Polyethylene-Based Knee Spacer for Infection Control: Design Concept and Pre-Clinical In Vitro Validations
Abstract
1. Introduction
2. Materials and Methods
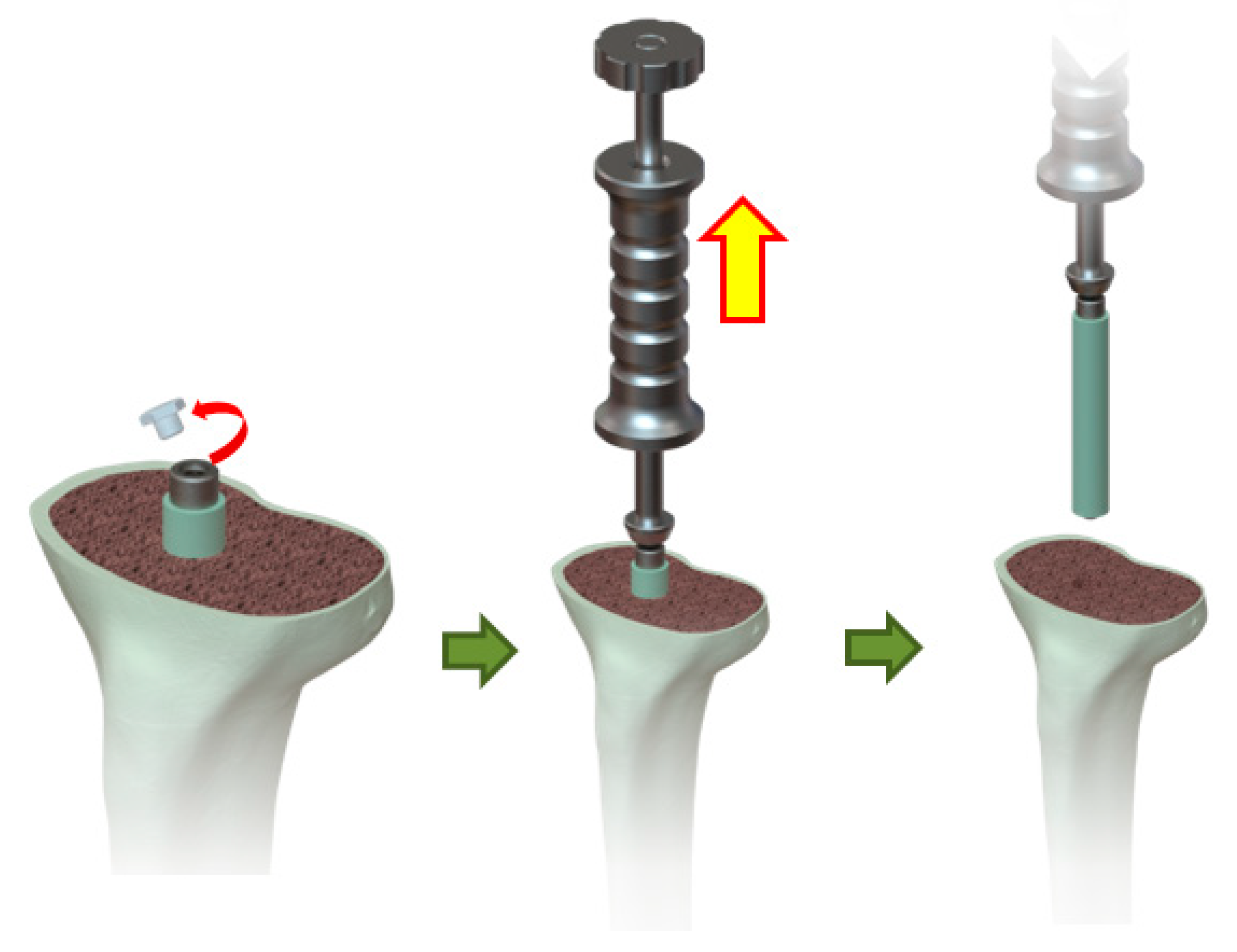
2.1. Device Description and Design Concept
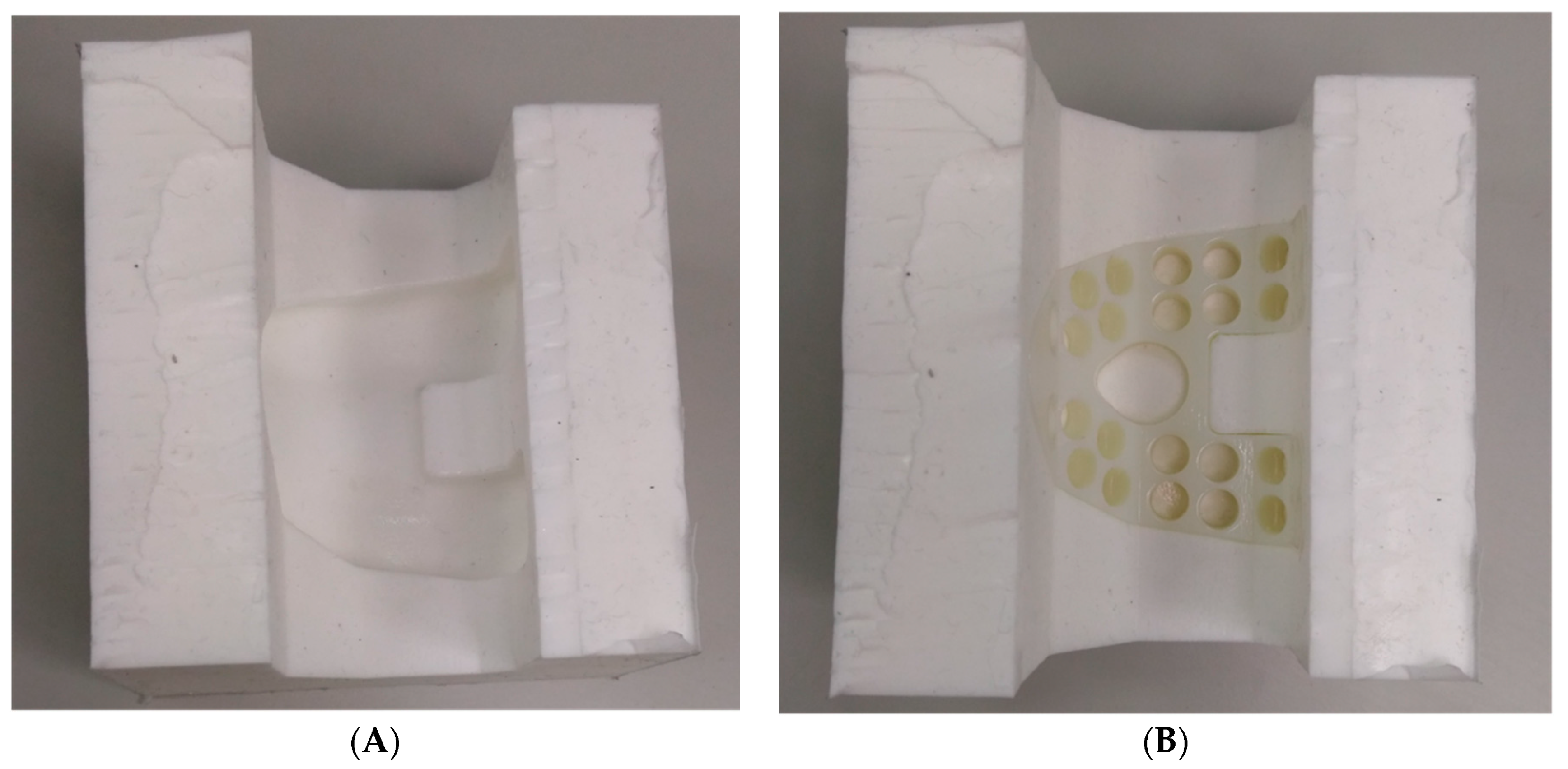
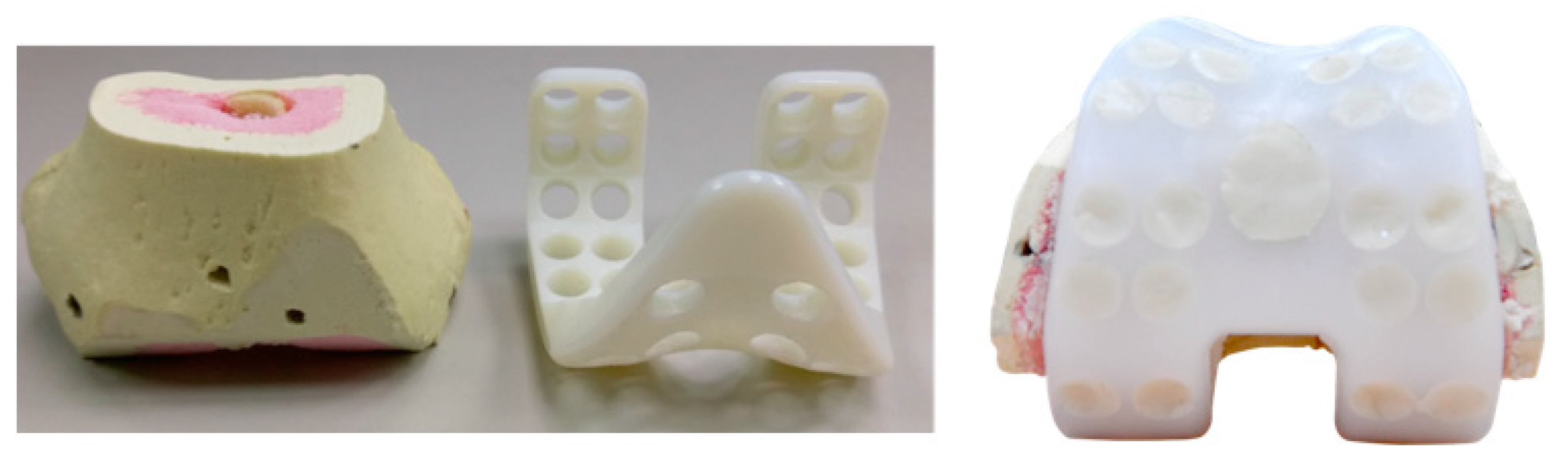
2.2. Preparation of Test Specimens
2.3. Tibial Spacer Fatigue Test
2.4. Wear Test
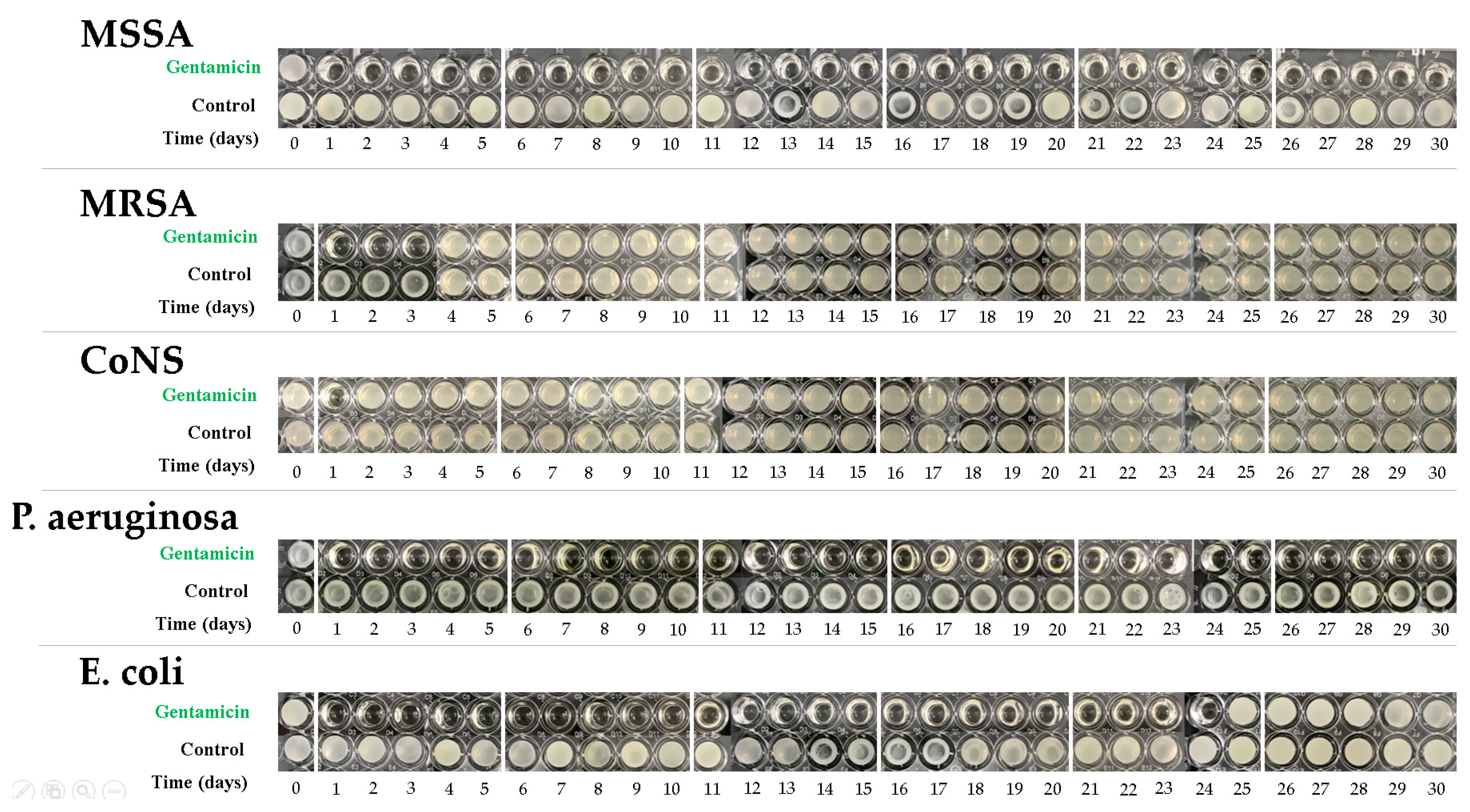
2.5. Antibiotic Elution Test
3. Results
3.1. Tibial Spacer Fatigue Test
3.2. Wear Test
3.2.1. Wear Rate—Loss of Specimen Weight
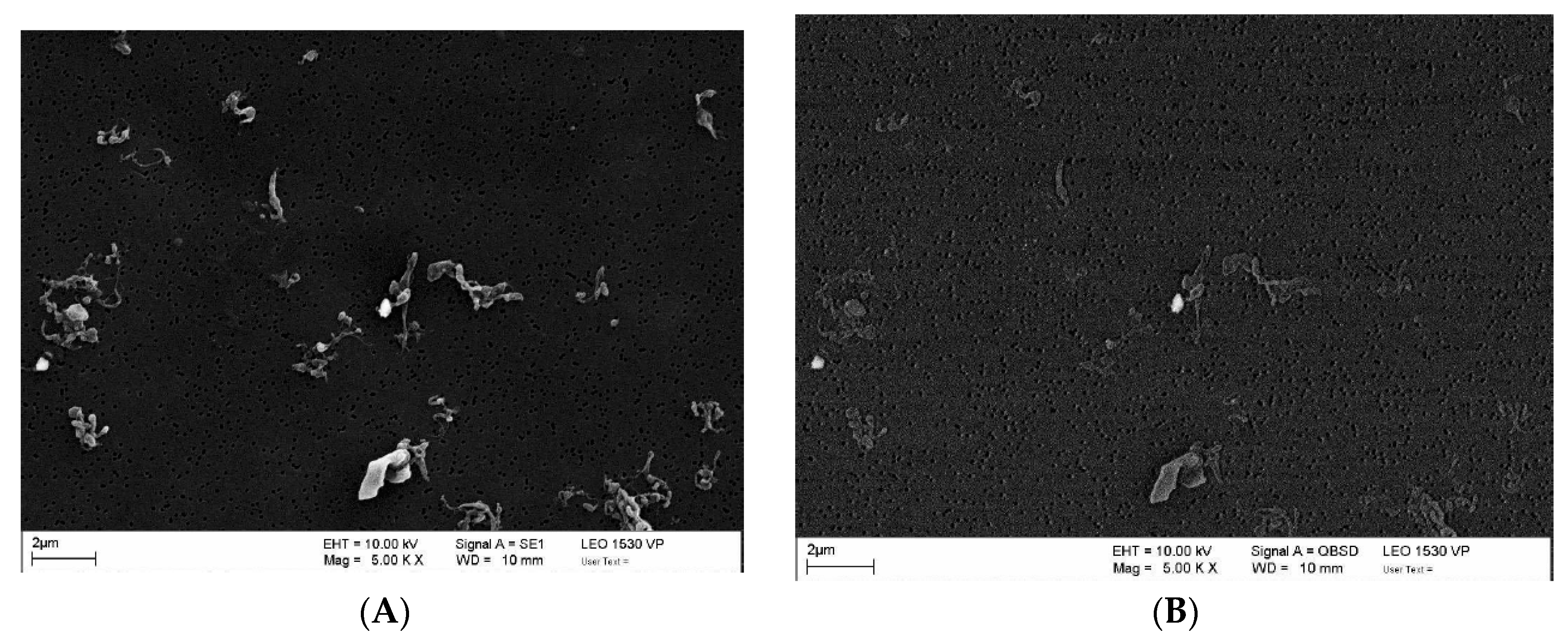
3.2.2. Morphology of Wear Particles
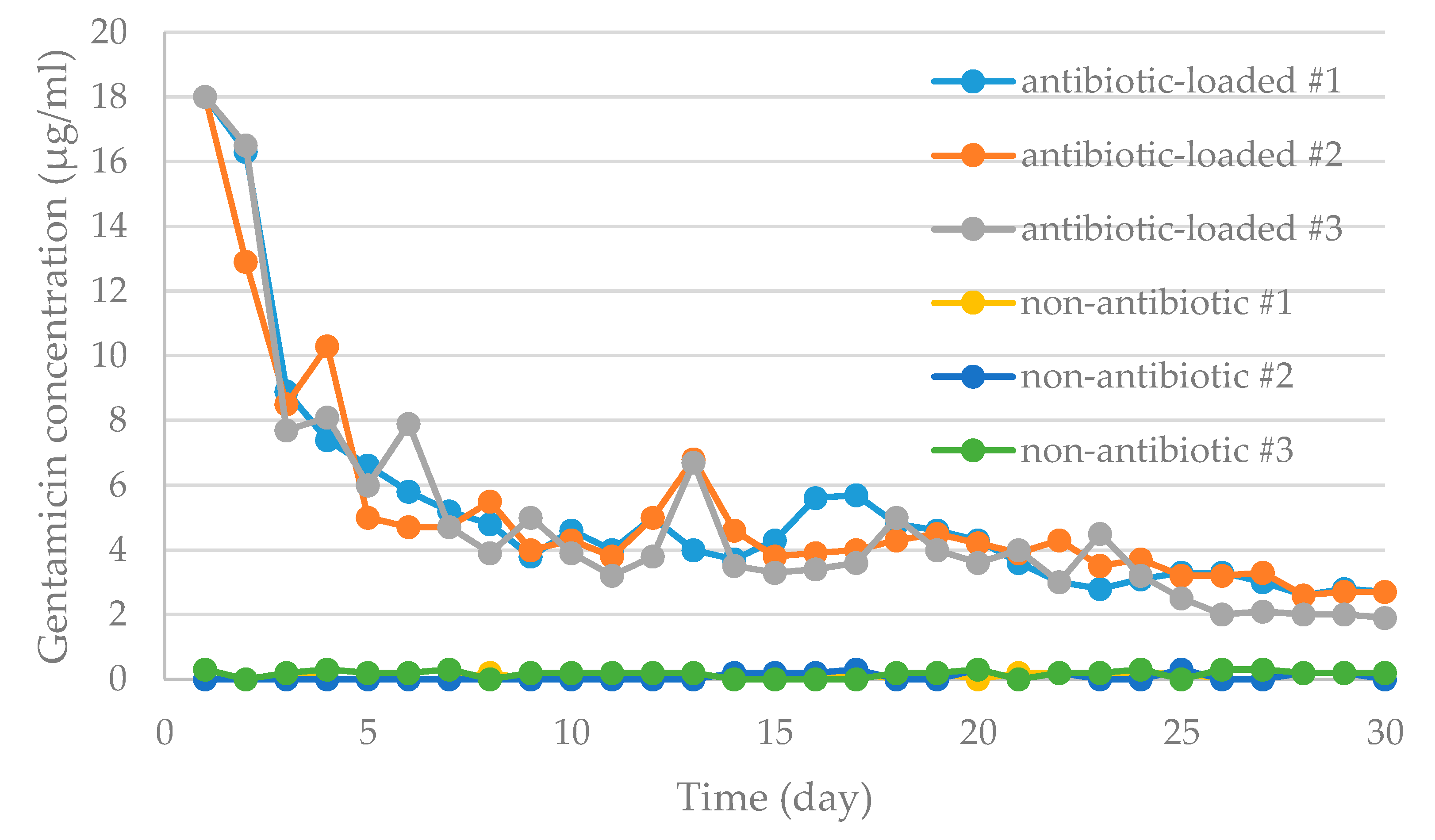
3.3. Antibiotic Elution Test
3.3.1. Antibiotic Concentration Elution Bioassay
3.3.2. Bioassay of Antibiotic Activity
4. Discussion
- -
- Concern of Bacterial Biofilm
- -
- The Antibiotic-Loaded Cement Applied
- -
- Modified Test Condition
- -
- Wear Rate Calculation
- -
- Surface Condition and Material of Molding Device
- -
- Unverified Canal Rod
5. Conclusions
6. Patents
- TW M465151U Porous artificial knee joint (patent of Taiwan, R.O.C.)
- CN203341865U Porous type artificial knee joint (patent of Mainland China)
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ethgen, O.; Bruyère, O.; Richy, F.; Dardennes, C.; Reginster, J.Y. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J. Bone Jt. Surg. Am. 2004, 86, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Konopka, J.F.; Lee, Y.Y.; Su, E.P.; McLawhorn, A.S. Quality-Adjusted Life Years after Hip and Knee Arthroplasty: Health-Related Quality of Life after 12,782 Joint Replacements. JBJS Open Access 2018, 3, e0007. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Mihalko, W.M.; Shields, J.S.; Ries, M.; Saleh, K.J. Antibiotic-impregnated cement spacers for the treatment of infection associated with total hip or knee arthroplasty. J. Bone Jt. Surg. Am. 2007, 89, 871–882. [Google Scholar] [CrossRef]
- Ritter, M.A.; Farris, A. Outcome of infected total joint replacement. Orthopedics 2010, 33, 33. [Google Scholar] [CrossRef]
- Delanois, R.E.; Mistry, J.B.; Gwam, C.U.; Mohamed, N.S.; Choksi, U.S.; Mont, M.A. Current Epidemiology of Revision Total Knee Arthroplasty in the United States. J. Arthroplast. 2017, 32, 2663–2668. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.P. Successful treatment of total hip and knee infection with articulating antibiotic components: A modified treatment method. Clin. Orthop. Relat. Res. 2004, 427, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.H.; Hu, C.C.; Hsieh, P.H.; Shih, H.N.; Ueng, S.W.; Chang, Y. Vancomycin and Ceftazidime in Bone Cement as a Potentially Effective Treatment for Knee Periprosthetic Joint Infection. J. Bone Jt. Surg. Am. 2017, 99, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Renz, N.; Perka, C.; Trampuz, A. Management of periprosthetic infections of the knee. Orthopade 2016, 45, 65–71. [Google Scholar] [CrossRef]
- Barrack, R.L.; Engh, G.; Rorabeck, C.; Sawhney, J.; Woolfrey, M. Patient satisfaction and outcome after septic versus aseptic revision total knee arthroplasty. J. Arthroplast. 2000, 15, 990–993. [Google Scholar] [CrossRef]
- Haleem, A.A.; Berry, D.J.; Hanssen, A.D. Mid-term to long-term followup of two-stage reimplantation for infected total knee arthroplasty. Clin. Orthop. Relat. Res. 2004, 428, 35–39. [Google Scholar] [CrossRef]
- Voleti, P.B.; Baldwin, K.D.; Lee, G.C. Use of static or articulating spacers for infection following total knee arthroplasty: A systematic literature review. J. Bone Jt. Surg. Am. 2013, 95, 1594–1599. [Google Scholar] [CrossRef] [PubMed]
- Haddad, F.S.; Masri, B.A.; Campbell, D.; McGraw, R.W.; Beauchamp, C.P.; Duncan, C.P. The PROSTALAC functional spacer in two-stage revision for infected knee replacements. Prosthesis of antibiotic-loaded acrylic cement. J. Bone Jt. Surg. Br. 2000, 82, 807–812. [Google Scholar] [CrossRef]
- Emerson, R.H., Jr.; Muncie, M.; Tarbox, T.R.; Higgins, L.L. Comparison of a static with a mobile spacer in total knee infection. Clin. Orthop. Relat. Res. 2002, 404, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.G.; Fehring, T.K.; Odum, S.M.; Fehring, K.; Griffin, W.L.; Mason, J.B. Functional advantage of articulating versus static spacers in 2-stage revision for total knee arthroplasty infection. J. Arthroplast. 2007, 22, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.S.; Huber, K.; Evangelopoulos, D.S.; Kleer, B.; Kohlhof, H.; Schär, M.; Eggli, S.; Kohl, S. The cement prosthesis-like spacer: An intermediate halt on the road to healing. Sci. World J. 2013, 2013, 763434. [Google Scholar] [CrossRef] [PubMed]
- Gooding, C.R.; Masri, B.A.; Duncan, C.P.; Greidanus, N.V.; Garbuz, D.S. Durable infection control and function with the PROSTALAC spacer in two-stage revision for infected knee arthroplasty. Clin. Orthop. Relat. Res. 2011, 469, 985–993. [Google Scholar] [CrossRef]
- Struelens, B.; Claes, S.; Bellemans, J. Spacer-related problems in two-stage revision knee arthroplasty. Acta Orthop. Belg. 2013, 79, 422–426. [Google Scholar]
- Van Thiel, G.S.; Berend, K.R.; Klein, G.R.; Gordon, A.C.; Lombardi, A.V.; Della Valle, C.J. Intraoperative molds to create an articulating spacer for the infected knee arthroplasty. Clin. Orthop. Relat. Res. 2011, 469, 994–1001. [Google Scholar] [CrossRef]
- Wan, Z.; Karim, A.; Momaya, A.; Incavo, S.J.; Mathis, K.B. Preformed articulating knee spacers in 2-stage total knee revision arthroplasty: Minimum 2-year follow-up. J. Arthroplast. Arthroplast. 2012, 27, 1469–1473. [Google Scholar] [CrossRef]
- Hofmann, A.A.; Tkach, T.K.; Evanich, C.J.; Camargo, M.P. Posterior stabilization in total knee arthroplasty with use of an ultracongruent polyethylene insert. J. Arthroplast. 2000, 15, 576–583. [Google Scholar] [CrossRef]
- Laskin, R.S.; Maruyama, Y.; Villaneuva, M.; Bourne, R. Deep-dish congruent tibial component use in total knee arthroplasty: A randomized prospective study. Clin. Orthop. Relat. Res. 2000, 380, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Hustedt, J.W.; Blizzard, D.J.; Baumgaertner, M.R.; Leslie, M.P.; Grauer, J.N. Current advances in training orthopaedic patients to comply with partial weight-bearing instructions. Yale J. Biol. Med. 2012, 85, 119–125. [Google Scholar] [PubMed]
- Villa, T.; Carnelli, D.; Pietrabissa, R. Fatigue Characterisation of the Preformed Knee Spacer. In Infection and Local Treatment in Orthopedic Surgery; Meani, E., Romanò, C., Crosby, L., Hofmann, G., Calonego, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 128–135. [Google Scholar]
- Mueller, U.; Reinders, J.; Smith-Romanski, S.; Kretzer, J.P. Wear Performance of Calcium Carbonate-Containing Knee Spacers. Materials (Basel) 2017, 10, 805. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.; Forster, H.; Jani, S.; Vadodaria, K.; Sauer, W.; Anthony, M. Validation of An Alternative Method for Isolating UHMWPE Wear Debris from Joint Simulator Serum; World Biomaterials Congress: Kamuela, HI, USA, 2000. [Google Scholar]
- Chang, Y.H.; Tai, C.L.; Hsu, H.Y.; Hsieh, P.H.; Lee, M.S.; Ueng, S.W. Liquid antibiotics in bone cement: An effective way to improve the efficiency of antibiotic release in antibiotic loaded bone cement. Bone Jt. Res. 2014, 3, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Tai, C.L.; Hsieh, P.H.; Ueng, S.W. Gentamicin in bone cement: A potentially more effective prophylactic measure of infection in joint arthroplasty. Bone Jt. Res. 2013, 2, 220–226. [Google Scholar] [CrossRef]
- Bertazzoni Minelli, E.; Benini, A. The Gentamicin-Vancomycin Spacer: A Pharmacological Study. In Infection and Local Treatment in Orthopedic Surgery; Meani, E., Romanò, C., Crosby, L., Hofmann, G., Calonego, G., Eds.; Springer: Berlin/Heidelberg, Germay, 2007; pp. 352–358. [Google Scholar]
- Mutimer, J.; Gillespie, G.; Lovering, A.M.; Porteous, A.J. Measurements of in vivo intra-articular gentamicin levels from antibiotic loaded articulating spacers in revision total knee replacement. Knee 2009, 16, 39–41. [Google Scholar] [CrossRef]
- Rogers, B.A.; Middleton, F.R.; Shearwood-Porter, N.; Kinch, S.; Roques, A.; Bradley, N.W.; Browne, M. Does cyclical loading affect the elution of antibiotics from articulating cement knee spacers? J. Bone Jt. Surg. Br. 2011, 93, 914–920. [Google Scholar] [CrossRef]
- Haenle, M.; Skripitz, C.; Mittelmeier, W.; Skripitz, R. Economic impact of infected total knee arthroplasty. Sci. World J. 2012, 2012, 196515. [Google Scholar] [CrossRef]
- Badia, J.M.; Casey, A.L.; Petrosillo, N.; Hudson, P.M.; Mitchell, S.A.; Crosby, C. Impact of surgical site infection on healthcare costs and patient outcomes: A systematic review in six European countries. J. Hosp. Infect. 2017, 96, 1–15. [Google Scholar] [CrossRef]
- Kuehn, K.D.; Ege, W.; Gopp, U. Acrylic bone cements: Mechanical and physical properties. Orthop. Clin. North Am. 2005, 36, 29–39. [Google Scholar] [CrossRef]
- Marks, K.E.; Nelson, C.L.; Lautenschlager, E.P. Antibiotic-impregnated acrylic bone cement. J. Bone Jt. Surg. Am. 1976, 58, 358–364. [Google Scholar] [CrossRef]
- Seldes, R.M.; Winiarsky, R.; Jordan, L.C.; Baldini, T.; Brause, B.; Zodda, F.; Sculco, T.P. Liquid gentamicin in bone cement: A laboratory study of a potentially more cost-effective cement spacer. J. Bone Jt. Surg. Am. 2005, 87, 268–272. [Google Scholar] [CrossRef]
- Jiranek, W.A.; Hanssen, A.D.; Greenwald, A.S. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. J. Bone Jt. Surg. Am. 2006, 88, 2487–2500. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, S.M.; Frondoza, C.G.; Lennox, D.W. Effects of polymethylmethacrylate exposure upon macrophages. J. Orthop. Res. 1988, 6, 827–832. [Google Scholar] [CrossRef]
- Mellman, I.S.; Plutner, H.; Steinman, R.M.; Unkeless, J.C.; Cohn, Z.A. Internalization and degradation of macrophage Fc receptors during receptor-mediated phagocytosis. J. Cell Biol. 1983, 96, 887–895. [Google Scholar] [CrossRef]
- Glant, T.T.; Jacobs, J.J.; Molnár, G.; Shanbhag, A.S.; Valyon, M.; Galante, J.O. Bone resorption activity of particulate-stimulated macrophages. J. Bone Miner. Res. 1993, 8, 1071–1079. [Google Scholar] [CrossRef]
- Sieving, A.; Wu, B.; Mayton, L.; Nasser, S.; Wooley, P.H. Morphological characteristics of total joint arthroplasty-derived ultra-high molecular weight polyethylene (UHMWPE) wear debris that provoke inflammation in a murine model of inflammation. J. Biomed. Mater. Res. A 2003, 64, 457–464. [Google Scholar] [CrossRef]
- Elke, R.; Rieker, C.B. Estimating the osteolysis-free life of a total hip prosthesis depending on the linear wear rate and head size. Proc. Inst. Mech. Eng. Part H 2018, 232, 753–758. [Google Scholar] [CrossRef]
- Berberich, C.; Sanz-Ruiz, P. Risk assessment of antibiotic resistance development by antibiotic-loaded bone cements: Is it a clinical concern? EFORT Open Rev. 2019, 4, 576–584. [Google Scholar] [CrossRef]
- Zacheus, O.M.; Iivanainen, E.K.; Nissinen, T.K.; Lehtola, M.J.; Martikainen, P.J. Bacterial biofilm formation on polyvinyl, chloride, polyethylene and stainless steel exposed to ozonated water. Water Res. 2000, 34, 67–70. [Google Scholar] [CrossRef]
- Sanz-Ruiz, P.; Matas-Diez, J.A.; Sanchez-Somolinos, M.; Villanueva-Martinez, M.; Vaquero-Martín, J. Is the Commercial Antibiotic-Loaded Bone Cement Useful in Prophylaxis and Cost Saving After Knee and Hip Joint Arthroplasty? The Transatlantic Paradox. J. Arthroplast. 2017, 32, 1095–1099. [Google Scholar] [CrossRef] [PubMed]








 | 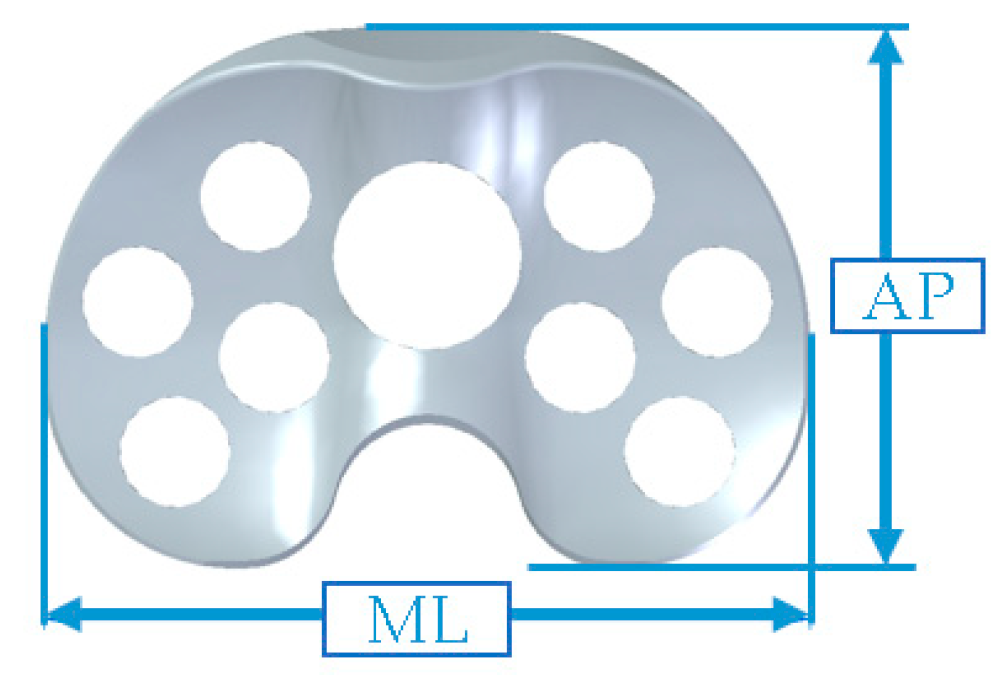 | |||
| Size | Femoral Spacer | Tibial Spacer | ||
| AP (mm) | ML (mm) | AP (mm) | ML (mm) | |
| #1 | 56 | 56 | 42 | 63 |
| #2 | 60 | 60 | 44.5 | 66 |
| #3 | 64 | 64 | 47 | 69 |
| #4 | 68 | 68 | 49.5 | 72 |
| #5 | 72 | 72 | 52.5 | 76 |
| Item | Condition |
|---|---|
| Test temperature | 37 ± 2 °C |
| Flexion/extension | 0° to 58° |
| Axial force | 84 N to 1300 N |
| AP force | −265 N to 110 N |
| Axial torque | −1 Nm to 6 Nm |
| Loss of Dry Weight | ||
|---|---|---|
| Specimen | Femoral Spacer (mg) | Tibial Spacer (mg) |
| #1 | 24.6 | 33.9 |
| #2 | 16.1 | 3.51 |
| #3 | 48.8 | 35.9 |
| #4 | −3.8* | 7.6 |
| #5 | 34.6 | 26.4 |
| #6 | 47.6 | 5.37 |
| Average | 27.98 ± 20.12 | 18.78 ± 14.96 |
| Average * | 34.34 ± 14.25 | 18.78 ± 14.96 |
| Commercial spacers [24] | 149.6 ± 10 | 226.0 ± 88.3 |
| Particle Material | Specimen | Equivalent Circle Diameter (μm) | Form Factor | Maximal Feret Diameter (μm) | Minimal Feret Diameter (μm) | Area (μm2) | Perimeter (μm) | Aspect Ratio | Particle Analyzed | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Range | Mean | Range | Mean | Range | Mean | Range | Mean | Range | Mean | Range | Mean | Range | |||
| UHMWPE | #1 | 0.49 | 0.12–1.97 | 0.54 | 0.07–1.00 | 0.92 | 0.14–4.61 | 0.47 | 0.09–2.81 | 0.3 | 0.01–3.06 | 2.96 | 0.38–19.36 | 1.85 | 1.05–5.65 | 105 |
| #2 | 0.45 | 0.13–1.52 | 0.56 | 0.07–1.00 | 0.81 | 0.16–3.17 | 0.45 | 0.11–2.44 | 0.24 | 0.01–1.82 | 2.67 | 0.41–13.87 | 1.74 | 1.07–3.10 | 109 | |
| #3 | 0.49 | 0.11–1.79 | 0.50 | 0.07–1.00 | 0.95 | 0.14–4.92 | 0.47 | 0.09–2.01 | 0.3 | 0.01–2.51 | 3.24 | 0.36–20.75 | 1.98 | 1.06–5.00 | 105 | |
| #4 | 0.54 | 0.11–2.13 | 0.46 | 0.06–0.99 | 1.04 | 0.14–5.14 | 0.54 | 0.11–2.05 | 0.32 | 0.01–3.56 | 3.59 | 0.38–24.10 | 1.93 | 1.08–5.03 | 87 | |
| #5 | 0.46 | 0.13–1.95 | 0.52 | 0.06–1.00 | 0.81 | 0.18–3.08 | 0.45 | 0.11–2.21 | 0.26 | 0.01–3.00 | 2.75 | 0.46–15.82 | 1.87 | 1.03–5.36 | 99 | |
| #6 | 0.54 | 0.13–1.64 | 0.42 | 0.05–1.00 | 1.11 | 0.18–3.69 | 0.56 | 0.11–1.77 | 0.32 | 0.01–2.11 | 3.87 | 0.44–13.51 | 2.00 | 1.08–5.61 | 93 | |
| Average | 0.50 ± 0.04 | 0.50 ± 0.05 | 0.94 ± 0.12 | 0.49 ± 0.05 | 0.29 ± 0.03 | 3.18 ± 0.48 | 1.89 ± 0.10 | - | ||||||||
| PMMA | #1 | 0.42 | 0.36–0.49 | 0.75 | 0.70–0.82 | 0.52 | 0.45–0.59 | 0.41 | 0.34–0.47 | 0.14 | 0.10–0.19 | 1.55 | 1.32–1.85 | 1.24 | 1.17–1.29 | 3 |
| #2 | 0.39 | 0.30–0.48 | 0.81 | 0.71–0.88 | 0.50 | 0.37–0.55 | 0.36 | 0.30–0.47 | 0.12 | 0.07–0.18 | 1.37 | 1.07–1.60 | 1.37 | 1.10–1.85 | 4 | |
| #3 | 0.68 | 0.29–0.90 | 0.57 | 0.42–0.72 | 1.00 | 0.52–1.25 | 0.62 | 0.22–0.83 | 0.4 | 0.07–0.64 | 2.86 | 1.26–3.79 | 1.72 | 1.45–2.38 | 4 | |
| #4 | 0.37 | 0.15–0.61 | 0.84 | 0.78–0.94 | 0.46 | 0.18–0.74 | 0.35 | 0.15–0.56 | 0.14 | 0.02–0.29 | 1.30 | 0.49–2.16 | 1.24 | 1.14–1.30 | 3 | |
| #5 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 0 | |
| #6 | 0.26 | 0.17–0.40 | 0.84 | 0.70–0.93 | 0.35 | 0.24–0.55 | 0.22 | 0.13–0.32 | 0.06 | 0.02–0.13 | 0.89 | 0.57–1.38 | 1.61 | 1.19–1.84 | 4 | |
| Average | 0.42 ± 0.15 | 0.76 ± 0.12 | 0.57 ± 0.25 | 0.39–0.15 | 0.17 ± 0.13 | 1.59 ± 0.75 | 1.43 ± 0.22 | - | ||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.; Lee, M.S.; Liau, J.-J.; Liu, Y.-L.; Chen, W.-C.; Ueng, S.W.N. Polyethylene-Based Knee Spacer for Infection Control: Design Concept and Pre-Clinical In Vitro Validations. Polymers 2020, 12, 2334. https://doi.org/10.3390/polym12102334
Chang Y, Lee MS, Liau J-J, Liu Y-L, Chen W-C, Ueng SWN. Polyethylene-Based Knee Spacer for Infection Control: Design Concept and Pre-Clinical In Vitro Validations. Polymers. 2020; 12(10):2334. https://doi.org/10.3390/polym12102334
Chicago/Turabian StyleChang, Yuhan, Mel S. Lee, Jiann-Jong Liau, Yu-Liang Liu, Wen-Chuan Chen, and Steve W. N. Ueng. 2020. "Polyethylene-Based Knee Spacer for Infection Control: Design Concept and Pre-Clinical In Vitro Validations" Polymers 12, no. 10: 2334. https://doi.org/10.3390/polym12102334
APA StyleChang, Y., Lee, M. S., Liau, J.-J., Liu, Y.-L., Chen, W.-C., & Ueng, S. W. N. (2020). Polyethylene-Based Knee Spacer for Infection Control: Design Concept and Pre-Clinical In Vitro Validations. Polymers, 12(10), 2334. https://doi.org/10.3390/polym12102334





