PLLA Honeycomb-Like Pattern on Fluorinated Ethylene Propylene as a Substrate for Fibroblast Growth
Abstract
1. Introduction
2. Materials and Methods
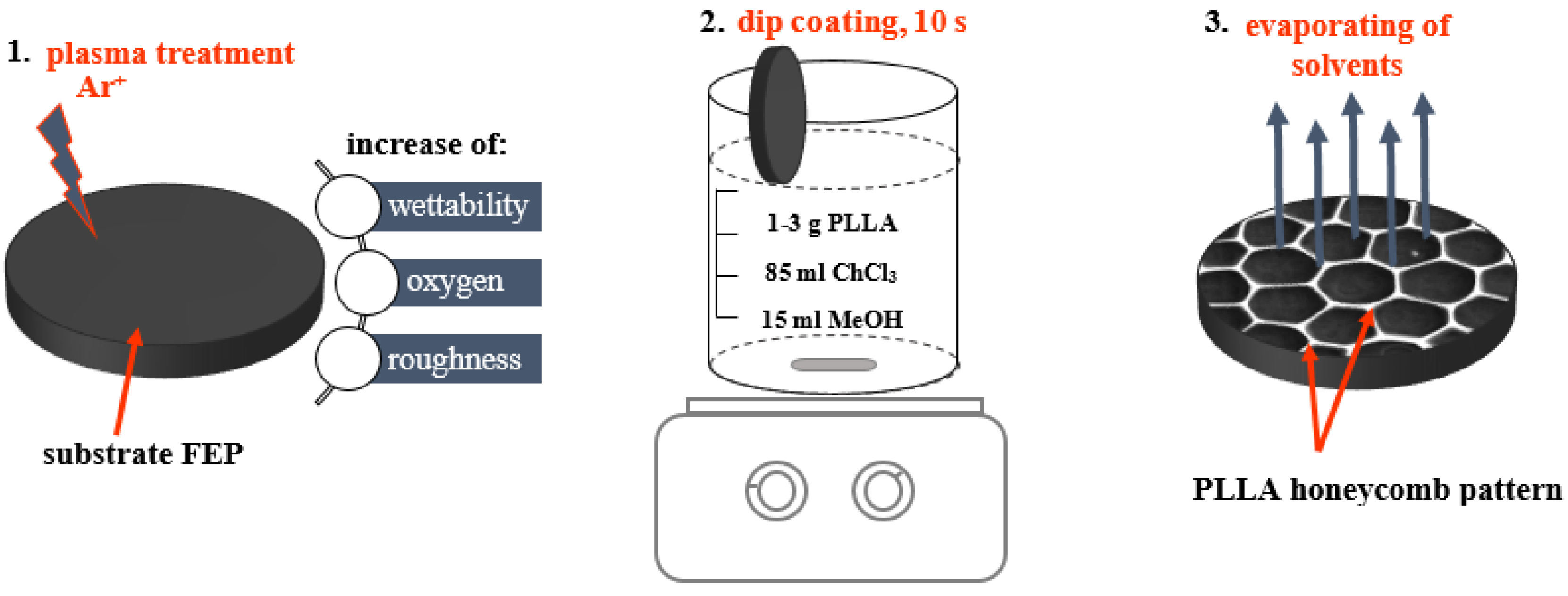
2.1. Preparation of the PLLA Film
2.2. Sample Characterization
2.3. Fibroblast Cultivation
2.4. Cell Viability Assay
2.5. Preparation of Cell Samples for Scanning Electron Microscopy
3. Results and Discussion
3.1. Surface Morphology–SEM Analysis
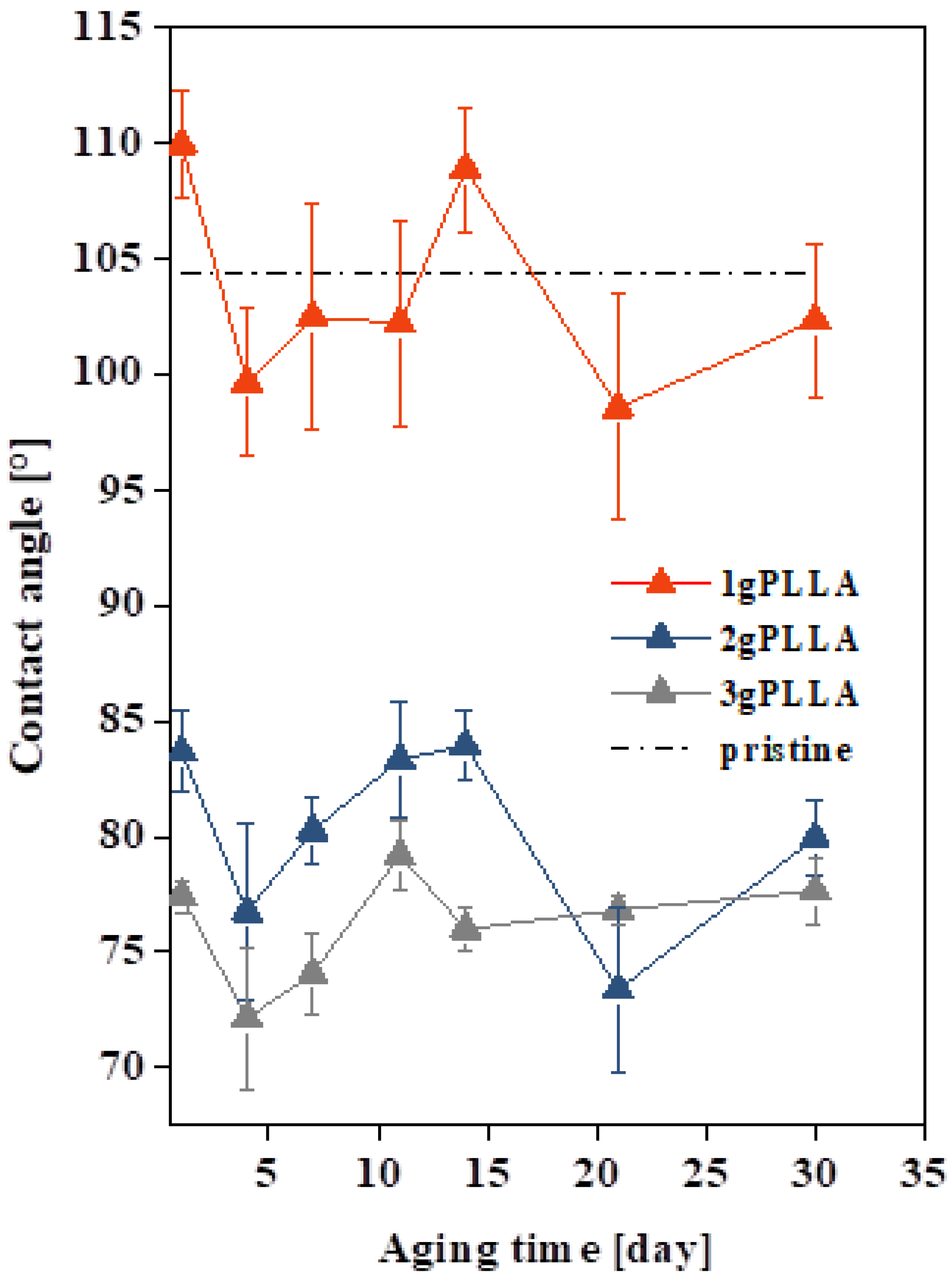
3.2. Surface Wettability
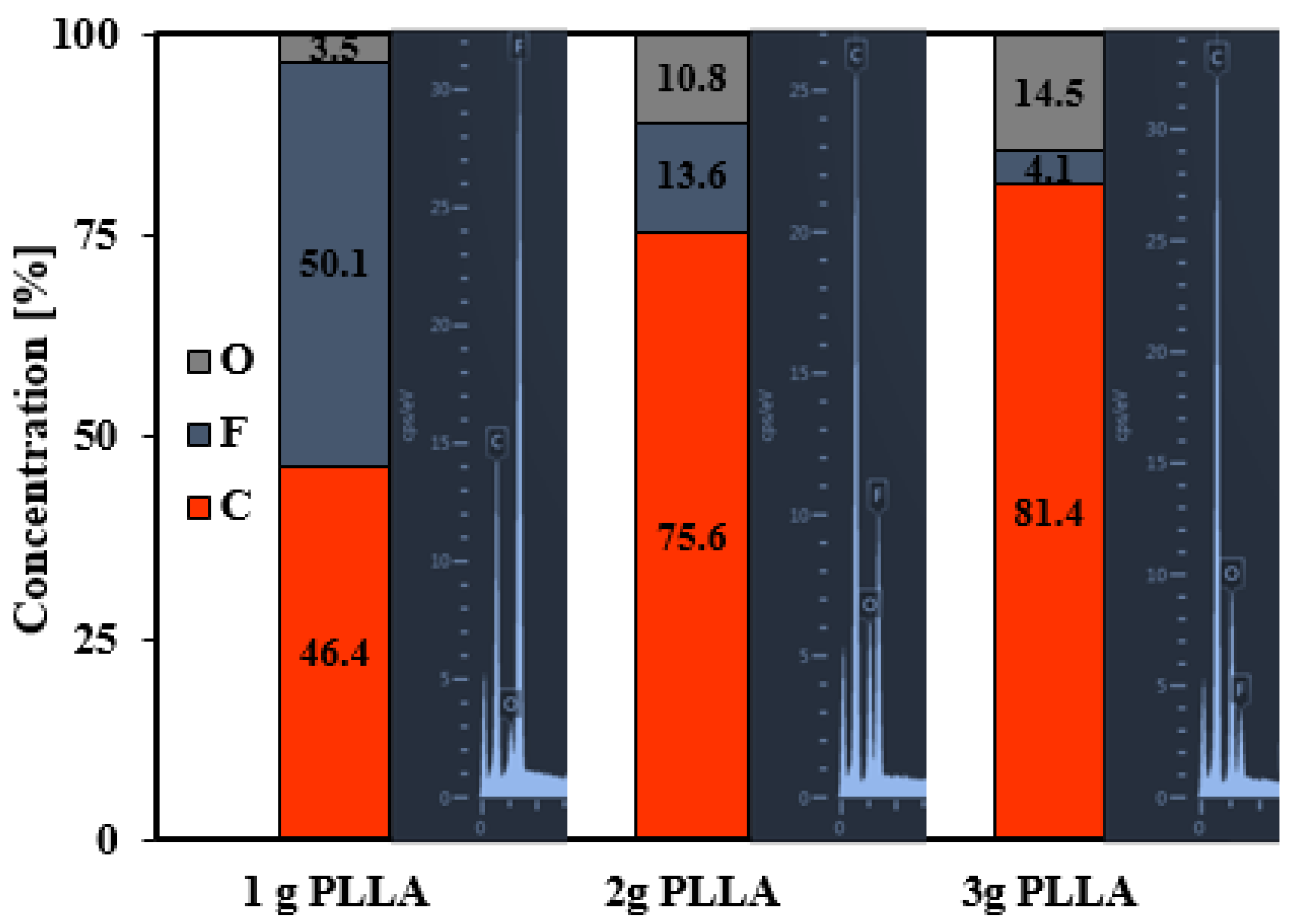
3.3. Surface Chemistry
3.4. Surface Roughness, Morphology and Surface Area–AFM Analysis
3.5. Cell Viability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Singhvi, R.; Stephanopoulos, G.; Wang, D.I.C. Review: Effects of substratum morphology on cell physiology. Biotechnol. Bioeng. 1994, 43, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yan, C.; Zheng, Z. Functional polymer surfaces for controlling cell behaviors. Mater. Today 2018, 21, 38–59. [Google Scholar] [CrossRef]
- Wu, X.; Wang, S. Regulating MC3T3-E1 cells on deformable poly(ε-caprolactone) honeycomb films prepared using a surfactant-free breath figure method in a water-miscible solvent. ACS Appl. Mater. Interfaces 2012, 4, 4966–4975. [Google Scholar] [CrossRef] [PubMed]
- Michaljaničová, I.; Slepička, P.; Rimpelová, S.; Slepičková Kasálková, N.; Švorčík, V. Regular pattern formation on surface of aromatic polymers and its cytocompatibility. Appl. Surf. Sci. 2016, 370, 131–141. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, X.; Li, P.; Huang, G.; Feng, S.; Shen, C.; Han, B.; Zhang, X.; Jin, F.; Xu, F.; et al. Bioinspired engineering of honeycomb structure—Using nature to inspire human innovation. Prog. Mater. Sci. 2015, 74, 332–400. [Google Scholar] [CrossRef]
- Nishikawa, T.; Arai, K.; Hayashi, J.; Hara, M.; Shimomura, M. “Honeycomb films”: Biointerface for tissue engineering. Int. J. Nanosci. 2002, 1, 415–418. [Google Scholar] [CrossRef]
- Kim, J.H.; Seo, M.; Kim, S.Y. Lithographically patterned breath figure of photoresponsive small molecules: Dual-patterned honeycomb lines from a combination of bottom-up and top-down lithography. Adv. Mater. 2009, 21, 4130–4133. [Google Scholar] [CrossRef]
- Calejo, M.T.; Ilmarinen, T.; Skottman, H.; Kellomäki, M. Breath figures in tissue engineering and drug delivery: State-of-the-art and future perspectives. Acta Biomater. 2018, 66, 44–66. [Google Scholar] [CrossRef]
- Bui, V.T.; Thuy, L.T.; Tran, Q.C.; Nguyen, V.T.; Dao, V.D.; Choi, J.S.; Choi, H.S. Ordered honeycomb biocompatible polymer films via a one-step solution-immersion phase separation used as a scaffold for cell cultures. Chem. Eng. J. 2017, 320, 561–569. [Google Scholar] [CrossRef]
- Place, E.S.; George, J.H.; Williams, C.K.; Stevens, M.M. Synthetic polymer scaffolds for tissue engineering. Chem. Soc. Rev. 2009, 38, 1139–1151. [Google Scholar] [CrossRef]
- Velema, J.; Kaplan, D. Biopolymer-based biomaterials as scaffolds for tissue engineering. In Tissue Engineering I. Advances in Biochemical Engineering/Biotechnology; Lee, K., Kaplan, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 102, pp. 187–238. [Google Scholar] [CrossRef]
- Savioli Lopes, M.; Jardini, A.L.; Maciel Filho, R. Poly (lactic acid) production for tissue engineering applications. Proc. Eng. 2012, 42, 1402–1413. [Google Scholar] [CrossRef]
- Liu, S.; Qin, S.; He, M.; Zhou, D.; Qin, Q.; Wang, H. Current applications of poly (lactic acid) composites in tissue engineering and drug delivery. Compos. Part B Eng. 2020, 199, 108238. [Google Scholar] [CrossRef]
- Bui, V.T.; Lee, H.S.; Choi, J.H.; Choi, H.S. Highly ordered and robust honeycomb films with tunable pore sizes fabricated via UV crosslinking after applying improved phase separation. Polymer 2015, 74, 46–53. [Google Scholar] [CrossRef]
- Li, L.; Zhong, Y.; Li, J.; Chen, C.; Zhang, A.; Xu, J.; Ma, Z. Thermally stable and solvent resistant honeycomb structured polystyrene films via photochemical cross-linking. J. Mater. Chem. 2009, 19, 7222–7227. [Google Scholar] [CrossRef]
- Cui, L.; Peng, J.; Ding, Y.; Li, X.; Han, Y. Ordered porous polymer films via phase separation in humidity environment. Polymer 2005, 46, 5334–5340. [Google Scholar] [CrossRef]
- Peng, J.; Han, Y.; Yang, Y.; Li, B. The influencing factors on the macroporous formation in polymer films by water droplet templating. Polymer 2004, 45, 447–452. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lin, K.Y.A.; Yang, T.W.; Chu Chen, Y.; Yang, H. Self-assembled hemispherical nanowell arrays for superhydrophobic antireflection coatings. J. Colloid Interface Sci. 2017, 490, 174–180. [Google Scholar] [CrossRef]
- Wong, T.; McGrath, J.A.; Navsaria, H. The role of fibroblasts in tissue engineering and regeneration. Br. J. Dermatol. 2007, 156, 1149–1155. [Google Scholar] [CrossRef]
- Slepička, P.; Neznalová, K.; Fajstavr, D.; Slepičková Kasálková, N.; Švorčík, V. Honeycomb-like pattern formation on perfluoroethylenepropylene enhanced by plasma treatment. Plasma Process. Polym. 2019, 16, 1900063. [Google Scholar] [CrossRef]
- Neznalová, K.; Slepička, P.; Sajdl, P.; Švorčík, V. Cellulose acetate honeycomb-like pattern created by improved phase separation. Express Polym. Lett. 2020, 14, 1078–1088. [Google Scholar] [CrossRef]
- Slepička, P.; Peterková, L.; Rimpelová, S.; Pinkner, A.; Slepičková Kasálková, N.; Kolská, Z.; Ruml, T.; Švorčík, V. Plasma activated perfluoroethylenepropylene for cytocompatibility enhancement. Polym. Degrad. Stabil. 2016, 130, 277–287. [Google Scholar] [CrossRef]
- Novotná, Z.; Řezníčková, A.; Rimpelová, S.; Veselý, M.; Kolská, Z.; Švorčík, V. Tailoring of PEEK bioactivity for improved cell interaction: Plasma treatment in action. RSC Adv. 2015, 5, 41428–41436. [Google Scholar] [CrossRef]
- Novotná, Z.; Rimpelová, S.; Juřík, P.; Veselý, M.; Kolská, Z.; Hubáček, T.; Švorčík, V. The interplay of plasma treatment and gold coating and ultra-high molecular weight polyethylene: On the cytocompatibility. Mater. Sci. Eng. C 2017, 71, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Švorčík, V.; Kolářová, K.; Slepička, P.; Macková, A.; Novotná, M.; Hnatowicz, V. Modification of surface properties of high and low density polyethylene by Ar plasma discharge. Polym. Degrad. Stabil. 2006, 91, 1219–1225. [Google Scholar] [CrossRef]
- Chau, T.T.; Bruckard, W.J.; Koh, P.T.L.; Nguyen, A.V. A review of factors that affect contact angle and implications for flotation practice. Adv. Coll. Interface Sci. 2009, 150, 106–115. [Google Scholar] [CrossRef]
- Kutasi, K.; Bibinov, N.; Von Keudell, A.; Wiesermann, K. Wettabilities of plasma deposited polymer films. J. Optoelectron. Adv. Mater. 2005, 7, 2549–2556. [Google Scholar]
- Brown, P.S.; Talbot, E.L.; Wood, T.J.; Bain, C.D.; Badyal, J.P.S. Superhydrophobic Hierarchical Honeycomb Surfaces. Langmuir 2012, 28, 13712–13719. [Google Scholar] [CrossRef]
- Sequeira, D.B.; Seabra, C.M.; Palma, P.J.; Cardoso, A.L.; Peça, J.; Santos, J.M. Effects of a New Bioceramic Material on Human Apical Papilla Cells. J. Funct. Biomater. 2018, 9, 74. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fajstavrová, K.; Rimpelová, S.; Fajstavr, D.; Švorčík, V.; Slepička, P. PLLA Honeycomb-Like Pattern on Fluorinated Ethylene Propylene as a Substrate for Fibroblast Growth. Polymers 2020, 12, 2436. https://doi.org/10.3390/polym12112436
Fajstavrová K, Rimpelová S, Fajstavr D, Švorčík V, Slepička P. PLLA Honeycomb-Like Pattern on Fluorinated Ethylene Propylene as a Substrate for Fibroblast Growth. Polymers. 2020; 12(11):2436. https://doi.org/10.3390/polym12112436
Chicago/Turabian StyleFajstavrová, Klára, Silvie Rimpelová, Dominik Fajstavr, Václav Švorčík, and Petr Slepička. 2020. "PLLA Honeycomb-Like Pattern on Fluorinated Ethylene Propylene as a Substrate for Fibroblast Growth" Polymers 12, no. 11: 2436. https://doi.org/10.3390/polym12112436
APA StyleFajstavrová, K., Rimpelová, S., Fajstavr, D., Švorčík, V., & Slepička, P. (2020). PLLA Honeycomb-Like Pattern on Fluorinated Ethylene Propylene as a Substrate for Fibroblast Growth. Polymers, 12(11), 2436. https://doi.org/10.3390/polym12112436





