1. Introduction
Microcapsule technology is a kind of packaging technology that uses natural or synthetic polymer film-forming materials to coat gas, liquid or solid into 1–1000-μm micro particles [
1]. Microcapsules are generally composed of a wall material (wrapping material) and core material (wrapped material). Due to the difference in the preparation methods and product requirements, the microcapsules have a variety of structures. The wall material determines the strength of microcapsules, the release characteristics of sustained-release core materials and the durability of microcapsules, which has an important impact on the performance of microcapsules [
2]. Microencapsulation technology has experienced a long development process, and has been widely used in the fields of medical treatment [
3], cosmetics, food, textile, coating and advanced materials. Although its application in wood coatings is still in the development stage, it has great application prospects [
4,
5,
6]. Self-healing technology, as a new effective way to suppress coating micro-cracks, has become a research hotspot in many application fields [
7,
8,
9]. Functionalized and application-oriented microcapsules are added to the coating to improve the repair ratio of the coating while enhancing the coating performance [
10]. The choice of microcapsule wall material and core material seriously affects the performance of microcapsules [
11,
12].
Mirabedini et al. [
13] applied a phase separation to prepare new oil-filled microcapsules with ethyl cellulose as the shell material, and investigated the relationship between the mass fraction of microcapsules and the tensile properties of the film. The results showed that the film had self-healing properties after adding microcapsules. Safaei et al. [
14] used microcapsules containing epoxy healing agents to develop economic and efficient self-healing epoxy coatings. The effects of the preparation process on the properties of microcapsules were studied, and optimal synthesis conditions were obtained. Wang et al. [
15] prepared microcapsules by in situ polymerization using poly(melamine-formaldehyde) as the wall materials and the mixture of bisphenol A diglycidyl ether and epoxy diluent as the core materials. The influences of epoxy diluent type, kinds of surfactant, mass fraction of emulsifier, and emulsification ratio on the physical performance of microcapsules were discussed. Zhang et al. [
16] synthesized polyurea formaldehyde microcapsules with tall oil fatty acid poxy ester as core materials by in situ polymerization. The self-healing coatings were prepared by adding the polyuria formaldehyde epoxy ester microcapsules into the epoxy coatings. As a common type of waterborne coating, waterborne acrylic coatings are widely used [
17]. The waterborne acrylic resin is a one-component repair agent that does not require a curing agent and a catalyst [
18,
19]. At present, there are many studies on the preparation of self-healing microcapsules using epoxy repair agents as the core material, while the research on the preparation of self-healing microcapsules for waterborne coatings with waterborne paint components as the core material is less extensive [
20,
21].
In this paper, microcapsules with urea-formaldehyde resin as the wall material and Dulux waterborne acrylic resin [
22,
23] as the core material were prepared by in situ polymerization. It was assumed that morphology, particle size, yield and encapsulation ratio of microcapsules had no relationship with the core–wall ratio. If the hypothesis was not true, it was considered that the performance of microcapsules was related to the core–wall ratio. The method of testing was proved by orthogonal experiment. Combined with the orthogonal test results, the preparation process of microcapsules was further optimized to determine the better morphology, yield and encapsulation performance of Dulux waterborne acrylic microcapsules, which provides a technical reference for the increased toughness of waterborne paint film dried at room temperature on the surface of the wood.
2. Materials and Methods
2.1. Materials
The 37.0% formaldehyde solution (Mw: 30.03 g/mol, CAS No.: 50-00-0), urea (Mw: 60.06 g/mol, CAS No.: 57-13-6), triethanolamine (Mw: 149.19 g/mol, CAS No.: 102-71-6), and ethyl acetate (Mw: 88.11 g/mol, CAS No.: 141-78-6) were provided by Nanjing Chemical Reagent Co., Ltd., Nanjing, China. Dulux waterborne acrylic resin was offered by Akzo Nobel Paints (Shanghai) Co., Ltd., Shanghai, China. Sodium dodecyl benzene sulfonate (Mw: 348.48 g/mol, CAS No.: 25155-30-0) was supplied by Beichen District Fangzheng Reagent Factory, Tianjin, China. Octanol (Mw: 130.23 g/mol, CAS No.: 111-87-5) was offered by Yatai United Chemical Co., Ltd., Wuxi, China. Citric acid (Mw: 210.14 g/mol, CAS No.: 5949-29-1) was provided by Beilian Fine Chemical Development Co., Ltd., Tianjin, China. Ethanol (Mw: 46.07 g/mol, CAS No.: 64-17-5) was provided by Outuopu Biotechnology Co., Ltd., Hangzhou, China. Tilia europaea (100 mm × 65 mm × 4 mm, color uniformity, after sanding pretreatment) was offered by Yihua Lifestyle Technology Co., Ltd., Guangdong, China.
2.2. Preparation of Microcapsules
The three-factor two-level orthogonal test was used to determine the factor that had the greatest impact on the performance of the microcapsules. Then, the single-factor independent test was conducted on the factor that had the greatest influence. In order to explore the most important factors affecting the morphology, particle size, yield and encapsulation ratio of microcapsules [
24], two levels of core–wall ratio, water bath temperature and depositing time were selected to conduct orthogonal experiments. The data of the orthogonal experiment arrangement are shown in
Table 1.
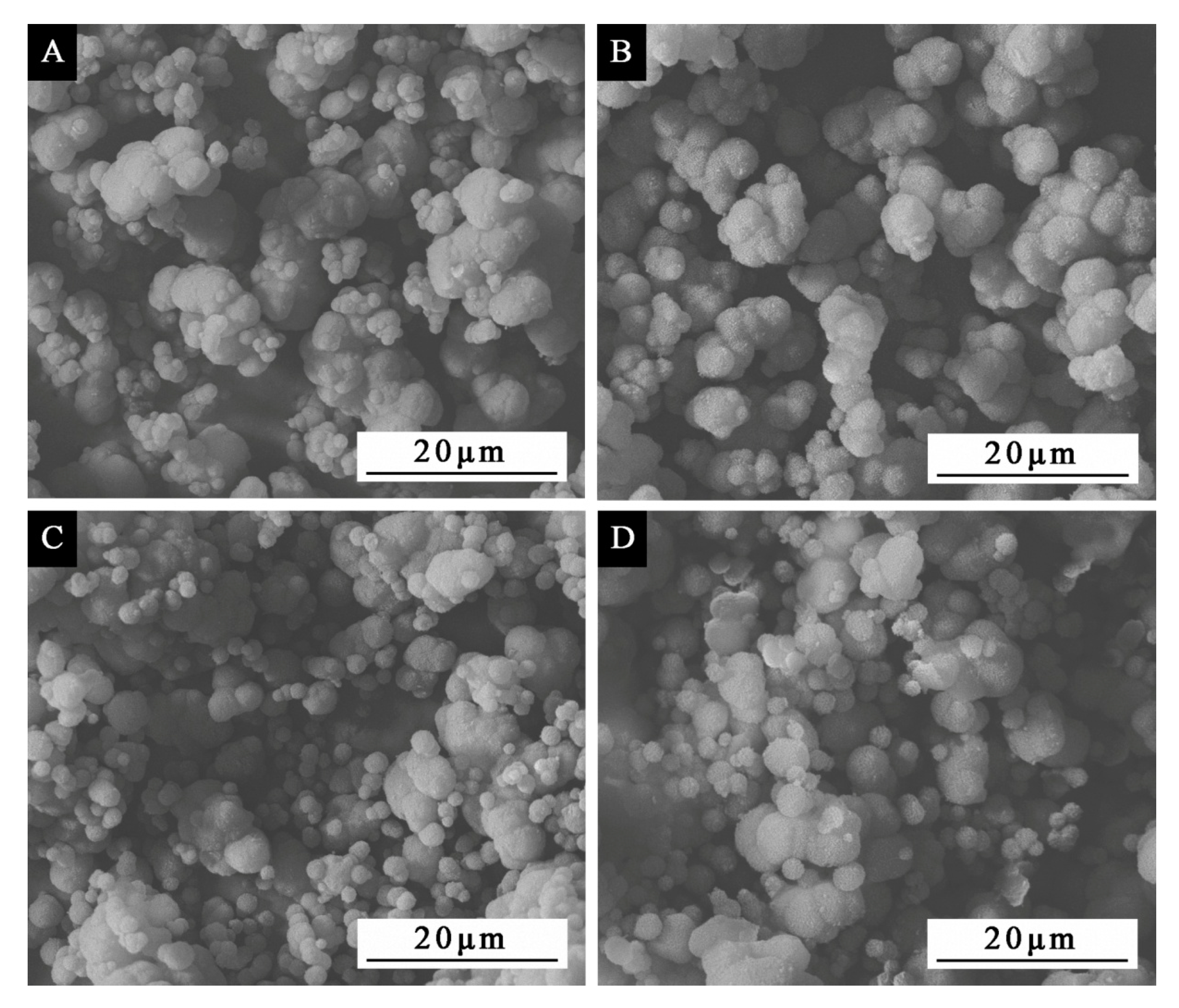
The preparation process of urea-formaldehyde, resin-coated waterborne acrylic microcapsules mainly includes three parts: preparation of wall material urea-formaldehyde, preparation of core material emulsion and microencapsulation. The first step was the preparation of the wall material: 20.0 g of urea and 27.0 g of 37.0% formaldehyde solution were added to the beaker and fully stirred with a magnetic stirrer at 100 rpm. After the urea was completely dissolved, the triethanolamine was laxly mixed to regulate the pH value to about 9.0. The mixed solution was then put in a 70 °C water bath and continuously stirred for 90 min to obtain a slightly viscous and transparent urea-formaldehyde prepolymer solution. The solution was cooled to room temperature for use. The second step was the preparation of the core material: the 0.975 g sodium dodecyl benzene sulfonate white powder was mixed with 96.52 mL of the distilled water, and the mixture was stirred until it was completely dissolved, to obtain a 1.0% aqueous solution of sodium dodecyl benzene sulfonate as an emulsifier. Then, the 12.5 g of Dulux waterborne acrylic resin was added to 97.0 mL of the 1.0% sodium dodecyl benzene sulfonate aqueous solution, and the mixed solution was then put in a 60 °C constant-temperature water bath and stirred at 1200 rpm for 30 min to gain the stable core material emulsion. The 1–2 drops of octanol were added for defoaming. The third step is microencapsulation: the urea-formaldehyde prepolymer was dropped into the prepared core material at the speed of 300 rpm, and then the citric acid was added gradually to adjust the pH to 2.5–3.0. The temperature was slowly raised to 50 °C and held for 3 h. Finally, the obtained product was filtered by suction and distilled water was added to rinse off the excess emulsifier. Then, the product was heated and dried at 80 °C for 4 h, and the white powder obtained was the sample 1# of the orthogonal test (
Table 2). The specific preparation progress of samples 2–4# is the same as that of sample 1#.
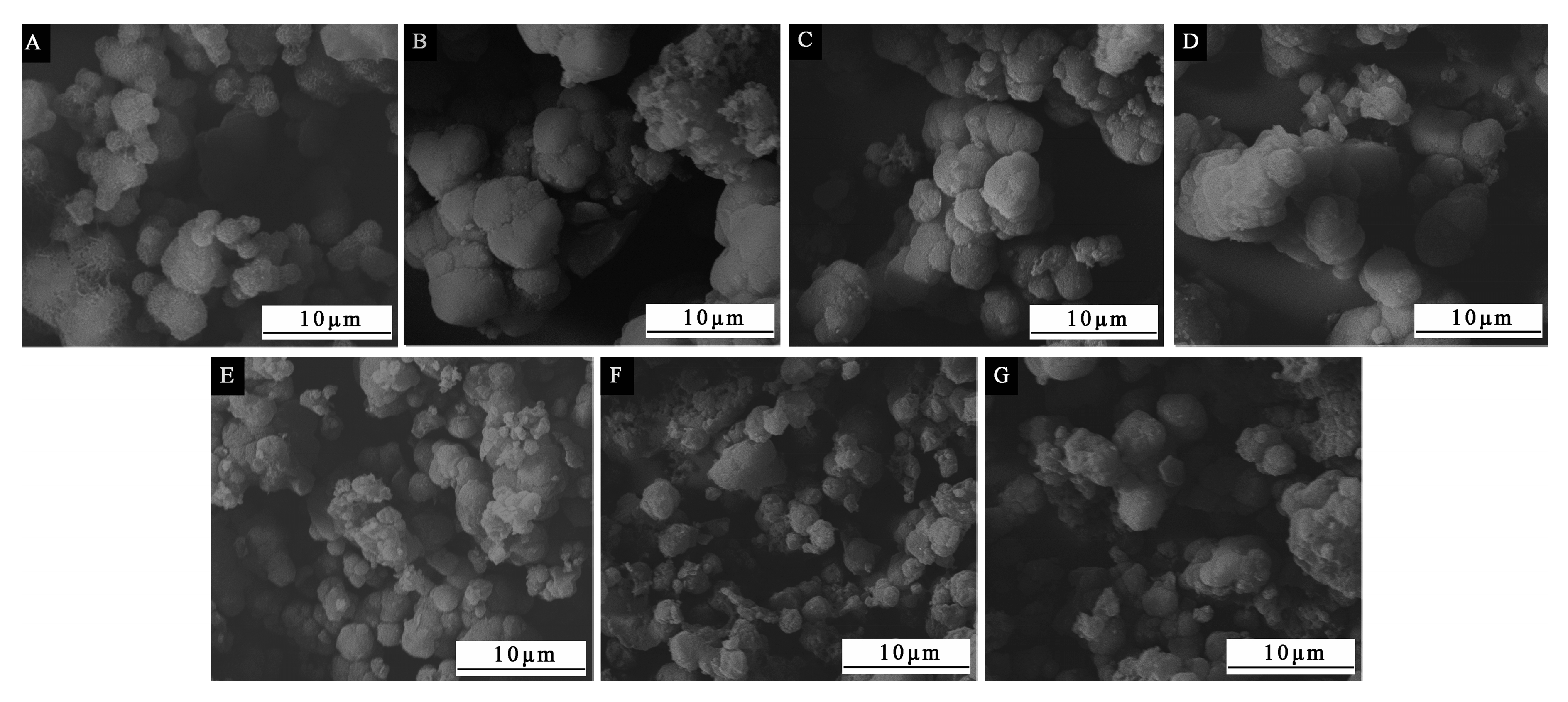
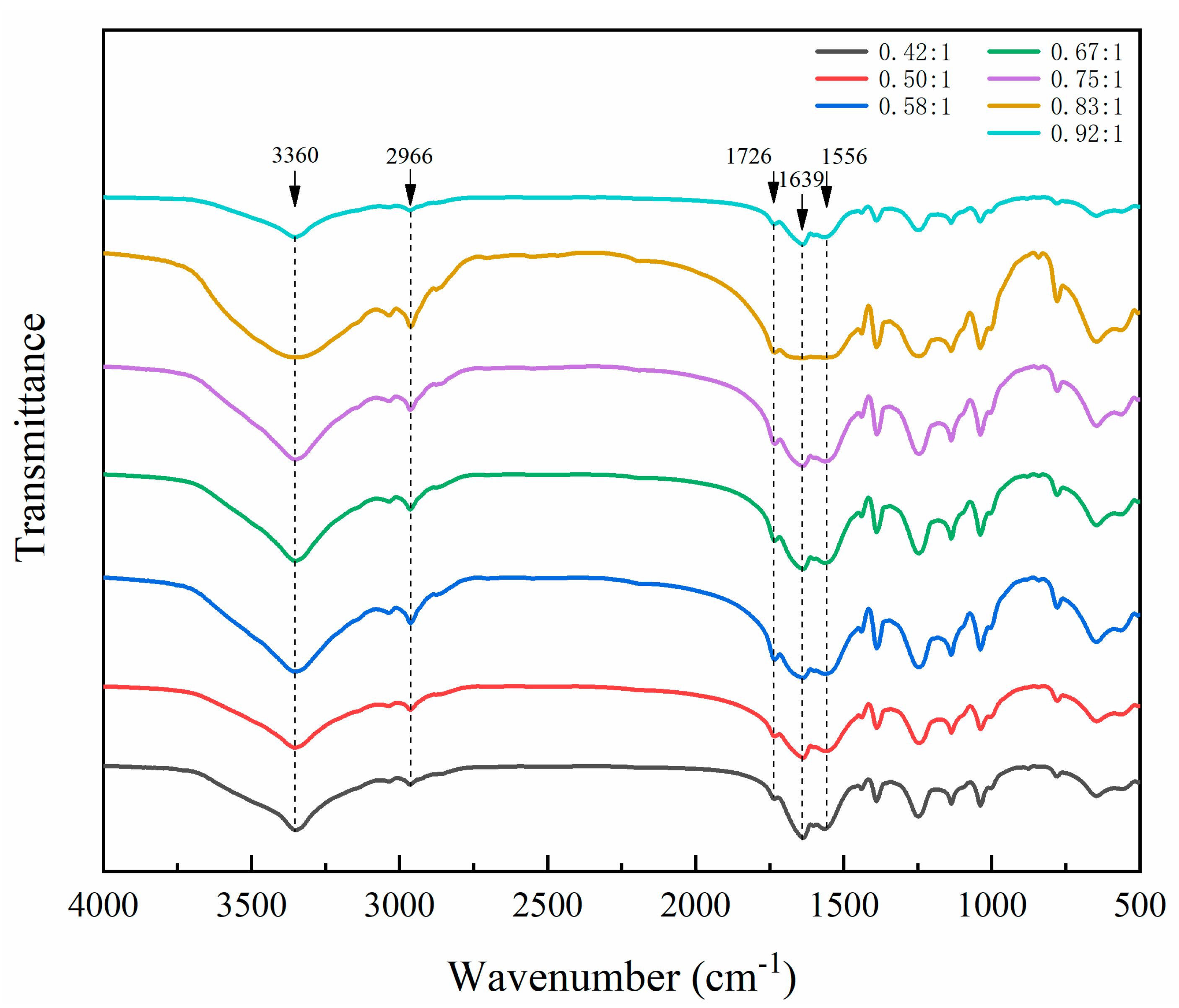
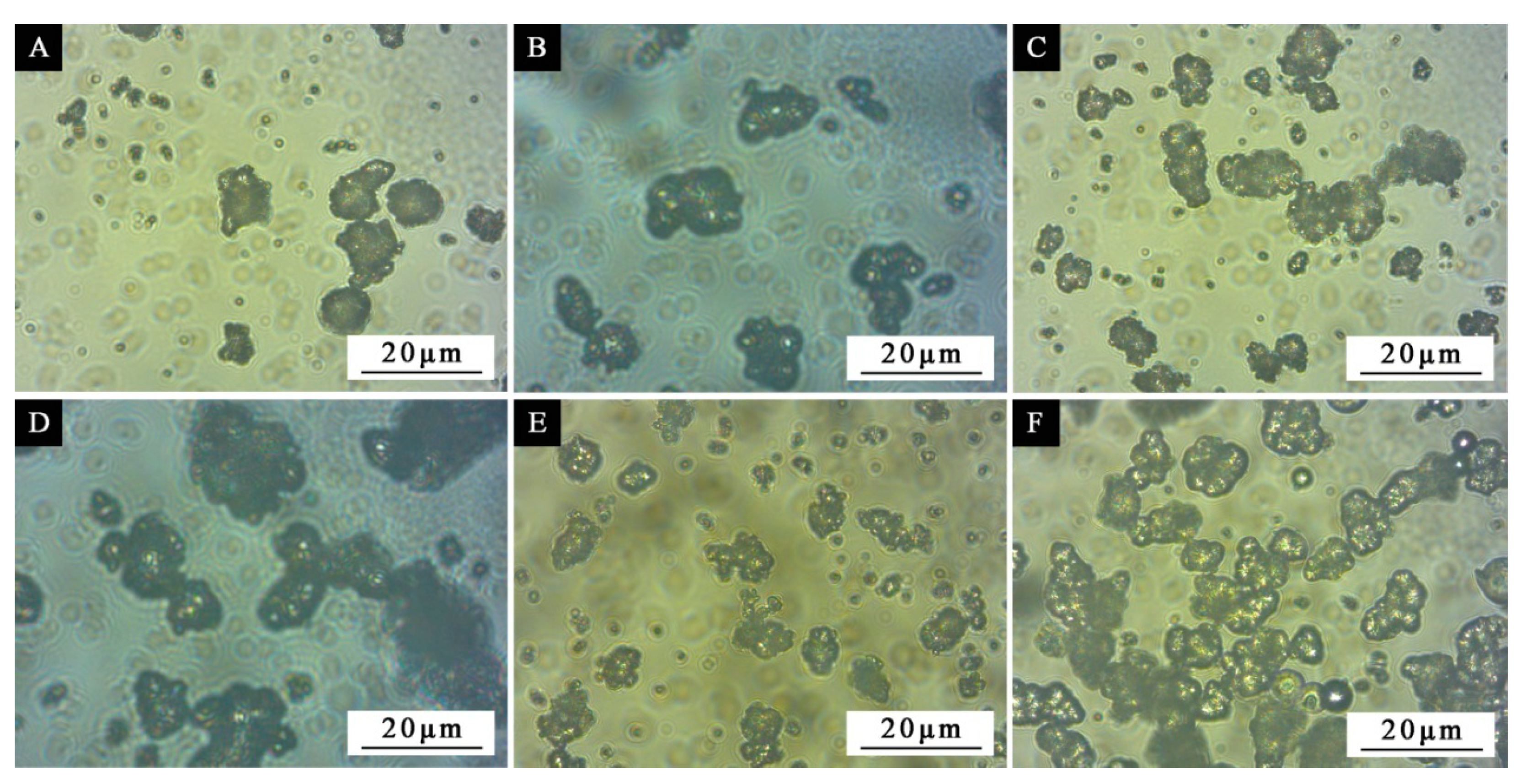
The most important factors affecting the performance of microcapsules were determined by the above orthogonal experiments. On this basis, the single-factor independent experiment was carried out to determine the optimal core wall ratio of microcapsules and further optimize the preparation scheme. In the single-factor independent experiment, the water bath temperature was set at 70 °C, the aging time was 5 d, and the core–wall ratio was set as 0.42:1, 0.50:1, 0.58:1, 0.67:1, 0.75:1, 0.83:1 and 0.92:1 (samples 5#−11#), respectively.
2.3. Preparation of Coatings
First, the 0.4 g microcapsules with 0.58:1 of core–wall ratio were added to 3.6 g waterborne acrylic resin coatings, and the mixture was evenly mixed to form a waterborne coating with 10.0% microcapsule concentration. The prepared paint was coated on the surface of the Tilia europaea panels using a SZQ tetrahedral fabricator (Jinghai Science and Technology Testing Machinery Factory, Tianjin, China), dried at room temperature for 30 min, then polished with 800 mesh sandpaper and wiped with a dry cloth. The above process was repeated three times, and the dry coating thickness was about 60 μm.
2.4. Testing and Characterization
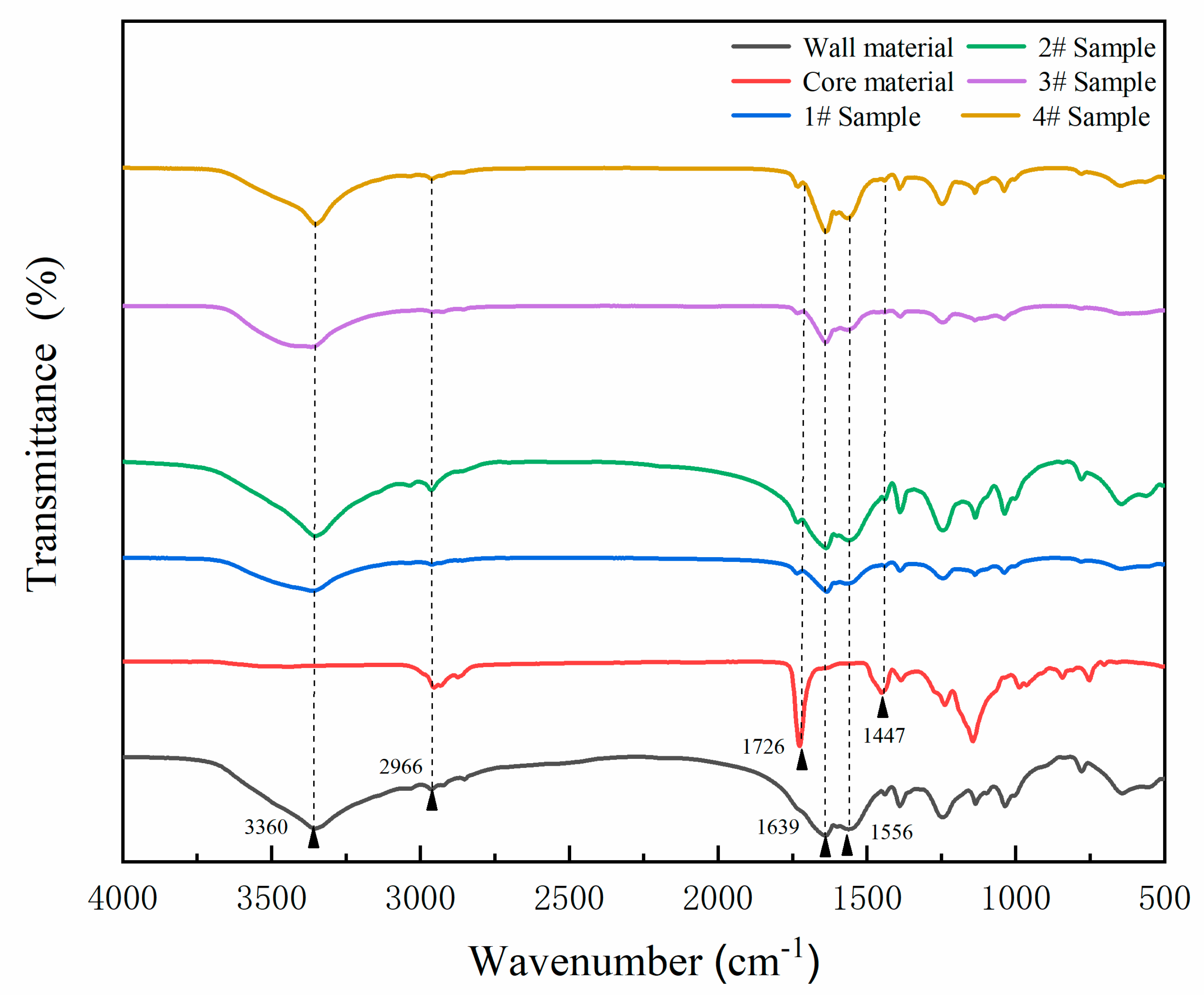
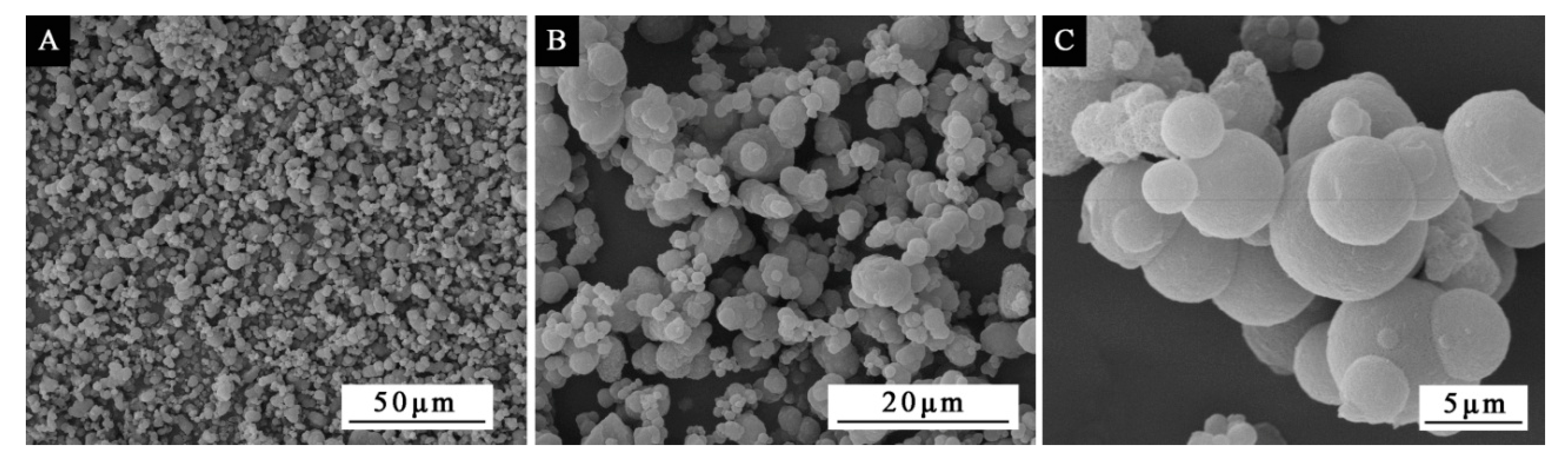
A ZEISS electron microscope AX10 (Carl Zeiss AG, Aalen, Germany) and a Quanta 200 environment scanning electron microscope (SEM), FEI Company, Hillsboro, OR, USA were used to characterize the microcapsule morphology [
25]. A VERTEX 80 V infrared spectrum analyzer (Germany Bruker Co., Ltd., Karlsruhe, Germany) was used to analyze the chemical composition of microcapsules [
26]. The encapsulation ratio of microcapsules can be tested as follows: the 1.0 g microcapsules (m
1) were fully ground in a mortar and placed in a sand core funnel. Then, a certain amount of ethyl acetate was added, the microcapsules were fully soaked for 72 h, and the ethyl acetate was changed every 24 h. The mixture was rinsed and filtered with deionized water, and the product was dried and weighed to obtain the residual wall mass (m
2). The encapsulation ratio (c) can be calculated by Formula (1) [
27].
The gloss of waterborne coatings was tested by HG268 gloss meter produced by 3NH Technology Co., Ltd., Shenzhen, China. The light incident angle of 60° was used. The hardness of paint film was measured by pencil hardness tester with a 6H-6B pencil. The pencil was pushed forward at an angle to the film. When the coating was not damaged, the maximum hardness of the pencil was the hardness of the paint film. The adhesion of the paint film was tested by QFH-HG600 film scriber (Tianjin Jingke Material Experiment Machine Factory, Tianjin, China), and the adhesion grade of the paint film was judged by the degree of damage. There are six grades: 0, 1, 2, 3, 4 and 5, of which grade 0 has the best adhesion and grade 5 has the worst adhesion. The impact resistance of paint film was measured by a QCJ film impactor tester (Tianjin Jingkelian Material Testing Machine Co., Ltd., Tianjin, China). The Model AG-IC100KN precision electronic universal capability experiment machine (Shimadzu Co., Ltd., Kyoto, Japan) was used to measure the elongation at break of the coating. The paint film was coated on the glass substrate and peeled off. After being stripped, the coatings were made into thin sheets, and then both ends of the coatings were fixed with clamps to prevent it from sliding. The coatings were destroyed at a tensile speed of 0.12 mm/min and a certain longitudinal load. The elongation at break was expressed by the ratio of the displacement value of the coating at break to the original length of the coating. Thermogravimetric analysis (STA8000) (PerkinElmer Inc., Waltham, MA, USA) was used to determine the thermal stability of the microcapsules at a heating rate of 20 °C·min−1 in a nitrogen atmosphere. The orthogonal design assistant version 3.1 was used to analyze the data of orthogonal experiment. The orthogonal design assistant version 3.1 is a professional software for orthogonal experimental design and analysis of experimental results. All the above measurements were repeated at least four times with the error lower than 5.0%.
4. Conclusions
The influences of the three factors (core–wall ratio, water bath temperature and depositing time) on the yield, encapsulation ratio, particle size and morphology of microcapsules are explored to determine the preferred level of 70 °C water bath temperature and 5 d depositing time. Among the three factors, the core–wall ratio is the factor that has the greatest impact on the performance of the microcapsule. In the single-factor test with the core–wall ratio as a variable, when the core–wall ratio is relatively small, the microcapsules have good morphological characteristics. The microcapsules with 0.58:1 of the core–wall ratio have a uniform and obvious particle size. When the core–wall ratio is relatively large, the yield of the microcapsules is higher. When the core–wall ratio is 0.58:1 or 0.67:1, it has a better encapsulation ratio. In short, the overall performance of microcapsules with 0.58:1 of the core–wall ratio is better in morphology, yield and encapsulation ratio. The microcapsules would not be completely deposited and agglomerated due to their long depositing time. When the waterborne acrylic microcapsules with 0.58:1 of the core–wall ratio were added into the waterborne coating with 10.0% concentration, the microcapsules have a good effect on the film properties, and the elongation at break of the film is significantly improved. The results provide a technical reference for improving the toughness of waterborne coatings cured at room temperature.