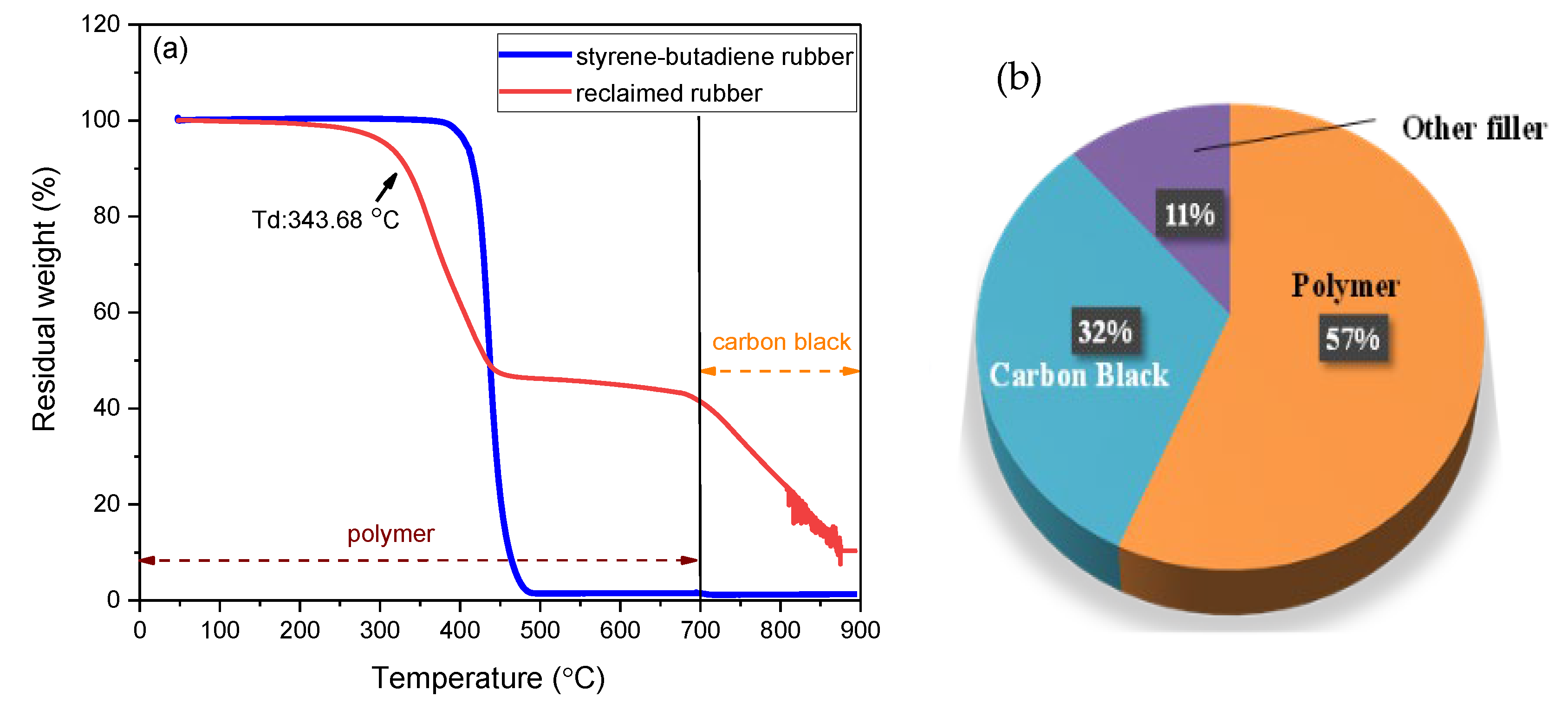
- The as-received reclaimed rubber was intensively investigated by TGA and FTIR analysis. The results revealed that the rubber type of reclaimed rubber is styrene–butadiene rubber, which accounted for more than half of the total components (57%).
- (b)
- The presence of carbon black and other filler was also found, and their presence decreased the thermal stability of rubber.
- (c)
- We measured the degree of devulcanization and the results showed that reclaimed rubber exhibited 45.5% devulcanization.
- (d)
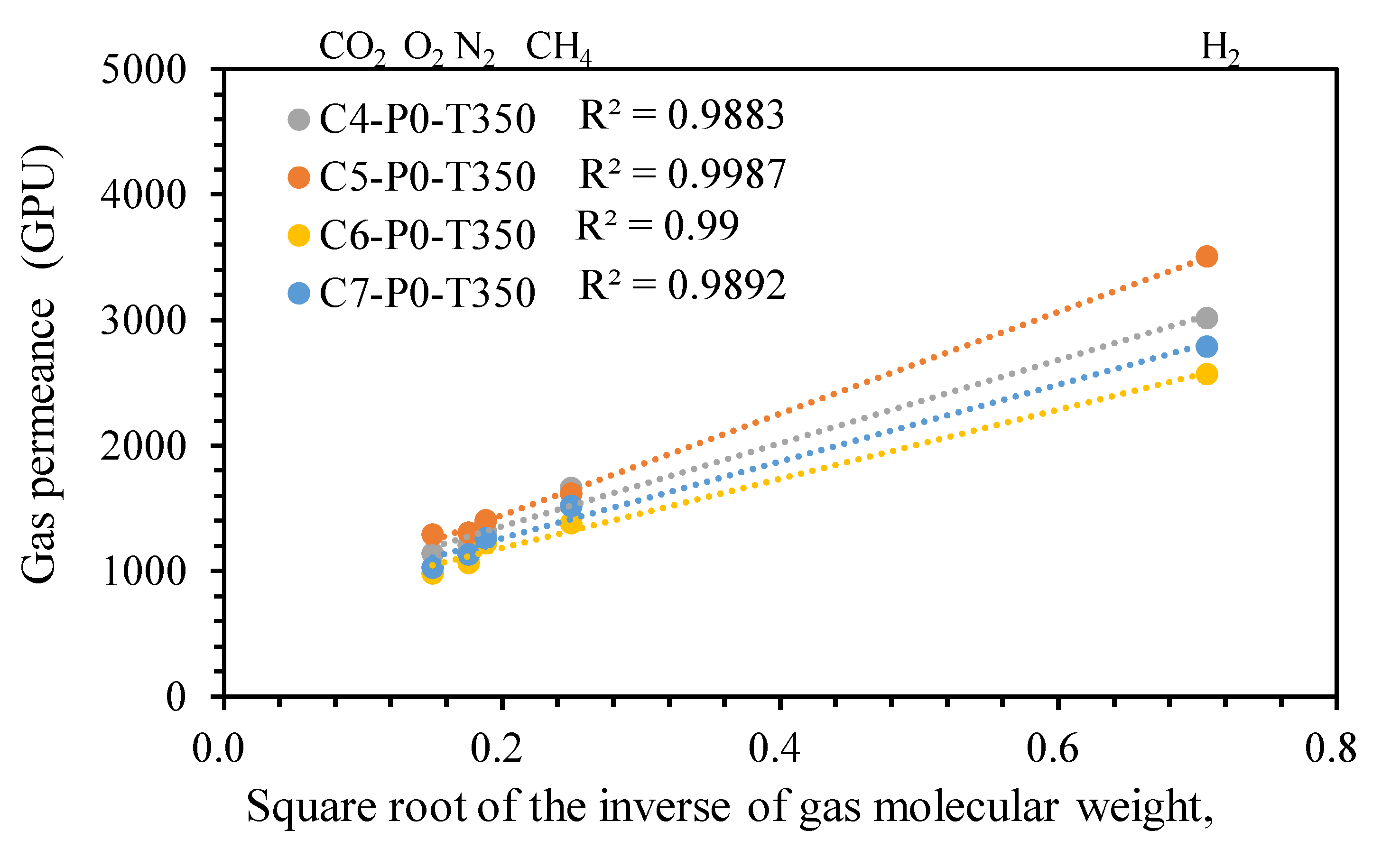
- According to the gas permeation tests, all rubber-derived TR membranes exhibited the Knudsen diffusion mechanism. In particular, the C7-P2.8-T250 membrane, with an ideal H2/CO2 selectivity of four and hydrogen permeability of 1333 Barrer, demonstrated a superior gas transport capability, mainly due to the conversion of the reclaimed rubber layer into a microporous membrane structure and the incorporation of a thermal stable filler.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Deng, S.; Zhuo, H.; Wang, Y.; Leng, S.; Zhuang, G.; Zhong, X.; Wei, Z.; Yao, Z.; Wang, J.G. Multiscale simulation on product distribution from pyrolysis of styrene-butadiene rubber. Polymers 2019, 11, 1967. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Dincer, I. Comparative assessment of various gasification fuels with waste tires for hydrogen production. Int. J. Hydrog. Energy 2019, 44, 18818–18826. [Google Scholar] [CrossRef]
- Xu, F.; Wang, B.; Yang, D.; Ming, X.; Jiang, Y.; Hao, J.; Qiao, Y.; Tian, Y. TG-FTIR and Py-GC/MS study on pyrolysis mechanism and products distribution of waste bicycle tire. Energy Convers. Manag. 2018, 175, 288–297. [Google Scholar] [CrossRef]
- Tsai, W.-T.; Chen, C.-C.; Lin, Y.-Q.; Hsiao, C.-F.; Tsai, C.-H.; Hsieh, M.-H. Status of waste tires’ recycling for material and energy resources in taiwan. J. Mater. Cycles Waste Manag. 2017, 19, 1288–1294. [Google Scholar] [CrossRef]
- Jankovská, Z.; Večeř, M.; Koutník, I.; Matějová, L. A Case Study of Waste Scrap Tyre-Derived Carbon Black Tested for Nitrogen, Carbon Dioxide, and Cyclohexane Adsorption. Molecules 2020, 25, 4445. [Google Scholar] [CrossRef] [PubMed]
- Rowhani, A.; Rainey, T.J. Scrap Tyre Management Pathways and Their Use as a Fuel—A Review. Energies 2016, 9, 888. [Google Scholar] [CrossRef]
- Kaewunruen, S.; Li, D.; Chen, Y.; Xiang, Z. Enhancement of Dynamic Damping in Eco-Friendly Railway Concrete Sleepers Using Waste-Tyre Crumb Rubber. Materials 2018, 11, 1169. [Google Scholar] [CrossRef]
- Czajczyńska, D.; Krzyżyńska, R.; Jouhara, H. Syngas Quality as a Key Factor in the Design of an Energy-Efficient Pyrolysis Plant for Scrap Tyres. Multidiscip. Digit. Publ. Inst. Proc. 2018, 2, 1455. [Google Scholar] [CrossRef]
- Park, H.B.; Han, S.H.; Jung, C.H.; Lee, Y.M.; Hill, A.J. Thermally rearranged (TR) polymer membranes for CO2 separation. J. Membr. Sci. 2010, 359, 11–24. [Google Scholar] [CrossRef]
- Jo, H.J.; Soo, C.Y.; Dong, G.; Do, Y.S.; Wang, H.H.; Lee, M.J.; Quay, J.R.; Murphy, M.K.; Lee, Y.M. Thermally rearranged poly(benzoxazole-co-imide) membranes with superior mechanical strength for gas separation obtained by tuning chain rigidity. Macromolecules 2015, 48, 2194–2202. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.M. Thermally rearranged (TR) polymer membranes with nanoengineered cavities tuned for CO2 separation. J. Nanopart. Res. 2012, 14, 265–275. [Google Scholar] [CrossRef]
- Patel, A.; Acharya, N. Thermally rearranged (TR) HAB-6FDA nanocomposite membranes for hydrogen separation. Int. J. Hydrog. Energy 2019, 45, 18685–18692. [Google Scholar] [CrossRef]
- Luo, K.; Zheng, W.; Zhao, X.; Wang, X.; Wu, S. Effects of antioxidant functionalized silica on reinforcement and anti-aging for solution-polymerized styrene butadiene rubber: Experimental and molecular simulation study. Mater. Des. 2018, 154, 312–325. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T. Fuel production from pyrolysis of natural and synthetic rubbers. Fuel 2017, 191, 403–410. [Google Scholar] [CrossRef]
- Fazli, A.; Rodrigue, D. Waste rubber recycling: A review on the evolution and properties of thermoplastic elastomers. Materials 2020, 13, 782. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, A. Sustainable Jute-Based Composite Materials: Mechanical and Thermomechanical Behaviour; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Williams, K.R. Rubber reclamation. J. Chem. Educ. 2007, 84, 217. [Google Scholar] [CrossRef]
- PerkinElmer. Compositional Analysis of Tire Elastomers Using Auto Stepwise TGA; PerkinElmer, Inc.: Waltham, MA, USA.
- Chen, Y.; Xu, C. Stress softening of NR reinforced by in situ prepared zinc dimethacrylate. J. Appl. Polym. Sci. 2012, 123, 833–841. [Google Scholar] [CrossRef]
- Xia, Z.; Patchan, M.; Maranchi, J.; Elisseeff, J.; Trexler, M. Determination of crosslinking density of hydrogels prepared from microcrystalline cellulose. J. Appl. Polym. Sci. 2013, 127, 4537–4541. [Google Scholar] [CrossRef]
- Wey, M.-Y.; Wang, C.-T.; Lin, Y.-T.; Lin, M.-D.; Uchytil, P.; Setnickova, K.; Tseng, H.-H. Interfacial interaction between CMS layer and substrate: Critical factors affecting membrane microstructure and H2 and CO2 separation performance from CH4. J. Membr. Sci. 2019, 580, 49–61. [Google Scholar] [CrossRef]
- Zhuang, G.-L.; Tseng, H.-H.; Wey, M.-Y. Facile synthesis of CO2-selective membrane derived from butyl reclaimed rubber (BRR) for efficient CO2 separation. J. CO2 Util. 2018, 25, 226–234. [Google Scholar] [CrossRef]
- Mangili, I.; Collina, E.; Anzano, M.; Pitea, D.; Lasagni, M. Characterization and supercritical CO2 devulcanization of cryo-ground tire rubber: Influence of devulcanization process on reclaimed material. Polym. Degrad. Stab. 2014, 102, 15–24. [Google Scholar] [CrossRef]
- Mann, D.C.; Cline, K.G. Confirmation of the Presence of Styrene Butadiene Resin (SBR) Polymer in Drywall Primer Applications; PerkinElmer, Inc.: Waltham, MA, USA, 2010. [Google Scholar]
- Willis, J.N.; Liu, X. A Study of Styrene Butadiene Rubber Using GPC-FTIR; International GPC Symposium; Waters Corporation: Florida, FL, USA, 1994. [Google Scholar]
- Wey, M.-Y.; Chen, H.-H.; Lin, Y.-T.; Tseng, H.-H. Thin carbon hollow fiber membrane with Knudsen diffusion for hydrogen/alkane separation: Effects of hollow fiber module design and gas flow mode. Int. J. Hydrog. Energy 2020, 45, 7290–7302. [Google Scholar] [CrossRef]
- Gilron, J.; Soffer, A. Knudsen diffusion in microporous carbon membranes with molecular sieving character. J. Membr. Sci. 2002, 209, 339–352. [Google Scholar] [CrossRef]
- Grosso, D. How to exploit the full potential of the dip-coating process to better control film formation. J. Mater. Chem. 2011, 21, 17033–17038. [Google Scholar] [CrossRef]
- Itta, A.K.; Tseng, H.-H.; Wey, M.-Y. Fabrication and characterization of PPO/PVP blend carbon molecular sieve membranes for H2/N2 and H2/CH4 separation. J. Membr. Sci. 2011, 372, 387–395. [Google Scholar] [CrossRef]
- Kim, Y.K.; Park, H.B.; Lee, Y.M. Carbon molecular sieve membranes derived from thermally labile polymer containing blend polymers and their gas separation properties. J. Membr. Sci. 2004, 243, 9–17. [Google Scholar] [CrossRef]
- Ozaki, J.; Endo, N.; Ohizumi, W.; Igarashi, K.; Nakahara, M. Novel preparation method for the production of mesoporous carbon fiber from a polymer blend. Carbon 1997, 35, 1031–1033. [Google Scholar] [CrossRef]
- Smith, S.J.; Hou, R.; Lau, C.H.; Konstas, K.; Kitchin, M.; Dong, G.; Lee, J.; Lee, W.H.; Seong, J.G.; Lee, Y.M. Highly permeable thermally rearranged mixed matrix membranes (TR-MMM). J. Membr. Sci. 2019, 585, 260–270. [Google Scholar] [CrossRef]
- Sanders, D.F.; Smith, Z.P.; Ribeiro, C.P., Jr.; Guo, R.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Gas permeability, diffusivity, and free volume of thermally rearranged polymers based on 3,3′-dihydroxy-4,4′-diamino-biphenyl (HAB) and 2,2′-bis-(3,4-dicarboxyphenyl) hexafluoropropane dianhydride (6FDA). J. Membr. Sci. 2012, 409, 232–241. [Google Scholar] [CrossRef]
- Han, S.H.; Lee, J.E.; Lee, K.J.; Park, H.B.; Lee, Y.M. Highly gas permeable and microporous polybenzimidazole membrane by thermal rearrangement. J. Membr. Sci. 2010, 357, 143–151. [Google Scholar] [CrossRef]
- Giel, V.; Morávková, Z.; Peter, J.; Trchová, M. Thermally treated polyaniline/polybenzimidazole blend membranes: Structural changes and gas transport properties. J. Membr. Sci. 2017, 537, 315–322. [Google Scholar] [CrossRef]
- Kumakiri, I.; Tamura, K.; Sasaki, Y.; Tanaka, K.; Kita, H. Influence of Iron Additive on the Hydrogen Separation Properties of Carbon Molecular Sieve Membranes. Ind. Eng. Chem. Res. 2018, 57, 5370–5377. [Google Scholar] [CrossRef]
- Richter, H.; Reger-Wagner, N.; Kämnitz, S.; Voigt, I.; Lubenau, U.; Mothes, R. Carbon membranes for bio gas upgrading. Energy Procedia. 2019, 158, 861–866. [Google Scholar] [CrossRef]
- CalRecovery, Inc. Evaluation of Waste Tire Devulcanization Technologies; Integrated Waste Management Board Public Affairs Office: Sacramento, CA, USA, 2004. [Google Scholar]
- Richard, W. Baker, Future Directions of Membrane Gas Separation Technology. Ind. Eng. Chem. Res. 2002, 41, 1393–1411. [Google Scholar]
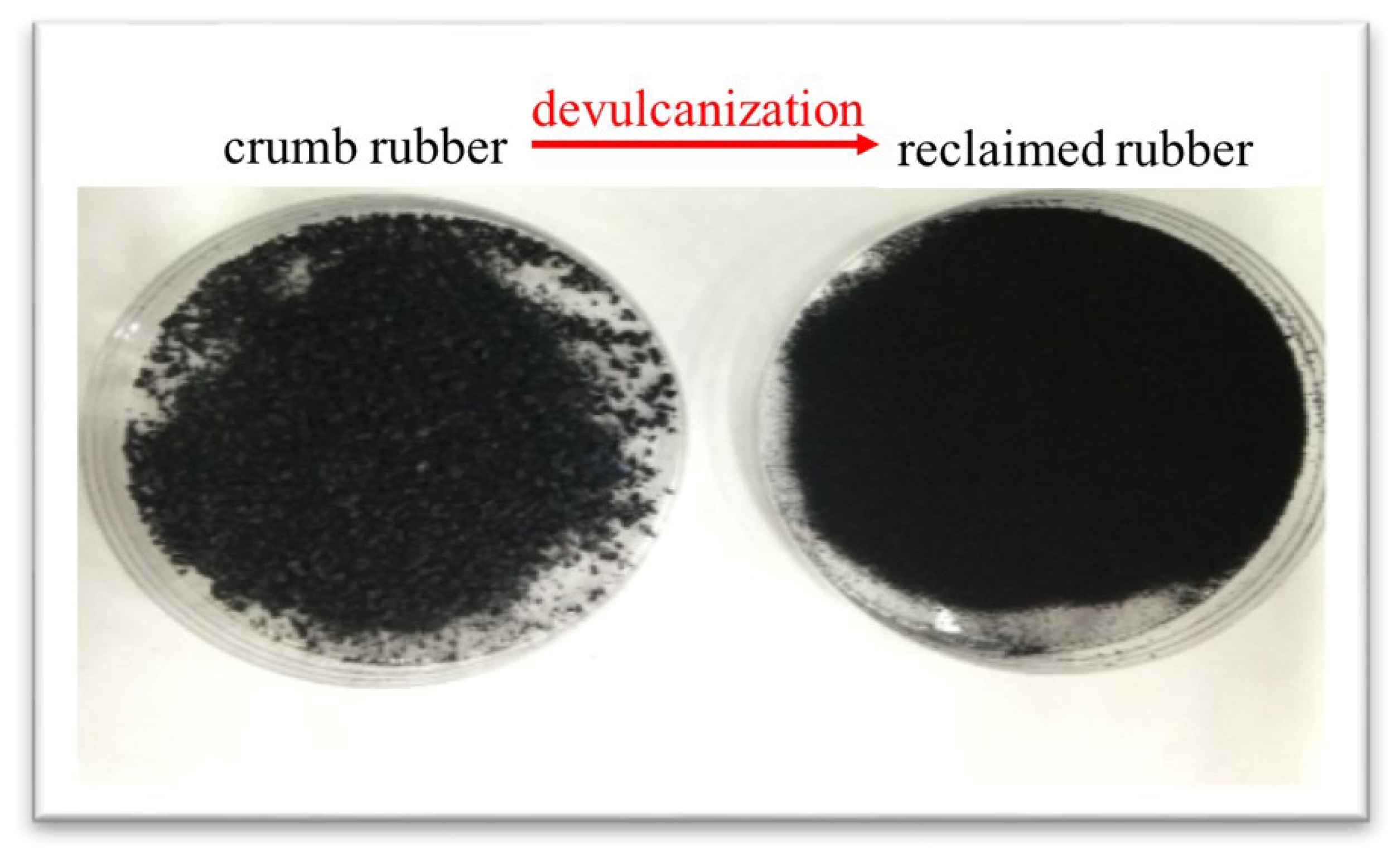





| Sample | Cross-Linking Density (10−7 mol/cm3) | Devulcanization (%) |
|---|---|---|
| Crumb Rubber | 2.233 | - |
| Reclaimed Rubber | 1.217 | 45.50 |
| Polymer | Thermal Rearrangement Protocols | Permeability (Barrer) * | H2/CO2 Selectivity | Reference | |
|---|---|---|---|---|---|
| H2 | CO2 | ||||
| 6FDA-HAB5DAM5 | 450 °C for 1 h | 1318 | 1607 | 0.82 | [32] |
| HAB-6FDA | 450 °C for 1 h | 530 | 410 | 1.29 | [33] |
| Aromatic Polyimides | 450 °C for 1 h | 738 | 295 | 2.50 | [9] |
| Poly(benzoxazole-co-imide) | 400 °C for 2 h | 222.1 | 172.8 | 1.29 | [10] |
| Polybenzimidazole (PBI) | 450 °C for 1 h | 1779 | 1624 | 1.1 | [34] |
| PANI/PBI | 300 °C for 3 h | 3.69 | 0.242 | 15.2 | [35] |
| C7-P2.8-T250 | 250 °C for 2 h | 1333 | 333.25 | 4.0 | This work |
| Item | |
|---|---|
| The price of reclaimed rubber [38] | USD 1555–2666/ton |
| The required polymer per unit membrane | 4–6 × 10−5 ton/m2 |
| The price of solvent (toluene) | USD 0.52/100 mL |
| The rubber solubility in solvent | 1.04 × 10−5 ton reclaimed/100 mL solvent |
| National aid | USD 100/ton reclaimed rubber |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-T.; Zhuang, G.-L.; Wey, M.-Y.; Tseng, H.-H. The Viable Fabrication of Gas Separation Membrane Used by Reclaimed Rubber from Waste Tires. Polymers 2020, 12, 2540. https://doi.org/10.3390/polym12112540
Lin Y-T, Zhuang G-L, Wey M-Y, Tseng H-H. The Viable Fabrication of Gas Separation Membrane Used by Reclaimed Rubber from Waste Tires. Polymers. 2020; 12(11):2540. https://doi.org/10.3390/polym12112540
Chicago/Turabian StyleLin, Yu-Ting, Guo-Liang Zhuang, Ming-Yen Wey, and Hui-Hsin Tseng. 2020. "The Viable Fabrication of Gas Separation Membrane Used by Reclaimed Rubber from Waste Tires" Polymers 12, no. 11: 2540. https://doi.org/10.3390/polym12112540
APA StyleLin, Y.-T., Zhuang, G.-L., Wey, M.-Y., & Tseng, H.-H. (2020). The Viable Fabrication of Gas Separation Membrane Used by Reclaimed Rubber from Waste Tires. Polymers, 12(11), 2540. https://doi.org/10.3390/polym12112540