Investigation of the Cytotoxicity of Electrospun Polysuccinimide-Based Fiber Mats
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Polysuccinimide (PSI), Poly(aspartic acid) (PASP) and Aallylamine-Modified Ppolysuccinimide (PSI-AA)
2.3. Electrospinning of PSI-AA and Plasma Treatment
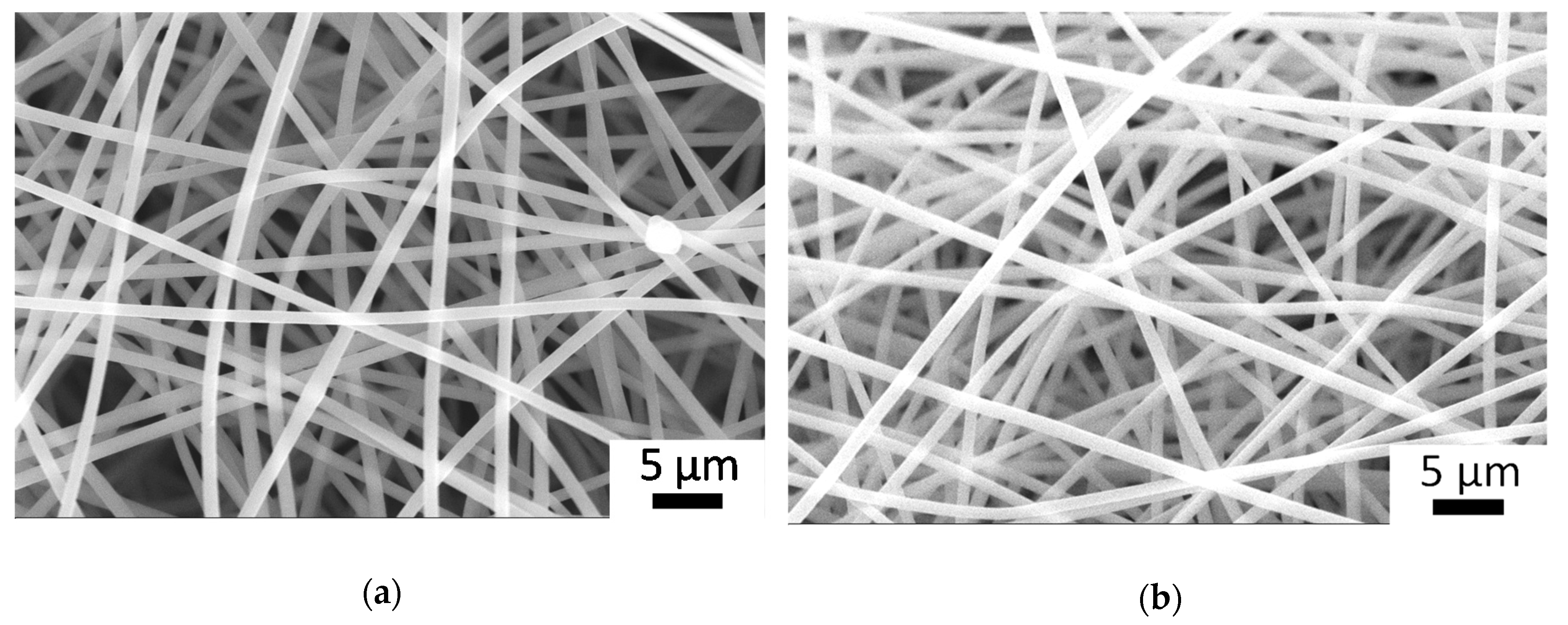
2.4. Scanning Electron Microscopy (SEM)
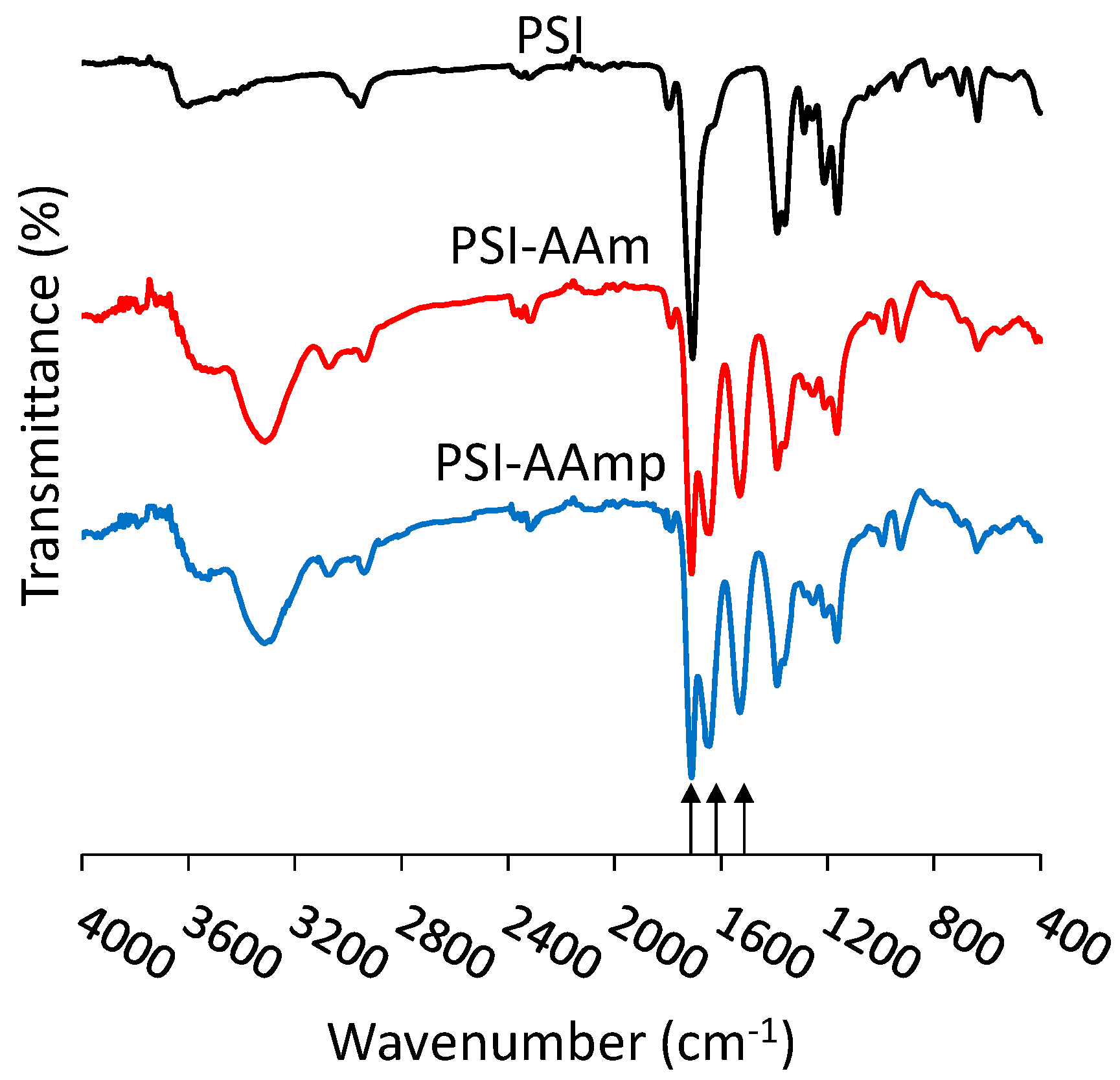
2.5. Attenuated Total Reflection Fourier Transform Infrared Spectroscopy (ATR-FTIR)
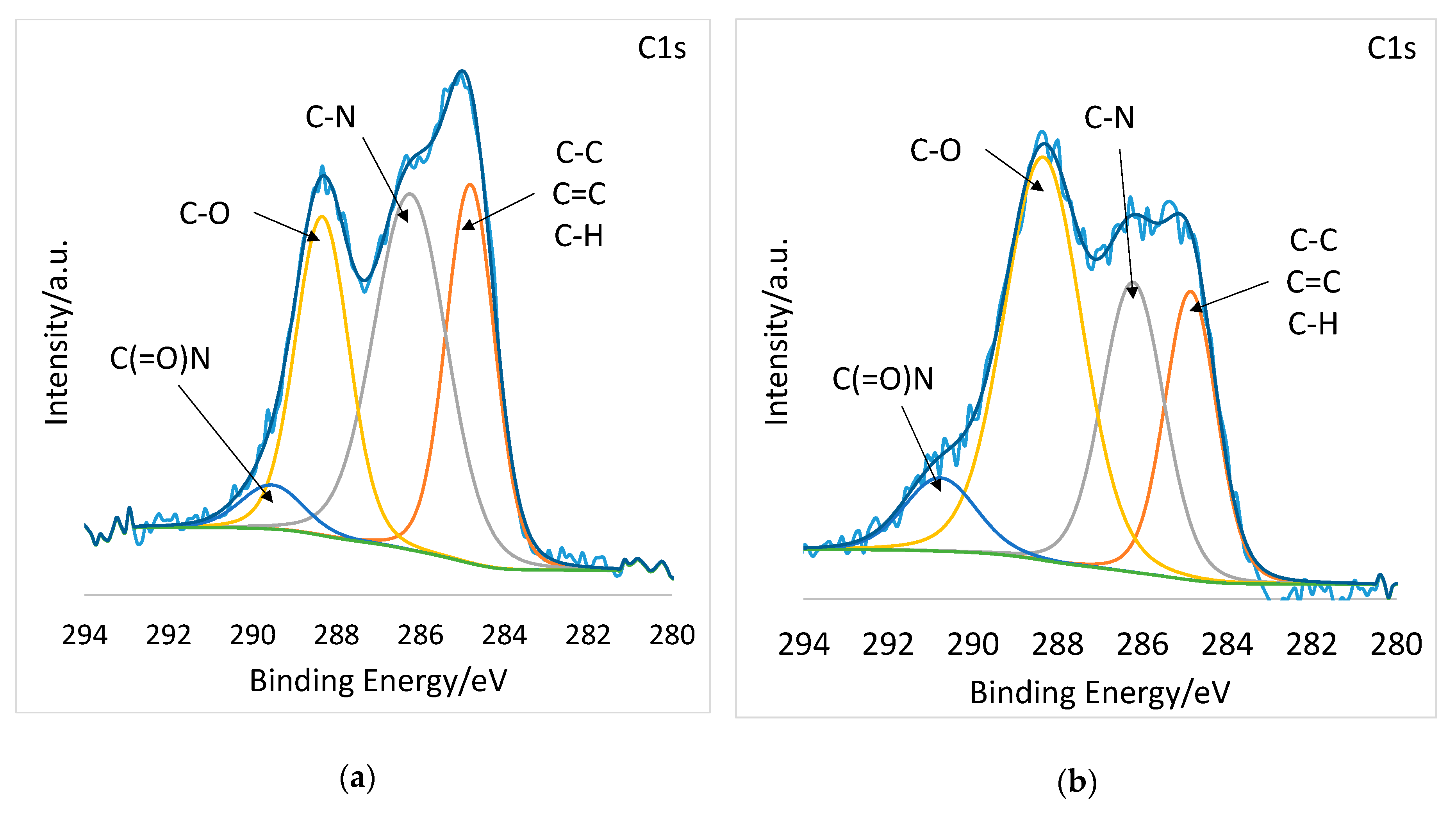
2.6. X-ray Photoelectron Spectroscopy (XPS)
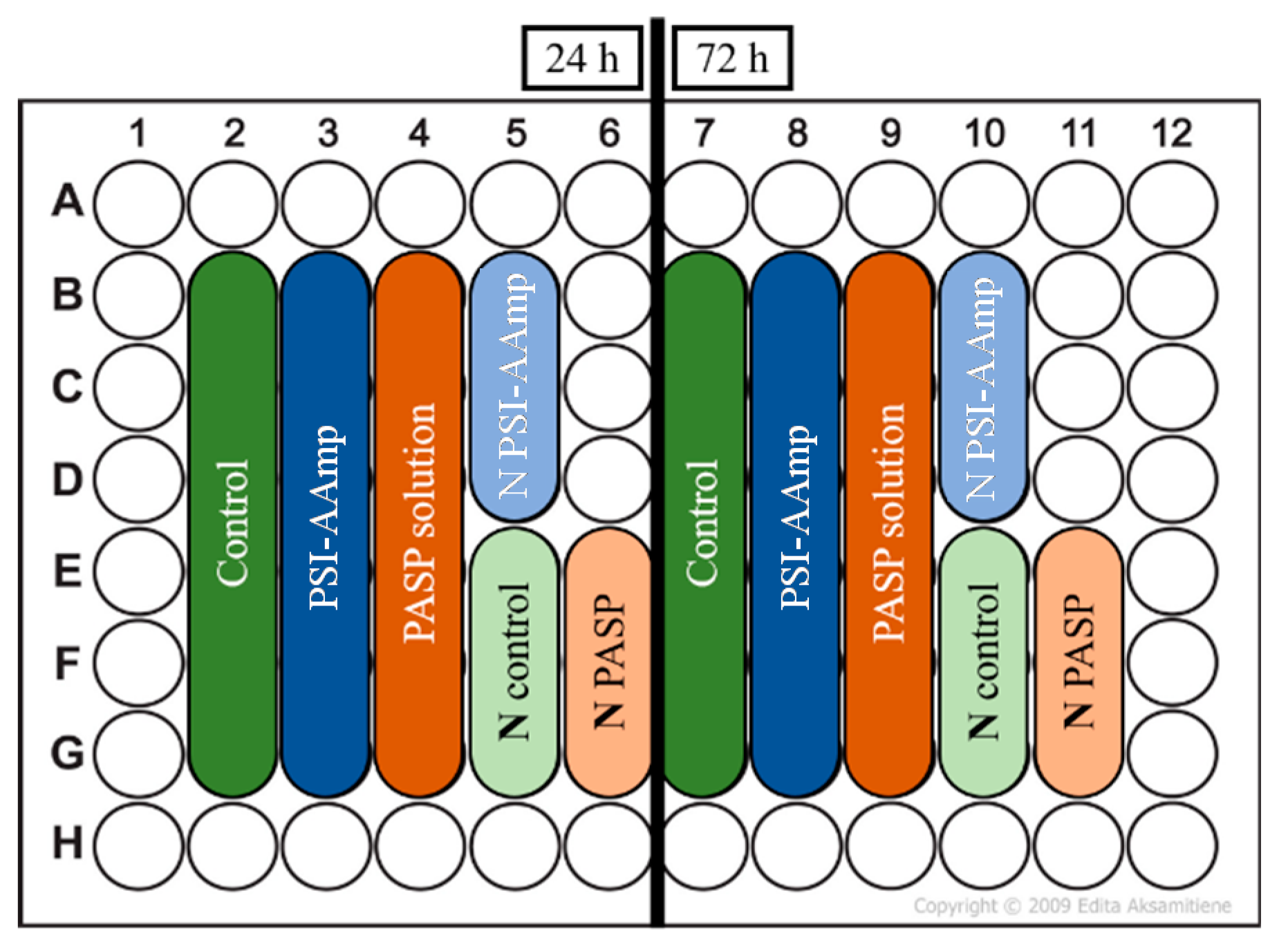
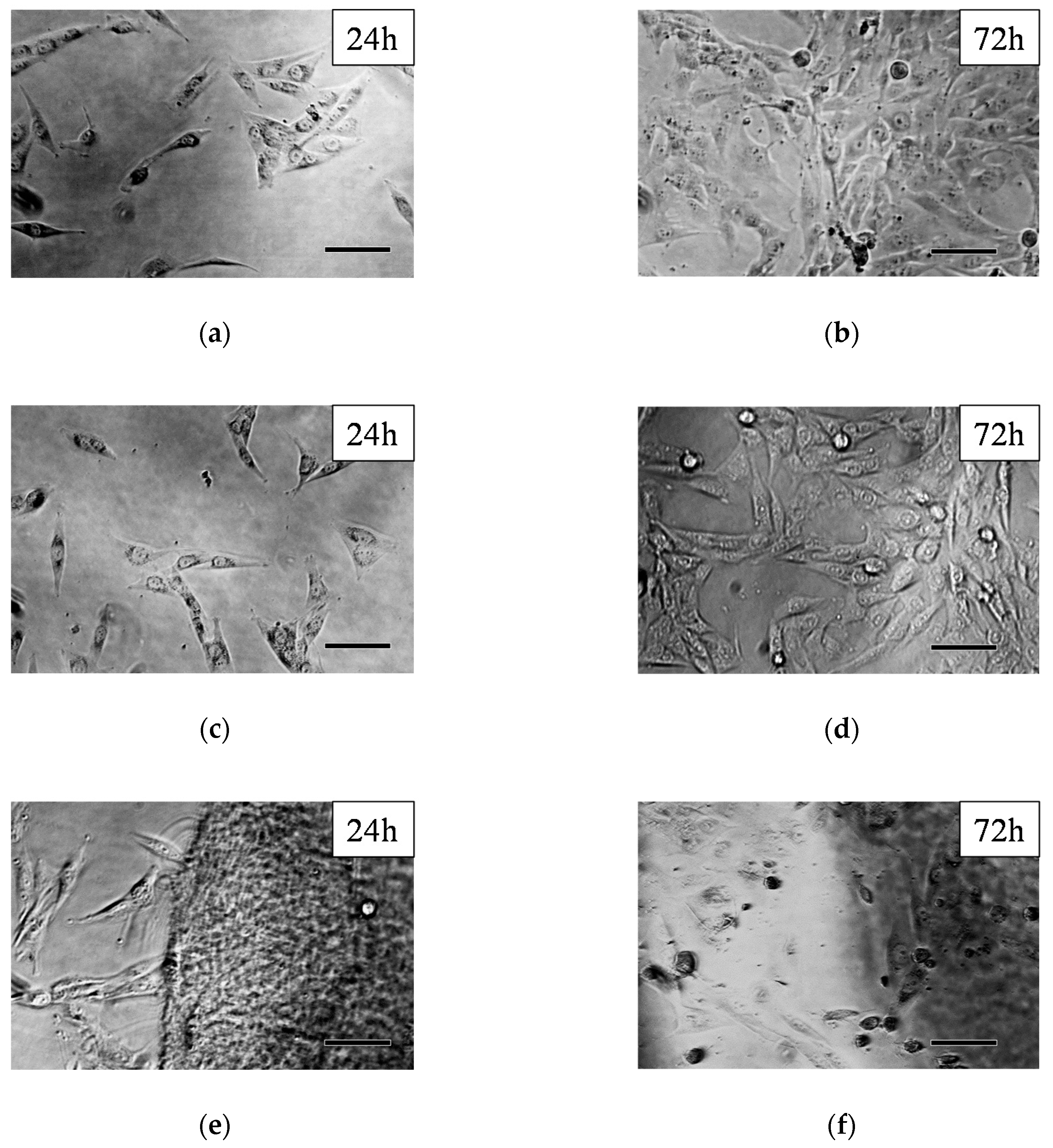
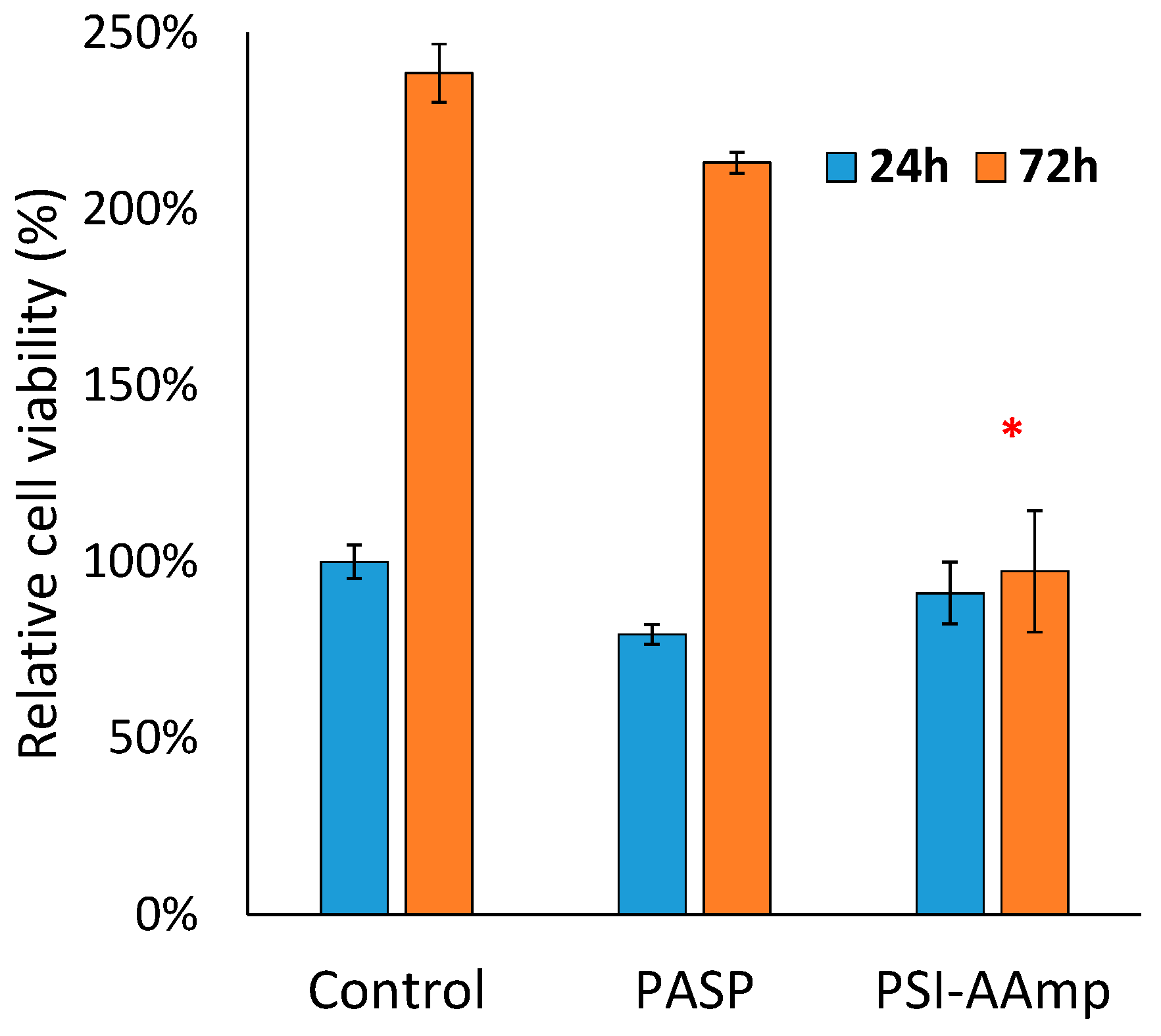
2.7. Cell Viability Assay
3. Results and Discussion
3.1. Electrospinning of PSI-AA and Crosslinking via Plasma Treatment
3.2. Cell Morphology and Viability Study
4. Conclusions
Author Contributions
Funding
 This work was also funded by The Ohio State University and the European Union’s Horizon 2020 Research And Innovation Program under the Marie Skłodowska-Curie grant agreement (No. 872152) (project GREEN-MAP). Additional support was received from the Hungarian Human Resources Development Operational Program (EFOP-3.6.2-16-2017-00006) and from the Higher Education Institutional Excellence Programme of the Ministry for Innovation and Technology in Hungary, within the framework of the Therapeutic Development Thematic Programme of the Semmelweis University.
This work was also funded by The Ohio State University and the European Union’s Horizon 2020 Research And Innovation Program under the Marie Skłodowska-Curie grant agreement (No. 872152) (project GREEN-MAP). Additional support was received from the Hungarian Human Resources Development Operational Program (EFOP-3.6.2-16-2017-00006) and from the Higher Education Institutional Excellence Programme of the Ministry for Innovation and Technology in Hungary, within the framework of the Therapeutic Development Thematic Programme of the Semmelweis University.Acknowledgments
Conflicts of Interest
References
- Tang, Z.; He, C.; Tian, H.; Ding, J.; Hsiao, B.S.; Chu, B.; Chen, X. Polymeric nanostructured materials for biomedical applications. Prog. Polym. Sci. 2016, 60, 86–128. [Google Scholar] [CrossRef]
- Annabi, N.; Tamayol, A.; Uquillas, J.A.; Akbari, M.; Bertassoni, L.E.; Cha, C.; Camci-Unal, G.; Dokmeci, M.R.; Peppas, N.A.; Khademhosseini, A. 25th Anniversary Article: Rational Design and Applications of Hydrogels in Regenerative Medicine. Adv. Mater. 2014, 26, 85–124. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Kowalczuk, M. Intrinsically Biocompatible Polymer Systems. Polymers 2020, 12, 272. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.-F.; Shakir, I.; Hussain, R. Electrospun fibers for tissue engineering, drug delivery, and wound dressing. J. Mater. Sci. 2013, 48, 3027–3054. [Google Scholar] [CrossRef]
- Ghobril, C.; Grinstaff, M.W. The chemistry and engineering of polymeric hydrogel adhesives for wound closure: A tutorial. Chem. Soc. Rev. 2015, 44, 1820–1835. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.-L.; Gong, R.-H. Manufacturing technologies of polymeric nanofibres and nanofibre yarns. Polym. Int. 2008, 57, 837–845. [Google Scholar] [CrossRef]
- Nayak, R.; Padhye, R.; Kyratzis, I.L.; Truong, Y.B.; Arnold, L. Recent advances in nanofibre fabrication techniques. Text. Res. J. 2012, 82, 129–147. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, J.; Yu, G.; Cardenas, R.; Wei, S.; Wujcik, E.K.; Guo, Z. Coaxial electrospun fibers: Applications in drug delivery and tissue engineering. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 654–677. [Google Scholar] [CrossRef]
- Tamayol, A.; Akbari, M.; Annabi, N.; Paul, A.; Khademhosseini, A.; Juncker, D. Fiber-based tissue engineering: Progress, challenges, and opportunities. Biotechnol. Adv. 2013, 31, 669–687. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.R.; Baptista-Silva, S.; de Oliveira, C.M.T.; Sousa, A.; Oliveira, A.L.; Bártolo, P.J.; Granja, P.L. In situ crosslinked electrospun gelatin nanofibers for skin regeneration. Eur. Polym. J. 2017, 95, 161–173. [Google Scholar] [CrossRef]
- Peng, Y.Y.; Glattauer, V.; Ramshaw, J.A.M. Stabilisation of Collagen Sponges by Glutaraldehyde Vapour Crosslinking. Int. J. Biomater. 2017, 2017, 8947823. [Google Scholar] [CrossRef] [PubMed]
- Campiglio, C.E.; Contessi Negrini, N.; Farè, S.; Draghi, L. Cross-Linking Strategies for Electrospun Gelatin Scaffolds. Materials 2019, 12, 2476. [Google Scholar] [CrossRef]
- Roweton, S.; Huang, S.J.; Swift, G. Poly(aspartic acid): Synthesis, biodegradation, and current applications. J. Environ. Polym. Degrad. 1997, 5, 175–181. [Google Scholar] [CrossRef]
- Molnar, K.; Juriga, D.; Nagy, P.M.; Sinko, K.; Jedlovszky-Hajdu, A.; Zrinyi, M. Electrospun poly(aspartic acid) gel scaffolds for artificial extracellular matrix. Polym. Int. 2014, 63, 1608–1615. [Google Scholar] [CrossRef]
- Krisch, E.; Messager, L.; Gyarmati, B.; Ravaine, V.; Szilágyi, A. Redox- and pH-Responsive Nanogels Based on Thiolated Poly(aspartic acid). Macromol. Mater. Eng. 2016, 301, 260–266. [Google Scholar] [CrossRef]
- Salakhieva, D.; Shevchenko, V.; Németh, C.; Gyarmati, B.; Szilágyi, A.; Abdullin, T. Structure–biocompatibility and transfection activity relationships of cationic polyaspartamides with (dialkylamino)alkyl and alkyl or hydroxyalkyl side groups. Int. J. Pharm. 2017, 517, 234–246. [Google Scholar] [CrossRef]
- Németh, C.; Szabó, D.; Gyarmati, B.; Gerasimov, A.; Varfolomeev, M.; Abdullin, T.; László, K.; Szilágyi, A. Effect of side groups on the properties of cationic polyaspartamides. Eur. Polym. J. 2017, 93, 805–814. [Google Scholar] [CrossRef]
- Huynh, N.; Jeon, Y.; Kim, D.; Kim, J. Preparation and swelling properties of “click” hydrogel from polyaspartamide derivatives using tri-arm PEG and PEG-co-poly(amino urethane) azides as crosslinking agents. Polymer 2013, 54, 1341–1349. [Google Scholar] [CrossRef]
- Sharma, S.; Dua, A.; Malik, A. Polyaspartic acid based superabsorbent polymers. Eur. Polym. J. 2014, 59, 363–376. [Google Scholar] [CrossRef]
- Adelnia, H.; Blakey, I.; Little, P.J.; Ta, H.T. Hydrogels Based on Poly(aspartic acid): Synthesis and Applications. Front. Chem. 2019, 7, 755. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, S.; Qin, X. Facile fabrication of novel pH-sensitive poly(aspartic acid) hydrogel by crosslinking nanofibers. Mater. Lett. 2014, 132, 393–396. [Google Scholar] [CrossRef]
- Molnar, K.; Jedlovszky-Hajdu, A.; Zrinyi, M.; Jiang, S.; Agarwal, S. Poly(amino acid)-Based Gel Fibers with pH Responsivity by Coaxial Reactive Electrospinning. Macromol. Rapid Commun. 2017, 38, 1700147. [Google Scholar] [CrossRef]
- Juriga, D.D.D.D.; Nagy, K.S.; Jedlovszky-Hajdú, A.; Perczel-Kovách, K.; Chen, Y.M.; Varga, G.G.G.; Zrínyi, M.; Jedlovszky-Hajdu, A.; Perczel-Kovach, K.; Chen, Y.M.; et al. Biodegradation and osteosarcoma cell cultivation on poly(aspartic acid) based hydrogels. ACS Appl. Mater. Interfaces 2016, 8, 23463–23476. [Google Scholar] [CrossRef]
- Molnar, K.; Jozsa, B.; Barczikai, D.; Krisch, E.; Puskas, J.E.; Jedlovszky-Hajdu, A. Plasma treatment as an effective tool for crosslinking of electrospun fibers. J. Mol. Liq. 2020, 303, 112628. [Google Scholar] [CrossRef]
- Gyarmati, B.; Mészár, E.Z.; Kiss, L.; Deli, M.A.; László, K.; Szilágyi, A. Supermacroporous chemically cross-linked poly(aspartic acid) hydrogels. Acta Biomater. 2015, 22, 32–38. [Google Scholar] [CrossRef]
- Németh, C.; Gyarmati, B.; Abdullin, T.; László, K.; Szilágyi, A. Poly(aspartic acid) with adjustable pH-dependent solubility. Acta Biomater. 2017, 49, 486–494. [Google Scholar] [CrossRef]
- Noszticzius, Z.; Wittmann, M.; Kály-Kullai, K.; Beregvári, Z.; Kiss, I.; Rosivall, L.; Szegedi, J. Chlorine dioxide is a size-selective antimicrobial agent. PLoS ONE 2013, 8, e79157. [Google Scholar] [CrossRef]
- Kim, M.; Shin, S.W.; Lim, C.W.; Kim, J.; Um, S.H.; Kim, D. Polyaspartamide-based graft copolymers encapsulating iron oxide nanoparticles for imaging and fluorescence labelling of immune cells. Biomater. Sci. 2017, 5, 305–312. [Google Scholar] [CrossRef]
- Shinoda, H.; Asou, Y.; Suetsugu, A.; Tanaka, K. Synthesis and Characterization of Amphiphilic Biodegradable Copolymer, Poly(aspartic acid-co-lactic acid). Macromol. Biosci. 2003, 3, 34–43. [Google Scholar] [CrossRef]
- Yalcinkaya, F. Effect of argon plasma treatment on hydrophilic stability of nanofiber webs. J. Appl. Polym. Sci. 2018, 135, 46751. [Google Scholar] [CrossRef]
- Peng, M.; Li, L.; Xiong, J.; Hua, K.; Wang, S.; Shao, T. Study on Surface Properties of Polyamide 66 Using. Coatings 2017, 7, 123. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, W.; Luo, Y.; Li, J. Poly(aspartic acid) surface modification of macroporous poly(glycidyl methacrylate) microspheres. J. Appl. Polym. Sci. 2019, 136, 47441. [Google Scholar] [CrossRef]
- Chai, C.; Xu, Y.Y.; Shi, S.; Zhao, X.; Wu, Y.; Xu, Y.Y.; Zhang, L. Functional polyaspartic acid derivatives as eco-friendly corrosion inhibitors for mild steel in 0.5 M H2SO4 solution. RSC Adv. 2018, 8, 24970–24981. [Google Scholar] [CrossRef]
- Hirsch, E.; Nacsa, M.; Ender, F.; Mohai, M.; Nagy, Z.K.; Marosi, G.J. Preparation and Characterization of Biocompatible Electrospun Nanofiber Scaffolds. Period. Polytech. Chem. Eng. 2018, 62, 510–518. [Google Scholar] [CrossRef]
- Ruijtenberg, S.; van den Heuvel, S. Coordinating cell proliferation and differentiation: Antagonism between cell cycle regulators and cell type-specific gene expression. Cell Cycle 2016, 15, 196–212. [Google Scholar] [CrossRef]
- Soufi, A.; Dalton, S. Cycling through developmental decisions: How cell cycle dynamics control pluripotency, differentiation and reprogramming. Development 2016, 143, 4301–4311. [Google Scholar] [CrossRef]
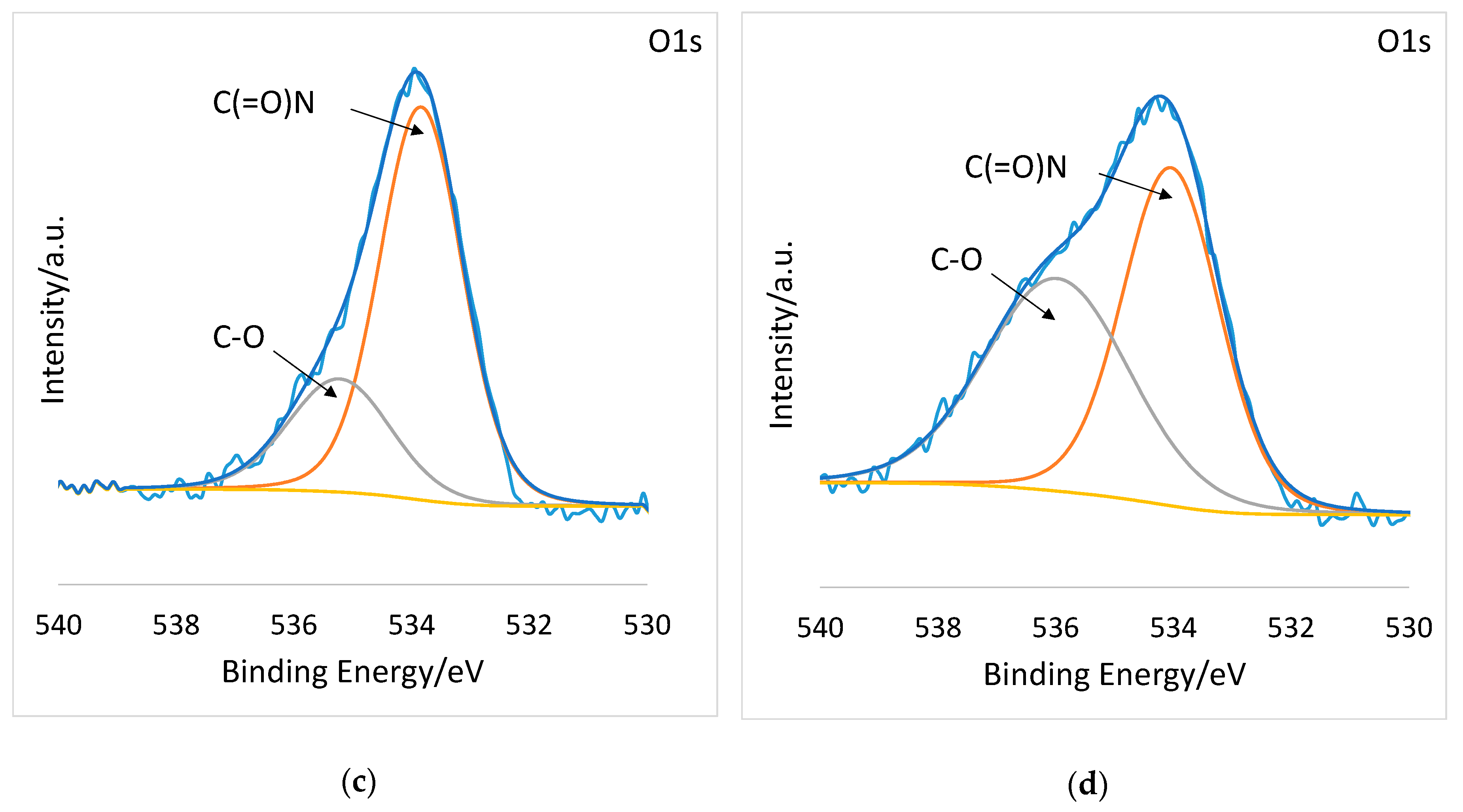
| Atoms | Theoretical Composition At % | PSI-AAm At % | PSI-AAmp At % |
|---|---|---|---|
| C | 61.11 | 66.41 | 63.10 |
| N | 16.67 | 14.56 | 14.83 |
| O | 22.22 | 19.03 | 22.07 |
| Bonds | Binding Energy (eV) | PSI-AAm At % | PSI-AAmp At % |
|---|---|---|---|
| C1s | |||
| C–C, C=C, C–H | 285 | 29.64 | 20.05 |
| C–N | 286 | 39.30 | 26.54 |
| C–O | 288 | 24.27 | 45.29 |
| C(=O)N | 290 | 6.79 | 8.12 |
| O1s | |||
| C(=O)N | 530 | 71.55 | 54.31 |
| C–O | 532 | 28.45 | 45.69 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molnar, K.; Varga, R.; Jozsa, B.; Barczikai, D.; Krisch, E.; Nagy, K.S.; Varga, G.; Jedlovszky-Hajdu, A.; Puskas, J.E. Investigation of the Cytotoxicity of Electrospun Polysuccinimide-Based Fiber Mats. Polymers 2020, 12, 2324. https://doi.org/10.3390/polym12102324
Molnar K, Varga R, Jozsa B, Barczikai D, Krisch E, Nagy KS, Varga G, Jedlovszky-Hajdu A, Puskas JE. Investigation of the Cytotoxicity of Electrospun Polysuccinimide-Based Fiber Mats. Polymers. 2020; 12(10):2324. https://doi.org/10.3390/polym12102324
Chicago/Turabian StyleMolnar, Kristof, Rita Varga, Benjamin Jozsa, Dora Barczikai, Eniko Krisch, Krisztina S. Nagy, Gabor Varga, Angela Jedlovszky-Hajdu, and Judit E. Puskas. 2020. "Investigation of the Cytotoxicity of Electrospun Polysuccinimide-Based Fiber Mats" Polymers 12, no. 10: 2324. https://doi.org/10.3390/polym12102324
APA StyleMolnar, K., Varga, R., Jozsa, B., Barczikai, D., Krisch, E., Nagy, K. S., Varga, G., Jedlovszky-Hajdu, A., & Puskas, J. E. (2020). Investigation of the Cytotoxicity of Electrospun Polysuccinimide-Based Fiber Mats. Polymers, 12(10), 2324. https://doi.org/10.3390/polym12102324






