Polymer-Based Materials Loaded with Curcumin for Wound Healing Applications
Abstract
1. Introduction
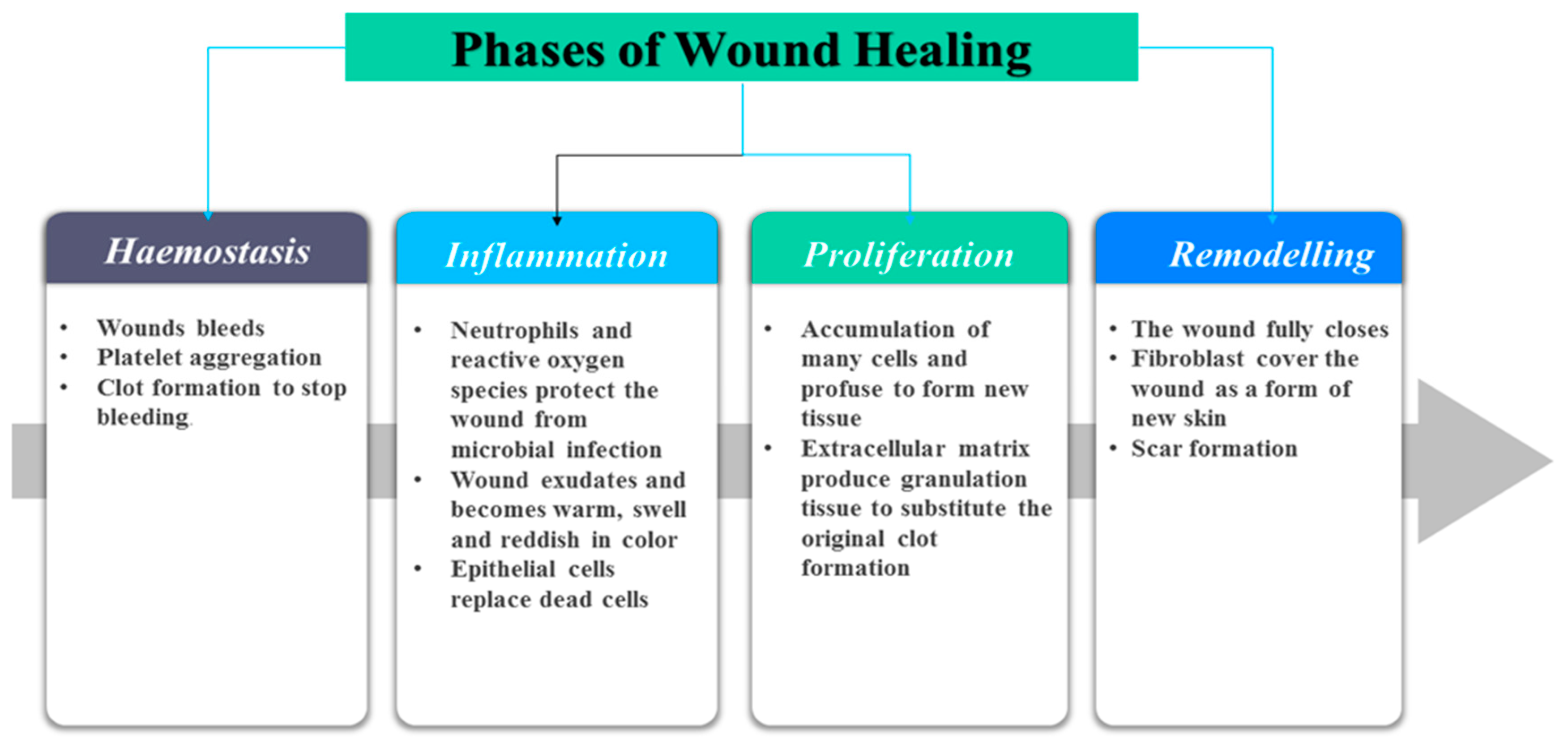
2. Classification of Wounds and Phases of Wound Healing Process
3. Classification of Wound Dressings
4. Biological Activity of Curcumin in Wound Healing
5. Curcumin-Loaded Wound Dressings
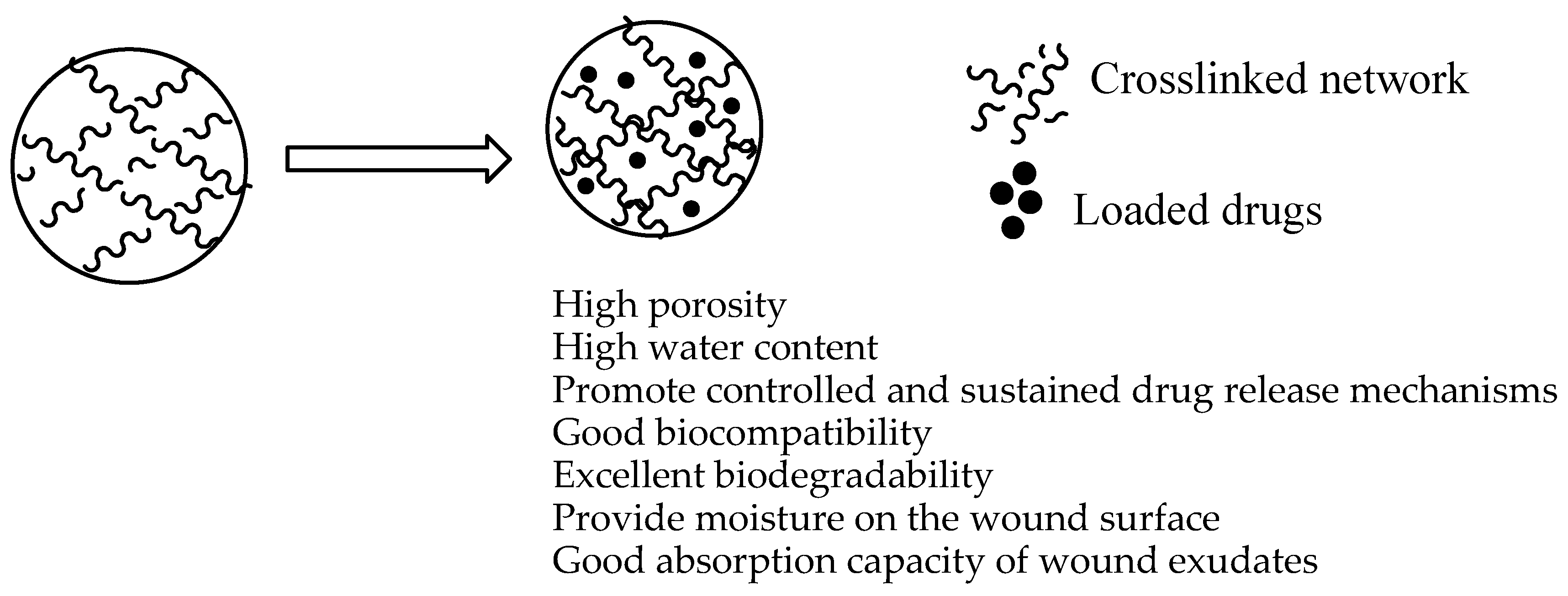
5.1. Hydrogels
5.2. Films/Membranes
5.3. Sponges/Bandages
5.4. Nanofibers
5.5. Other Wound Dressing Materials
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Malini, R.I.; Lesage, J.; Toncelli, C.; Fortunato, G.; Rossi, R.M.; Spano, F. Crosslinking dextran electrospun nanofibers via borate chemistry: Proof of concept for wound patches. Eur. Polym. J. 2019, 110, 276–282. [Google Scholar] [CrossRef]
- Zhou, S.; Hokugo, A.; Mcclendon, M.; Zhang, Z.; Bakshi, R.; Wang, L.; Segovia, L.A.; Rezzadeh, K.; Stupp, S.I.; Jarrahy, R. Bioactive peptide amphiphile nanofiber gels enhance burn wound healing. Burns 2019, 45, 1112–1121. [Google Scholar] [CrossRef]
- Abou-Okel, A.; Fahmy, H.M.; El-Bisi, M.; Ahmed-Farid, O. Hyaluronic acid/Na-alginate films as topical bioactive wound dressings. Eur. Polym. J. 2018, 109, 101–109. [Google Scholar] [CrossRef]
- Lee, S.J.; Heo, D.N.; Moon, J.; Ko, W.; Lee, J.B.; Bae, M.S.; Park, S.W.; Kim, J.E.; Lee, D.H.; Kim, E.; et al. Electrospun chitosan nanofibers with controlled levels of silver nanoparticles. Preparation, characterization and antibacterial activity. Carbohydr. Polym. 2014, 111, 530–537. [Google Scholar] [CrossRef]
- Liu, R.; Dai, L.; Si, C.; Zeng, Z. Antibacterial and hemostatic hydrogel via nanocomposite from cellulose nanofibers. Carbohydr. Polym. 2018, 195, 63–70. [Google Scholar] [CrossRef]
- Anisha, B.S.; Sankar, D.; Mohandas, A.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. Chitosan—Hyaluronan/nano chondroitin sulfate ternary composite sponges for medical use. Carbohydr. Polym. 2013, 92, 1470–1476. [Google Scholar] [CrossRef]
- Li, S.; Li, L.; Guo, C.; Qin, H.; Yu, X. A promising wound dressing material with excellent cytocompatibility and proangiogenesis action for wound healing: Strontium loaded Silk fibroin/Sodium alginate (SF/SA) blend films. Int. J. Biol. Macromol. 2017, 104, 969–978. [Google Scholar] [CrossRef]
- Ye, S.; Jiang, L.; Su, C.; Zhu, Z.; Wen, Y.; Shao, W. Development of gelatin/bacterial cellulose composite sponges as potential natural wound dressings. Int. J. Biol. Macromol. 2019, 133, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Miguel, S.P.; Moreira, A.F.; Correia, I.J. Chitosan based-asymmetric membranes for wound healing: A review. Int. J. Biol. Macromol. 2019, 127, 460–475. [Google Scholar] [CrossRef] [PubMed]
- Aderibigbe, B.A.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Aycan, D.; Selmi, B.; Kelel, E.; Yildirim, T.; Alemdar, N. Conductive polymeric fi lm loaded with ibuprofen as a wound dressing material. Eur. Polym. J. 2019, 121, 109308. [Google Scholar] [CrossRef]
- Türe, H. Characterization of hydroxyapatite-containing alginate—Gelatin composite films as a potential wound dressing. Int. J. Biol. Macromol. 2019, 123, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Kaygusuz, H.; Torlak, E.; Akim-Evingur, G.; Ozen, I.; von Kitzing, R.; Erim, F.B. Antimicrobial cerium ion-chitosan crosslinked alginate biopolymer films: A novel and potential wound dressing. Int. J. Biol. Macromol. 2017, 105, 1161–1165. [Google Scholar] [CrossRef] [PubMed]
- Hubner, P.; Donati, N.; Kelin, L.; Quines, D.M.; Tessaro, I.C.; Marcilio, N.R. Gelatin-based films containing clinoptilolite-Ag for application as wound dressing. Mater. Sci. Eng. C 2020, 107, 110215. [Google Scholar] [CrossRef] [PubMed]
- Poonguzhali, R.; Basha, S.K.; Kumari, V.S. Synthesis and characterization of chitosan-PVP-nanocellulose composites for in-vitro wound dressing application. Int. J. Biol. Macromol. 2017, 105, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Bayat, S.; Amiri, N.; Pishavar, E.; Kalalinia, F.; Movaffagh, J.; Hashemi, M. Bromelain-loaded chitosan nanofibers prepared by electrospinning method for burn wound healing in animal models. Life Sci. 2019, 229, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zhu, X.; Wang, N.; Zhang, X.; Yang, D.; Nie, J.; Ma, G. Biodegradable core-shell electrospun nanofibers based on PLA and γ-PGA for wound healing. Eur. Polym. J. 2019, 116, 30–37. [Google Scholar] [CrossRef]
- Shi, R.; Geng, H.; Gong, M.; Ye, J.; Wu, C.; Hu, X.; Zhang, L. Long-acting broad-spectrum antimicrobial electrospun poly (e-caprolactone)/gelatin micro/nanofibers for wound dressing. J. Colloid Interface Sci. 2018, 509, 275–284. [Google Scholar] [CrossRef]
- Xia, J.; Wang, H.; Zhang, Q.-M.; Zheng, Z.; Han, Z.-M. The therapeutic effect of curcumin in male albino rats and its putative mechanisms on cerebral microvascular flow. Brain Res. 2016, 1642, 131–135. [Google Scholar] [CrossRef]
- Kerdsakunde, N.; Wiwattanapatapee, R.; Mahattanadul, S. Floating gellan gum-based in situ gels containing curcumin for specific delivery to the stomach. Thai J. Pharm. Sci. (TJPS) 2016, 40, 33–36. [Google Scholar]
- Pecora, T.M.G.; Cianciolo, S.; Guidi, G.D.; Ruozi, B.; Chiara, M.; Paolino, D.; Graziano, A.C.E.; Fresta, M.; Pignatello, R. Preparation, characterization and photostability assessment of curcumin microencapsulated within methacrylic copolymers. J. Drug Deliv. Sci. Technol. 2016, 33, 88–97. [Google Scholar] [CrossRef]
- Yadav, S.K.; Sah, A.K.; Jha, R.K.; Sah, P.; Shah, D.K. Turmeric (curcumin) remedies gastroprotective action. Pharmacogn. Rev. 2013, 7, 42–46. [Google Scholar]
- Ghalandarlaki, N.; Alizadeh, A.M.; Ashkani-esfahani, S. Nanotechnology-Applied Curcumin for Different Diseases Therapy. J. BioMed Res. Int. 2014, 2014, 1–23. [Google Scholar] [CrossRef]
- Velasques, K.; Maciel, T.R.; Forno, H.A.H.C.D.; Teixeira, F.E.G.; Fonseca, A.L.F.; Varotti, F.P.; Fajaro, A.R.; Avila, D.S.; Haas, S.E. Co-nanoencapsulation of antimalarial drugs increases their in vitro efficacy against Plasmodium falciparum and decreases their toxicity to Caenorhabditis elegans. Eur. J. Pharm. Sci. 2018, 118, 1–12. [Google Scholar] [CrossRef]
- Aditya, N.P.; Chimote, G.; Gunalan, K.; Banerjee, R.; Patankar, S.; Madhusudhan, B. Curcuminoids-loaded liposomes in combination with arteether protects against Plasmodium berghei infection in mice. Exp. Parasitol. 2012, 131, 292–299. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Shajahan, A.; Kalaichelvan, P.T.; Kaviyarasan, V. Fungal chitosan based nanocomposites sponges—An alternative medicine for wound dressing. Int. J. Biol. Macromol. 2017, 104, 1905–1915. [Google Scholar] [CrossRef]
- Schreml, S.; Szeimies, R.; Prantl, L.; Karrer, S.; Landthaler, M.; Babilas, P. Oxygen in acute and chronic wound healing. Braz. J. Dermatol. 2010, 163, 257–268. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharmacother. 2019, 112, 108615. [Google Scholar] [CrossRef]
- Fredric, S.; Gowda, D.V.; Yashashwini, M. Wafers for wound healing. J. Chem. Pharm. Res. 2015, 7, 450–468. [Google Scholar]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. Biomedicine 2015, 5, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Rivera, E.A.; Spencer, J.M. Clinical aspects of full-thickness wound healing. Clin. Dermatol. 2007, 25, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, P.; Rezaeian, I.; Ranaei-Siadat, S.O.; Jafari, S.H.; Supaphol, P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Adv. Technol. 2010, 21, 77–95. [Google Scholar] [CrossRef]
- Zuliani-Alvarez, L.; Midwood, K.S. Fibrinogen-related proteins in tissue repair: How a unique domain with a common structure controls diverse aspects of wound healing. Adv. Wound Care 2015, 4, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Abousamra, M.M. Nanoparticles as Safe and Effective Drug Delivery Systems for Wound Healing. Austin J. Nanomed. Nanotechnol. 2019, 7, 1056. [Google Scholar]
- Frykberg, R.G.; Banks, J. Challenges in the treatment of chronic wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef]
- Gainza, G.; Villullas, S.; Pedraz, J.; Hernandez, R.; Igartua, M. Advances in drug delivery systems (DDSs) to release growth factors for wound healing and skin regeneration. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1551–1573. [Google Scholar] [CrossRef]
- Mihai, M.M.; Dima, M.B.; Dima, B.; Holban, A.M. Nanomaterials forWound Healing and Infection Control. Materials 2019, 12, 2176. [Google Scholar] [CrossRef]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Martin, P.; Nunan, R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br. J. Dermatol. 2015, 173, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, N.K.; Sundar, S.; Kumar, S.S.D.; Houreld, N.N.; Abrahamse, H. A review on nanoparticle based treatment for wound healing. J. Drug Deliv. Sci. Technol. 2018, 44, 421–430. [Google Scholar] [CrossRef]
- Wang, P.; Huang, B.; Horng, H.; Yeh, C.; Chen, Y.-J. Wound healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lu, K.; Yu, C.; Huang, Q.-L.; Du, Y.Z. Nano-drug delivery systems in wound treatment and skin regeneration. J. Nanobiotech. 2019, 17, 82. [Google Scholar] [CrossRef] [PubMed]
- Horn, T. Wound dressings. Overview and classification. Unfallchirurg 2012, 115, 774–782. [Google Scholar] [CrossRef]
- Kenawy, E.; Omer, A.M.; Tamer, T.M.; Elmeligy, M.A.; Eldin, M.S.M. Fabrication of biodegradable gelatin/chitosan/cinnamaldehyde crosslinked membranes for antibacterial wound dressing applications. Int. J. Biol. Macromol. 2019, 139, 440–448. [Google Scholar] [CrossRef]
- Contardi, M.; Heredia-guerrero, J.A.; Perotto, G.; Valentini, P.; Paolo, P.; Goldoni, L.; Bertorelli, R.; Athanassiou, A.; Bayer, I.S. Transparent ciprofloxacin-povidone antibiotic films and nanofiber mats as potential skin and wound care dressings. Eur. J. Pharm. Sci. 2017, 104, 133–144. [Google Scholar] [CrossRef]
- Hansson, C. Interactive wound dressings. A practical guide to their use in older patients. Drugs Aging 1997, 11, 271–284. [Google Scholar] [CrossRef]
- Hussain, Z.; Thu, H.E.; Shuid, A.N.; Katas, H.; Hussain, F. Recent Advances in Polymer-based Wound Dressings for the Treatment of Diabetic Foot Ulcer: An Overview of State-of-the-art. Curr. Drug Targets 2017, 19, 527–550. [Google Scholar] [CrossRef]
- Oro, F.B.; Sikka, R.S.; Wolters, B.; Graver, R.; Boyd, J.L.; Nelson, B.; Swiontkowski, M.F. Autograft versus allograft: An economic cost comparison of anterior cruciate ligament reconstruction. Arthroscopy 2011, 27, 1219–1225. [Google Scholar] [CrossRef]
- Liu, L.; Sun, L.; Wu, Q.; Guo, W.; Li, L.; Chen, Y.; Li, Y.; Gong, C.; Qian, Z.; Wei, Y. Curcumin loaded polymeric micelles inhibit breast tumor growth and spontaneous pulmonary metastasis. Int. J. Pharm. 2013, 443, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.; Shivakumar, H.G. A review on curcumin: Wound healing properties and biomarkers of wound healing. Int. Res. J. Pharm. 2018, 9, 1–5. [Google Scholar] [CrossRef]
- Meng, B.; Li, J.; Cao, H. Antioxidant and Antiinflammatory Activities of Curcumin on Diabetes Mellitus and its Complications. Curr. Pharm. Des. 2013, 19, 2101–2113. [Google Scholar] [PubMed]
- Akbik, D.; Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Curcumin as a wound healing agent. Life Sci. 2014, 116, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, C.; Sahoo, S.K. Curcumin and its topical formulations for wound healing applications. Drug Discov. Today 2017, 22, 1582–1592. [Google Scholar] [CrossRef]
- Menon, V.; Sudheer, A. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar]
- Mohanty, C.; Sahoo, S. The in vitro stability and in vivo pharmacokinetics of curcumin prepared as an aqueous nanoparticulate formulation. Biomaterials 2010, 31, 6597–6611. [Google Scholar] [CrossRef]
- Alven, S.; Aderibigbe, B.A. Combination Therapy Strategies for the Treatment. Molecules 2019, 24, 3601. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, A.; Sharma, A.; Dhiman, A. Design of Antibiotic Drug Loaded Carbopol-Crosslinked-Poly (2-Hydroxyethylmethacrylate) Hydrogel for Wound Dressing Applications. Am. J. Drug Deliv. Ther. 2017, 4, 1. [Google Scholar]
- Zafalon, A.T.; dos Santos, V.J.; Esposito, F.; Lincopan, N.; Rangari, V.; Lugão, A.B.; Parra, D.F. Synthesis of polymeric hydrogel loaded with antibiotic drug for wound healing applications. Charact. Miner. Met. Mater. 2018, 2018, 165–176. [Google Scholar] [CrossRef]
- Shefa, A.A.; Sultana, T.; Park, M.K.; Lee, S.Y.; Gwon, J.; Lee, B. Curcumin incorporation into an oxidized cellulose nanofiber-polyvinyl alcohol hydrogel system promotes wound healing. Mater. Des. 2020, 186, 108313. [Google Scholar] [CrossRef]
- Pham, L.; Dang, L.H.; Truong, M.D.; Nguyen, T.H.; Le, L.; Le, V.T.; Nam, N.D.; Bach, L.G.; Nguyen, V.T.; Tran, N.Q. A dual synergistic of curcumin and gelatin on thermal-responsive hydrogel based on Chitosan-P123 in wound healing application. Biomed. Pharmacother. 2019, 117, 109183. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Liu, M.; Zhou, C. Cellulose—Halloysite nanotube composite hydrogels for curcumin delivery. Cellulose 2017, 24, 2861–2875. [Google Scholar] [CrossRef]
- Anjum, S.; Gupta, A.; Sharma, D.; Gautam, D.; Bhan, S.; Sharma, A.; Kapil, A.; Gupta, B. Development of novel wound care systems based on nanosilver nanohydrogels of polymethacrylic acid with Aloe vera and curcumin. Mater. Sci. Eng. C 2016, 64, 157–166. [Google Scholar] [CrossRef]
- Cirillo, G.; Curcio, M.; Spizzirri, U.G.; Vittorio, O.; Tucci, P.; Picci, N.; Iemma, F.; Hampel, S.; Nicoletta, F.P. Carbon nanotubes hybrid hydrogels for electrically tunable release of Curcumin. Eur. Polym. J. 2017, 90, 1–12. [Google Scholar] [CrossRef]
- Gong, C.; Wu, Q.; Wang, Y.; Zhang, D.; Luo, F.; Zhao, X.; Wei, Y.; Qian, Z. A biodegradable hydrogel system containing curcumin encapsulated in micelles for cutaneous wound healing. Biomaterials 2013, 34, 6377–6387. [Google Scholar] [CrossRef] [PubMed]
- Alibolandi, M.; Mohammadi, M.; Mohammad, S.; Abnous, K.; Ramezani, M. Synthesis and preparation of biodegradable hybrid dextran hydrogel incorporated with biodegradable curcumin nanomicelles for full thickness wound healing. Int. J. Pharm. 2017, 532, 466–477. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Zhang, B.; Li, M.; Diao, K.; Zhang, Z.; Li, J.; Xu, Y.; Wang, X.; Chen, H. In situ injectable nano-composite hydrogel composed of curcumin, N, O-carboxymethyl chitosan and oxidized alginate for wound healing application. Int. J. Pharm. 2012, 437, 110–119. [Google Scholar] [CrossRef]
- Gupta, A.; Keddie, D.J.; Kannappan, V.; Gibson, H.; Khalil, I.R.; Kowalczuk, M.; Martin, C.; Shuai, X.; Radecka, I. Production and characterisation of bacterial cellulose hydrogels loaded with curcumin encapsulated in cyclodextrins as wound dressings. Eur. Polym. J. 2019, 118, 437–450. [Google Scholar] [CrossRef]
- Du, L.; Feng, X.; Xiang, X.; Jin, Y. Wound Healing Effect of an in situ Forming Hydrogel Loading Curcumin-Phospholipid Complex. Curr. Drug Deliv. 2016, 13, 76–82. [Google Scholar] [CrossRef]
- Kumar, S.S.; Abdel-kader, D.H.; Rashed, L.A. Beneficial effect of Curcumin Nanoparticles-Hydrogel on excisional skin wound healing in type-I diabetic rat: Histological and immunohistochemical studies. Ann. Anat. 2019, 222, 94–102. [Google Scholar] [CrossRef]
- Ravindra, S.; Mula-Bafubiandi, A.F.; Rajinkanth, V.; Varaprasad, K.; Reddy, N.N.; Raju, K.M. Development and Characterization of Curcumin Loaded Silver Nanoparticle Hydrogels for Antibacterial and Drug Delivery Applications. J. Inorg. Organomet. Polym. 2012, 22, 1254–1262. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.-G.; Chen, W.-M.; Yu, A.-C. Efficacy of thermosensitive chitosan/β-glycerophosphate hydrogel loaded with β-cyclodextrin-curcumin for the treatment of cutaneous wound infection in rats. Exp. Ther. Med. 2018, 14, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ye, X.; Qi, J.; Fan, R.; Wu, Y.; Zhou, L.; Tong, A.; Gou, G. EGF and curcumin co-encapsulated nanoparticle / hydrogel system as potent skin regeneration agent. Int. J. Nanomed. 2016, 11, 3993–4009. [Google Scholar]
- Rezvan, G.; Pircheraghi, G.; Reza, B. Curcumin incorporated PVA-borax dual delivery hydrogels as potential wound dressing materials—Correlation between viscoelastic properties and curcumin release rate. J. Appl. Polym. Sci. 2018, 135, 46735. [Google Scholar] [CrossRef]
- Juan, L.; Zhiqiang, C.; Jie, W.; Ruihong, L.; Tingting, L.; Mingyang, C.; Fang, Y.; Yunfang, W. Encapsulation of Curcumin Nanoparticles with MMP9-Responsive and Thermo-Sensitive Hydrogel Improves Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 16315–16326. [Google Scholar]
- Zhang, M.; Zhuang, B.; Du, G.; Han, G.; Jin, Y. Curcumin solid dispersion-loaded in situ hydrogels for local treatment of injured vaginal bacterial infection and improvement of vaginal wound healing. J. Pharm. Pharmacol. 2019, 71, 1044–1054. [Google Scholar] [CrossRef]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef]
- Salguero, Y.; Valenti, L.; Rojas, R.; García, M.C. Ciprofloxacin-intercalated layered double hydroxide-in-hybrid films as composite dressings for controlled antimicrobial topical delivery. Mater. Sci. Eng. C 2020, 111, 110859. [Google Scholar] [CrossRef]
- Gupta, B.; Agarwal, R.; Alam, M. Textile-based smart wound dressings. Indian J. Fibre Text. Res. 2020, 35, 174–184. [Google Scholar]
- Sasikala, L.; Rathinamoorthy, R.; Dhurai, B. Optimization of process conditions for chitosan-manuka honey film as wound contact layer for wound dressings. Wound Med. 2018, 23, 11–21. [Google Scholar] [CrossRef]
- Colobatiu, L.; Gavan, A.; Mocan, A.; Bogdan, C.; Mirel, S.; Tomuta, I. Development of bioactive compounds-loaded chitosan films by using a QbD approach—A novel and potential wound dressing material. React. Funct. Polym. 2019, 138, 46–54. [Google Scholar] [CrossRef]
- Li, X.; Nan, K.; Li, L.; Zhang, Z.; Chen, H. In vivo evaluation of curcumin nanoformulation loaded methoxy poly (ethylene glycol)-graft-chitosan composite film for wound healing application. Carbohydr. Polym. 2012, 88, 84–90. [Google Scholar] [CrossRef]
- Dhurai, B.; Saraswathy, N.; Maheswaran, R.; Sethupathi, P.; Vanitha, P.; Vigneshwaran, S.; Rameshababu, V. Electrospinning of curcumin loaded chitosan/poly (lactic acid) nanofilm and evaluation of its medicinal characteristics. Front. Mater. Sci. 2013, 7, 350–361. [Google Scholar] [CrossRef]
- Khamrai, M.; Lal, S.; Paul, S.; Samanta, S.; Kundu, P.P. Curcumin entrapped gelatin/ionically modified bacterial cellulose based self-healable hydrogel film: An eco-friendly sustainable synthesis method of wound healing patch. Int. J. Biol. Macromol. 2019, 122, 940–953. [Google Scholar] [CrossRef]
- Tong, W.Y.; Abdullah, A.Y.K.; Rozman, N.A.S.; Wahid, M.I.A.B.; Hossain, M.S.; Ring, L.C.; Lazim, Y.; Wen-Nee, T. Antimicrobial wound dressing film utilizing cellulose nanocrystal as drug delivery system for curcumin. Cellulose 2018, 25, 631–638. [Google Scholar] [CrossRef]
- Wathoni, N.; Motoyama, K.; Higashi, T.; Okajima, M.; Kuneko, T.; Arima, H. Enhancement of curcumin wound healing ability by complexation with 2-hydroxypropyl-?-cyclodextrin in sacran hydrogel film. Int. J. Biol. Macromol. 2017, 98, 268–276. [Google Scholar] [CrossRef]
- Manna, P.J.; Mitra, T.; Pramanik, N.; Kavitha, V.; Gnanamani, A.; Kundu, P.P. Potential use of curcumin loaded carboxymethylated guar gum grafted gelatin film for biomedical applications. Int. J. Biol. Macromol. 2015, 75, 437–446. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Ahuja, S.; Chand, N.; Bajpai, M. Nanocellulose dispersed chitosan film with Ag NPs/Curcumin: An in vivo study on Albino Rats for wound dressing. Int. J. Biol. Macromol. 2017, 104, 1012–1019. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Chand, N.; Ahuja, S. Investigation of curcumin release from chitosan/cellulose micro crystals (CMC) antimicrobial films. Int. J. Biol. Macromol. 2015, 79, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Varaprasad, K.; Vimala, K.; Ravindra, S.; Reddy, N.N.; Reddy, G.V.S.; Raju, K.M. Fabrication of silver nanocomposite films impregnated with curcumin for superior antibacterial applications. J. Mater. Sci. Mater. Med. 2011, 22, 1863–1872. [Google Scholar] [CrossRef] [PubMed]
- Naseri-Nosar, M.; Salehi, M.; Farzamfar, S.; Azami, M. The single and synergistic effects of montmorillonite and curcumin-loaded chitosan microparticles incorporated onto poly (lactic acid) electrospun film on. J. Bioact. Compat. Polym. 2018, 33, 239–253. [Google Scholar] [CrossRef]
- Vimala, K.; Mohan, Y.M.; Varaprasad, K.; Narayana, N. Fabrication of Curcumin Encapsulated Chitosan-PVA Silver Nanocomposite Films for Improved Antimicrobial Activity. J. Biomater. Nanobiotechnol. 2011, 2011, 55–64. [Google Scholar] [CrossRef]
- Liu, X.; You, L.; Tarafder, S.; Zou, L.; Fang, Z.; Chen, J.; Lee, C.H.; Zhang, Q. Curcumin-releasing chitosan/aloe membrane for skin regeneration. Chem. Eng. J. 2019, 359, 1111–1119. [Google Scholar] [CrossRef]
- Ponnanikajamideen, M.; Priya, S.D.; Vanaja, M.; Paulkumar, K.; Rajeshkumar, S.; Annadurai, G. In-Vivo Wound Healing Efficiency of Curcumin Loaded on Chitosan Polyvinyl Propylene Nanofilm. Adv. Sci. Eng. Med. 2016, 8, 763–770. [Google Scholar] [CrossRef]
- Reshmi, C.; Suja, P.; Manaf, O.; Sanu, P.; Sujith, A. Nanochitosan enriched poly ԑ-caprolactone electrospun wound dressing membranes: A fine tuning of physicochemical properties, hemocompatibility and curcumin release profile. Int. J. Biol. Macromol. 2018, 108, 1261–1272. [Google Scholar]
- Baldino, L.; Cardea, S.; Reverchon, E. Biodegradable membranes loaded with curcumin to be used as engineered independent devices in active packaging. J. Taiwan Inst. Chem. Eng. 2017, 71, 518–526. [Google Scholar] [CrossRef]
- Marulasiddeshwara, R.; Jyothi, M.S.; Soontarapa, K.; Keri, R.S.; Velmurugan, R. Nonwoven fabric supported, chitosan membrane anchored with curcumin/TiO 2 complex: Scaffolds for MRSA infected wound skin reconstruction. Int. J. Biol. Macromol. 2020, 144, 85–93. [Google Scholar] [CrossRef]
- Cardea, S.; Baldino, L.; Reverchon, E. Comparative study of PVDF-HFP-curcumin porous structures produced by supercritical assisted processes. J. Supercrit. Fluids 2018, 133, 270–277. [Google Scholar] [CrossRef]
- Yang, X.; Liu, W.; Xi, G.; Wang, M.; Liang, B.; Shi, Y.; Fenga, Y.; Ren, X.; Shi, C. Fabricating antimicrobial peptideimmobilized starch sponges for hemorrhage control and antibacterial treatment. Carbohydr. Polym. 2019, 222, 115012. [Google Scholar] [CrossRef]
- Feng, Y.; Li, X.; Zhang, Q.; Yan, S.; Guo, Y.; Li, M.; You, R. Mechanically robust and flexible silk protein/polysaccharide composite sponges for wound dressing. Carbohydr. Polym. 2019, 216, 17–24. [Google Scholar] [CrossRef]
- Ma, R.; Wang, Y.; Qi, H.; Shi, C.; Wei, G.; Xiao, L.; Huang, Z.; Liu, S.; Yu, H.; Teng, C.; et al. Nanocomposite sponges of sodium alginate/graphene oxide/polyvinyl alcohol as potential wound dressing: In vitro and in vivo evaluation. Compos. Part B 2019, 167, 396–405. [Google Scholar] [CrossRef]
- Khan, Z.A.; Jamil, S.; Akhtar, A.; Bashir, M.M.; Yar, M. Chitosan based hybrid materials used for wound healing applications- A short review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 419–436. [Google Scholar] [CrossRef]
- Chen, C.; Liu, L.; Huang, T.; Wang, Q.; Fang, Y. Bubble template fabrication of chitosan/poly (vinyl alcohol) sponges for wound dressing applications. Int. J. Biol. Macromol. 2013, 62, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.C.; Nguyen, V.B.; Hsieh, M. Curcumin-Loaded Chitosan/Gelatin Composite Sponge for Wound Healing Application. Int. J. Polym. Sci. 2013, 2013, 17–23. [Google Scholar] [CrossRef]
- Momin, M.; Kurhade, S.; Khanekar, P.; Mhatre, S. Novel biodegradable hydrogel sponge containing curcumin and honey for wound healing. J. Wound Care 2016, 25, 364–372. [Google Scholar] [CrossRef]
- Zhao, Y.; Dai, C.; Wang, Z.; Chen, W.; Liu, J.; Zhou, R.; Yu, A.; Huang, S. A novel curcumin-loaded composite dressing facilitates wound healing due to its natural antioxidant effect. Drug Des. Dev. Ther. 2019, 13, 3269–3280. [Google Scholar] [CrossRef]
- Mohanty, C.; Das, M.; Sahoo, S.K. Sustained Wound Healing Activity of Curcumin Loaded Oleic Acid Based Polymeric Bandage in a Rat Model. Mol. Pharm. 2012, 9, 2801–2811. [Google Scholar] [CrossRef]
- Mohanty, C.; Pradhan, J. A human epidermal growth factor-curcumin bandage bioconjugate loaded with mesenchymal stem cell for in vivo diabetic wound healing. Mater. Sci. Eng. C 2020, 111, 110751. [Google Scholar] [CrossRef]
- Zhao, R.; Li, X.; Sun, B.; Zhang, Y.; Zhang, D.; Tang, Z.; Chen, X.; Wang, C. Electrospun chitosan/sericin composite nanofibers with antibacterial property as potential wound dressings. Int. J. Biol. Macromol. 2014, 68, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Abrigo, M.; McArthur, S.L.; Kingshott, P. Electrospun nanofibers as dressings for chronic wound care: Advances, challenges, and future prospects. Macromol. Biosci. 2014, 14, 772–792. [Google Scholar] [CrossRef]
- Zhang, Y.; Lim, C.T.; Ramakrishna, S.; Huang, Z.M. Recent development of polymer nanofibers for biomedical and biotechnological applications. J. Mater. Sci. Mater. Med. 2005, 16, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, R.L.; Kasper, F.K.; Mikos, A.G. Polymeric nanofibers in tissue engineering. Tissue Eng. Part B Rev. 2011, 17, 349–364. [Google Scholar] [CrossRef]
- Merrell, J.G.; McLaughlin, S.W.; Tie, L.; Laurencin, C.T.; Chen, A.F.; Nair, L.S. Curcumin Loaded Poly(ε-Caprolactone) Nanofibers: Diabetic Wound Dressing with Antioxidant and Anti-inflammatory Properties. Clin. Exp. Pharmacol. Physiol. 2009, 36, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, N.; Natarajan, T.S.; Rajiv, S. Preparation and characterization of electrospun curcumin loaded poly(2-hydroxyethyl methacrylate) nanofiber—A biomaterial for multidrug resistant organisms. J. Biomed. Mater. Res. 2015, 103, 16–24. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Ghosh, C.; Hwang, S.-G.; Tran, L.D.; Park, J.S. Characteristics of curcumin-loaded poly (lactic acid) nanofibers for wound healing. J. Mater. Sci. 2013, 48, 7125–7133. [Google Scholar] [CrossRef]
- Ravikumar, R.; Ganesh, M.; Ubaidulla, U.; Choi, E.Y.; Jang, H.T. Preparation, characterization, and in vitro diffusion study of nonwoven electrospun nanofiber of curcumin-loaded cellulose acetate phthalate polymer. Saudi Pharm. J. 2017, 25, 921–926. [Google Scholar] [CrossRef]
- Ranjbar-mohammadi, M.; Rabbani, S.; Bahrami, S.H.; Joghataei, M.T.; Moayer, F. Antibacterial performance and in vivo diabetic wound healing of curcumin loaded gum tragacanth/poly (ε-caprolactone) electrospun nanofibers. Mater. Sci. Eng. C 2016, 69, 1183–1191. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Bahrami, S.H. Electrospun curcumin loaded poly (ԑ-caprolactone)/gum tragacanth nanofibers for biomedical application. Int. J. Biol. Macromol. 2016, 84, 448–456. [Google Scholar] [CrossRef]
- Ghaee, A.; Bagheri-khoulenjani, S.; Afshar, H.A.; Bogheiri, H. Biomimetic nanocomposite scaffolds based on surface modified PCL-nanofibers containing curcumin embedded in chitosan/gelatin for skin regeneration. Compos. Part B 2019, 177, 107339. [Google Scholar] [CrossRef]
- Moradkhannejhad, L.; Abdouss, M.; Nikfarjam, N.; Shahriari, M.H.; Heidary, V. The effect of molecular weight and content of PEG on in vitro drug release of electrospun curcumin loaded PLA/PEG nano fibers. J. Drug Deliv. Sci. Technol. 2020, 56, 101554. [Google Scholar] [CrossRef]
- Mutlu, G.; Calamak, S.; Ulubayram, K.; Guven, E. Curcumin-loaded electrospun PHBV nanofibers as potential wound-dressing material. J. Drug Deliv. Sci. Technol. 2018, 43, 185–193. [Google Scholar] [CrossRef]
- Bui, H.T.; Chung, O.H.; Cruz, J.D.; Park, J.S. Fabrication and Characterization of Electrospun Curcumin-Loaded Polycaprolactone-Polyethylene Glycol Nanofibers for Enhanced Wound Healing. Macromol. Res. 2014, 22, 1288–1296. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Zak, M.S.; Majdi, H.; Mostafavi, E.; Barati, M.; Lotfimehr, H.; Ghaseminasab, K.; Pazoki-Toroudi, H.; Webster, T.J.; Akbarzadeh, A. The effect of chrysin—Curcumin-loaded nanofibres on the wound-healing process in male rats. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1642–1652. [Google Scholar] [CrossRef] [PubMed]
- Perumal, G.; Pappuru, S.; Chakraborty, D.; Nandkumar, A.M.; Chand, D.K.; Doble, M. Synthesis and characterization of curcumin loaded PLA-Hyperbranched polyglycerol electrospun blend for wound dressing applications. Mater. Sci. Eng. C 2017, 76, 1196–1204. [Google Scholar] [CrossRef]
- Rramaswamy, R.; Mani, G.; Venkatachalam, S.; Venkata, R.Y.; Lavanya, J.S.; young Choi, E. Tetrahydro curcumin loaded PCL-PEG electrospun transdermal nanofiber patch: Preparation, characterization, and in vitro diff usion evaluations. J. Drug Deliv. Sci. Technol. 2018, 44, 342–348. [Google Scholar]
- Shababdoust, A.; Zandi, M.; Ehsani, M.; Shokrollahi, P.; Foudazi, R. Controlled curcumin release from nano fi bers based on amphiphilic-block segmented polyurethanes. Int. J. Pharm. 2020, 575, 118947. [Google Scholar] [CrossRef]
- Ahmed, R.; Tariq, M.; Ali, I.; Asghar, R.; Khanam, P.N.; Augustine, R.; Hasan, A. Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nano fi brous mats with antibacterial and antioxidant properties for diabetic wound healing. Int. J. Biol. Macromol. 2018, 120, 385–393. [Google Scholar] [CrossRef]
- Mohan, C.; Purwar, R.; Pal, A. Enhanced potential of biomimetic, silver nanoparticles functionalized Antheraea mylitta (tasar) silk fi broin nano fi brous mats for skin tissue engineering. Int. J. Biol. Macromol. 2019, 130, 437–453. [Google Scholar]
- Shahzad, S.; Yar, M.; Anwar, S. Chitosan-based electrospun nanofibrous mats, hydrogels and cast films: Novel anti-bacterial wound dressing matrices. J. Mater. Sci. 2015, 26, 136. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Wang, B.; Li, J.; Jansen, J.A.; Walboomers, X.F.; Yang, F. Antibacterial e ff ect and wound healing ability of silver nanoparticles incorporation into chitosan-based nanofibrous membranes. Mater. Sci. Eng. C 2019, 8, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Wang, X.; Liu, Y.; Kong, C.; Liu, N.; Wan, Y.; Guo, Q.; Liu, K.; Lu, Z.; Li, M.; et al. Ag nanoparticles decorated PVA-co-PE nanofiber-based membrane with antifouling surface for highly efficient inactivation and interception of bacteria. Appl. Surf. Sci. 2020, 506, 144664. [Google Scholar] [CrossRef]
- Yildiz, A.; Bayramol, D.V.; Atav, R.; Agirgan, A.Ö.; Kurc, M.A.; Ergunay, U.; Mayer, C.; Hadimani, R.L. Synthesis and characterization of Fe3O4@Cs@Ag nanocomposite and its use in the production of magnetic and antibacterial nanofibrous membranes. Appl. Surf. Sci. 2020, 521, 146332. [Google Scholar] [CrossRef]
- Fu, S.; Meng, X.; Fan, J.; Yang, L.; Wen, Q.; Ye, S.; Lin, S.; Wang, B.; Chen, L.; Wu, J.; et al. Acceleration of dermal wound healing by using electrospun poly (e -caprolactone) fibrous mats. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 533–542. [Google Scholar] [CrossRef]
- Lian, Y.; Zhan, J.-C.; Zhang, K.-H.; Mo, X.-M. Fabrication and characterization of curcumin-loaded silk fi broin / P ( LLA-CL) nanofibrous scaffold. Front. Mater. Sci. 2014, 8, 354–362. [Google Scholar] [CrossRef]
- Tsekova, P.B.; Spasova, M.G.; Manolova, N.E.; Markova, D.; Rashkov, I.B. Electrospun curcumin-loaded cellulose acetate/polyvinylpyrrolidone fi brous materials with complex architecture and antibacterial activity. Mater. Sci. Eng. C 2017, 73, 206–214. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Fast-dissolving antioxidant curcumin/cyclodextrin inclusion complex electrospun nanofibrous webs. Food Chem. 2020, 317, 126397. [Google Scholar] [CrossRef]
- Saeed, S.M.; Mojgan, M.; Jalal, Z.; Jalal, B. Designing and fabrication of curcumin loaded PCL/PVA multi-layer nanofibrous electrospun structures as active wound dressing. Prog. Biomater. 2017, 6, 39–48. [Google Scholar] [CrossRef]
- Esmaeili, E.; Eslami-arshaghi, T.; Hosseinzadeh, S.; Elahirad, E.; Jamalpoor, Z.; Hatamie, S.; Soleimani, M. The biomedical potential of cellulose acetate/polyurethane nanofibrous mats containing reduced graphene oxide/silver nanocomposites and curcumin: Antimicrobial performance and cutaneous wound healing. Int. J. Biol. Macromol. 2020, 152, 418–427. [Google Scholar] [CrossRef]
- Pankongadisak, P.; Sangklin, S.; Chuysinuan, P.; Suwantong, O.; Supaphol, P. The use of electrospun curcumin-loaded poly (L-lactic acid) fi ber mats as wound dressing materials. J. Drug Deliv. Sci. Technol. 2019, 53, 101121. [Google Scholar] [CrossRef]
- Mahmud, M.M.; Zaman, S.; Perveen, A.; Jahan, R.A.; Islam, M.F.; Arafat, M.T. Controlled release of curcumin from electrospun fiber mats with antibacterial activity. J. Drug Deliv. Sci. Technol. 2020, 55, 101386. [Google Scholar] [CrossRef]
- Suwantong, O.; Opanasopit, P.; Ruktanonchai, U.; Supaphol, P. Electrospun cellulose acetate fiber mats containing curcumin and release characteristic of the herbal substance. Polymer 2007, 48, 7546–7557. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, S.; Zhao, Z.; Wu, T.; Wang, R.; Xu, S.; Liu, L.; Xie, R.; Zheng, Z.; Li, G.; et al. Silk fibroin/polyethylene glycol nanofibrous membranes loaded with curcumin. Therm. Sci. 2017, 21, 1587–1594. [Google Scholar] [CrossRef][Green Version]
- Zahiri, M.; Khanmohammadi, M.; Goodarzi, A.; Ababzadeh, S.; Frahani, M.S.; Mohandesnezhad, S.; Bahrami, N.; Nabipour, I.; Ai, J. Encapsulation of curcumin loaded chitosan nanoparticle within poly (e-caprolactone) and gelatin fiber mat for wound healing and layered dermal reconstitution. Int. J. Biol. Macromol. 2020, 153, 1241–1250. [Google Scholar] [CrossRef]
- Hegge, A.B.; Andersen, T.; Melvik, J.E.; Bruzell, E.; Kristensen, S.; Tønnesen, H.H. Formulation and bacterial phototoxicity of curcumin loaded alginate foams for wound treatment applications: Studies on curcumin and curcuminoides XLII. J. Pharm. Sci. 2011, 100, 174–185. [Google Scholar] [CrossRef]
- Niranjan, R.; Kaushik, M.; Prakash, J.; Venkataprasanna, K.S.; Christy, A.; Pannerselvam, B.; Venkatasubbu, G.D. Enhanced wound healing by PVA/Chitosan/Curcumin patches: In vitro and in vivo study. Colloids Surf. B Biointerfaces 2019, 182, 110339. [Google Scholar]
- Bulbake, U.; Jain, S.; Kumar, N.; Mittal, A. Curcumin loaded biomimetic composite graft for faster regeneration of skin in diabetic wounds. J. Drug Deliv. Sci. Technol. 2018, 47, 12–21. [Google Scholar] [CrossRef]
- Shah, S.A.A.; Imran, M.; Lian, Q.; Shehzad, F.K.; Athir, N.; Zhang, J.; Cheng, J. Curcumin incorporated polyurethane urea elastomers with tunable thermo-mechanical properties. React. Funct. Polym. 2018, 128, 97–103. [Google Scholar] [CrossRef]
- Ternullo, S.; Gagnat, E.; Julin, K.; Johannessen, M.; Basnet, P.; Vanic, Z.; Skalko-Basnet, N. Liposomes augment biological benefits of curcumin for multitargeted skin therapy. Eur. J. Pharm. Biopharm. 2019, 144, 154–164. [Google Scholar] [CrossRef]
- Nguyen, M.; Lee, S.E.; Tran, T.; Bui, C.; Nguyen, T.; Vu, N.-B.-D.; Tran, T.-T.; Nguyen, T.-H.-P.; Nguyen, T.-T.; Hadinito, K. A simple strategy to enhance the in vivo wound-healing activity of curcumin in the form of self-assembled nanoparticle complex of curcumin and oligochitosan. Mater. Sci. Eng. C 2019, 98, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Chereddy, K.K.; Coco, R.; Memvanga, P.B.; Ucakar, B.; Rieux, A.; Vandermeulen, G.; Préat, V. Combined effect of PLGA and curcumin on wound healing activity. J. Control. Release 2013, 171, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Karri, V.V.S.; Kuppusamy, G.; Talluri, S.V.; Mannemala, S.S.; Kollipara, R.; Wadhwani, A.D.; Mulukutla, S.; Raju, H.R.S.; Malayandi, R. Curcumin loaded chitosan nanoparticles impregnated into collagen-alginate scaffolds for diabetic wound healing. Int. J. Biol. Macromol. 2016, 93, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Rezaii, M.; Oryan, S.; Javeri, A. Curcumin nanoparticles incorporated collagen-chitosan scaffold promotes cutaneous wound healing through regulation of TGF-β1/Smad7 gene expression. Mater. Sci. Eng. C 2019, 98, 347–357. [Google Scholar] [CrossRef]
- Venkatasubbu, G.D.; Anusuya, T. Investigation on Curcumin nanocomposite for wound dressing. Int. J. Biol. Macromol. 2017, 98, 366–378. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alven, S.; Nqoro, X.; Aderibigbe, B.A. Polymer-Based Materials Loaded with Curcumin for Wound Healing Applications. Polymers 2020, 12, 2286. https://doi.org/10.3390/polym12102286
Alven S, Nqoro X, Aderibigbe BA. Polymer-Based Materials Loaded with Curcumin for Wound Healing Applications. Polymers. 2020; 12(10):2286. https://doi.org/10.3390/polym12102286
Chicago/Turabian StyleAlven, Sibusiso, Xhamla Nqoro, and Blessing Atim Aderibigbe. 2020. "Polymer-Based Materials Loaded with Curcumin for Wound Healing Applications" Polymers 12, no. 10: 2286. https://doi.org/10.3390/polym12102286
APA StyleAlven, S., Nqoro, X., & Aderibigbe, B. A. (2020). Polymer-Based Materials Loaded with Curcumin for Wound Healing Applications. Polymers, 12(10), 2286. https://doi.org/10.3390/polym12102286







