Nacre-Mimetic Green Flame Retardant: Ultra-High Nanofiller Content, Thin Nanocomposite as an Effective Flame Retardant
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Characterization
2.3. Preparation of Main-Chain Benzoxazine Oligomers 8,8′-((1,1,3,3,5,5-Hexamethyltrisiloxane-1,5-Diyl) bis(Propane-3,1-Diyl))bis(3-(Furan-2-Ylmethyl)-2,3,4,7,8,9-Hexahydrobenzo [1 –e:4,3-e’]bis([1,3]Oxazine)), (Abbreviated as CA-Pdms-Fu)
2.4. Preparation of Benzoxazine Oligomer Films
2.5. Preparation of Elastomeric Benzoxazine/Laponite Nanocomposite
2.6. Single Dip Coating of Very-Thin Film Nanocomposite on Polyurethane Foam
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Camino, G.; Costa, L.; Luda di Cortemiglia, M.P. Overview of Fire Retardant Mechanism. Polym. Degrad. Stab. 1991, 33, 131–154. [Google Scholar] [CrossRef]
- Bourbigot, S.; Duquesne, S. Fire Retardant Polymers: Recent Developments and Opportunities. J. Mater. Chem. 2007, 17, 2283–2300. [Google Scholar] [CrossRef]
- Zaikov, G.E.; Lomakin, S.M. Ecological Issue of Polymer Flame Retardancy. J. Appl. Polym. Sci. 2002, 86, 2449–2462. [Google Scholar] [CrossRef]
- Dasari, A.; Yu, Z.Z.; Cai, G.P.; Mai, Y.W. Recent Developments in the Fire Retardancy of Polymeric Materials. Prog. Polym. Sci. 2013, 38, 1357–1387. [Google Scholar] [CrossRef]
- Van der Veen, I.; de Boer, J. Phosphorus Flame Retardants: Properties, Production, Environmental Occurrence, Toxicity and Analysis. Chemosphere 2012, 88, 1119–1153. [Google Scholar] [CrossRef]
- Alongi, J.; Han, Z.; Bourbigot, S. Intumescence: Tradition versus Novelty. A Comprehensive Review. Prog. Polym. Sci. 2014, 51, 28–73. [Google Scholar] [CrossRef]
- Villamil Watson, D.A.; Schiraldi, D.A. Biomolecules as Flame Retardant Additives for Polymers: A Review. Polymers (Basel) 2020, 12, 849. [Google Scholar] [CrossRef]
- Marty, A.; Ben, E. Fire Loss in the United States during 2018; 2019. Available online: https://www.nfpa.org/-/media/Files/News-and-Research/Fire-statistics-and-reports/US-Fire-problem/Old-FL-LL-and-Cat/FireLoss2019.ashx (accessed on 15 March 2020).
- George, J.; Ishida, H. A Review on the Very High Nanofiller-Content Nanocomposites: Their Preparation Methods and Properties with High Aspect Ratio Fillers. Prog. Polym. Sci. 2018, 86, 1–39. [Google Scholar] [CrossRef]
- Laufer, G.; Kirkland, C.; Morgan, A.B.; Grunlan, J.C. Intumescent Multilayer Nanocoating, Made with Renewable Polyelectrolytes, for Flame-Retardant Cotton. Biomacromolecules 2012, 13, 2843–2848. [Google Scholar] [CrossRef]
- Kim, Y.S.; Harris, R.; Davis, R. Innovative Approach to Rapid Growth of Highly Clay-Filled Coatings on Porous Polyurethane Foam. ACS Macro Lett. 2012, 1, 820–824. [Google Scholar] [CrossRef]
- Holder, K.M.; Smith, R.J.; Grunlan, J.C. A Review of Flame Retardant Nanocoatings Prepared Using Layer-by-Layer Assembly of Polyelectrolytes. J. Mater. Sci. 2017, 52, 12923–12959. [Google Scholar] [CrossRef]
- Das, P.; Malho, J.M.; Rahimi, K.; Schacher, F.H.; Wang, B.; Demco, D.E.; Walther, A. Nacre-Mimetics with Synthetic Nanoclays up to Ultrahigh Aspect Ratios. Nat. Commun. 2015, 6, 5967. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cheng, Q.; Lin, L.; Chen, L.; Jiang, L. Understanding the Relationship of Performance with Nanofiller Content in the Biomimetic Layered Nanocomposites. Nanoscale 2013, 5, 6356–6362. [Google Scholar] [CrossRef] [PubMed]
- Wegst, U.G.K.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired Structural Materials. Nat. Mater. 2015, 14, 23–36. [Google Scholar] [CrossRef]
- Wahab, R.; Mustafa, M.T.; Salam, M.A.; Sudin, M.; Samsi, H.W.; Rasat, M.S.M. Chemical Composition of Four Cultivated Tropical Bamboo in Genus Gigantochloa. J. Agric. Sci. 2013, 5. [Google Scholar] [CrossRef]
- Huang, C.; Cheng, Q. Learning from Nacre: Constructing Polymer Nanocomposites. Compos. Sci. Technol. 2017, 150, 141–166. [Google Scholar] [CrossRef]
- Kakisawa, H.; Sumitomo, T. The Toughening Mechanism of Nacre and Structural Materials Inspired by Nacre. Sci. Technol. Adv. Mater. 2012, 12, 064710. [Google Scholar] [CrossRef]
- Barthelat, F.; Espinosa, H.D. An Experimental Investigation of Deformation and Fracture of Nacre-Mother of Pearl. Exp. Mech. 2007, 47, 311–324. [Google Scholar] [CrossRef]
- Xie, H.; Lai, X.; Li, H.; Gao, J.; Zeng, X.; Huang, X.; Lin, X. A Highly Efficient Flame Retardant Nacre-Inspired Nanocoating with Ultrasensitive Fire-Warning and Self-Healing Capabilities. Chem. Eng. J. 2019, 369, 8–17. [Google Scholar] [CrossRef]
- Ming, P.; Song, Z.; Gong, S.; Zhang, Y.; Duan, J.; Zhang, Q.; Jiang, L.; Cheng, Q. Nacre-Inspired Integrated Nanocomposites with Fire Retardant Properties by Graphene Oxide and Montmorillonite. J. Mater. Chem. A. 2015, 3, 21194–21200. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, X.; Zheng, H.; Shang, W. Bio-Inspired Fabrication of Nacre-Mimetic Hybrid Nanocoating for Eco-Friendly Fire-Resistant Precious Cellulosic Chinese Xuan Paper. Carbohydr. Polym. 2020, 235, 115782. [Google Scholar] [CrossRef] [PubMed]
- Jaber, M.; Komarneni, S.; Zhou, C.H. Handbook of Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 223–241. [Google Scholar]
- CMS Nomenclature Committee. The Clay Minerals Society Glossary for Clay Science; Part 1; The Clay Minerals Society: Chantilly, VA, USA, 2018. [Google Scholar]
- Ishida, H.; Low, H.Y. A Study on the Volumetric Expansion of Benzoxazine-Based Phenolic Resin. Macromolecules 1997, 30, 1099–1106. [Google Scholar] [CrossRef]
- Tuzub, A.; Kiskan, B.; Alemdar, N.; Erciyes, A.T.; Yaggi, Y. Benzoxazine Containing Polyester Thermosets with Inproved Adhesion and Flexibility. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 4279–4284. [Google Scholar]
- Velez-Herrera, P.; Doyama, K.; Abe, H.; Ishida, H. Synthesis and Characterization of Highly Fluorinated Polymer with the Benzoxazine Moiety in the Main Chain. Macromolecules 2008, 41, 9704–9714. [Google Scholar] [CrossRef]
- Velez-Herrera, P.; Ishida, H. Synthesis and Characterization of Highly Fluorinated Diamines and Benzoxazines Derived Therefrom. J. Fluor. Chem. 2009, 130, 573–580. [Google Scholar] [CrossRef]
- Lin, C.H.; Chang, S.L.; Lee, H.H.; Chang, H.C.; Hwang, K.Y.; Tu, A.P.; Su, W.C. Fluorinated Benzoxazines and the Structure-Property Relationship of Resulting Polybenzoxazines. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 4970–4983. [Google Scholar] [CrossRef]
- Shieh, J.Y.; Lin, C.Y.; Huang, C.L.; Wang, C.S. Synthesis and Characterization of Novel Dihydrobenzoxazine Resins. J. Appl. Polym. Sci. 2006, 101, 342–347. [Google Scholar] [CrossRef]
- Shan, F.; Ohashi, S.; Erlichman, A.; Ishida, H. Non- Flammable Thiazole-Functional Monobenzoxazines: Synthesis, Polymerization, Thermal and Thermomechanical Properties and Flammability Studies. Polymer (Guildf.) 2018, 157, 38–49. [Google Scholar] [CrossRef]
- Walters, R.N.; Lyon, R.E. Molar Group Contributions to Polymer Flammability. J. Appl. Polym. Sci. 2002, 87, 548–563. [Google Scholar] [CrossRef]
- Zhao, C.; Li, P.; He, D.; Li, Y.; Lei, F.; Sue, H.J. Flame Retardation Behavior of Polybenzoxazine/α-ZrP Nanocomposites. RSC Adv. 2016, 6, 73485–73495. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, C.; Guo, C.; Li, Y.; Li, S.; Sun, L.; Li, H.; Xiang, D. Polybenzoxazine Resins with Polyphosphazene Microspheres: Synthesis, Flame Retardancy, Mechanisms, and Applications. ACS Omega 2019, 4, 20275–20284. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Neisius, N.M.; Gaan, S. Recent Developments in Flame Retardant Polymeric Coatings. Prog. Org. Coat. 2013, 76, 1642–1665. [Google Scholar] [CrossRef]
- Lu, S.Y.; Hamerton, I. Recent Developments in the Chemistry of Halogen-Free Flame Retardant Polymers. Prog. Polym. Sci. 2002, 27, 1661–1712. [Google Scholar] [CrossRef]
- Choi, S.W.; Ohba, S.; Brunovska, Z.; Hemvichian, K.; Ishida, H. Synthesis, Characterization and Thermal Degradation of Functional Benzoxazine Monomers and Polymers Containing Phenylphosphine Oxide. Polym. Degrad. Stab. 2006, 91, 1166–1178. [Google Scholar] [CrossRef]
- Zhang, T.T.; Men, W.W.; Liu, Y.; Lu, Z.J. Synthesis and Characterization of Polybenzoxazine Containing Phosphorus. Chinese, J. Polym. Sci. 2012, 30, 250–257. [Google Scholar] [CrossRef]
- Li, H.; Xu, J.; Zeng, K.; Li, Y.; Li, C.Z.G. Synthesis and Characterization of Siloxane-Containing Benzoxazines with High Thermal Stability. High Perform. Polym. 2019, 32, 268–275. [Google Scholar] [CrossRef]
- Liu, Y.L.; Chou, C.I. High Performance Benzoxazine Monomers and Polymers Containing Furan Groups. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 5267–5282. [Google Scholar] [CrossRef]
- Yee Low, H.; Ishida, H. Structural Effects of Phenols on the Thermal and Thermo-Oxidative Degradation of Polybenzoxazines. Polymer (Guildf.) 1999, 40, 4365–4376. [Google Scholar] [CrossRef]
- Chernykh, A.; Liu, J.; Ishida, H. Synthesis and Properties of a New Crosslinkable Polymer Containing Benzoxazine Moiety in the Main Chain. Polymer (Guildf) 2006, 47, 7664–7669. [Google Scholar] [CrossRef]
- Takeichi, T.; Kano, T.; Agag, T. Synthesis and Thermal Cure of High Molecular Weight Polybenzoxazine Precursors and the Properties of the Thermosets. Polymer 2005, 46, 12172–12180. [Google Scholar] [CrossRef]
- Han, L.; Iguchi, D.; Gil, P.; Heyl, T.R.; Sedwick, V.M.; Arza, C.R.; Ohashi, S.; Lacks, D.J.; Ishida, H. Oxazine Ring-Related Vibrational Modes of Benzoxazine Monomers Using Fully Aromatically Substituted, Deuterated, 15N Isotope Exchanged, and Oxazine-Ring-Substituted Compounds and Theoretical Calculations. J. Phys. Chem. A 2017, 121, 6269–6282. [Google Scholar] [CrossRef] [PubMed]
- Ishida, H. Handbook of Benzoxazine Resins; Ishida, H., Agag, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 3–81. [Google Scholar]
- He, Y.; Gao, S.; Lu, Z. A Mussel-Inspired Polybenzoxazine Containing Catechol Groups. Polymer 2018, 158, 53–58. [Google Scholar] [CrossRef]
- Takeichi, T.; Kano, T.; Agag, T.; Kawauchi, T.; Nobuyuki, F. Preparation of High Molecular Weight Polybenzoxazine Prepolymers Containing Siloxane Unites and Properties of Their Thermosets. Polym. Chem. 2010, 48, 5945–5952. [Google Scholar] [CrossRef]
- Ohashi, S.; Iguchi, D.; Heyl, T.R.; Froimowicz, P.; Ishida, H. Quantitative Studies on the: P -Substituent Effect of the Phenolic Component on the Polymerization of Benzoxazines. Polym. Chem. 2018, 9, 4194–4204. [Google Scholar] [CrossRef]
- Zhang, K.; Han, L.; Nie, Y.; Szigeti, M.L.; Ishida, H. Examining the Effect of Hydroxyl Groups on the Thermal Properties of Polybenzoxazines: Using Molecular Design and Monte Carlo Simulation. RSC Adv. 2018, 8, 18038–18050. [Google Scholar] [CrossRef]
- Baqar, M.; Agag, T.; Huang, R.; Maia, J.; Qutubuddin, S.; Ishida, H. Mechanistic Pathways for the Polymerization of Methylol-Functional Benzoxazine Monomers. Macromolecules 2012, 45, 8119–8125. [Google Scholar] [CrossRef]
- Zhang, K.; Froimowicz, P.; Han, L.; Ishida, H. Hydrogen-Bonding Characteristics and Unique Ring-Opening Polymerization Behavior of Ortho-Methylol Functional Benzoxazine. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 3635–3642. [Google Scholar] [CrossRef]
- Owen, M.J. The Surface Activity of Silicones: A Short Review. Ind. Eng. Chem. Prod. Res. Dev. 1980, 191, 97–103. [Google Scholar] [CrossRef]
- Liu, J.; Ishida, H. Anomalous Isomeric Effect on the Properties of Bisphenol F-Based Benzoxazines: Toward the Molecular Design for Higher Performance. Macromolecules 2014, 47, 5682–5690. [Google Scholar] [CrossRef]
- Bagherifam, S.; Kiskan, B.; Aydogan, B.; Yagci, Y.; Hacaloglu, J. Thermal Degradation of Polysiloxane and Polyetherester Containing Benzoxazine Moieties in the Main Chain. J. Anal. Appl. Pyrolysis 2011, 90, 155–163. [Google Scholar] [CrossRef]
- Bagherifam, S.; Uyar, T.; Ishida, H.; Hacaloglu, J. The Use of Pyrolysis Mass Spectrometry to Investigate Polymerization and Degradation Processes of Methyl Amine-Based Benzoxazine. Polym. Test. 2010, 29, 520–526. [Google Scholar] [CrossRef]
- Shen, X.; Dai, J.; Liu, Y.; Liu, X.; Zhu, J. Synthesis of High Performance Polybenzoxazine Networks from Bio-Based Furfurylamine: Furan vs Benzene Ring. Polymer 2017, 122, 258–269. [Google Scholar] [CrossRef]
- Xu, J.Z.; Ma, R.; Jiao, Y.H.; Xie, J.X.; Wang, R.; Su, L. Synthesis, Characterization, and Thermal Degradation Property of Aniline Polyphosphazene. J. Macromol. Sci. Part B Phys. 2011, 50, 897–906. [Google Scholar] [CrossRef]
- Ardhyananta, H.; Wahid, M.H.; Sasaki, M.; Agag, T.; Kawauchi, T.; Ismail, H.; Takeichi, T. Performance Enhancement of Polybenzoxazine by Hybridizationwith Polysiloxane.Pdf. Polymer (Guildf.) 2008, 49, 4585–4591. [Google Scholar] [CrossRef]
- Ardhyananta, H.; Kawauchi, T.; Takeichi, T.; Ismail, H. Preparation and Properties of Polybenzoxazinepoly(Dimethylsiloxane-Co- Diphenylsiloxane) Hybrids as High Performance Polymers. High Perform. Polym. 2010, 22, 609–632. [Google Scholar] [CrossRef]
- Ardhyananta, H.; Kawauchi, T.; Ismail, H.; Takeichi, T. Effect of Pendant Group of Polysiloxanes on the Thermal and Mechanical Properties of Polybenzoxazine Hybrids. Polymer (Guildf.) 2009, 50, 5959–5969. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. General Treatment of the Thermogravimetry of Polymers. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1966, 70, 487–523. [Google Scholar] [CrossRef]
- Li, Y.; Qiang, Z.; Chen, X.; Ren, J. Understanding Thermal Decomposition Kinetics of Flame-Retardant Thermoset Polylactic Acid. RSC Adv. 2019, 9, 3128–3139. [Google Scholar] [CrossRef]
- Van Krevelen, D.W. Some Basic Aspects of Flame Resistance of Polymeric Materials. Polymer (Guildf) 1975, 16, 615–620. [Google Scholar] [CrossRef]
- Sudha; Sarojadevi. Synthesis, Characterization, Curing, and Thermal Properties of Bifunctional Phenol-Based Polybenzoxazines. High Perform. Polym. 2016, 28, 331–339. [Google Scholar] [CrossRef]
- Afriyie, R.; Qiang, M.; Solomon, X.; Jin, A.C. Correlation Analysis of Cone Calorimetry and Microscale Combustion Calorimetry Experiments Correlation Analysis of Cone Calorimetry and Microscale Combustion Calorimetry Experiments. J. Therm. Anal. Calorim. 2018, 136, 589–599. [Google Scholar]
- Lyon, R.E.; Takemori, M.T.; Safronava, N.; Stoliarov, S.I.; Walters, R.N. A Molecular Basis for Polymer Flammability. Polymer (Guildf.) 2009, 50, 2608–2617. [Google Scholar] [CrossRef]
- Lyon, R.E.; Safronava, N.; Quintiere, J.G.; Stoliarov, S.I.; Walters, R.N.; Crowley, S. Material Properties and Fire Test Results. Fire Mater. 2014, 38, 264–278. [Google Scholar] [CrossRef]
- Lyon, R.E.; Walters, R.N. Pyrolysis Combustion Flow Calorimetry. J. Anal. Appl. Pyrolysis 2004, 71, 27–46. [Google Scholar] [CrossRef]
- Lyon, R.E.; Louise, S.; Walters, R.N.; Sean, C. Fire-Resistant Elastomers. Fire Mater. 2003, 27, 195–208. [Google Scholar] [CrossRef]
- Pálková, H.; Madejová, J.; Zimowska, M.; Serwicka, E.M. Laponite-Derived Porous Clay Heterostructures: II. FTIR Study of the Structure Evolution. Microporous Mesoporous Mater. 2010, 127, 237–244. [Google Scholar] [CrossRef]
- Lee, J.H.; Han, W.J.; Jang, H.S.; Choi, H.J. Highly Tough, Biocompatible, and Magneto-Responsive Fe3O4/Laponite/PDMAAm Nanocomposite Hydrogels. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Cummins, H.Z. Liquid, Glass, Gel: The Phases of Colloidal Laponite. J. Non. Cryst. Solids 2007, 353, 3891–3905. [Google Scholar] [CrossRef]
- Ruzicka, B.; Zaccarelli, E. A Fresh Look at the Laponite Phase Diagram. Soft Matter 2011, 7, 1268–1286. [Google Scholar] [CrossRef]
- Xu, P.; Lan, Y.; Xing, Z.; Eiser, E. Liquid Crystalline Behaviour of Self-Assembled LAPONITE®/PLL-PEG Nanocomposites. Soft Matter 2018, 14, 2782–2788. [Google Scholar] [CrossRef]
- Hemvichian, K.; Laobuthee, A.; Chirachanchai, S.; Ishida, H. Thermal Decomposition Processes in Polybenzoxazine Model Dimers Investigated by TGA–FTIR and GC–MS.Pdf. Polym. Degrad. Stab. 2002, 76, 1–15. [Google Scholar] [CrossRef]
- Hemvichian, K.; Ishida, H. Thermal Decomposition Processes in Aromatic Amine-Based Polybenzoxazines Investigated by TGA and GC–MS. Polymer (Guildf.) 2002, 43, S0032–S3861. [Google Scholar] [CrossRef]
- Corcione, C.E.; Frigione, M. Characterization of Nanocomposites by Thermal Analysis. Materials (Basel) 2012, 5, 2960–2980. [Google Scholar] [CrossRef]
- Krämer, R.H.; Zammarano, M.; Linteris, G.T.; Gedde, U.W.; Gilman, J.W. Heat Release and Structural Collapse of Flexible Polyurethane Foam. Polym. Degrad. Stab. 2010, 95, 1115–1122. [Google Scholar] [CrossRef]
- Wilkens Flecknoe-Brown, K.; van Hees, P. Sensitivity Analysis on the Microscale Combustion Calorimeter for Polyurethane Foam Using a Full Factorial Design Methodology. J. Fire Sci. 2018, 36, 453–471. [Google Scholar] [CrossRef]
- Nam, S.; Condon, B.D.; Xia, Z.; Nagarajan, R.; Hinchliffe, D.J.; Madison, C.A. Intumescent Flame-Retardant Cotton Produced by Tannic Acid and Sodium Hydroxide. J. Anal. Appl. Pyrolysis 2017, 126, 239–246. [Google Scholar] [CrossRef]
- Lefebvre, J.; Bastin, B.; Le Bras, M.; Duquesne, S.; Ritter, C.; Paleja, R.; Poutch, F. Flame Spread of Flexible Polyurethane Foam: Comprehensive Study. Polym. Test. 2004, 23, 281–290. [Google Scholar] [CrossRef]
- Pan, Y.; Pan, H.; Yuan, B.; Hong, N.; Zhang, J.; Wang, B.; Song, L.; Hu, Y. Construction of Organic-Inorganic Hybrid Nano-Coating α-Zirconium Phosphate with High Efficiency for Reducing Fire Hazards of Flexible Polyurethane Foam. Mater. Chem. Phys. 2015, 163, 107–115. [Google Scholar] [CrossRef]
- Nabipour, H.; Wang, X.; Song, L.; Hu, Y. Laponite-Based Inorganic-Organic Hybrid Coating to Reduce Fire Risk of Flexible Polyurethane Foams. Appl. Clay Sci. 2020, 189, 105525. [Google Scholar] [CrossRef]
- Zammarano, M.; Krämer, R.H.; Harris, R.; Ohlemiller, T.J.; Shields, J.R.; Rahatekar, S.S.; Lacerda, S.; Gilman, J.W. Flammability Reduction of Flexible Polyurethane Foams via Carbon Nanofiber Network Formation. Polym. Adv. Technol. 2008, 19, 588–595. [Google Scholar] [CrossRef]
- Stefanidou, M.; Athanaselis, S.; Spiliopoulou, C. Health Impacts of Fire Smoke Inhalation. Inhal. Toxicol. 2008, 20, 761–766. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |

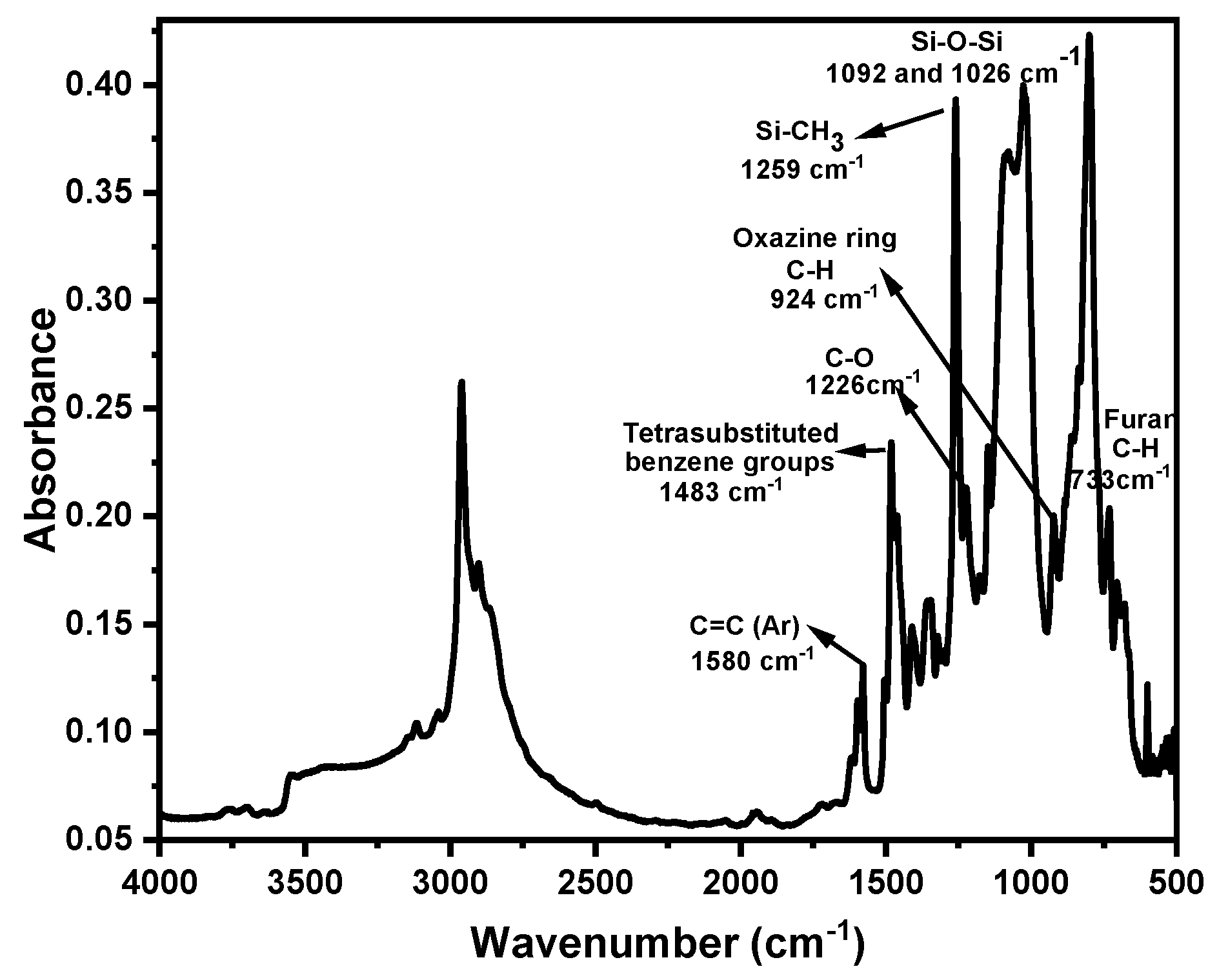
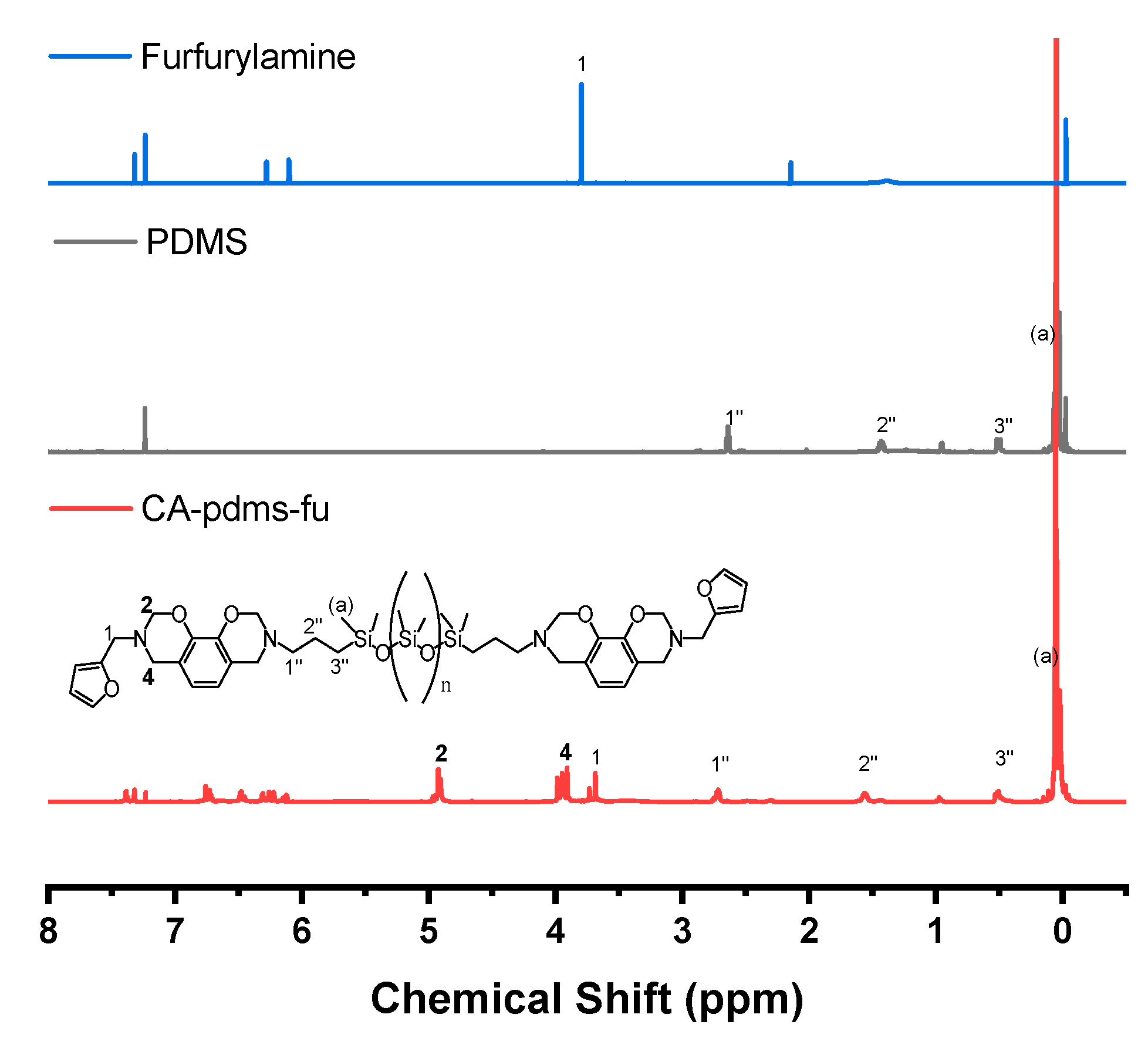
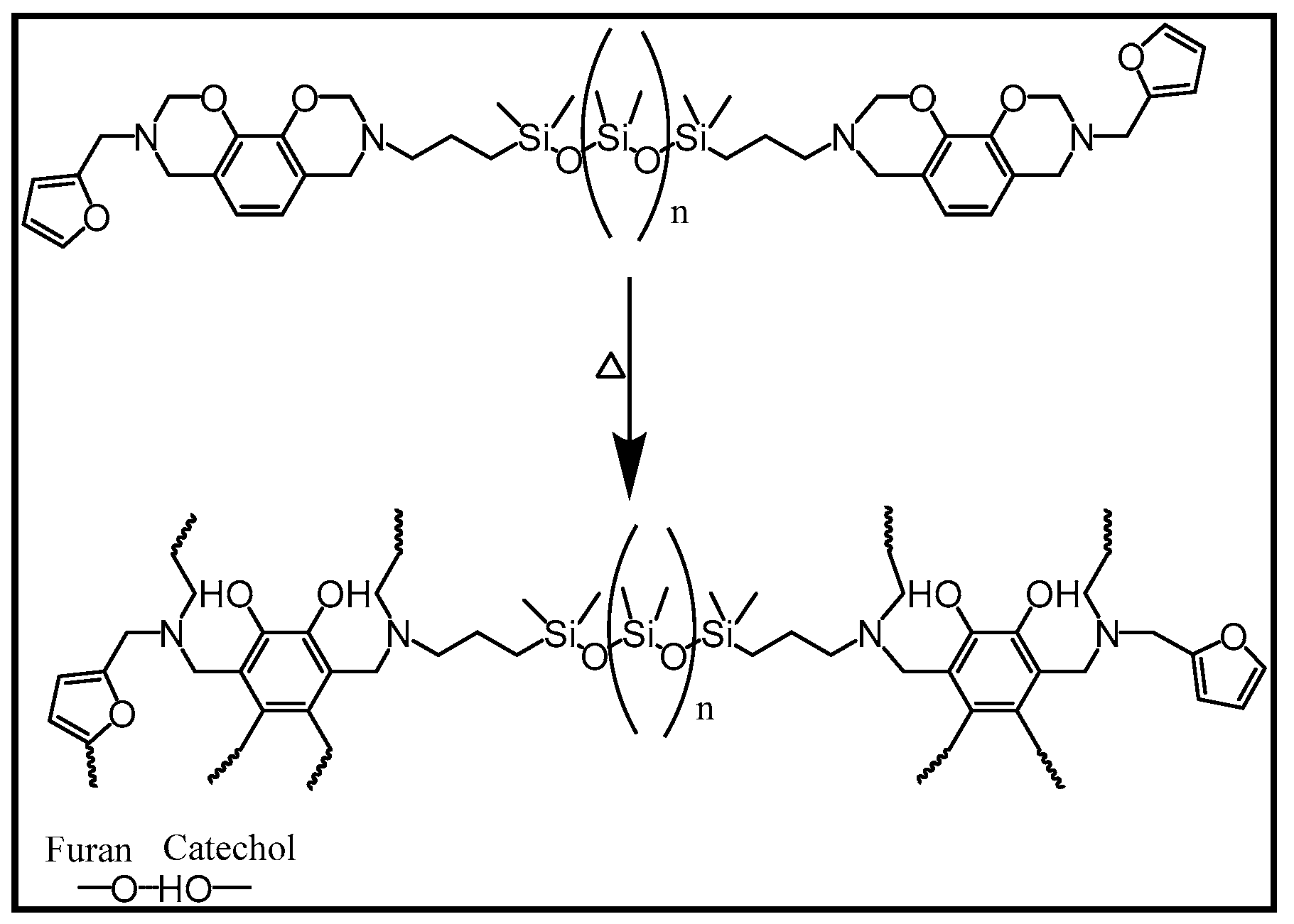
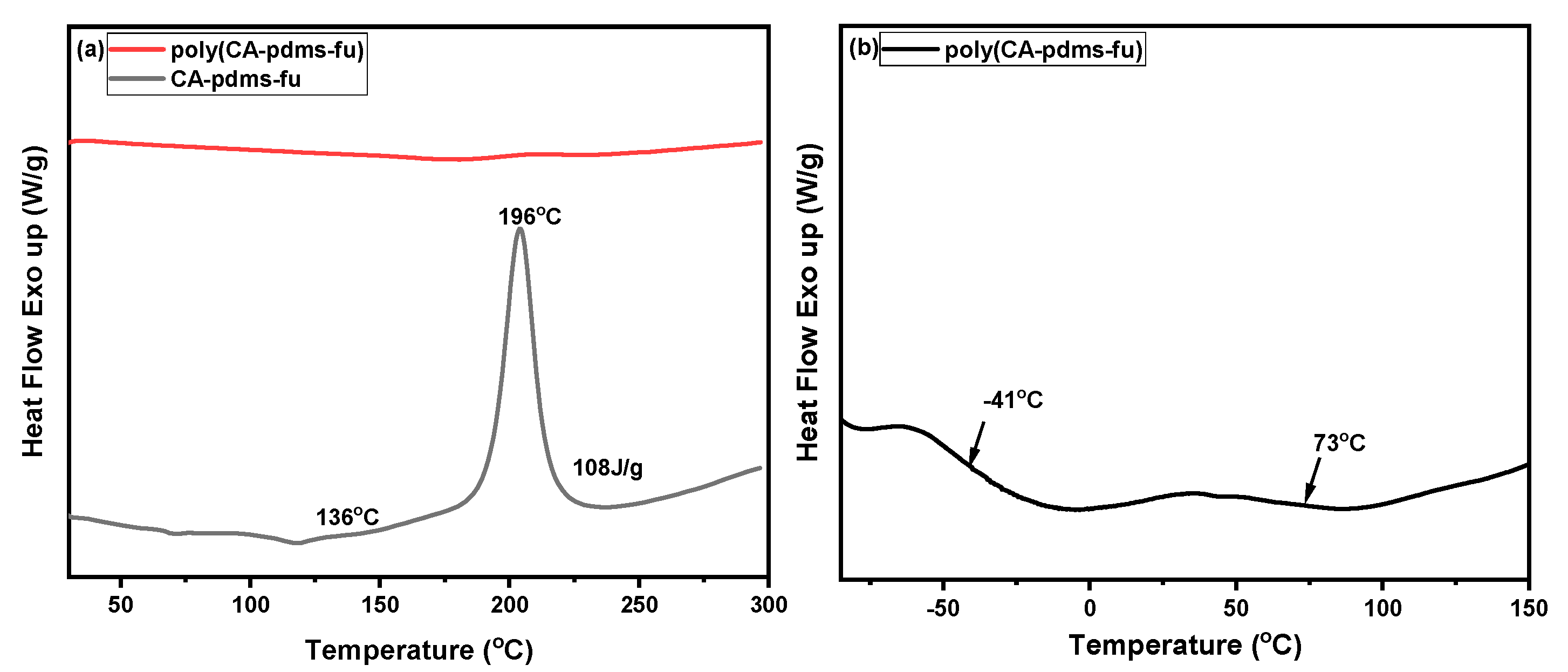
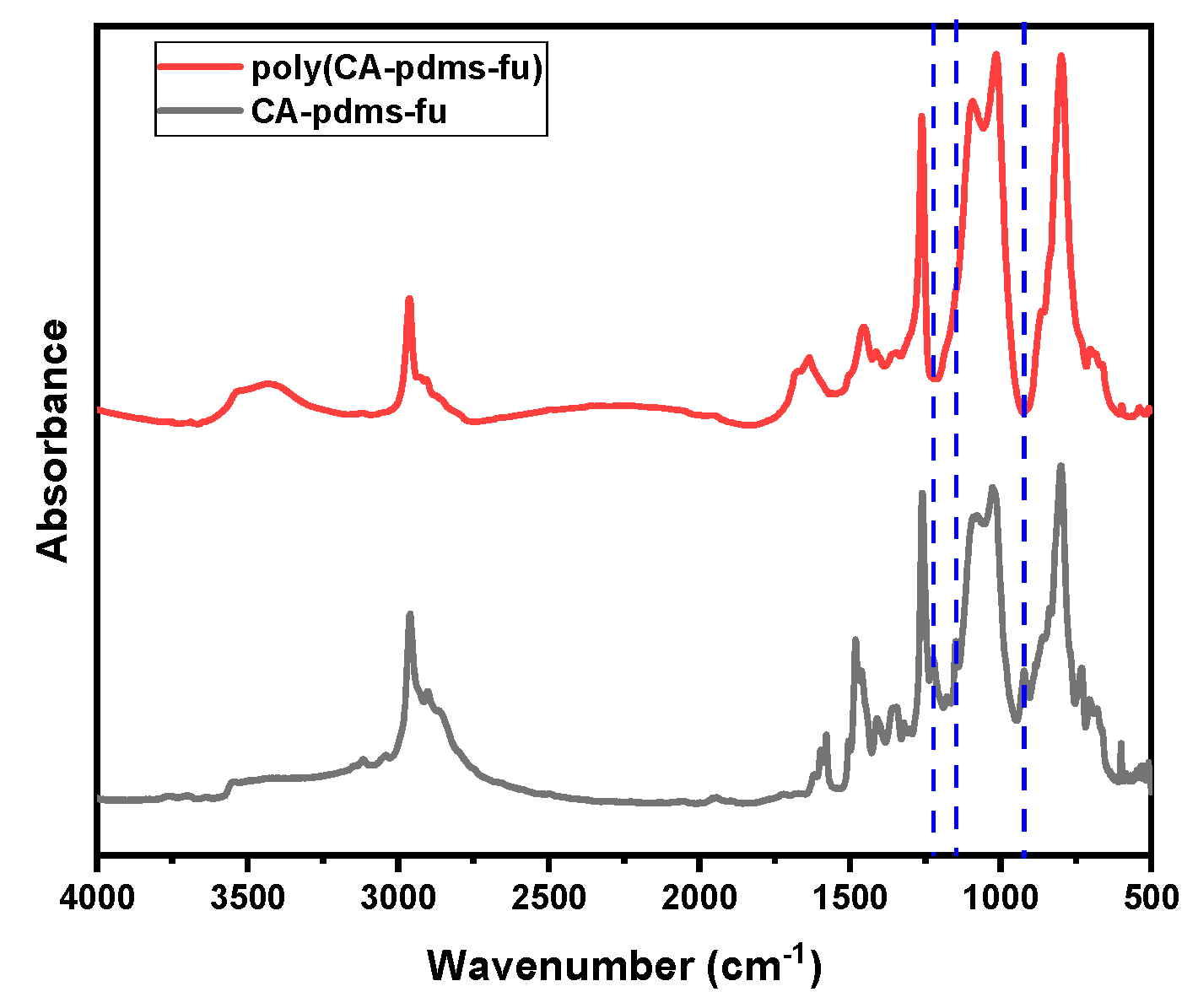
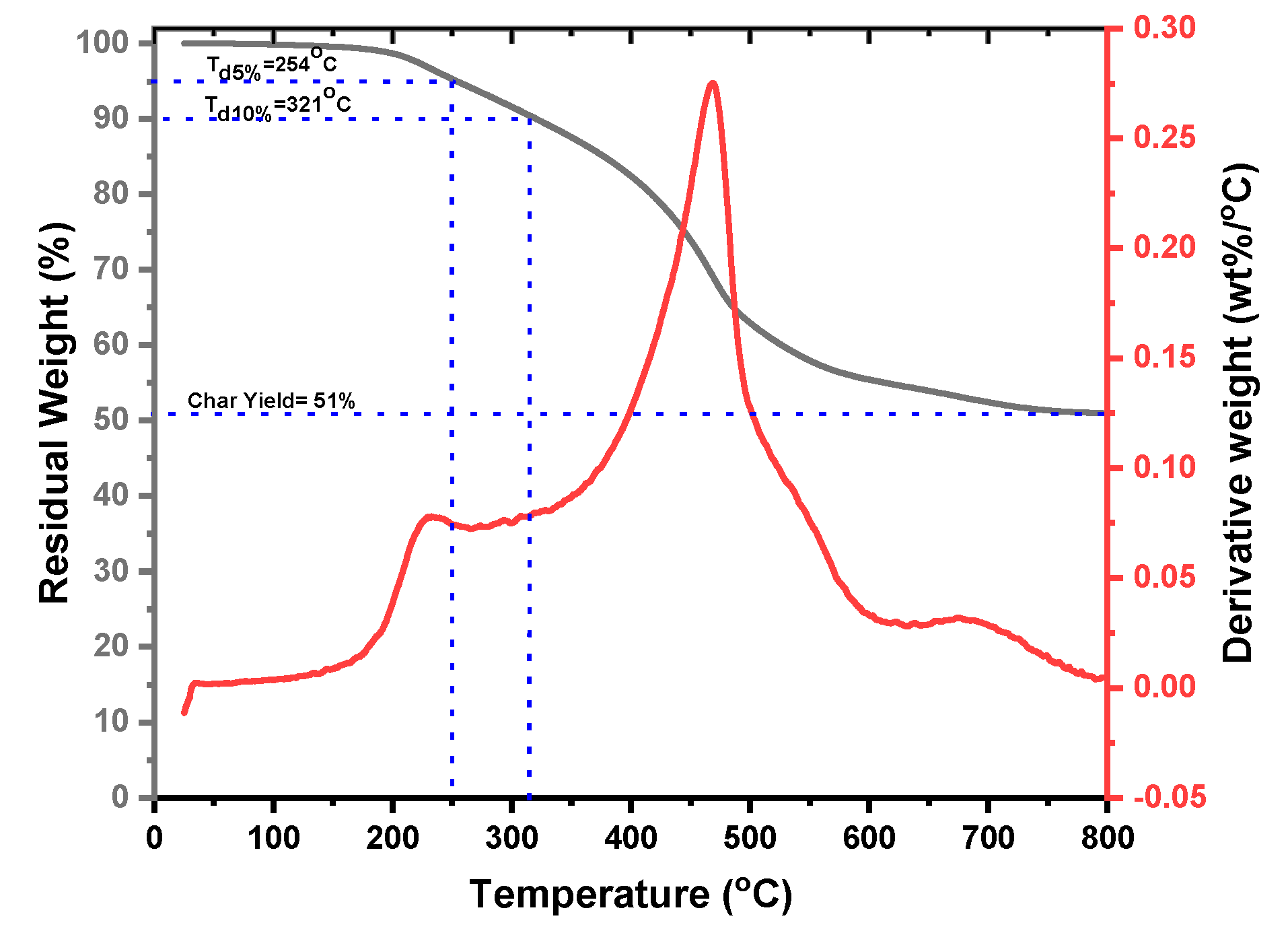
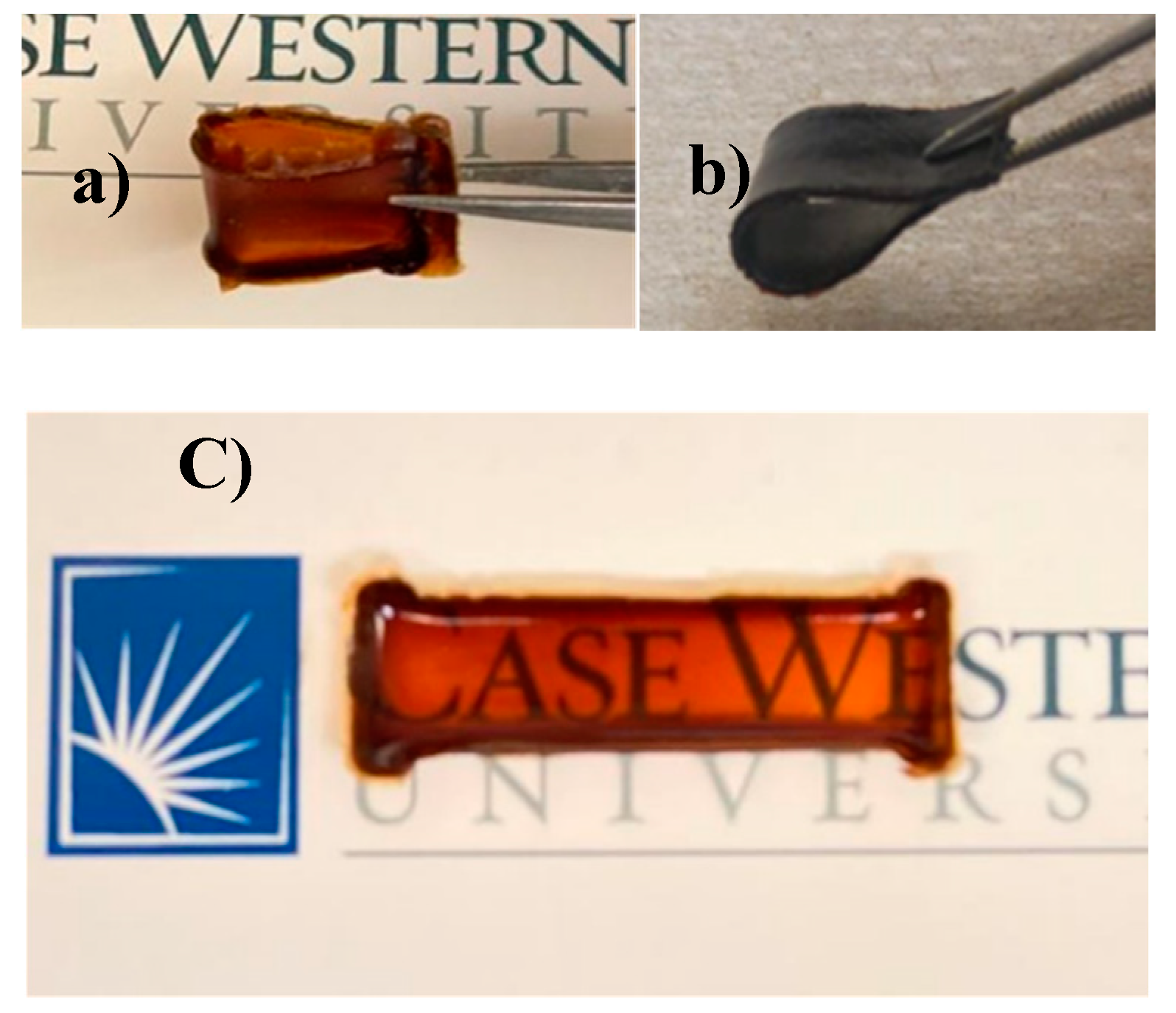
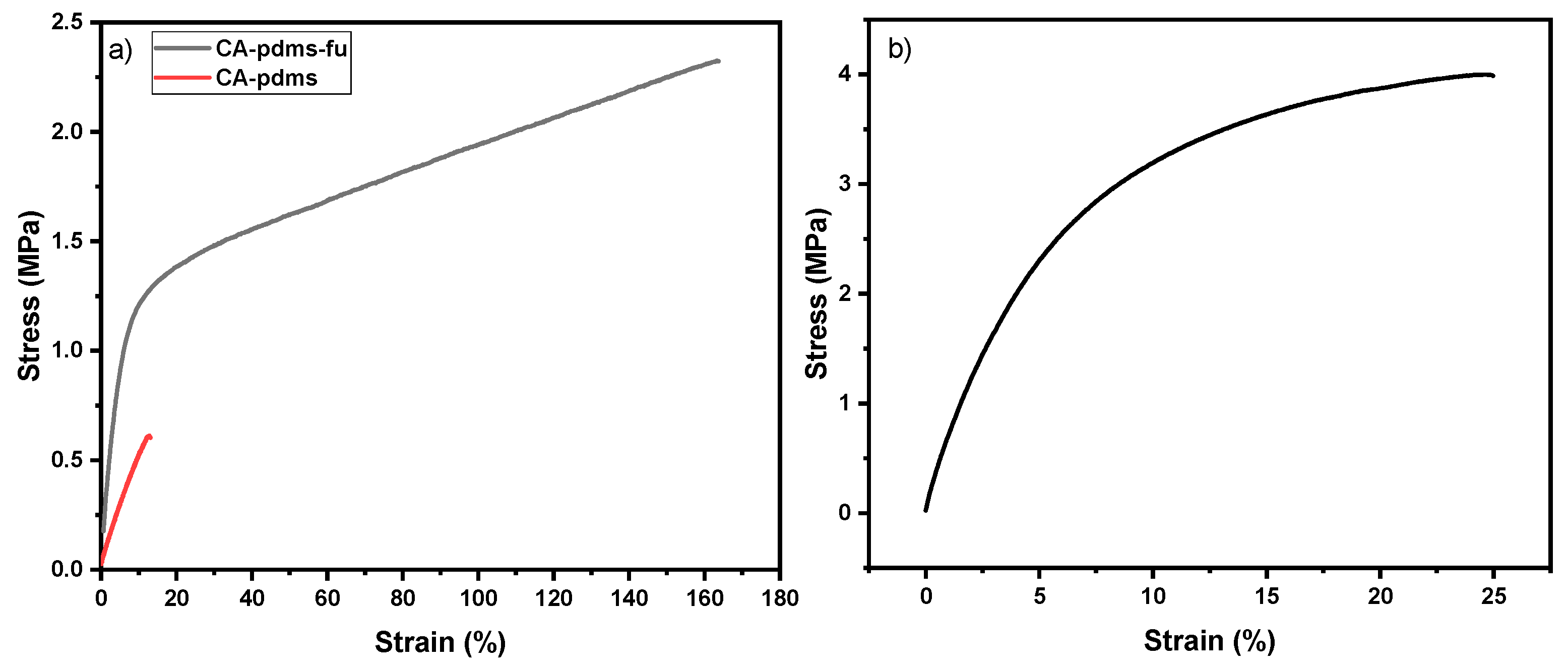


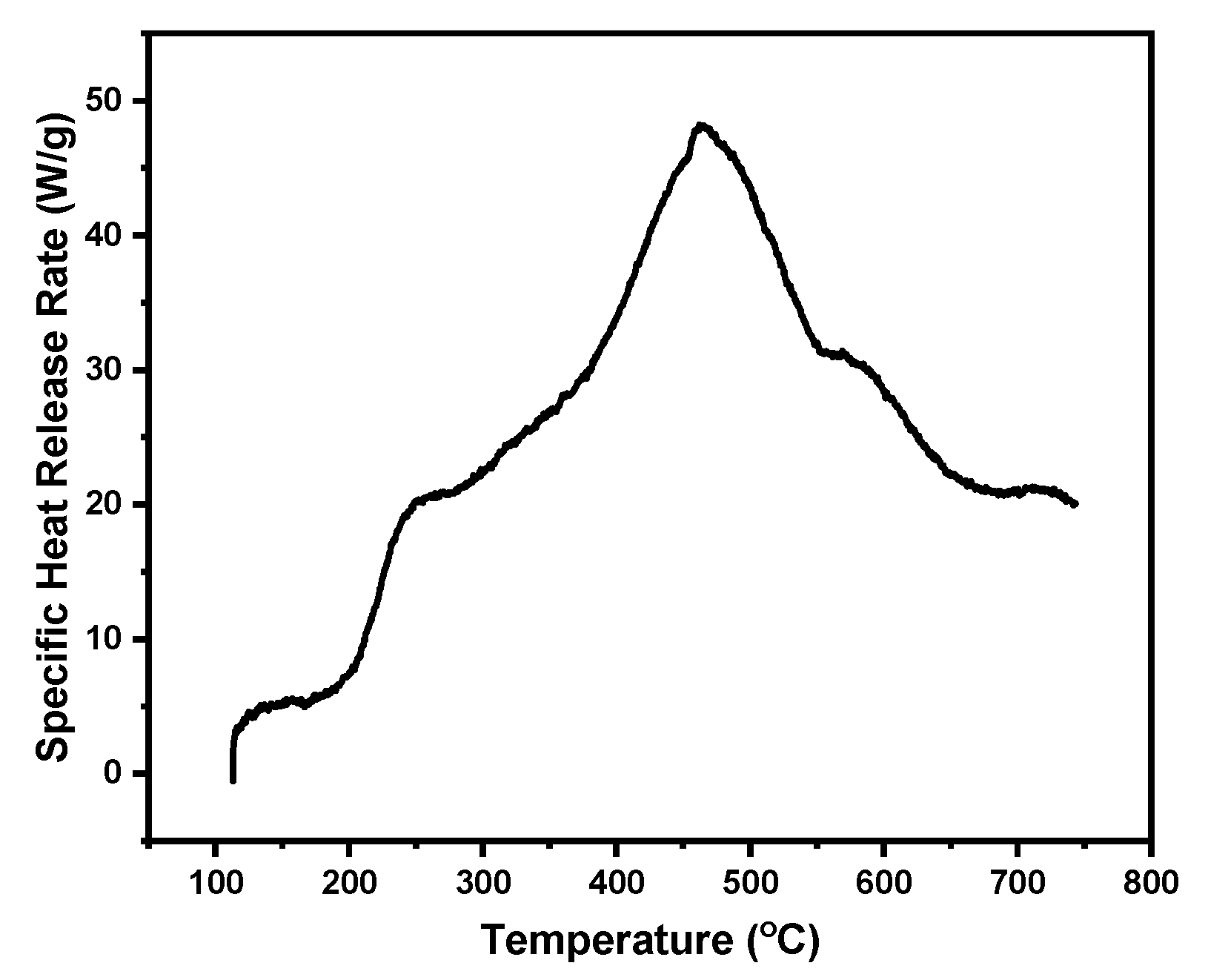

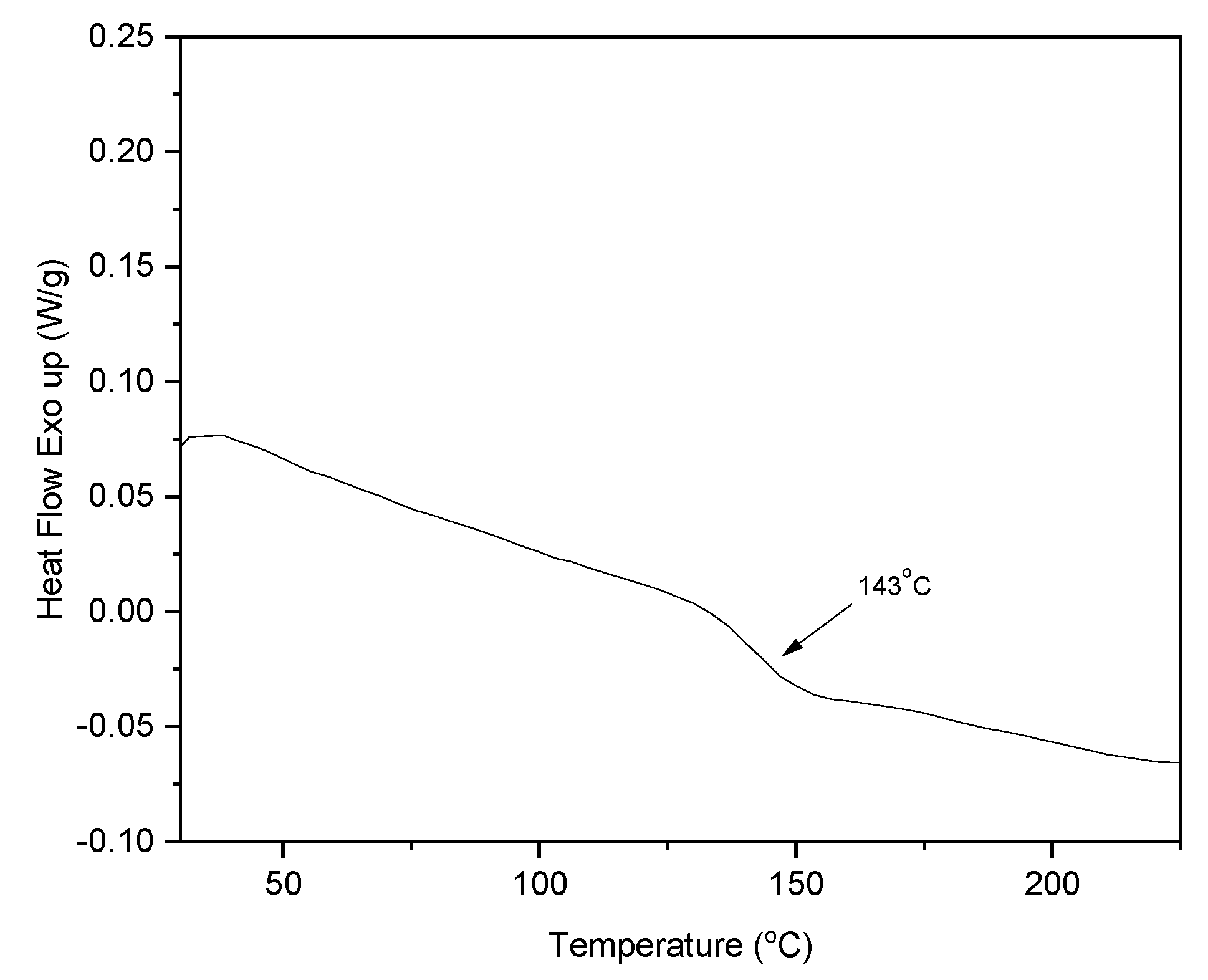
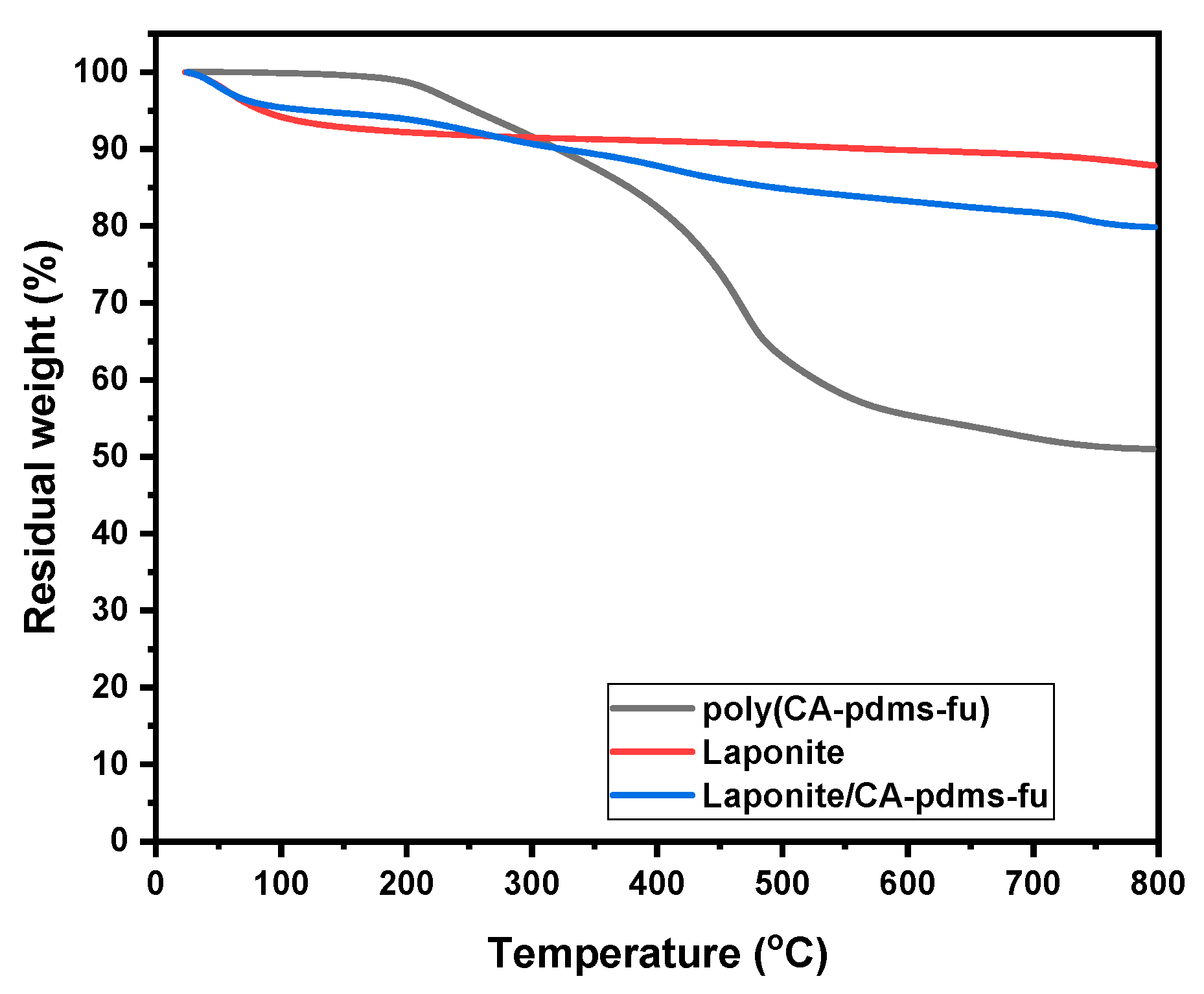
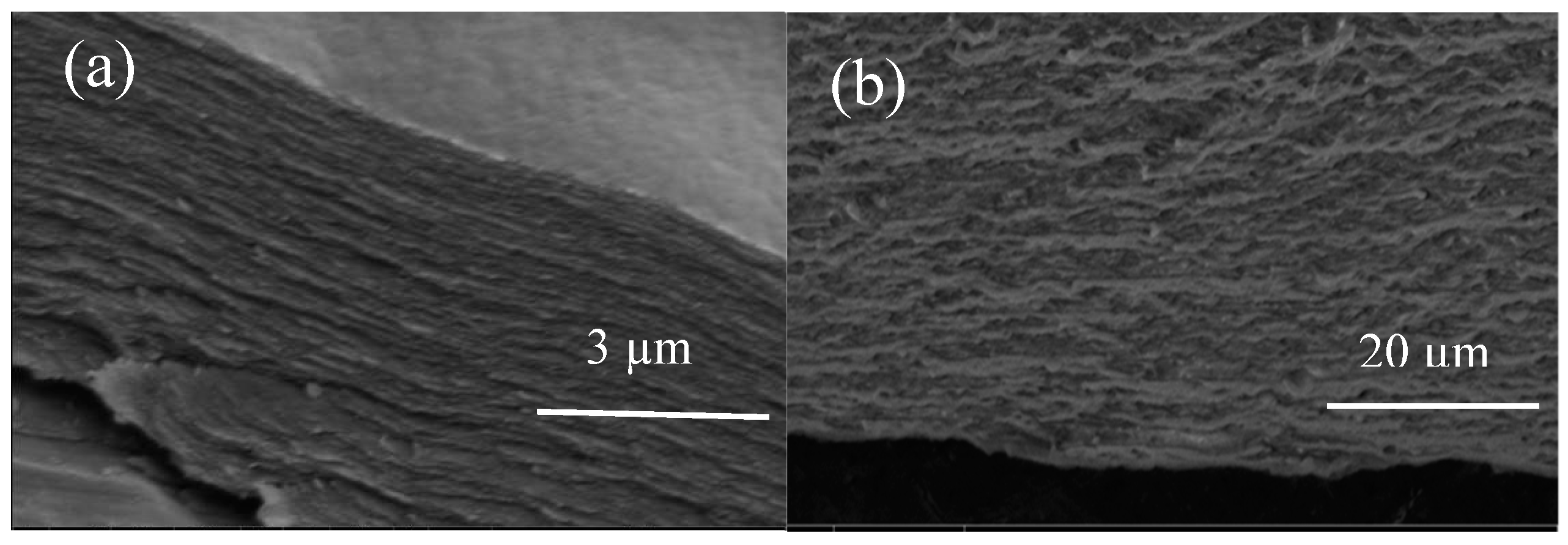
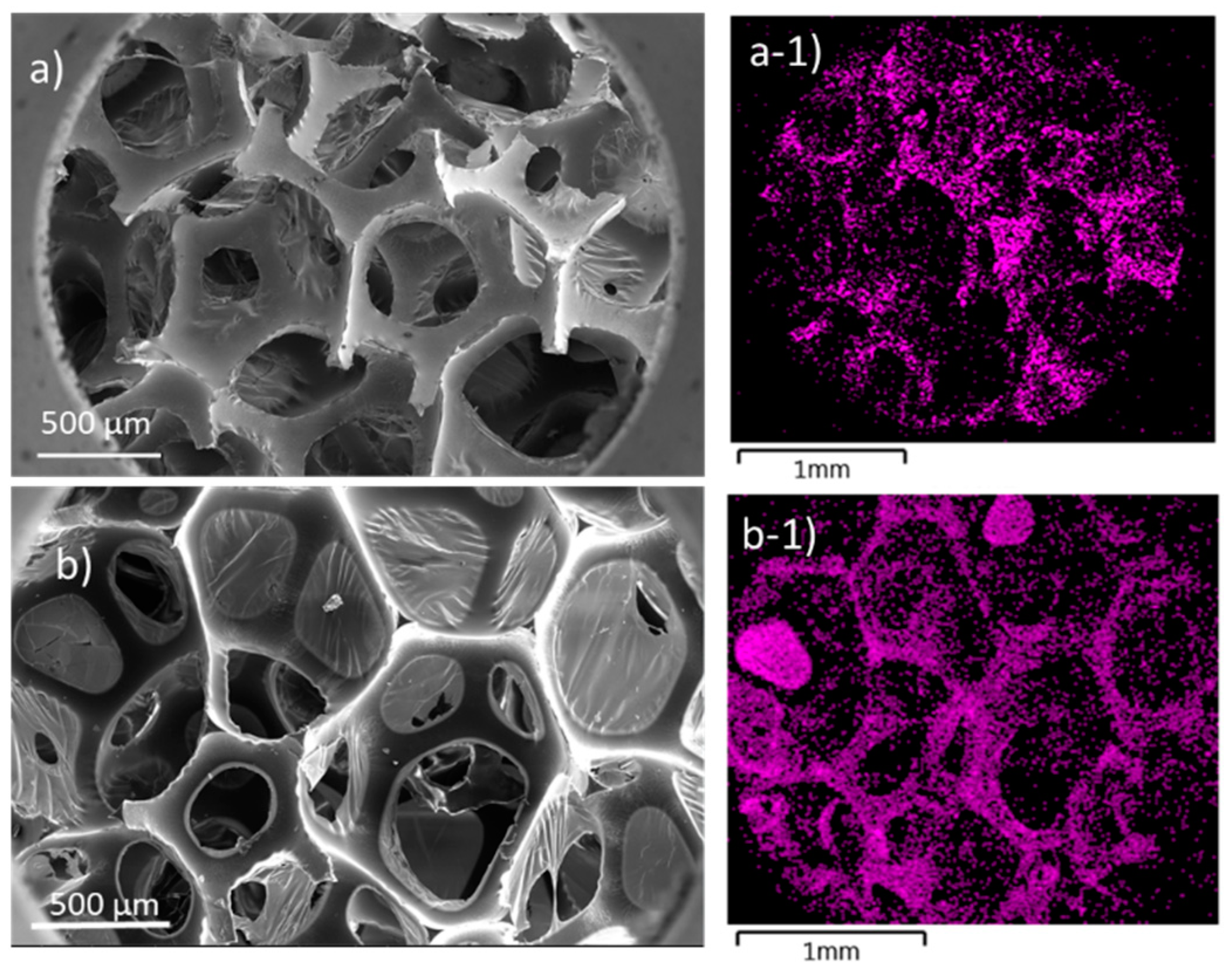

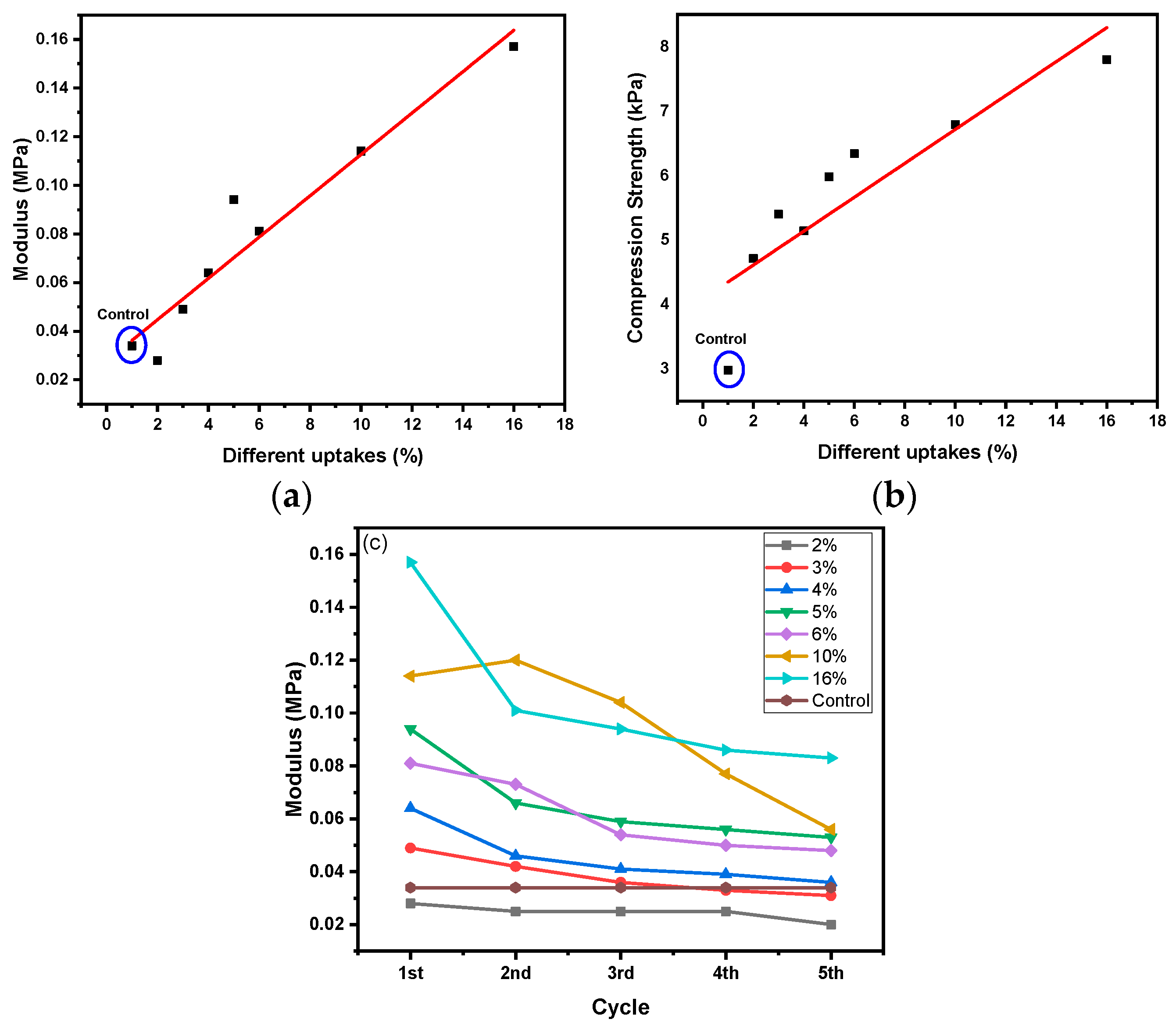
| Code | Elastic Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break (%) | Approach for Toughness |
|---|---|---|---|---|
| Poly (BA-a) [47] | 3300 | 35 | 1.6 | - |
| Poly (CA-pdms-fu) | 65 | 4 | 25 | Copolymerization |
| Poly (BA-a)/PDMS (850) [47] * | 330 | 9 | 3.8 | Copolymerization |
| Poly (BA-a)/PDMS (1622) [47] ** | 20 | 0.04 | 7.1 | Copolymerization |
| Poly (BA-a)/PDMS (7%) [58] † | 2600 | 42 | 3.5 | Hybridization |
| Poly (BA-a)/PDMS (13%) [58] † | 2200 | 48 | 4.4 | Hybridization |
| Poly (BA-a)/PDMS (5%) [60] ‡ | 2800 | 38 | 2.6 | Hybridization |
| Poly (BA-a)/PDMS (10%) [60] ‡ | 2600 | 42 | 3.5 | Hybridization |
| Poly (BA-a)/PDMS (20%) [60] ‡ | 2200 | 48 | 4.4 | Hybridization |
| Poly (BA-a)/PDMS (30%) [60] ‡ | Macroscopic phase separation | Hybridization | ||
| Poly (BA-a)/PDMS (20%) [59] ⁑ | 2200 | 36 | 4.3 | Hybridization |
| Poly (BA-a)/PDMS (30%) [59] ⁑ | 2200 | 56 | 4.6 | Hybridization |
| Poly (BA-a)/PDMS (40%) [59] ⁑ | 2200 | 56 | 4.4 | Hybridization |
| Nanocomposite/Uptake | Char Yield (%) |
|---|---|
| Polyurethane Foam | 0.36 |
| Laponite/CA-PDMS-fu—2 wt% | 2.26 |
| Laponite/CA-PDMS-fu—4 wt% | 4.49 |
| Laponite/CA-PDMS-fu—5 wt% | 10 |
| Laponite/CA-PDMS-fu—6 wt% | 10.73 |
| Laponite/CA-PDMS-fu—7 wt% | 5.51 |
| Laponite/CA-PDMS-fu—10 wt% | 7.8 |
| Laponite/CA-PDMS-fu—18 wt% | 12.97 |
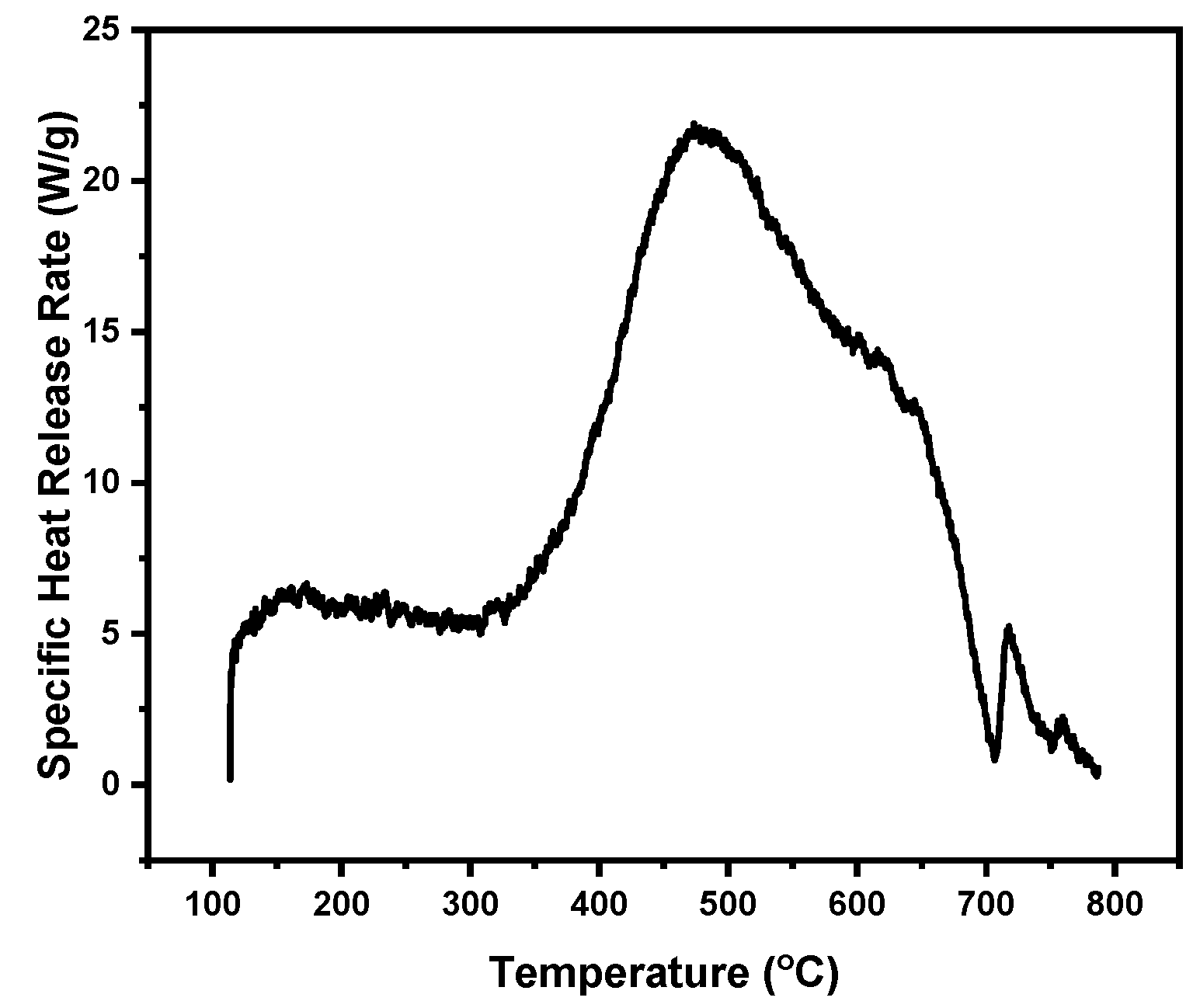
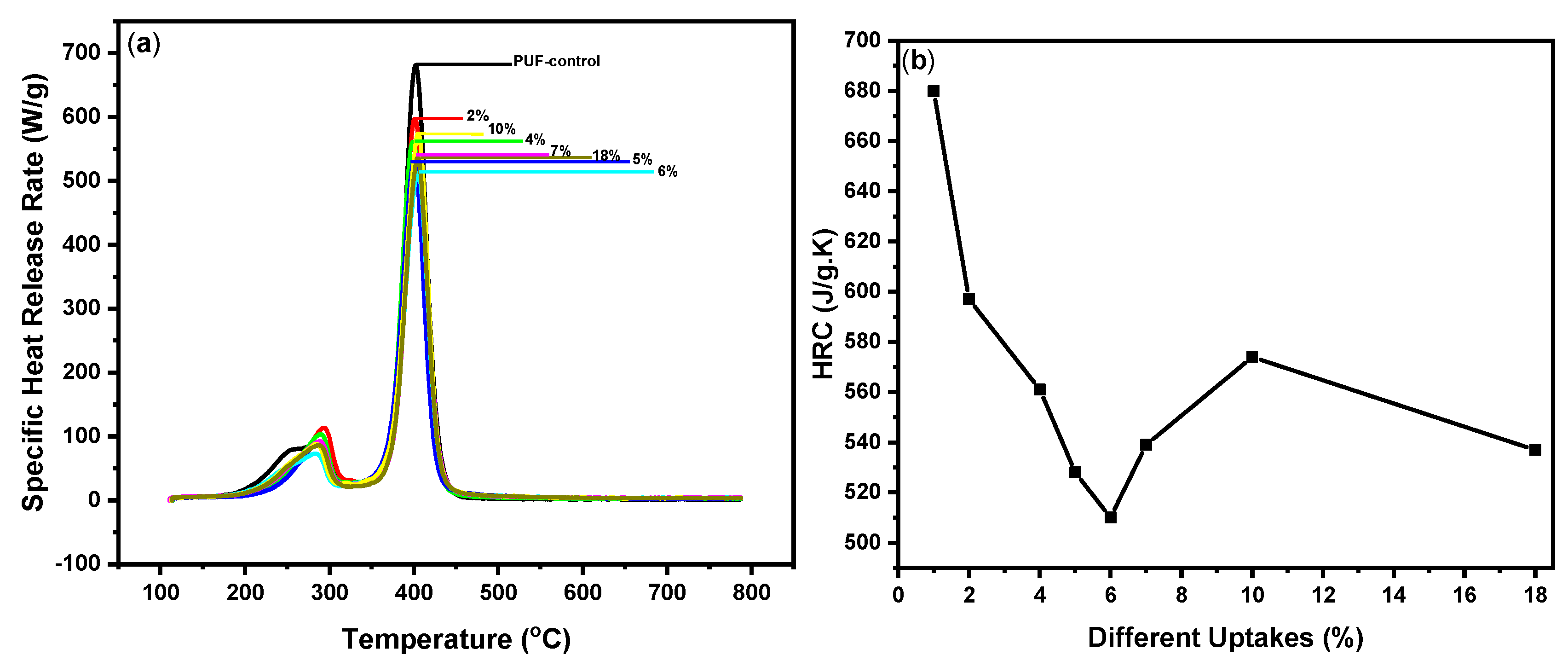
| Sample | Equation (3) | First Peak Max. T(°C) | Equation (3) | Second Peak Max. T(°C) | Char Yield (%) |
|---|---|---|---|---|---|
| 1st Peak HRC (J/g.K) | 2nd Peak HRC (J/g.K) | ||||
| Control PUF | 85 | 287 | 680 | 403 | 9.8 |
| Coated PUF 2% | 113 | 294 | 597 | 400 | 10.2 |
| Coated PUF 4% | 103 | 292 | 561 | 398 | 76 |
| Coated PUF 5% | 89 | 289 | 528 | 401 | 82 |
| Coated PUF 6% | 71 | 287 | 510 | 406 | 84 |
| Coated PUF 7% | 92 | 289 | 539 | 405 | 83 |
| Coated PUF 10% | 86 | 287 | 574 | 405 | 88 |
| Coated PUF 18% | 86 | 288 | 537 | 404 | 88 |
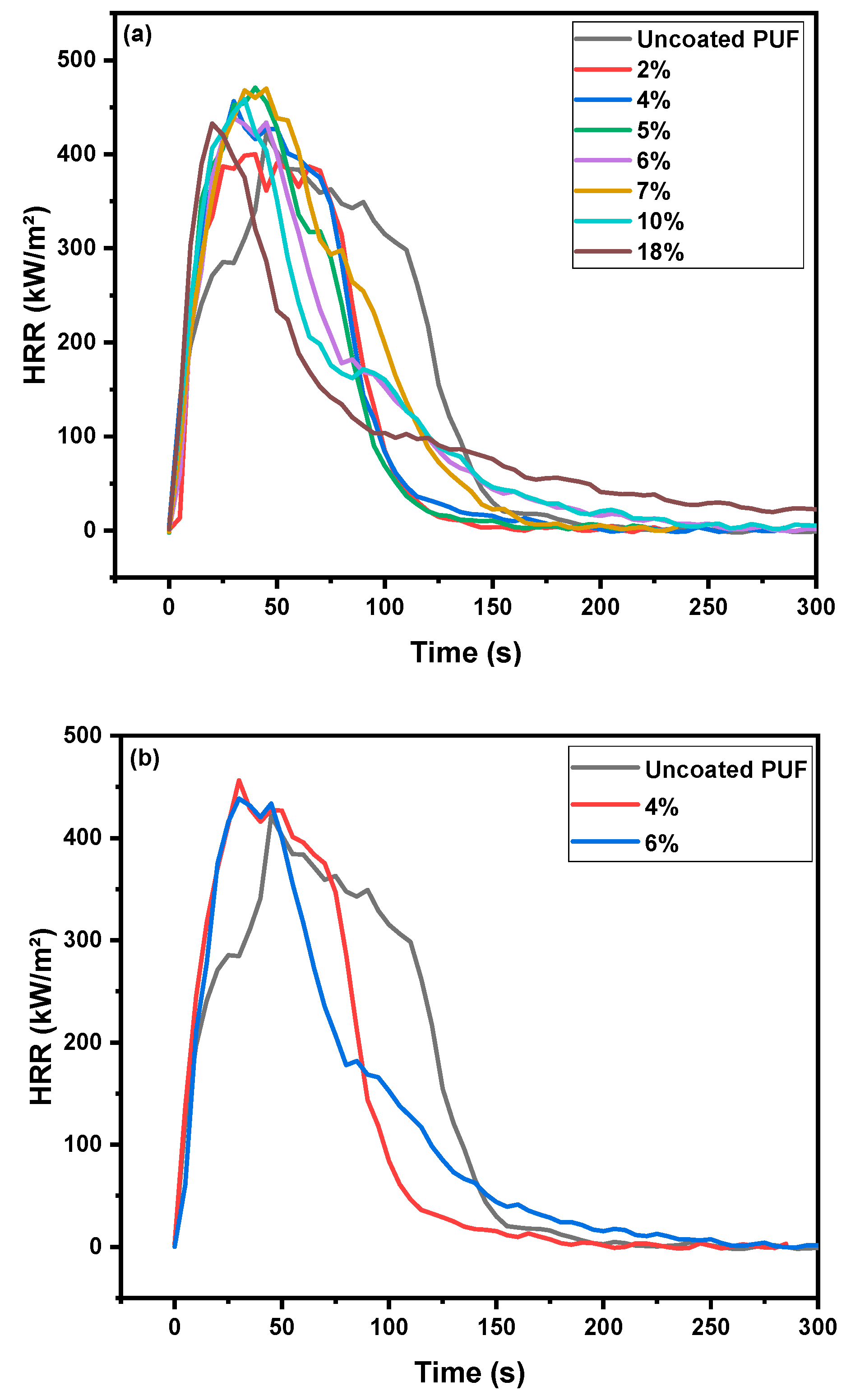
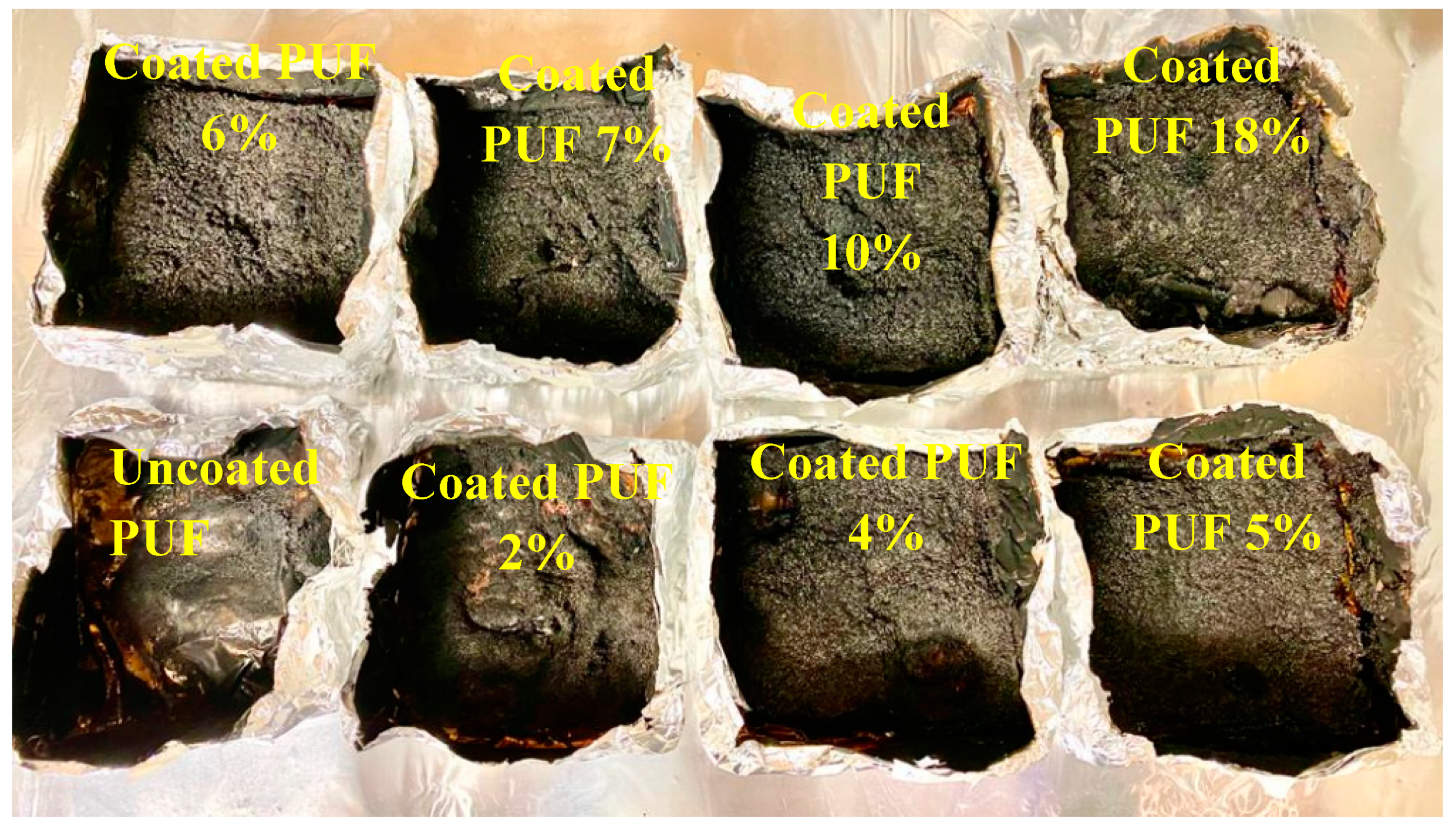
| Sample | 1st PHRR (kW/m2) | 2nd PHRR (kW/m2) | TSR (m2/m2) | THR (MJ/m2) | Char Residue (%) |
|---|---|---|---|---|---|
| Uncoated PUF | 285 | 483 | 227 | 41 | 1 |
| Coated PUF 2% | 307 | 400 | 215 | 31 | 7 |
| Coated PUF 4% | 456 | - | 235 | 34 | 9 |
| Coated PUF 5% | 470 | 318 | 215 | 32 | 12 |
| Coated PUF 6% | 438 | 182 | 215 | 34 | 15 |
| Coated PUF 7% | 468 | 298 | 272 | 38 | 12 |
| Coated PUF 10% | 458 | 172 | 196 | 34 | 15 |
| Coated PUF 18% | 432 | - | 173 | 34 | 23 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, I.; Hsieh, I.; Calado, V.; Chapin, T.; Ishida, H. Nacre-Mimetic Green Flame Retardant: Ultra-High Nanofiller Content, Thin Nanocomposite as an Effective Flame Retardant. Polymers 2020, 12, 2351. https://doi.org/10.3390/polym12102351
Machado I, Hsieh I, Calado V, Chapin T, Ishida H. Nacre-Mimetic Green Flame Retardant: Ultra-High Nanofiller Content, Thin Nanocomposite as an Effective Flame Retardant. Polymers. 2020; 12(10):2351. https://doi.org/10.3390/polym12102351
Chicago/Turabian StyleMachado, Irlaine, Isabel Hsieh, Veronica Calado, Thomas Chapin, and Hatsuo Ishida. 2020. "Nacre-Mimetic Green Flame Retardant: Ultra-High Nanofiller Content, Thin Nanocomposite as an Effective Flame Retardant" Polymers 12, no. 10: 2351. https://doi.org/10.3390/polym12102351
APA StyleMachado, I., Hsieh, I., Calado, V., Chapin, T., & Ishida, H. (2020). Nacre-Mimetic Green Flame Retardant: Ultra-High Nanofiller Content, Thin Nanocomposite as an Effective Flame Retardant. Polymers, 12(10), 2351. https://doi.org/10.3390/polym12102351








