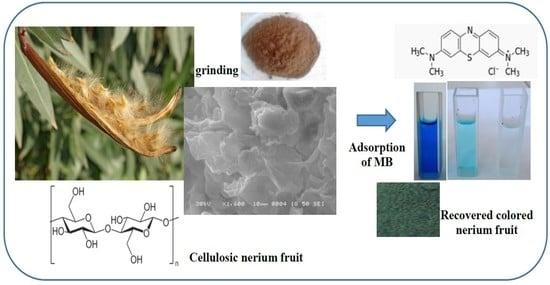
A Cellulosic Fruit Derived from Nerium oleander Biomaterial: Chemical Characterization and Its Valuable Use in the Biosorption of Methylene Blue in a Batch Mode
Abstract
1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Preparation of Nerium oleander Fruit
2.3. Characterization Instruments
2.4. Biosorption Experiments
3. Results and Discussion
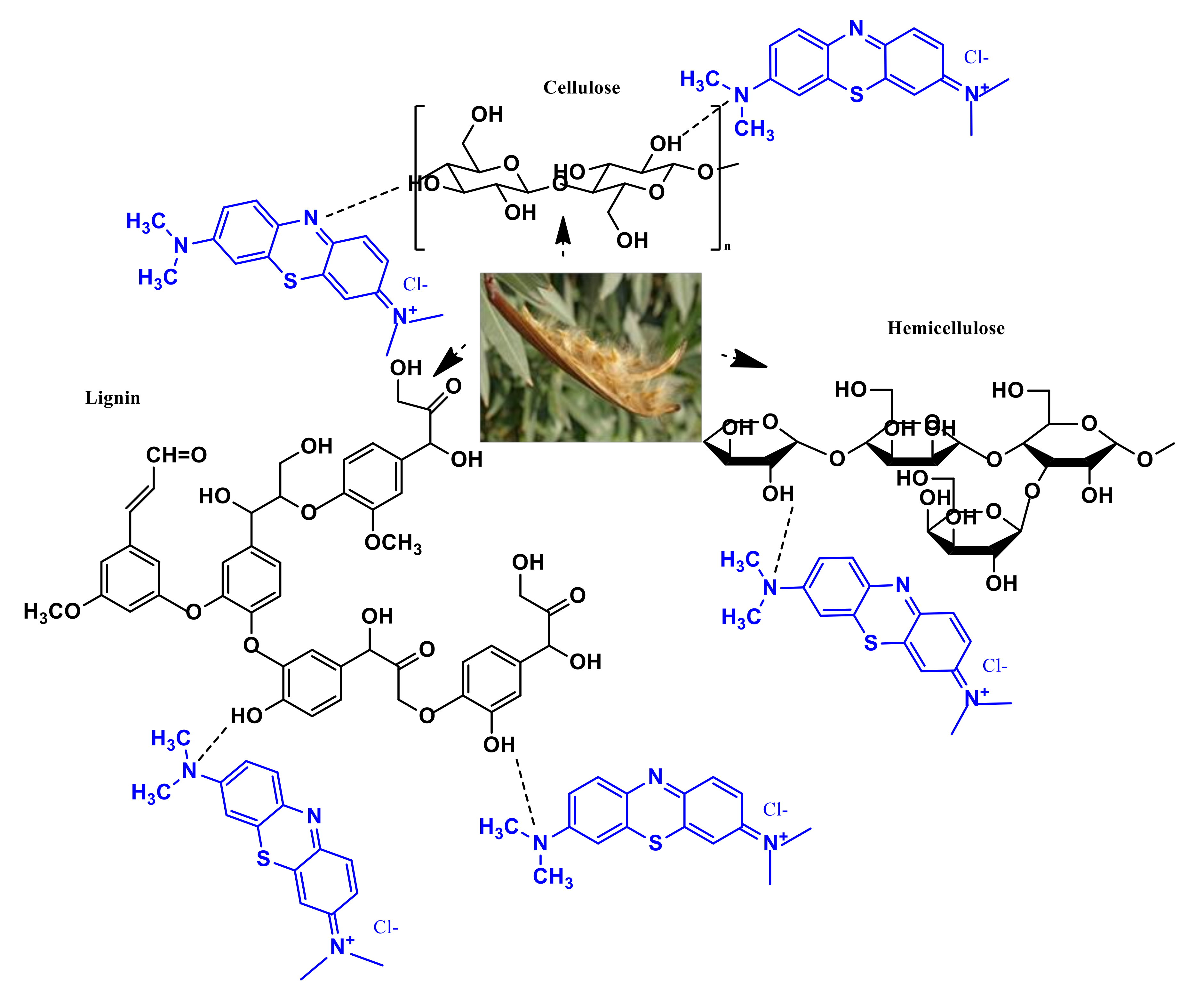
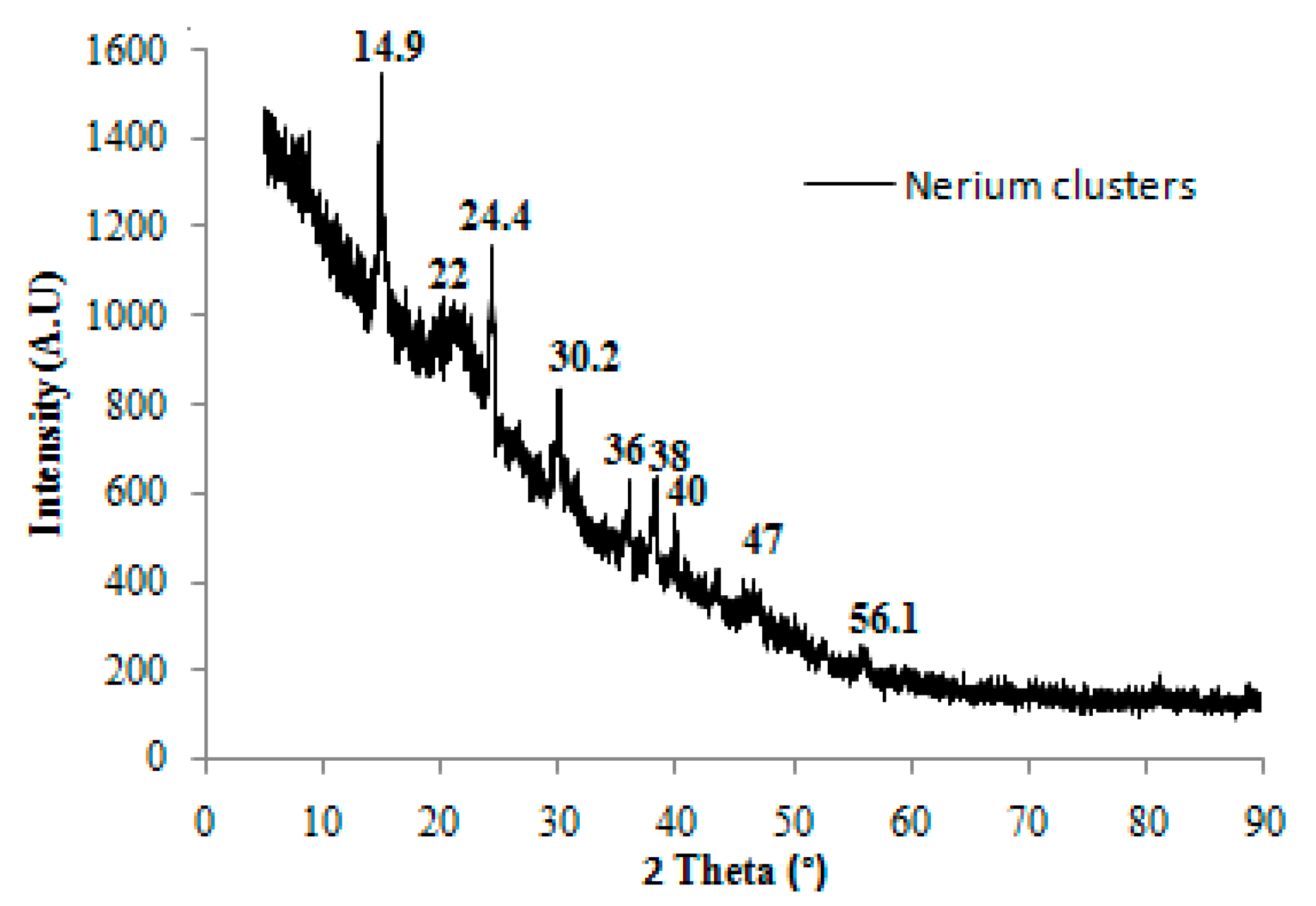
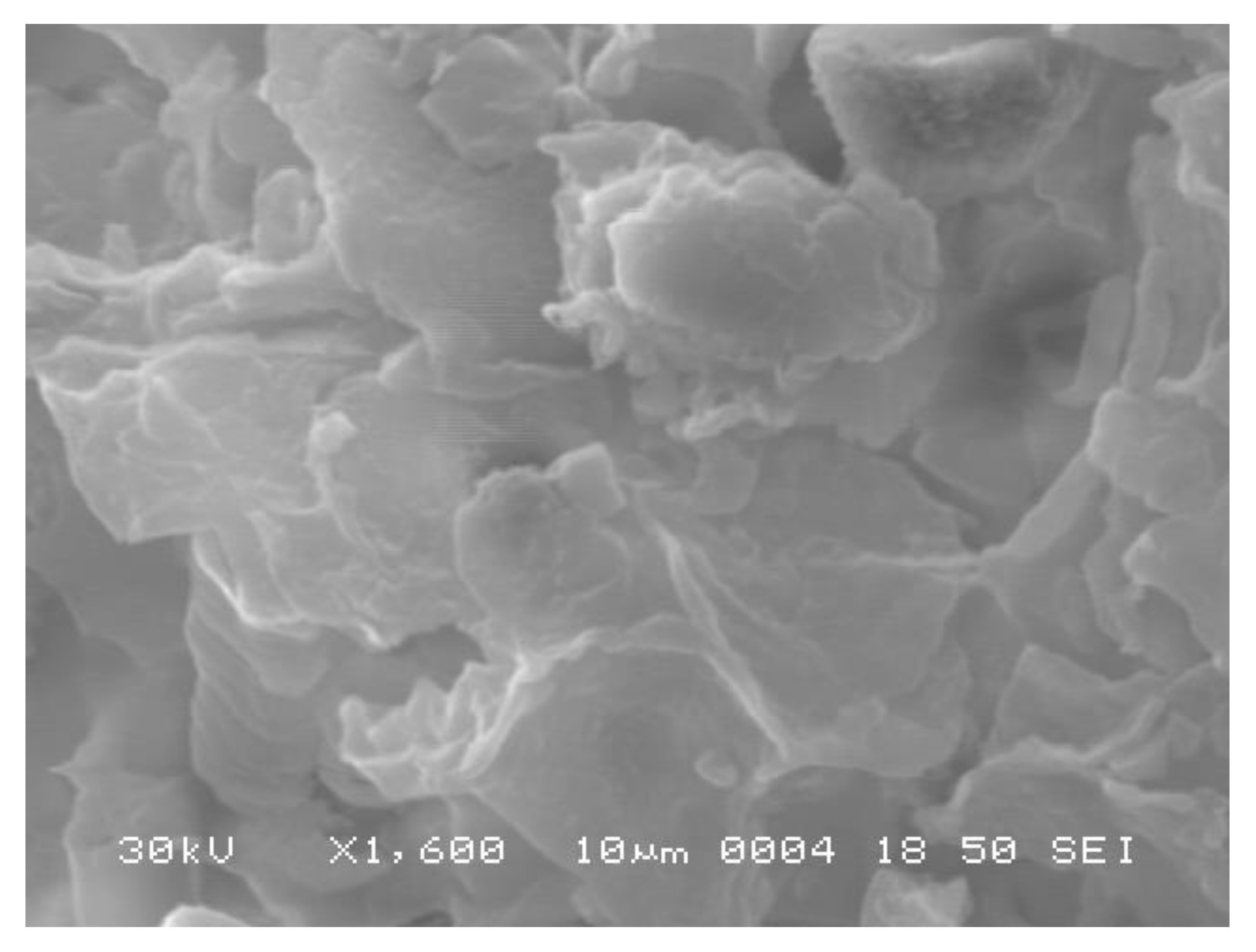
3.1. Characterization of Nerium oleander Fruit
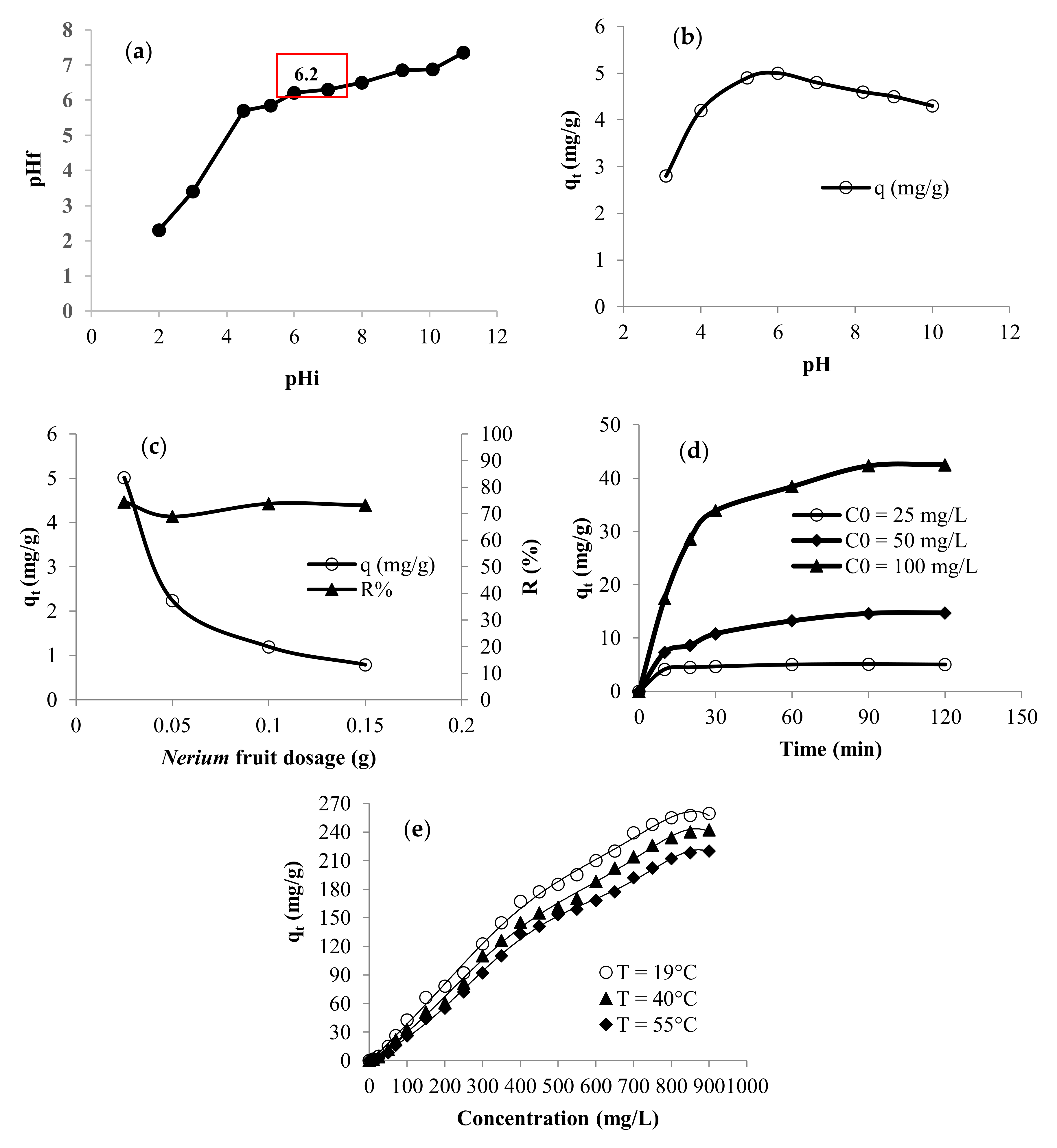
3.2. Factors Affecting the Adsorption of Methylene Blue
3.2.1. Effect of Experimental Parameters on Adsorption Performance
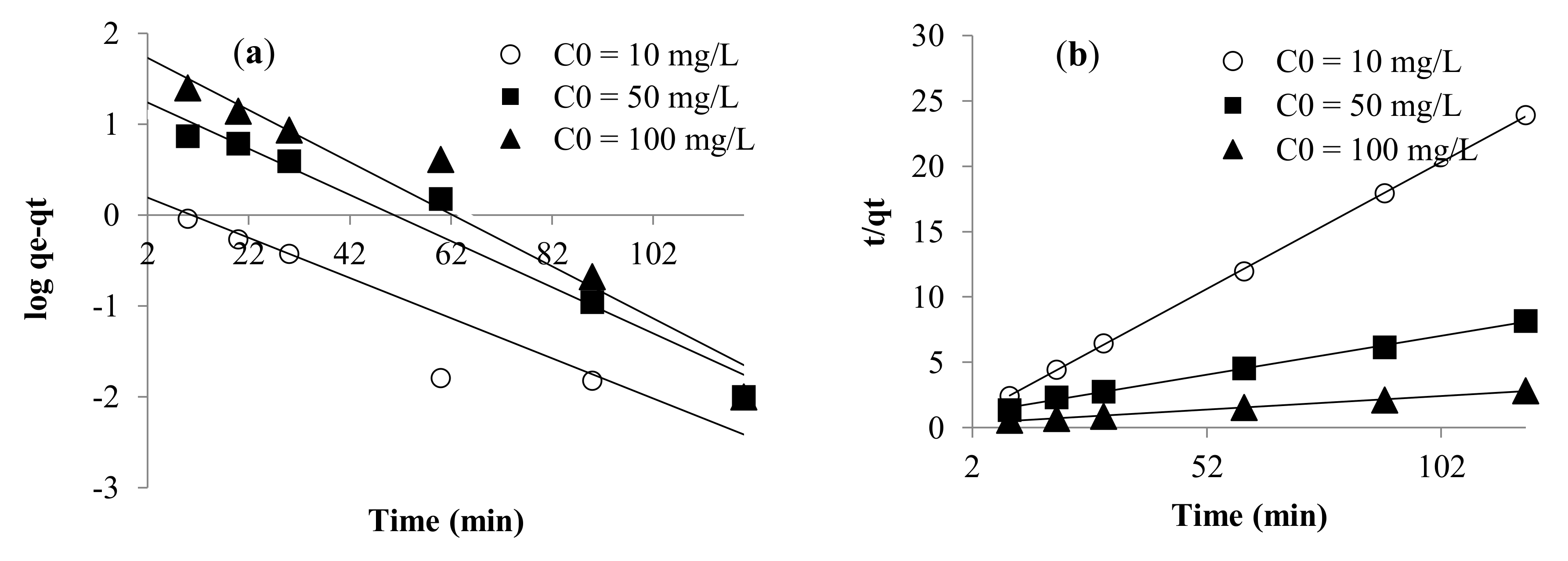
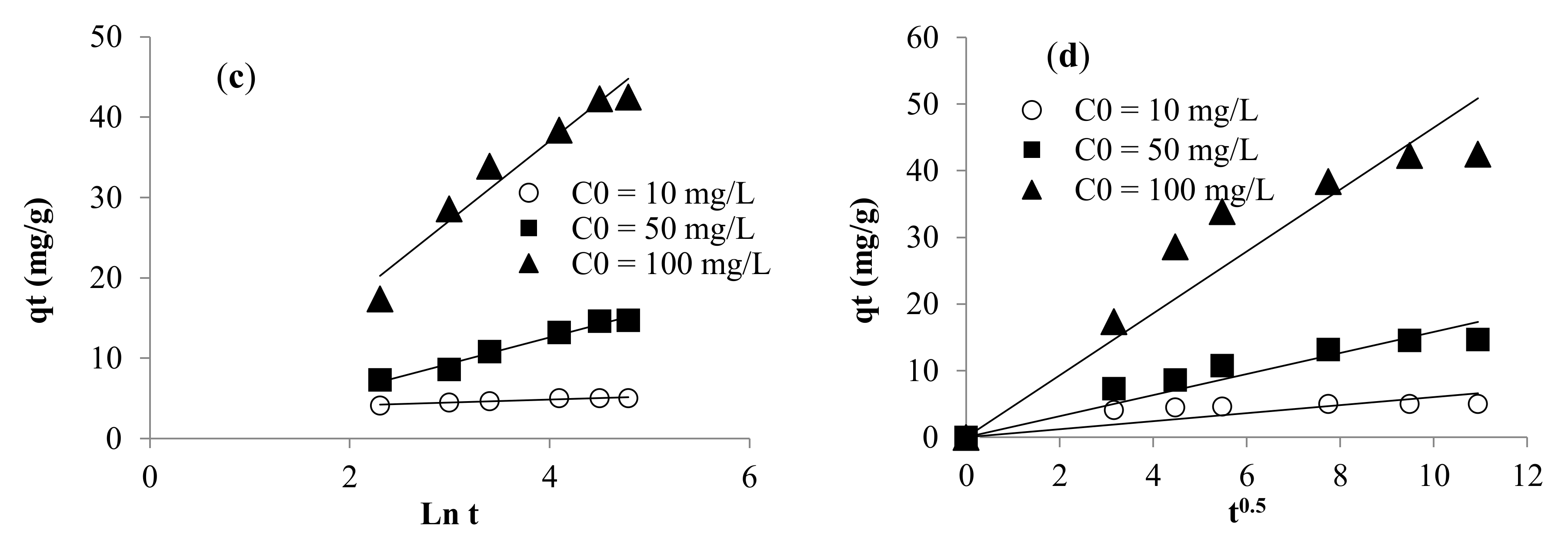
3.2.2. Kinetic Modeling
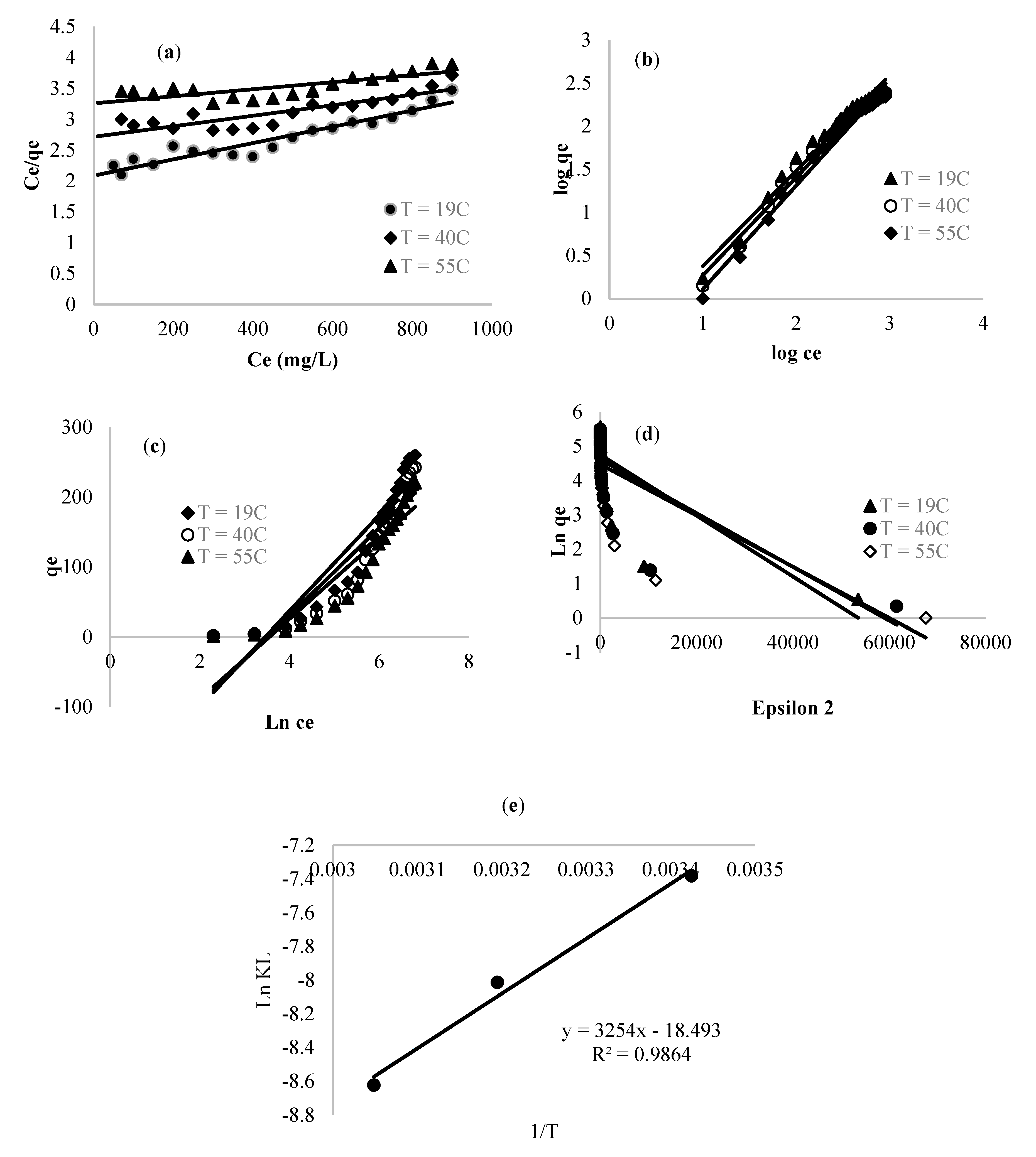
3.2.3. Isotherm Study and Thermodynamic Parameter Calculation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Deng, H.; Lua, J.; Li, G.; Zhang, G.; Wang, X. Adsorption of methylene blue on adsorbent materials produced from cotton stalk. Chem. Eng. J. 2011, 172, 326–334. [Google Scholar] [CrossRef]
- Gupta, N.; Kushwaha, A.K.; Chattopadhyaya, M.C. Application of potato (Solanum tuberosum) plant wastes for the removal of methylene blue and malachite green dye from aqueous solution. Arab. J. Chem. 2011, 9, 707–716. [Google Scholar] [CrossRef]
- Markovic, S.; Stankovic, A.; Lopicic, Z.; Lazarevic, S.; Stojanovic, M.; Uskoković, D. Application of raw peach shell particles for removal of methylene blue. J. Environ. Chem. Eng. 2015, 3, 716–724. [Google Scholar] [CrossRef]
- Jabli, M.; Gamha, E.; Sebeia, N.; Hamdaoui, M. Almond shell waste (Prunus dulcis): Functionalization with [dimethy-diallyl-ammonium-chloride-diallylamin-co-polymer] and chitosan polymer and its investigation in dye adsorption. J. Mol. Liq. 2017, 240, 35–44. [Google Scholar] [CrossRef]
- Pavan, F.A.; Gushikem, Y.; Mazzocato, A.S.; Dias, S.L.P.; Lima, E.C. Statistical design of experiments as tool for optimizing the batch conditions to methylene blue adsorption on yellow passion fruit and mandarin peels. Dye. Pigment. 2007, 72, 256–266. [Google Scholar] [CrossRef]
- Jain, S.; Jayaram, R.V. Removal of basic dyes from aqueous solution by low-cost adsorbent: Wood apple shell (Feronia acidissima). Desalination 2010, 250, 921–927. [Google Scholar] [CrossRef]
- Saeed, A.; Sharif, M.; Iqbal, M. Application potential of grapefruit peel as dye sorbent: Kinetics, equilibrium and mechanism of crystal violet adsorption. J. Hazard. Mater. 2010, 179, 564–572. [Google Scholar] [CrossRef]
- Gong, R.M.; Li, M.; Yang, C.; Sun, Y.Z.; Chen, J. Removal of cationic dyes from aqueous solution by adsorption on peanut hull. J. Hazard. Mater. 2005, 121, 247–250. [Google Scholar] [CrossRef]
- Allen, S.J.; Mckay, G.; Porter, J.F. Adsorption isotherm models for basic dye adsorption by peat in single and binary component systems. J. Colloid Interface Sci. 2004, 280, 322–333. [Google Scholar] [CrossRef]
- Han, R.P.; Ding, D.D.; Xu, Y.F.; Zou, W.H.; Wang, Y.F.; Li, Y.F.; Zou, L.N. Use of rice husk for the adsorption of Congo red from aqueous solution in column mode. Bioresour. Technol. 2008, 99, 2938–2946. [Google Scholar] [CrossRef]
- Jain, R.; Sikarwar, S. Removal of hazardous dye Congo red from waste material. J. Hazard. Mater. 2008, 152, 942–948. [Google Scholar] [CrossRef]
- Sadaf, S.; Bhatti, H.N. Batch and fixed bed column studies for the removal of Indosol Yellow BG dye by peanut husk. J. Taiwan Inst. Chem. Eng. 2014, 45, 541–553. [Google Scholar] [CrossRef]
- Ozacar, M.; Sengil, I.A. Adsorption of metal complex dyes from aqueous solution by pine sawdust. Bioresour. Technol. 2005, 96, 791–795. [Google Scholar] [CrossRef]
- Aramiab, M.; Limaeea, N.Y.; Mahmoodia, N.M.; Tabrizia, N.S. Removal of dyes from colored textile wastewater by orange peel adsorbent: Equilibrium and kinetic studies. J. Colloid Interface Sci. 2005, 288, 371–376. [Google Scholar] [CrossRef]
- Nascimento, G.E.; Campos, N.F.; Silva, J.J.; Barbosa, C.M.B.M.; Duarte, M.M.M.B. Adsorption of anionic dyes from an aqueous solution by banana peel and green coconut mesocarp. Desalination Water Treat. 2015, 57, 14093–14108. [Google Scholar] [CrossRef]
- Erdremoglu, N.; Küpeli, E.; Yeşilada, E. Anti-inflammatory and antinociceptive activity assessment of plants used as remedy in Turkish folk medicine. J. Ethnopharmacol. 2003, 89, 123–129. [Google Scholar] [CrossRef]
- Kumar, S.; Anand, G.R. Evaluation of anti-inflammatory activity of Nerium oleander. Pharmacia 2010, 1, 33–36. [Google Scholar]
- El Sawi, N.M.; Geweely, N.S.; Qusti, S.; Mohamed, M.; Kamel, A. Cytotoxicity and antimicrobial activity of Nerium oleander extracts. J. App. Anim. Res. 2010, 37, 25–31. [Google Scholar] [CrossRef]
- Abu-El-Halawa, R.; Zabin, S.A.; Abu-Sittah, H.H. Investigation of Methylene Blue Dye Adsorption from Polluted Water Using Oleander Plant (Al Defla) Tissues as Sorbent. Am. J. Environ. Sci. 2016, 12, 213–224. [Google Scholar] [CrossRef]
- Jabli, M.; Tka, N.; Salman, G.A.; Elaissi, A.; Sebeia, N.; Hamdaoui, M. Rapid interaction, in aqueous media, between anionic dyes and cellulosic Nerium oleander fibers modified with Ethylene-Diamine and Hydrazine. J. Mol. Liq. 2017, 242, 272–283. [Google Scholar] [CrossRef]
- Jabli, M.; Tka, N.; Ramzi, K.; Saleh, T.A. Physicochemical characteristics and dyeing properties of lignin-cellulosic fibers derived from Nerium oleander. J. Mol. Liq. 2018, 249, 1138–1144. [Google Scholar] [CrossRef]
- Sebeia, N.; Jabli, M.; Ghith, A.; ElGhoul, Y.; Alminderej, F.M. Populus tremula, Nerium oleander and Pergularia tomentosa seed fibers as sources of cellulose and lignin for the bio-sorption of methylene blue. Int. J. Biol. Macromol. 2019, 121, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Sebeia, N.; Jabli, M.; Ghith, A. Biological synthesis of copper nanoparticles, using Nerium oleander leaves extract: Characterization and study of their interaction with organic dyes. Inorg. Chem. Commun. 2019, 105, 36–46. [Google Scholar] [CrossRef]
- Boumaza, S.; Yenounne, A.; Hachi, W.; Kaouah, F.; Bouhamidi, Y.; Trari, M. Application of Typha angustifolia (L.) Dead Leaves Waste as Biomaterial for the Removal of Cationic Dye from Aqueous Solution. Int. J. Environ. Res. 2018, 12, 561–573. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, Y.; Xue, L.; Sun, H.; Guo, Z.; Zhang, Y.; Yang, L. Carboxylic acid functionalized sesame straw: A sustainable cost-effective bio adsorbent with superior dye adsorption capacity. Bioresour. Technol. 2017, 238, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.N.; Mancini, M.C.; Oliveira, F.C.S.; Passos, T.M.; Quility, B.; Thire, R.M.S.; Mcguiness, G.B. FTIR analysis and quantification of phenols and flavonoids of five commercially available plants extracts used in wound healing. Rev. Matéria 2016, 21, 767–779. [Google Scholar] [CrossRef]
- Poletto, M.; Zattera, A.J.; Santana, R.M.C. Structural differences between wood species: Evidence from chemical composition, FTIR spectroscopy, and thermogravimetric analysis. J. Appl. Polym. Sci. 2012, 126, 1–8. [Google Scholar] [CrossRef]
- Chen, X.; Xu, R.; Xu, Y.; Hu, H.; Pan, S.; Pan, H. Natural adsorbent based on sawdust for removing impurities in waste lubricants. J. Hazard. Mater. 2018, 350, 38–45. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Reddy, K.O.; Maheswari, C.U.; Dhlamini, M.S.; Mothudi, B.M.; Zhang, J.; Zhang, J.; Nagarajan, R.; Rajulu, A.V. Preparation and characterization of regenerated cellulose films using borassus fruit fibers and an ionic liquid. Carbohydr. Polym. 2017, 160, 203–211. [Google Scholar] [CrossRef]
- Maaloul, N.; Arfi, R.B.; de la Vega, M.R.; Ghorbal, A.; Díaz, M. Dialysis-free extraction and characterization of cellulose crystals from almond (Prunus dulcis) shells. J. Mater. Environ. Sci. 2017, 8, 4171–4181. [Google Scholar]
- Dong, Z.; Hou, X.; Sun, F.; Zhang, L.; Yang, Y. Textile grade long natural cellulose fibers from bark of cotton stalks using steam explosion as a pretreatment. Cellulose 2014, 21, 3851–3860. [Google Scholar] [CrossRef]
- Thygesen, A.; Oddershede, J.; Lilholt, H.; Thomsen, A.B.; Stahl, K. On the determination of crystallinity and cellulose content in plant fibres. Cellulose 2005, 12, 563–576. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ahmad, I.; Abdullah, I.; Dufresne, A.; Zainudin, S.Y.; Sheltami, R.M. Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose 2012, 19, 855–866. [Google Scholar] [CrossRef]
- Trache, D.; Donnot, A.; Khimeche, K.; Benelmir, R.; Brosse, N. Physicochemicalproperties and thermal stability of microcrystalline cellulose isolated from Alfafibres. Carbohydr. Polym. 2014, 104, 223–230. [Google Scholar] [CrossRef]
- Bettaieba, F.; Khiari, R.; Hassand, M.L.; Belgacemb, M.N.; Bras, J.; Dufresne, A.; Mhenni, M.F. Preparation and characterization of new cellulose nanocrystals from marine biomass Posidoniaoceanica. Ind. Crop. Prod. 2015, 72, 175–182. [Google Scholar] [CrossRef]
- Thambiraj, S.; Shankaran, D.R. Preparation and physicochemicalcharacterization of cellulose nanocrystals from industrial waste cotton. Appl. Surf. Sci. 2017, 412, 405–416. [Google Scholar] [CrossRef]
- Trache, D.; Khimeche, K.; Donnot, A.; Benelmir, R. FTIR spectroscopy and X-raypowder diffraction characterization of microcrystalline cellulose obtainedfrom alfa fibers. MATEC Web Conf. 2013, 3, 1023. [Google Scholar] [CrossRef]
- Celekli, A.; Birecikligil, S.S.; Geyik, F.; Bozkurt, H. Prediction of removal efficiency of Lanaset Red G on walnut husk using artificial neural network model. Bioresour. Technol. 2012, 103, 64–70. [Google Scholar] [CrossRef]
- Momcilovic, M.; Purenovic, M.; Miljkovic, M.; Bojic, A.; Randjelovic, M. Adsorption of cationic dye methylene blue onto activated carbon obtained from horse chestnut kernel. Hem. Ind. 2011, 65, 123–129. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Zhang, X.; He, X.; Zhang, W.; Zhang, X.; Lu, C. In situ synthesis of mno2 coated cellulose nanofibers hybrid for effective removal of methylene blue. Carbohydr. Polym. 2014, 110, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Huang, J.; He, B.; Zhang, F.; Li, H. Peach gumfor efficient removal of methylene blue and methyl violet dyes from aqueous solution. Carbohydr. Polym. 2014, 101, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Mitrogiannis, D.; Markou, G.; Celekli, A.; Bozkurt, H. Biosorption of methylene blue onto Arthrospira platensis biomass: Kinetic, equilibrium and thermodynamic studies. J. Environ. Chem. Eng. 2015, 3, 670–680. [Google Scholar] [CrossRef]
- Ai, L.; Li, M.; Li, L. Adsorption of methylene blue from aqueous solution with activated carbon/cobalt ferrite/alginate composite beads: Kinetics, isotherms, and thermodynamics. J. Chem. Eng. Data 2011, 56, 3475–3483. [Google Scholar] [CrossRef]
- Zeng, L.; Xie, M.; Zhang, Q.; Kang, Y.; Guo, X.; Xiao, H.; Peng, Y.; Luo, J. Chitosan/organic rectorite composite for the magnetic uptake of methylene blue and methyl orange. Carbohydr. Polym. 2015, 123, 89–98. [Google Scholar] [CrossRef]
- Hameed, B.H.; Krishni, R.R.; Sata, S.A. A novel agricultural waste adsorbent for the removal of cationic dye from aqueous solutions. J. Hazard. Mater. 2009, 162, 305–311. [Google Scholar] [CrossRef]
- Han, X.; Han, X.; Wang, W.; Ma, X. Adsorption characteristics of methylene blue onto low cost biomass material lotus leaf. Chem. Eng. J. 2011, 171, 1–8. [Google Scholar] [CrossRef]
- Belala, Z.; Jeguirim, M.; Belhachemi, M.; Addoun, F.; Trouve, G. Biosorption of basic dye from aqueous solutions by date palm trees: Kinetic, equilibrium and thermodynamic studies. Desalination 2011, 271, 80–87. [Google Scholar] [CrossRef]
- Salazar-Rabago, J.J.; Leyva-Ramos, R.; Rivera-Utrilla, J.; Ocampo-Perez, R.; Cerino-Cordova, F.J. Biosorption mechanism of Methylene Blue from aqueous solution onto white pine (Pinus durangensis) sawdust. Effect of operating conditions. Sustain. Environ. Res. 2016, 27, 32–40. [Google Scholar] [CrossRef]
- Khodabandehloo, A.; Shayesteh, H.; Rahbar-Kelishami, A. Methylene blue removal using Salix babylonica (Weeping willow) leaves powder as a low-cost biosorbent in batch mode: Kinetic, equilibrium, and thermodynamic studies. J. Mol. Liq. 2017, 244, 540–548. [Google Scholar] [CrossRef]
- Sajab, M.S.; Chia, C.H.; Zakaria, S.; Jani, S.M.; Ayob, M.K.; Chee, K.L.; Khiew, P.S.; Chiu, W.S. Citric acid modified kenaf core fibres for removal of methylene blue from aqueous solution. Bioresour. Technol. 2011, 102, 7237–7243. [Google Scholar] [CrossRef] [PubMed]
- Abdelkarim, S.; Mohammed, H.; Nouredine, B. Sorption of Methylene Blue Dye from Aqueous Solution Using an Agricultural Waste. Trends Green Chem. 2017, 3, 1–7. [Google Scholar] [CrossRef]
- Rubin, E.; Rodriguez, P.; Herrero, R.; Cremades, J.; Barbara, I.; de Vicente, M.E.S. Removal of methylene blue from aqueous solutions using as biosorbent Sargassum muticum: An invasive macroalga in Europe. J. Chem. Technol. Biotechnol. 2005, 80, 291–298. [Google Scholar] [CrossRef]
- Hameed, B.H.; El-Khaiary, M.I. Sorption kinetics and isotherm studies of a cationic dye using agricultural waste: Broad bean peels. J. Hazard. Mater. 2008, 154, 639–648. [Google Scholar] [CrossRef]
- Vilar, V.J.; Botelho, C.M.; Boaventura, R.A. Methylene blue adsorption by algal biomass based materials: Biosorbents characterization and process behaviour. J. Hazard. Mater. 2007, 147, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Nasuha, N.; Hameed, B.H.; Din, A.T. Rejected tea as a potential low-cost adsorbent for the removal of methylene blue. J. Hazard. Mater. 2010, 175, 126–132. [Google Scholar] [CrossRef]
- Kumar, K.V.; Kumaran, A. Removal of methylene blue by mango seed kernel powder. Biochem. Eng. J. 2005, 27, 83–93. [Google Scholar] [CrossRef]
- Rebitanim, N.Z.; Ghani, W.A.W.A.K.; Mahmoud, D.K.; Rebitanim, N.A.; Salleh, M.A.M. Adsorption capacity of raw empty fruit bunch biomass onto methylene 516 blue dye in aqueous solution. J. Purity Util. React. Environ. 2012, 1, 45–60. [Google Scholar]
- Vadivelan, V.; Kumar, K.V. Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J. Colloid Interface Sci. 2005, 286, 90–100. [Google Scholar] [CrossRef]
- Annadurai, G.; Juang, R.-S.; Lee, D.-J. Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J. Hazard. Mater. 2002, 92, 263–274. [Google Scholar] [CrossRef]
- Banerjee, S.; Dastidar, M.G. Use of jute processing wastes for treatment of wastewater contaminated with dye and other organics. Bioresour. Technol. 2005, 96, 1919–1928. [Google Scholar] [CrossRef]
- Hameed, B.H.; Ahmad, A.A. Batch adsorption of methylene blue from aqueous solution by garlic peel, an agricultural waste biomass. J. Hazard. Mater. 2009, 164, 870–875. [Google Scholar] [CrossRef]
- Banat, F.; Al-Asheh, S.; Al-Makhadmeh, L. Evaluation of the use of raw and activated date pits as potential adsorbents for dye containing waters. Proc. Biochem. 2003, 39, 193–202. [Google Scholar] [CrossRef]
- Miyah, Y.; Lahrichi, A.; Idrissi, M.; Khalil, A.; Zerrouq, F. Adsorption of methylene blue dye from aqueous solutions onto walnut shells powder: Equilibrium and kinetic studies. Surf. Interfaces 2018, 11, 74–81. [Google Scholar] [CrossRef]
- Mazengarb, S.; Roberts, G.A.F. Studies on the diffusion of direct dyes in chitosan film. Prog. Chem. App. Chitin Derivat. 2009, 14, 25–32. [Google Scholar]
- Gucek, A.; Sener, S.; Bilgen, S.; Mazmanci, A. Adsorption and kinetic studies of cationic and anionic dyes on pyrophyllite from aqueous solutions. J. Coll. Interf. Sci. 2005, 286, 53–60. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. The kinetics of sorption of basic dyes from aqueous solution by sphagnum moss peat. Can. J. Chem. Eng. 1998, 76, 822–827. [Google Scholar] [CrossRef]
- Ngulube, T.; Gumbo, J.R.; Masindi, V.; Maity, A. Calcined magnesite as an adsorbent for cationic and anionic dyes: Characterization, adsorption parameters, isotherms and kinetics study. Heliyon 2018, 4, 838. [Google Scholar] [CrossRef]
- Runping, H.; Pan, H.; Zhaohui, C.; Zhenhui, Z.; Mingsheng, T. Kinetics and isotherms of neutral red adsorption on peanut husk. J. Envrion. Sci. 2008, 20, 1035–1041. [Google Scholar]




| Adsorbent | Target | Maximum Adsorption Capacity (mg g−1) | Reference |
|---|---|---|---|
| Nerium fruits | Methylene blue | 259 | Current work |
| Pineapple stem | Methylene blue | 119 | [46] |
| Lotus leaf | Methylene blue | 221.7 | [47] |
| Palm tree waste | Methylene blue | 39.47 | [48] |
| Pinus durangensis | Methylene blue | 85 | [49] |
| Salix babylonica leaves | Methylene blue | 60.97 | [50] |
| Kenaf core fibers | Methylene blue | 131.6 | [51] |
| Almond shell | Methylene blue | 84.9 | [4] |
| Prickly (peel) bark of cactus fruit | Methylene blue | 222 | [52] |
| Algae Sargassum muticum | Methylene blue | 279.2 | [53] |
| Broad been peals | Methylene blue | 192.7 | [54] |
| Algae Gelidium | Methylene blue | 171 | [55] |
| Rejected tea | Methylene blue | 147 | [56] |
| Mango seed kernel | Methylene blue | 142.9 | [57] |
| Empty fruit bunch | Methylene blue | 50.76 | [58] |
| Rice husk | Methylene blue | 40.6 | [59] |
| Orange peel | Methylene blue | 18.6 | [60] |
| Jute processing waste | Methylene blue | 22.47 | [61] |
| Garlic peel | Methylene blue | 82.64 | [62] |
| Date pits | Methylene blue | 80.31 | [63] |
| Walnut shell powder | Methylene blue | 178.9 | [64] |
| Dead Typha angustifolia (L.) leaves | Methylene blue | 106.75 | [24] |
| Peach shells | Methylene blue | 183.6 | [3] |
| Kinetic equations | Constants | Dye concentration (mg/L) | Isotherms | Parameters | Temperature (°C) | ||||
| Pseudo-first-order | 10 | 50 | 100 | 19 | 40 | 55 | |||
| K1 (min−1) | 0.022 | 0.025 | 0.028 | qm (mg g−1) | 769.23 | 1111.11 | 1666.67 | ||
| q (mg g−1) | 1.716 | 19.40 | 61.23 | Langmuir | KL (L g−1) | 0.000623 | 0.000331 | 0.00018 | |
| R2 | 0.86 | 0.96 | 0.95 | R2 | 0.91 | 0.74 | 0.61 | ||
| Pseudo-second-order | K2 | 0.0735 | 0.0037 | 0.0014 | Thermodynamic parameters | ΔH° (KJ mol−1) | −27.05 | ||
| q (mg g−1) | 5.154 | 16.806 | 48.309 | ΔS° (J mol−1) | −153.75 | ||||
| h | 1.954 | 1.052 | 3.245 | ΔG° (KJ mol−1) | 17.84 | 21.07 | 23.37 | ||
| R2 | 0.999 | 0.997 | 0.997 | Freundlich | KF (L g−1) | 0.185 | 0.135 | 0.080 | |
| Elovich | (mg g−1 min−1) | 2656.25 | 2.807 | 7.648 | n | 0.902 | 0.878 | 0.831 | |
| β (mg g−1 min−1) | 2.65 | 0.305 | 0.1011 | R2 | 0.98 | 0.98 | 0.98 | ||
| R2 | 0.938 | 0.978 | 0.948 | Temkin | B (J/mol) | 68.407 | 62.665 | 57.173 | |
| Intraparticular diffusion | K (mg g−1 min1/2) | 0.606 | 1.583 | 4.645 | A (L g−1) | 0.0314 | 0.0296 | 0.0287 | |
| bt (J/mol) | 35.48 | 41.53 | 47.69 | ||||||
| R2 | 0.339 | 0.876 | 0.848 | R2 | 0.87 | 0.85 | 0.84 | ||
| Dubinin | q (mg g−1) | 115.85 | 100.35 | 87.11 | |||||
| E (KJ/mol) | 74.536 | 79.057 | 84.52 | ||||||
| KDR (mol2/kJ2) | 9 × 10−5 | 8 × 10−5 | 7 × 10−5 | ||||||
| R2 | 0.55 | 0.54 | 0.53 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ghamdi, Y.O.; Jabli, M.; Soury, R.; Ali Khan, S. A Cellulosic Fruit Derived from Nerium oleander Biomaterial: Chemical Characterization and Its Valuable Use in the Biosorption of Methylene Blue in a Batch Mode. Polymers 2020, 12, 2539. https://doi.org/10.3390/polym12112539
Al-Ghamdi YO, Jabli M, Soury R, Ali Khan S. A Cellulosic Fruit Derived from Nerium oleander Biomaterial: Chemical Characterization and Its Valuable Use in the Biosorption of Methylene Blue in a Batch Mode. Polymers. 2020; 12(11):2539. https://doi.org/10.3390/polym12112539
Chicago/Turabian StyleAl-Ghamdi, Youssef O., Mahjoub Jabli, Raoudha Soury, and Shahid Ali Khan. 2020. "A Cellulosic Fruit Derived from Nerium oleander Biomaterial: Chemical Characterization and Its Valuable Use in the Biosorption of Methylene Blue in a Batch Mode" Polymers 12, no. 11: 2539. https://doi.org/10.3390/polym12112539
APA StyleAl-Ghamdi, Y. O., Jabli, M., Soury, R., & Ali Khan, S. (2020). A Cellulosic Fruit Derived from Nerium oleander Biomaterial: Chemical Characterization and Its Valuable Use in the Biosorption of Methylene Blue in a Batch Mode. Polymers, 12(11), 2539. https://doi.org/10.3390/polym12112539