Innovative Hyperbranched Polybenzoxazine-Based Graphene Oxide—Poly(amidoamines) Nanomaterials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Carboxylated GO (GO-COOH)
2.3. Synthesis of GO-COOH Functionalized with Poly(amidoamine) Dendrimer (GO-PAMAM)
2.4. Synthesis of Benzoxazine-Functionalized GO-PAMAM
2.5. Measurements
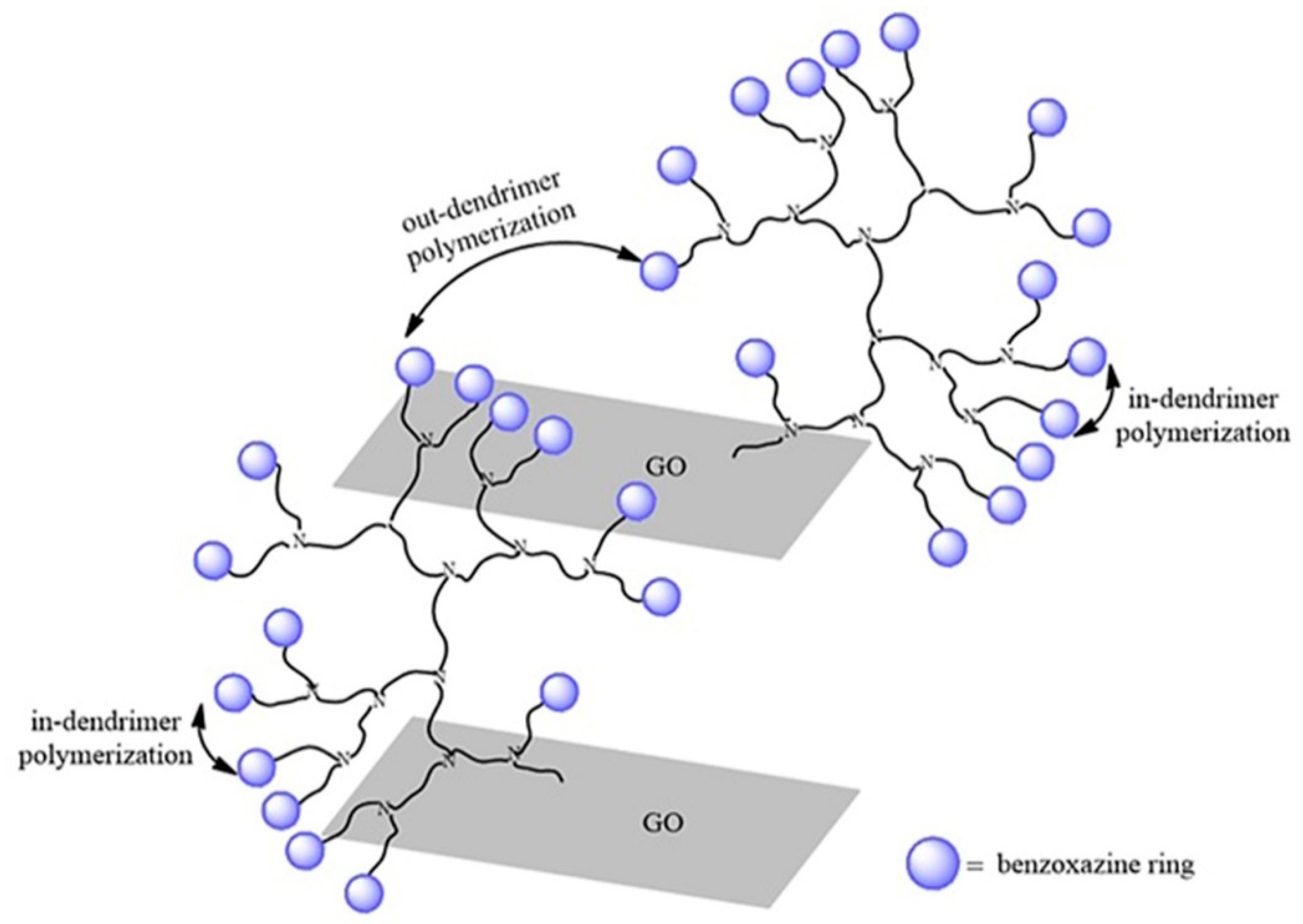
3. Results and Discussion
3.1. XPS Investigation
3.2. FT-IR Analyses
3.3. 1H-NMR Results
3.4. Raman Spectrometry
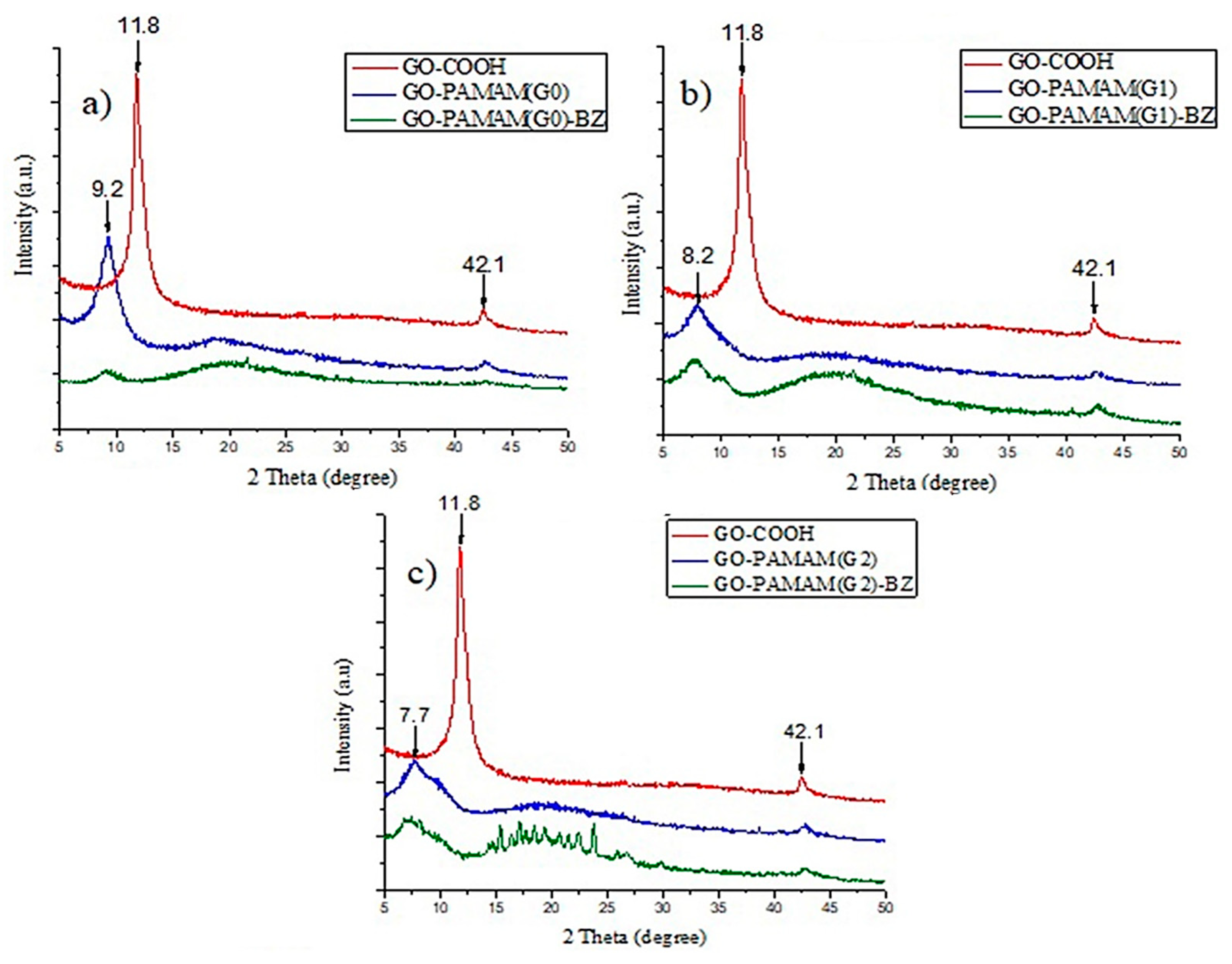
3.5. XRD Tests
3.6. TGA Data
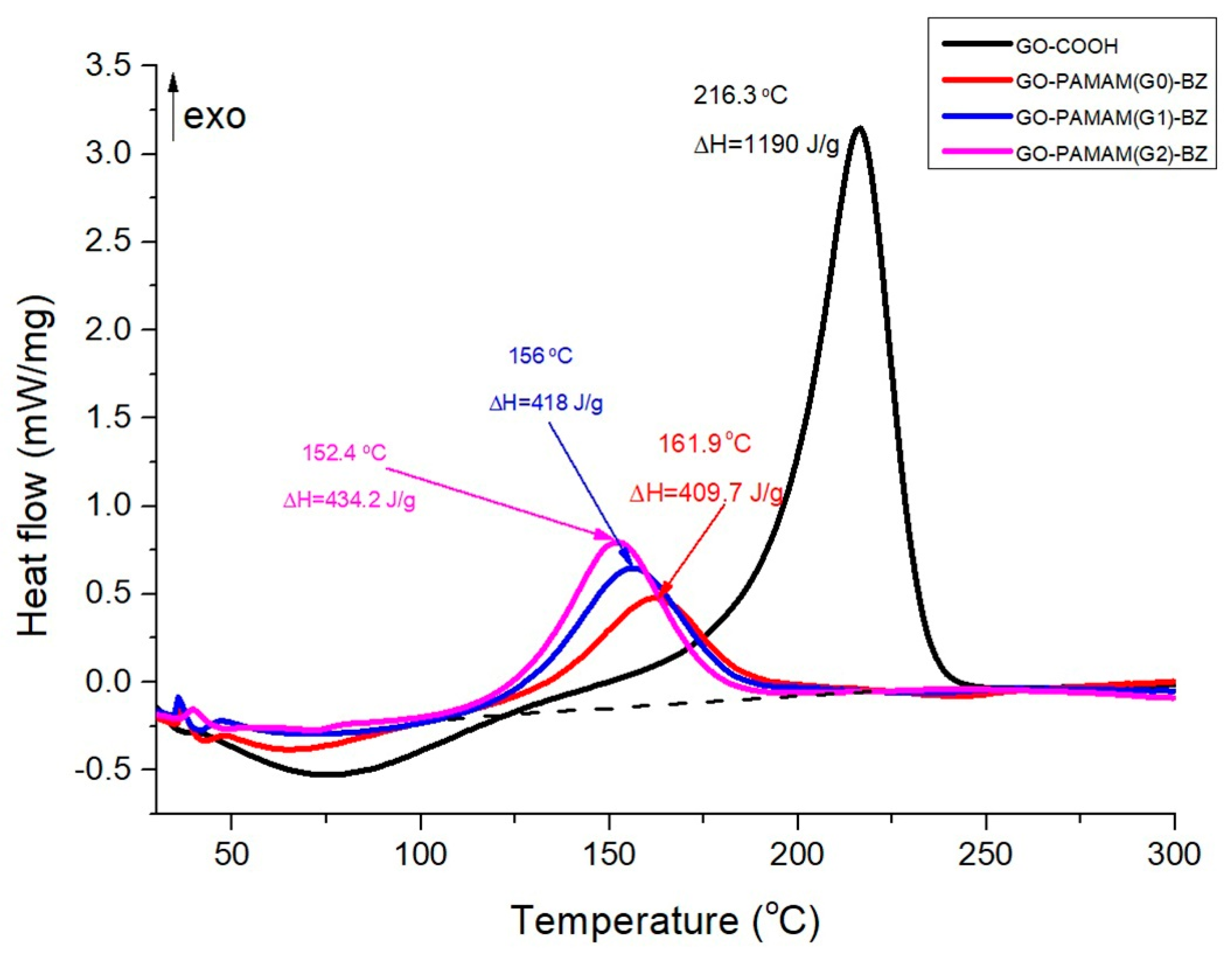
3.7. DSC Results
3.8. Nanoindentation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, K.S.; Nair, C.R. Polybenzoxazine—New generation phenolics. In Handbook of Thermoset Plastics, 3rd ed.; Dodiuk, H., Goodman, S.H., Eds.; William Andrew Publishing: Boston, MA, USA, 2014; pp. 45–73. [Google Scholar] [CrossRef]
- Zhang, K.; Han, L.; Froimowicz, P.; Ishida, H. Synthesis, polymerization kinetics and thermal properties of para-methylol functional benzoxazine. React. Funct. Polym. 2018, 129, 23–28. [Google Scholar] [CrossRef]
- Yen, Y.-C.; Cheng, C.-C.; Chu, Y.-L.; Chang, F.-C. A new benzoxazine containing uracil, complementary functionality. Polym. Chem. 2011, 2, 1648–1653. [Google Scholar] [CrossRef]
- Higginson, C.J.; Malollari, K.G.; Xu, Y.; Kelleghan, A.V.; Ricapito, N.G.; Messersmith, P.B. Bioinspired Design Provides High-Strength Benzoxazine Structural Adhesives. Angew. Chem. Int. Ed. 2019, 58, 12271–12279. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, N.N.; Kiskan, B.; Yagci, Y. Polybenzoxazines—New high performance thermosetting resins: Synthesis and properties. Prog. Polym. Sci. 2007, 32, 1344–1391. [Google Scholar] [CrossRef]
- Liu, C.; Shen, D.; Sebastián, R.M.; Marquet, J.; Schönfeld, R. Mechanistic Studies on Ring-Opening Polymerization of Benzoxazines: A Mechanistically Based Catalyst Design. Macromolecules 2011, 44, 4616–4622. [Google Scholar] [CrossRef]
- Bai, Y.; Yang, P.; Song, Y.; Zhu, R.; Gu, Y. Effect of hydrogen bonds on the polymerization of benzoxazines: Influence and control. RSC Adv. 2016, 6, 45630–45635. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Han, M.; Froimowicz, P. Smart and sustainable design of latent catalyst-containing benzoxazine-bio-resins and application studies. Green Chem. 2020, 22, 1209–1219. [Google Scholar] [CrossRef]
- Zhang, K.; Tan, X.; Wang, Y.; Ishida, H. Unique self-catalyzed cationic ring-opening polymerization of a high performance deoxybenzoin-based 1,3-benzoxazine monomer. Polymer 2019, 168, 8–15. [Google Scholar] [CrossRef]
- Martos, A.; Sebastián, R.M.; Marquet, J. Studies on the ring-opening polymerization of benzoxazines: Understanding the effect of the substituents. Eur. Polym. J. 2018, 108, 20–27. [Google Scholar] [CrossRef]
- Zhang, K.; Han, L.; Nie, Y.; Szigeti, M.L.; Ishida, H. Examining the effect of hydroxyl groups on the thermal properties of polybenzoxazines: Using molecular design and Monte Carlo simulation. RSC Adv. 2018, 8, 18038–18050. [Google Scholar] [CrossRef]
- Xu, M.; Ren, D.; Chen, L.; Li, K.; Liu, X. Understanding of the polymerization mechanism of the phthalonitrile-based resins containing benzoxazine and their thermal stability. Polymer 2018, 143, 28–39. [Google Scholar] [CrossRef]
- Akkus, B.; Kiskan, B.; Yagci, Y. Cyanuric chloride as a potent catalyst for the reduction of curing temperature of benzoxazines. Polym. Chem. 2020, 11, 1025–1032. [Google Scholar] [CrossRef]
- Sun, J.; Wei, W.; Xu, Y.; Qu, J.; Liu, X.; Endo, T. A curing system of benzoxazine with amine: Reactivity, reaction mechanism and material properties. RSC Adv. 2015, 5, 19048–19057. [Google Scholar] [CrossRef]
- Sudo, A.; Yamashita, H.; Endo, T. Ring-opening polymerization of 1,3-benzoxazines by p-toluenesulfonates as thermally latent initiators. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 3631–3636. [Google Scholar] [CrossRef]
- Liu, C.; Shen, D.; Sebastián, R.M.; Marquet, J.; Schönfeld, R. Catalyst effects on the ring-opening polymerization of 1,3-benzoxazine and on the polymer structure. Polymer 2013, 54, 2873–2878. [Google Scholar] [CrossRef]
- Akkus, B.; Kiskan, B.; Yagci, Y. Counterion Effect of Amine Salts on Ring-Opening Polymerization of 1,3-Benzoxazines. Macromol. Chem. Phys. 2019, 220, 1800268. [Google Scholar] [CrossRef]
- Kocaarslan, A.; Kiskan, B.; Yagci, Y. Ammonium salt catalyzed ring-opening polymerization of 1,3-benzoxazines. Polymer 2017, 122, 340–346. [Google Scholar] [CrossRef]
- Machado, B.F.; Serp, P. Graphene-based materials for catalysis. Catal. Sci. Technol. 2012, 2, 54–75. [Google Scholar] [CrossRef]
- Nováček, M.; Jankovský, O.; Luxa, J.; Sedmidubský, D.; Pumera, M.; Fila, V.; Lhotka, M.; Klímová, K.; Matějková, S.; Sofer, Z. Tuning of graphene oxide composition by multiple oxidations for carbon dioxide storage and capture of toxic metals. J. Mater. Chem. A 2017, 5, 2739–2748. [Google Scholar] [CrossRef]
- Ambrosi, A.; Chua, C.K.; Latiff, N.M.; Loo, A.H.; Wong, C.H.A.; Eng, A.Y.S.; Bonanni, A.; Pumera, M. Graphene and its electrochemistry—An update. Chem. Soc. Rev. 2016, 45, 2458–2493. [Google Scholar] [CrossRef]
- Afzali, M.; Mostafavi, A.; Shamspur, T. Developing a novel sensor based on ionic liquid molecularly imprinted polymer/gold nanoparticles/graphene oxide for the selective determination of an anti-cancer drug imiquimod. Biosens. Bioelectron. 2019, 143, 111620. [Google Scholar] [CrossRef]
- Liang, Y.; Yu, L.; Yang, R.; Li, X.; Qu, L.; Li, J. High sensitive and selective graphene oxide/molecularly imprinted polymer electrochemical sensor for 2,4-dichlorophenol in water. Sens. Actuators B Chem. 2017, 240, 1330–1335. [Google Scholar] [CrossRef]
- Dehghani, M.; Nasirizadeh, N.; Yazdanshenas, M.E. Determination of cefixime using a novel electrochemical sensor produced with gold nanowires/graphene oxide/electropolymerized molecular imprinted polymer. Mater. Sci. Eng. C 2019, 96, 654–660. [Google Scholar] [CrossRef]
- Dhanabalan, S.C.; Dhanabalan, B.; Chen, X.; Ponraj, J.S.; Zhang, H. Hybrid carbon nanostructured fibers: Stepping stone for intelligent textile-based electronics. Nanoscale 2019, 11, 3046–3101. [Google Scholar] [CrossRef]
- Kim, J.I.; Cho, J.S.; Wang, D.H.; Park, J.H. Highly dispersible graphene oxide nanoflakes in pseudo-gel-polymer porous separators for boosting ion transportation. Carbon 2020, 166, 427–435. [Google Scholar] [CrossRef]
- Othman, N.H.; Che Ismail, M.; Mustapha, M.; Sallih, N.; Kee, K.E.; Ahmad Jaal, R. Graphene-based polymer nanocomposites as barrier coatings for corrosion protection. Prog. Org. Coat. 2019, 135, 82–99. [Google Scholar] [CrossRef]
- Massoud, A.; Farid, O.M.; Maree, R.M.; Allan, K.F.; Tian, Z.R. An improved metal cation capture on polymer with graphene oxide synthesized by gamma radiation. React. Funct. Polym. 2020, 151, 104564. [Google Scholar] [CrossRef]
- Zeng, M.; Wang, J.; Li, R.; Liu, J.; Chen, W.; Xu, Q.; Gu, Y. The curing behavior and thermal property of graphene oxide/benzoxazine nanocomposites. Polymer 2013, 54, 3107–3116. [Google Scholar] [CrossRef]
- Xu, Q.; Zeng, M.; Feng, Z.; Yin, D.; Huang, Y.; Chen, Y.; Yan, C.; Li, R.; Gu, Y. Understanding the effects of carboxylated groups of functionalized graphene oxide on the curing behavior and intermolecular interactions of benzoxazine nanocomposites. RSC Adv. 2016, 6, 31484–31496. [Google Scholar] [CrossRef]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent Functionalization of Graphene and Graphene Oxide for Energy Materials, Biosensing, Catalytic, and Biomedical Applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef]
- Meng, F.; Ishida, H.; Liu, X. Introduction of benzoxazine onto the graphene oxide surface by click chemistry and the properties of graphene oxide reinforced polybenzoxazine nanohybrids. RSC Adv. 2014, 4, 9471–9475. [Google Scholar] [CrossRef]
- Biru, I.; Damian, C.M.; Gârea, S.A.; Iovu, H. Benzoxazine-functionalized graphene oxide for synthesis of new nanocomposites. Eur. Polym. J. 2016, 83, 244–255. [Google Scholar] [CrossRef]
- Bîru, E.I.; Gârea, S.A.; Nicolescu, A.; Vasile, E.; Iovu, H. Advanced Polybenzoxazine Structures Based on Modified Reduced Graphene Oxide. Polymers 2018, 10, 941. [Google Scholar] [CrossRef]
- Wang, X.; Li, N.; Wang, J.; Li, G.; Zong, L.; Liu, C.; Jian, X. Hyperbranched polyether epoxy grafted graphene oxide for benzoxazine composites: Enhancement of mechanical and thermal properties. Compos. Sci. Technol. 2018, 155, 11–21. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, J.; Lu, Y.; Gai, P.; Zhong, H. Synthesis of poly(amido amine)-derived dendrimers with pendant benzoxazine groups and their thermal behavior. J. Appl. Polym. Sci. 2013, 127, 282–288. [Google Scholar] [CrossRef]
- Lin, R.-C.; Mohamed, M.G.; Kuo, S.-W. Benzoxazine/Triphenylamine-Based Dendrimers Prepared through Facile One-Pot Mannich Condensations. Macromol. Rapid Commun. 2017, 38, 1700251. [Google Scholar] [CrossRef]
- Lin, R.-C.; Kuo, S.-W. Well-defined benzoxazine/triphenylamine-based hyperbranched polymers with controlled degree of branching. RSC Adv. 2018, 8, 13592–13611. [Google Scholar] [CrossRef]
- Park, K.-W. Carboxylated graphene oxide–Mn2O3 nanorod composites for their electrochemical characteristics. J. Mater. Chem. A 2014, 2, 4292–4298. [Google Scholar] [CrossRef]
- Cividanes, L.S.; Simonetti, E.A.N.; Moraes, M.B.; Fernandes, F.W.; Thim, G.P. Influence of carbon nanotubes on epoxy resin cure reaction using different techniques: A comprehensive review. Polym. Eng. Sci. 2014, 54, 2461–2469. [Google Scholar] [CrossRef]
- Xiao, W.; Yan, B.; Zeng, H.; Liu, Q. Dendrimer functionalized graphene oxide for selenium removal. Carbon 2016, 105, 655–664. [Google Scholar] [CrossRef]
- Yu, S.; Liu, J.; Zhu, W.; Hu, Z.-T.; Lim, T.-T.; Yan, X. Facile room-temperature synthesis of carboxylated graphene oxide-copper sulfide nanocomposite with high photodegradation and disinfection activities under solar light irradiation. Sci. Rep. 2015, 5, 16369. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.V.; Cividanes, L.D.S.; Brito, F.S.; de Menezes, B.R.C.; Franceschi, W.; Simonetti, E.A.N.; Thim, G.P. Functionalization of Graphene and Applications. In Functionalizing Graphene and Carbon Nanotubes: A Review; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–29. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Brito, F.S.; Franceschi, W.; Simonetti, E.A.N.; Cividanes, L.S.; Chipara, M.; Lozano, K. Functionalized graphene oxide as reinforcement in epoxy based nanocomposites. Surf. Interfaces 2018, 10, 100–109. [Google Scholar] [CrossRef]
- Gu, Y.; Guo, Y.; Wang, C.; Xu, J.; Wu, J.; Kirk, T.B.; Ma, D.; Xue, W. A polyamidoamne dendrimer functionalized graphene oxide for DOX and MMP-9 shRNA plasmid co-delivery. Mater. Sci. Eng. C 2017, 70, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Kiskan, B.; Yagci, Y.; Ishida, H. Synthesis, characterization, and properties of new thermally curable polyetheresters containing benzoxazine moieties in the main chain. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 414–420. [Google Scholar] [CrossRef]
- Maio, A.; Fucarino, R.; Khatibi, R.; Rosselli, S.; Bruno, M.; Scaffaro, R. A novel approach to prevent graphene oxide re-aggregation during the melt compounding with polymers. Compos. Sci. Technol. 2015, 119, 131–137. [Google Scholar] [CrossRef]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’homme, R.K.; Aksay, I.A.; Car, R. Raman Spectra of Graphite Oxide and Functionalized Graphene Sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron Spectrosc. Relat. Phenom. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Samadaei, F.; Salami-Kalajahi, M.; Roghani-Mamaqani, H.; Banaei, M. A structural study on ethylenediamine- and poly(amidoamine)-functionalized graphene oxide: Simultaneous reduction, functionalization, and formation of 3D structure. RSC Adv. 2015, 5, 71835–71843. [Google Scholar] [CrossRef]
- Qiu, Y.; Collin, F.; Hurt, R.H.; Külaots, I. Thermochemistry and kinetics of graphite oxide exothermic decomposition for safety in large-scale storage and processing. Carbon 2016, 96, 20–28. [Google Scholar] [CrossRef]
- Arslan, M.; Kiskan, B.; Yagci, Y. Ring-Opening Polymerization of 1,3-Benzoxazines via Borane Catalyst. Polymers 2018, 10, 239. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Gómez-Fatou, M.A.; Ania, F.; Flores, A. Nanoindentation in polymer nanocomposites. Progr. Mater. Sci. 2015, 67, 1–94. [Google Scholar] [CrossRef]


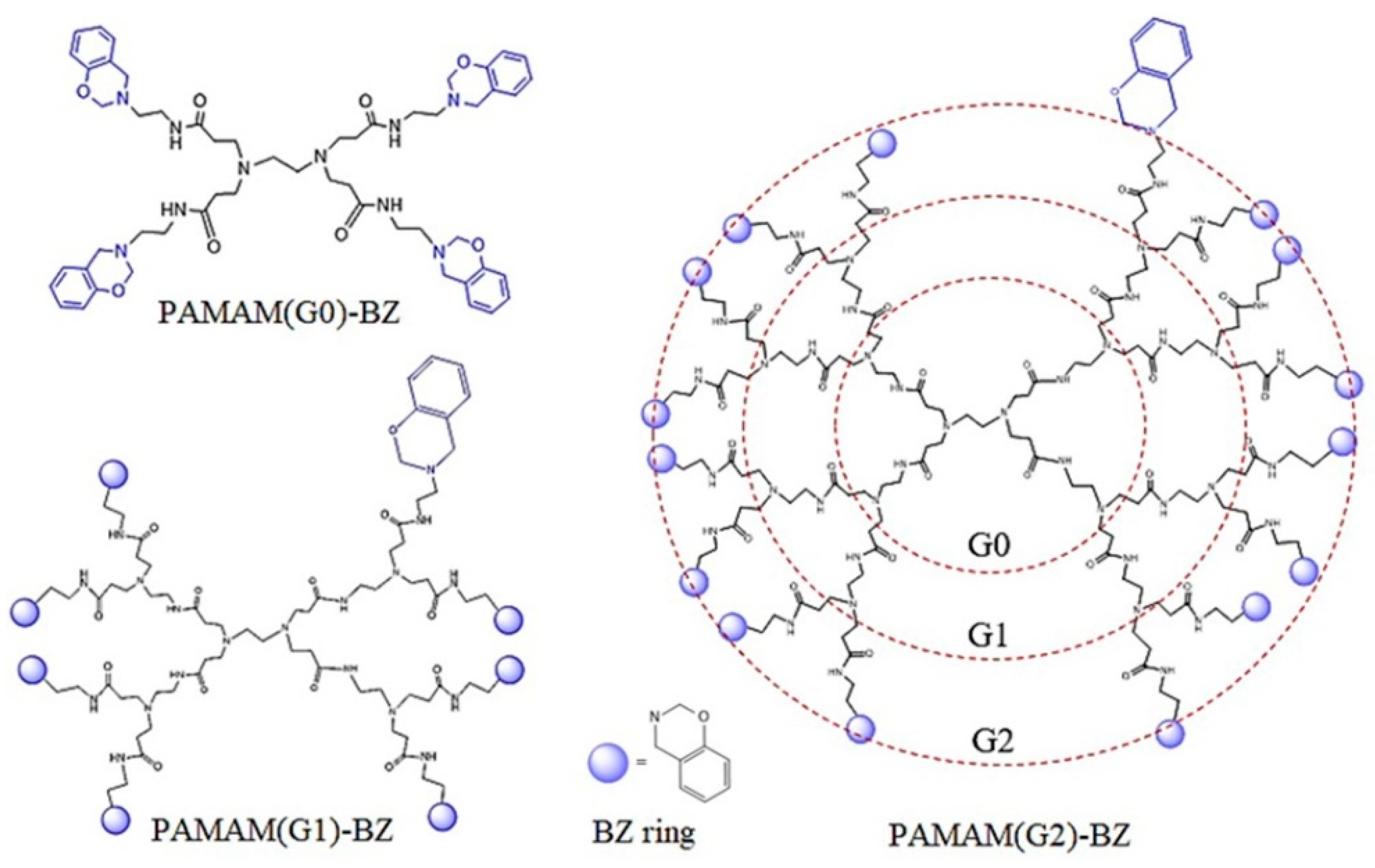
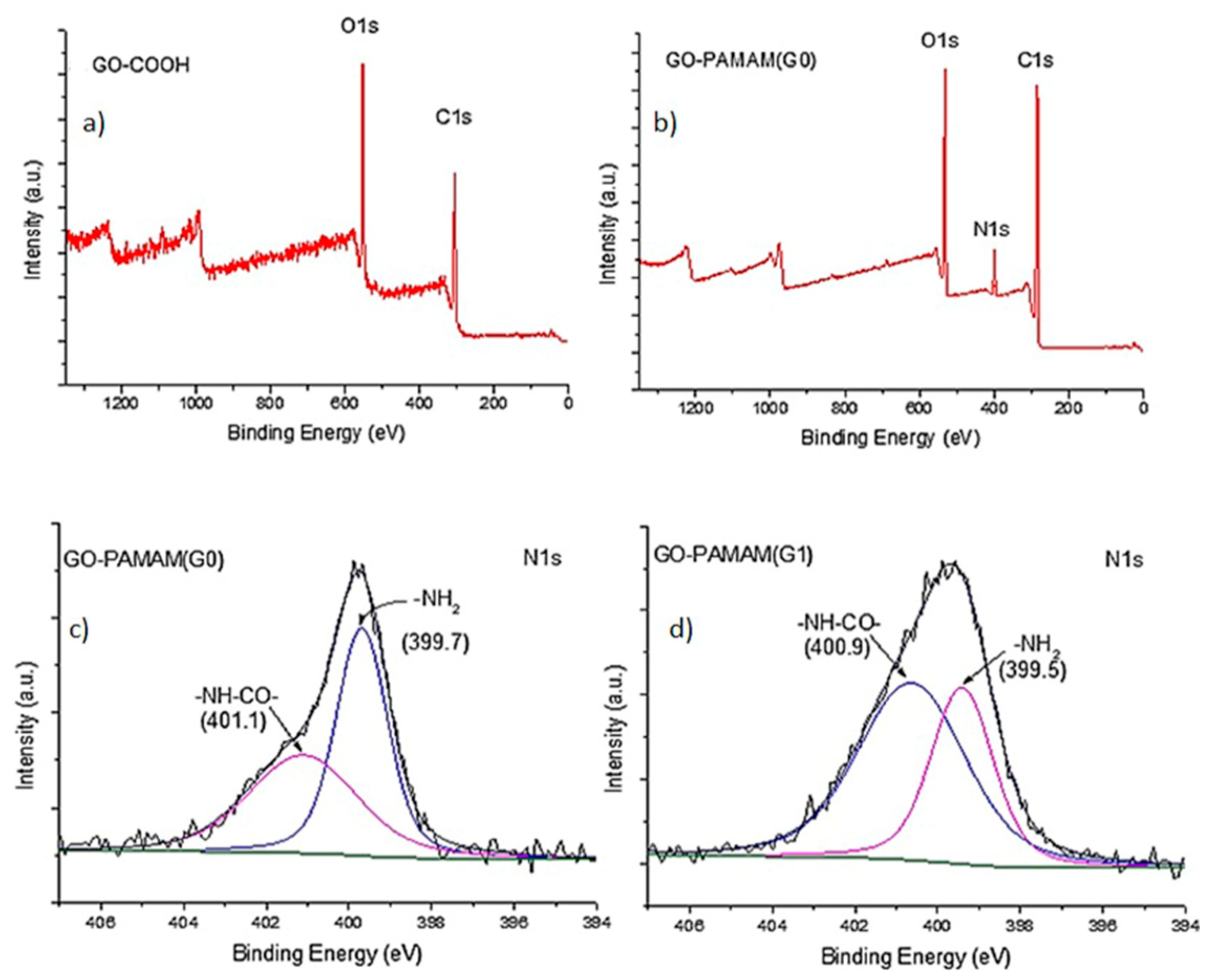
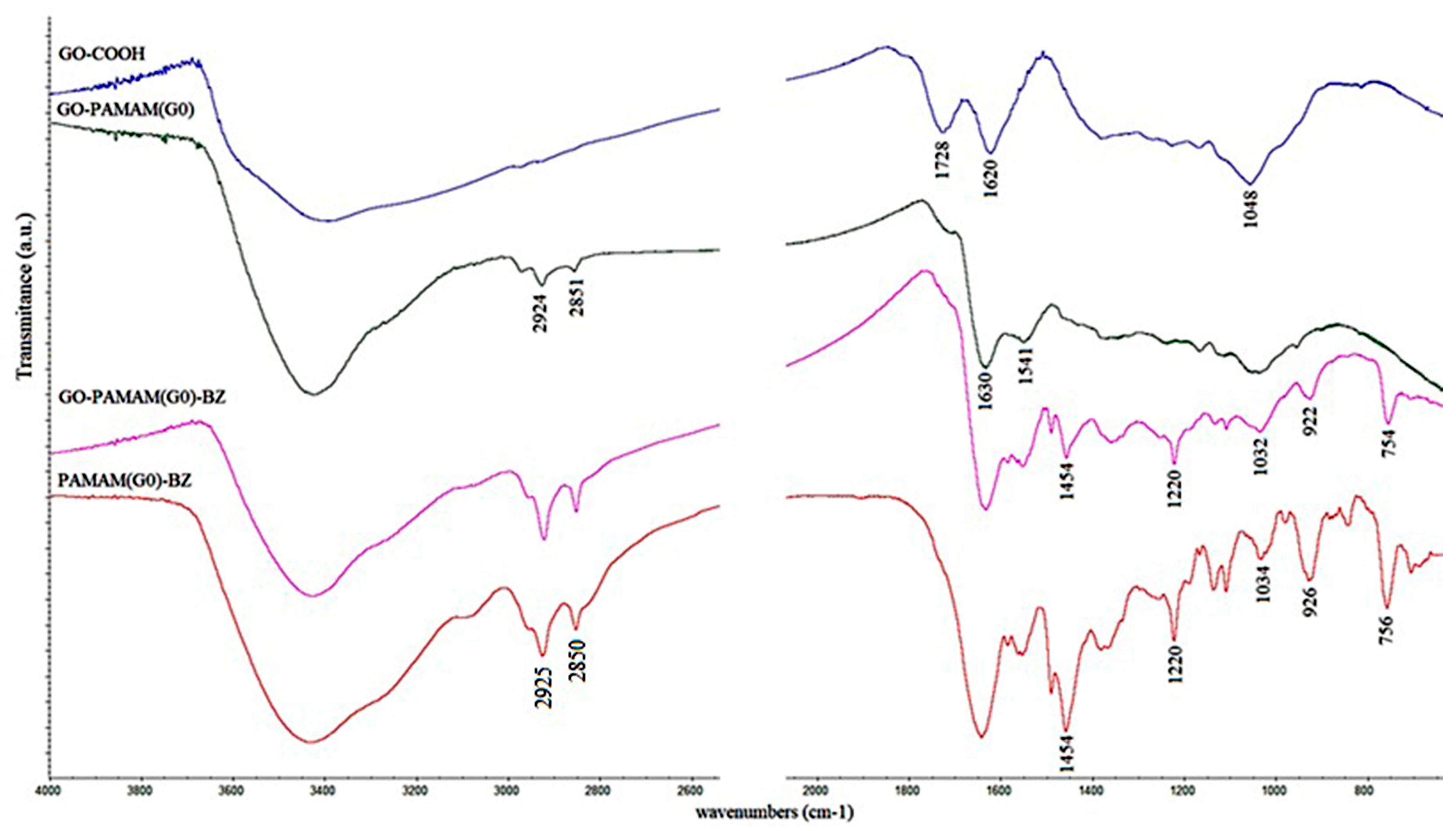
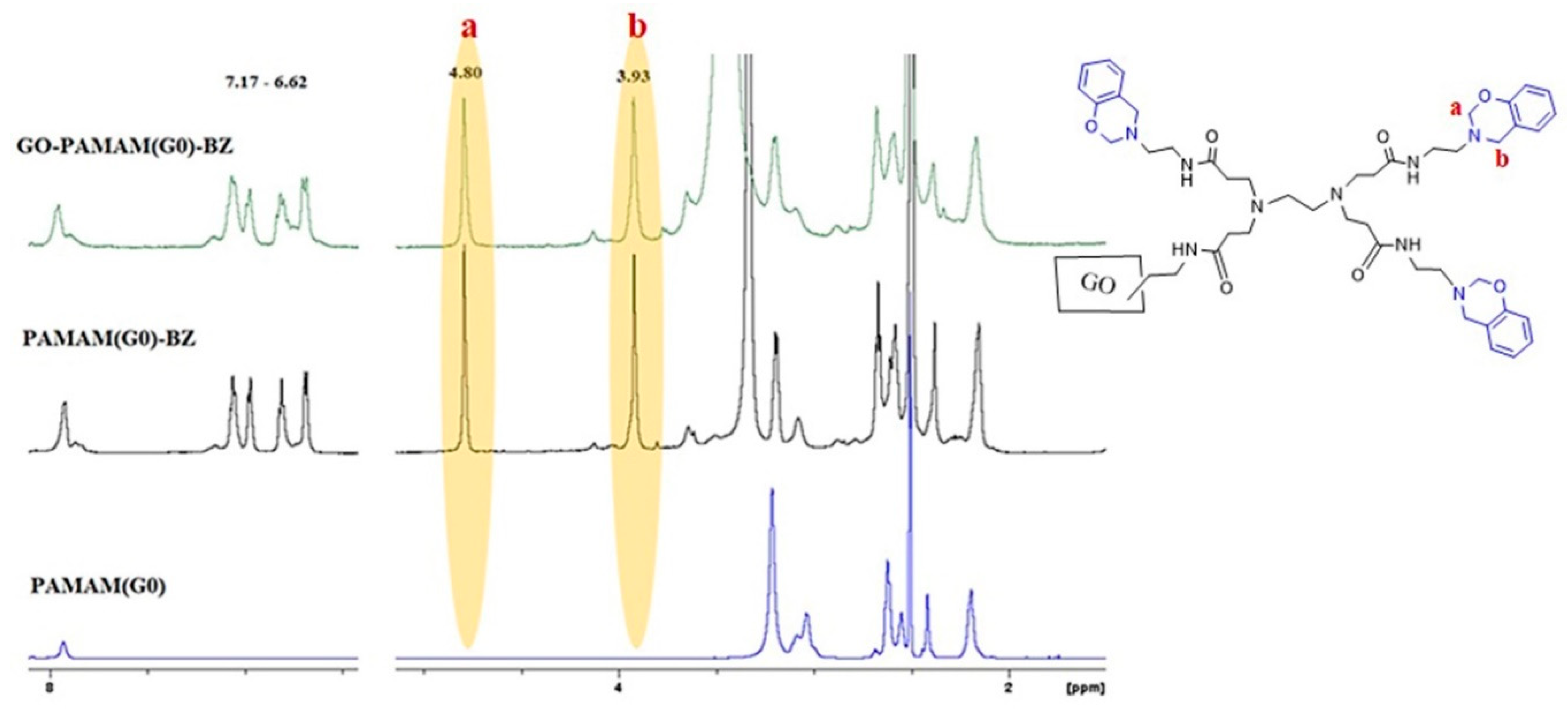




| Sample | C (%) | O (%) | N (%) |
|---|---|---|---|
| GO-COOH | 67 | 33 | 0 |
| GO-PAMAM(G0) | 76 | 20 | 4 |
| GO-PAMAM(G1) | 72 | 20 | 8 |
| GO-PAMAM(G2) | 71 | 20 | 9 |
| Sample | ID/IG (473nm) |
|---|---|
| GO-COOH | 0.78 |
| GO-PAMAM(G0) | 0.85 |
| GO-PAMAM(G1) | 0.86 |
| GO-PAMAM(G2) | 0.86 |
| GO-PAMAM(G0)-BZ | 1.02 |
| GO-PAMAM(G1)-BZ | 0.88 |
| GO-PAMAM(G2)-BZ | 0.83 |
| Sample | 2θ Position (°) | d-spacing (Å) |
|---|---|---|
| GO-COOH | 11.85 | 7.46 |
| GO-PAMAM(G0) | 9.24 | 9.61 |
| GO-PAMAM(G1) | 8.22 | 10.74 |
| GO-PAMAM(G2) | 7.72 | 11.44 |
| GO-PAMAM(G0)-BZ | 9.19 | 9.65 |
| GO-PAMAM(G1)-BZ | 7.7 | 11.46 |
| GO-PAMAM(G2)-BZ | 7.01 | 12.61 |
| Sample | Weight Loss, % (25–800 °C) | Td3%, °C | Td5%, °C |
|---|---|---|---|
| GO-COOH | 90 | 58.8 | 76.7 |
| GO-PAMAM(G0)-BZ | 59.7 | 84.9 | 118.6 |
| GO-PAMAM(G1)-BZ | 61.4 | 80.8 | 109.3 |
| GO-PAMAM(G2)-BZ | 54.9 | 113.1 | 136.8 |
| Sample | E (GPa) | Hardness (GPa) | Stiffness (N/m) |
|---|---|---|---|
| GO-COOH | 0.614 | 0.057 | 4416 |
| GO-PAMAM(G0)-BZ | 0.536 | 0.036 | 3617 |
| GO-PAMAM(G1)-BZ | 0.763 | 0.078 | 5117 |
| GO-PAMAM(G2)-BZ | 1.418 | 0.157 | 9621 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bîru, E.I.; Gârea, S.A.; Iovu, H. Innovative Hyperbranched Polybenzoxazine-Based Graphene Oxide—Poly(amidoamines) Nanomaterials. Polymers 2020, 12, 2424. https://doi.org/10.3390/polym12102424
Bîru EI, Gârea SA, Iovu H. Innovative Hyperbranched Polybenzoxazine-Based Graphene Oxide—Poly(amidoamines) Nanomaterials. Polymers. 2020; 12(10):2424. https://doi.org/10.3390/polym12102424
Chicago/Turabian StyleBîru, Elena Iuliana, Sorina Alexandra Gârea, and Horia Iovu. 2020. "Innovative Hyperbranched Polybenzoxazine-Based Graphene Oxide—Poly(amidoamines) Nanomaterials" Polymers 12, no. 10: 2424. https://doi.org/10.3390/polym12102424
APA StyleBîru, E. I., Gârea, S. A., & Iovu, H. (2020). Innovative Hyperbranched Polybenzoxazine-Based Graphene Oxide—Poly(amidoamines) Nanomaterials. Polymers, 12(10), 2424. https://doi.org/10.3390/polym12102424






