Preparation of Poly-1-butene Nanofiber Mat and Its Application as Shutdown Layer of Next Generation Lithium Ion Battery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Electrospinning Solution and Polymer Fibers
2.3. Characterization
2.4. Preparation of PB Fibrous Separator and Shutdown Test
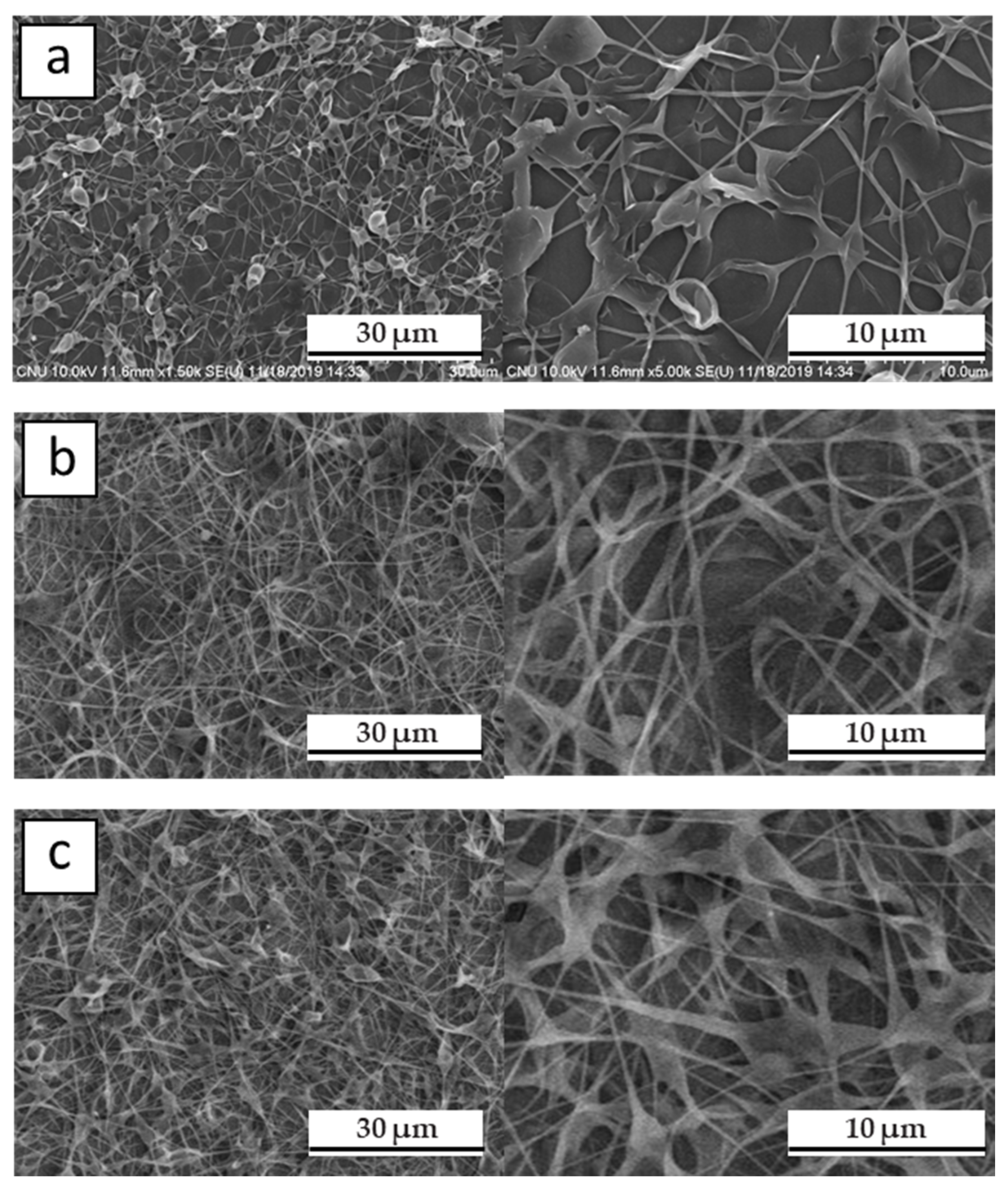
3. Result and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, F.; Maier, J.; Yu, Y. Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem. Soc. Rev. 2020, 49, 1569–1614. [Google Scholar] [CrossRef] [PubMed]
- Arya, A.; Sharma, A. Polymer electrolytes for lithium ion batteries: A critical study. Ionics 2017, 23, 497–540. [Google Scholar] [CrossRef]
- Yoshino, A. Development of the Lithium-Ion Battery and Recent Technological Trends. In Lithium-Ion Batteries; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–20. [Google Scholar]
- Deng, D. Li-ion batteries: Basics, progress, and challenges. Energy Sci. Eng. 2015, 3, 385–418. [Google Scholar] [CrossRef]
- Weber, C.J.; Geiger, S.; Falusi, S.; Roth, M. Material review of Li ion battery separators. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2014. [Google Scholar]
- Prasanna, K.; Subburaj, T.; Lee, W.J.; Lee, C.W. Polyethylene separator: Stretched and coated with porous nickel oxide nanoparticles for enhancement of its efficiency in Li-ion batteries. Electrochim. Acta 2014, 137, 273–279. [Google Scholar] [CrossRef]
- Wu, T.; Xiang, M.; Cao, Y.; Kang, J.; Yang, F. Influence of lamellar structure on double yield behavior and pore size distribution in β nucleated polypropylene stretched membranes. RSC Adv. 2014, 4, 43012–43023. [Google Scholar] [CrossRef]
- Wu, T.; Xiang, M.; Cao, Y.; Kang, J.; Yang, F. Pore formation mechanism of β nucleated polypropylene stretched membranes. RSC Adv. 2014, 4, 36689–36701. [Google Scholar] [CrossRef]
- Zeng, F.; Xu, R.; Ye, L.; Xiong, B.; Kang, J.; Xiang, M.; Li, L.; Sheng, X.; Hao, Z. Effects of Heat Setting on the Morphology and Performance of Polypropylene Separator for Lithium Ion Batteries. Ind. Eng. Chem. Res. 2019, 58, 2217–2224. [Google Scholar] [CrossRef]
- Prasanna, K.; Lee, C.W. Physical, thermal, and electrochemical characterization of stretched polyethylene separators for application in lithium-ion batteries. J. Solid State Electrochem. 2013, 17, 1377–1382. [Google Scholar] [CrossRef]
- Wan, C.; Cao, T.; Chen, X.; Meng, L.; Li, L. Fabrication of polyethylene nanofibrous membranes by biaxial stretching. Mater. Today Commun. 2018, 17, 24–30. [Google Scholar] [CrossRef]
- Venugopal, G.; Moore, J.; Howard, J.; Pendalwar, S. Characterization of microporous separators for lithium-ion batteries. J. Power Sources 1999, 77, 34–41. [Google Scholar] [CrossRef]
- Li, Z.; Xiong, Y.; Sun, S.; Zhang, L.; Li, S.; Liu, X.; Xu, Z.; Xu, S. Tri-layer nonwoven membrane with shutdown property and high robustness as a high-safety lithium ion battery separator. J. Membr. Sci. 2018, 565, 50–60. [Google Scholar] [CrossRef]
- Prasanna, K.; Kim, C.-S.; Lee, C.W. Effect of SiO2 coating on polyethylene separator with different stretching ratios for application in lithium ion batteries. Mater. Chem. Phys. 2014, 146, 545–550. [Google Scholar] [CrossRef]
- Gwon, S.-J.; Choi, J.-H.; Sohn, J.-Y.; Lim, Y.-M.; Nho, Y.-C.; Ihm, Y.-E. Battery performance of PMMA-grafted PE separators prepared by pre-irradiation grafting technique. J. Ind. Eng. Chem. 2009, 15, 748–751. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Y.; Hu, X.; Yao, J.; Chen, Z.; Hao, M.; Tian, W.; Huang, Z.; Li, F. Multilayer Nanofiber Composite Separator for Lithium-Ion Batteries with High Safety. Polymers 2019, 11, 1671. [Google Scholar] [CrossRef]
- Cai, H.; Tong, X.; Chen, K.; Shen, Y.; Wu, J.; Xiang, Y.; Wang, Z.; Li, J. Electrospun polyethylene terephthalate nonwoven reinforced polypropylene separator: Scalable synthesis and its lithium ion battery performance. Polymers 2018, 10, 574. [Google Scholar] [CrossRef]
- Cho, T.-H.; Tanaka, M.; Onishi, H.; Kondo, Y.; Nakamura, T.; Yamazaki, H.; Tanase, S.; Sakai, T. Battery performances and thermal stability of polyacrylonitrile nano-fiber-based nonwoven separators for Li-ion battery. J. Power Sources 2008, 181, 155–160. [Google Scholar] [CrossRef]
- Choi, S.W.; Jo, S.M.; Lee, W.S.; Kim, Y.R. An electrospun poly (vinylidene fluoride) nanofibrous membrane and its battery applications. Adv. Mater. 2003, 15, 2027–2032. [Google Scholar] [CrossRef]
- Bansal, D.; Meyer, B.; Salomon, M. Gelled membranes for Li and Li-ion batteries prepared by electrospinning. J. Power Sources 2008, 178, 848–851. [Google Scholar] [CrossRef]
- Liang, X.; Yang, Y.; Jin, X.; Huang, Z.; Kang, F. The high performances of SiO2/Al2O3-coated electrospun polyimide fibrous separator for lithium-ion battery. J. Membr. Sci. 2015, 493, 1–7. [Google Scholar] [CrossRef]
- Sheng, J.; Wang, R.; Yang, R. Physicochemical properties of cellulose separators for lithium ion battery: Comparison with Celgard2325. Materials 2019, 12, 2. [Google Scholar] [CrossRef]
- Gao, X.; Sheng, W.; Wang, Y.; Lin, Y.; Luo, Y.; Li, B.G. Polyethylene battery separator with auto-shutdown ability, thermal stability of 220 °C, and hydrophilic surface via solid-state ultraviolet irradiation. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Kritzer, P. Nonwoven support material for improved separators in Li–polymer batteries. J. Power Sources 2006, 161, 1335–1340. [Google Scholar] [CrossRef]
- Yi, W.; Huaiyu, Z.; Jian, H.; Yun, L.; Shushu, Z. Wet-laid non-woven fabric for separator of lithium-ion battery. J. Power Sources 2009, 189, 616–619. [Google Scholar] [CrossRef]
- Byun, S.; Lee, S.H.; Song, D.; Ryou, M.-H.; Lee, Y.M.; Park, W.H. A crosslinked nonwoven separator based on an organosoluble polyimide for high-performance lithium-ion batteries. J. Ind. Eng. Chem. 2019, 72, 390–399. [Google Scholar] [CrossRef]
- Yesil, Y.; Bhat, G.S. Porosity and barrier properties of polyethylene meltblown nonwovens. J. Text. Inst. 2017, 108, 1035–1040. [Google Scholar] [CrossRef]
- Barhate, R.S.; Ramakrishna, S. Nanofibrous filtering media: Filtration problems and solutions from tiny materials. J. Membr. Sci. 2007, 296, 1–8. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, H.; Yu, J.; Luo, W.; Ding, B. Microwave structured polyamide-6 nanofiber/net membrane with embedded poly (m-phenylene isophthalamide) staple fibers for effective ultrafine particle filtration. J. Mater. Chem. A 2016, 4, 6149–6157. [Google Scholar] [CrossRef]
- Lee, J.; Seo, E.; Yoo, M.; Kim, S.; Choi, J.; Jung, H.; Lee, H.W.; Lee, H.M.; Kim, H.Y.; Lee, B.; et al. Preparation of non-woven nanofiber webs for detoxification of nerve gases. Polymer 2019, 179, 121664. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Tan, Z. A review of electrospun nanofiber-based separators for rechargeable lithium-ion batteries. J. Power Sources 2019, 443, 227262. [Google Scholar] [CrossRef]
- Calhoun, A. Polypropylene, in Multilayer Flexible Packaging; Elsevier: Amsterdam, The Netherlands, 2016; pp. 35–45. [Google Scholar]
- Peacock, A. Handbook of Polyethylene: Structures: Properties, and Applications; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Watanabe, K.; Kim, B.-S.; Kim, I.-S. Development of polypropylene nanofiber production system. Polym. Rev. 2011, 51, 288–308. [Google Scholar] [CrossRef]
- Luciani, L.; Seppälä, J.; Löfgren, B. Poly-1-butene: Its preparation, properties and challenges. Prog. Polym. Sci. 1988, 13, 37–62. [Google Scholar] [CrossRef]
- Lee, K.-H.; Givens, S.R.; Snively, C.M.; Chase, B.; Rabolt, J.F. Crystallization behavior of electrospun PB/PMP blend fibrous membranes. Macromolecules 2008, 41, 3144–3148. [Google Scholar] [CrossRef]
- Gooch, J.W. (Ed.) Polybutadiene. In Encyclopedic Dictionary of Polymers; Springer: New York, NY, USA, 2007; pp. 740–741. [Google Scholar]
- Lee, Y.; Lee, J.; Kimura, D.; Kim, B.S.; Koh, J.; Kim, I.S. Effects of mechanical force on crystalline structure of electrospun poly (1-butene) membranes. Polym. Int. 2011, 60, 1442–1445. [Google Scholar] [CrossRef]
- Shao, H.-f.; Ma, Y.-p.; Nie, H.-r.; He, A.-h. Solvent vapor annealing induced polymorphic transformation of polybutene-1. Chin. J. Polym. Sci. 2016, 34, 1141–1149. [Google Scholar] [CrossRef]
- Roth, E.; Doughty, D.; Pile, D. Effects of separator breakdown on abuse response of 18650 Li-ion cells. J. Power Sources 2007, 174, 579–583. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, L.; Sutton, D.; Wang, X.; Lin, T. Needleless Melt-Electrospinning of Polypropylene Nanofibres. J. Nanomater. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Rajandas, H.; Parimannan, S.; Sathasivam, K.; Ravichandran, M.; Su Yin, L. A novel FTIR-ATR spectroscopy based technique for the estimation of low-density polyethylene biodegradation. Polym. Test. 2012, 31, 1094–1099. [Google Scholar] [CrossRef]
- Zhong, Z.; Ge, H.; Su, Z. Direct formation of form I’ crystals in polybutene-1/polypropylene blend enhanced by cold crystallization. Polymer 2018, 156, 30–38. [Google Scholar] [CrossRef]
- Ukita, M. The vibrational spectra and vibrational assignments of isotactic polybutene-1. Bull. Chem. Soc. Jpn. 1966, 39, 742–749. [Google Scholar] [CrossRef]
- Othman, N.; Ismail, H.; Mariatti, M. Effect of compatibilisers on mechanical and thermal properties of bentonite filled polypropylene composites. Polym. Degrad. Stab. 2006, 91, 1761–1774. [Google Scholar] [CrossRef]
- Luo, C.; Stride, E.; Edirisinghe, M. Mapping the influence of solubility and dielectric constant on electrospinning polycaprolactone solutions. Macromolecules 2012, 45, 4669–4680. [Google Scholar] [CrossRef]
- Evans, H.; Young, D. Solubility of Polybutene in Pure Solvents. Ind. Eng. Chem. 1942, 34, 461–466. [Google Scholar] [CrossRef]
- Sastry, K.S.; Patel, R. Dilute solution properties of isotactic polybutene-1. Eur. Polym. J. 1969, 5, 79–91. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Kass, M.D.; Theiss, T.; Janke, C.; Pawel, S. Compatibility Study for Plastic, Elastomeric, and Metallic Fueling Infrastructure Materials Exposed to Aggressive Formulations of Ethanol-Blended Gasoline; ORNL/TM-2012/88; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2012.
- Van Krevelen, D.W.; Nijenhuis, K.T. Properties of Polymers: Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Wilski, H.; Grewer, T. The specific heat of poly-1-butene. In Journal of Polymer Science Part C: Polymer Symposia; Wiley Online Library: New York, NY, USA, 1964. [Google Scholar]
- Zhang, D. Advances in Filament Yarn Spinning of Textiles and Polymers; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Masuelli, M.A. Mark-Houwink parameters for aqueous-soluble polymers and biopolymers at various temperatures. J. Polym. Biopolym. Phys. Chem. 2014, 2373–3411. [Google Scholar]
- Norzita, Y.; Norhashidah, T.; Maznah, M. Determination of viscosity-average molecular weight of chitosan using intrinsic viscosity measurement. In Proceedings of the Nuclear Technical Convention 2011, Bangi, Malaysia, 13–15 September 2011. [Google Scholar]
- Wu, X.-F.; Salkovskiy, Y.; Dzenis, Y.A. Modeling of solvent evaporation from polymer jets in electrospinning. Appl. Phys. Lett. 2011, 98, 223108. [Google Scholar] [CrossRef]
- Cheremisinoff, N.P. Industrial Solvents Handbook, Revised and Expanded; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Averbukh, M.; Lugovskoy, S. Theoretical Description of Carbon Felt Electrical Properties Affected by Compression. Appl. Sci. 2019, 9, 4030. [Google Scholar] [CrossRef]
- Chaudhari, A.; More, N.; Mehrotra, S. Static dielectric constant and relaxation time for the binary mixture of water, ethanol, N, N-dimethylformamide, dimethylsulphoxide, and N, N-dimethylacetamide with 2-methoxyethanol. Bull.-Korean Chem. Soc. 2001, 22, 357–361. [Google Scholar]
- Rudin, A.; Choi, P. Practical Aspects of Molecular Weight Measurements. In Modern Methods of Polymer Characterization; John Wiley & Sons: New York, NY, USA, 1991; pp. 103–112. [Google Scholar]
- Gong, W.; Wei, S.; Ruan, S.; Shen, C. Electrospun coaxial PPESK/PVDF fibrous membranes with thermal shutdown property used for lithium-ion batteries. Mater. Lett. 2019, 244, 126–129. [Google Scholar] [CrossRef]
- Wagner, J.R., Jr. Multilayer Flexible Packaging; William Andrew: Amsterdam, The Netherlands, 2016. [Google Scholar]



| PE | PP | PB | |||
|---|---|---|---|---|---|
| hPB | cPB (With PP) | ||||
| Structure | 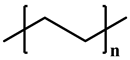 | 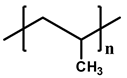 | 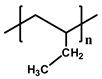 |  | |
| Tm (°C) | 118 | 180 | 108 | 96 | |
| Solubility | N.A for any electrospinning solvent | N.A for any electrospinning solvent | Volume ratio of cosolvent (Cyclohexane/THF/DMF) | ||
| 1:1:0.1 | 1:1:0.2 | 1:1:0.3 | |||
| soluble | soluble | soluble | |||
| Spinnability | N.A.* | N.A. | Poor | Good | Best |
| Materials | Wavenumber(cm−1) | Vibration Type | Assignment |
|---|---|---|---|
| PP, PB | a (2955) | Asymmetrical stretching | CH3 |
| PP, PE, PB | b (2912) | Asymmetrical stretching | CH2 |
| PP, PB | c (2867) | stretching | CH |
| d (2844) | Symmetrical stretching | CH2 | |
| PP, PE, PB | e (1461) | Symmetrical bending | CH2 |
| PP, PB | f (1370) | Symmetrical bending | CH3 |
| PP | g (1163) | Rocking | CH3 |
| PB | h (921) | Rocking | CH2 |
| i (762) | Rocking | CH3 |
| Solubility Parameter | Hansen | Hoftyzer-Van Krevelen | |||
|---|---|---|---|---|---|
| PE a | PP a | PE b | PP b | PB b | |
| δd | 18 | 18 | 16.4 | 19.7 | 18.9 |
| δp | 0 | 0 | 0 | 0 | 0 |
| δh | 2 | 1 | 0 | 0 | 0 |
| R0 | 2 | 6 | |||
| Solvent | δd | δp | δh | Ra | Solvent Behavior |
|---|---|---|---|---|---|
| Methanol | 14.7 | 12.3 | 22.3 | 26.8 | insoluble |
| DMF | 17.4 | 13.7 | 11.3 | 18.0 | insoluble |
| Acetone | 15.5 | 10.4 | 7 | 14.2 | insoluble |
| Methyl Acetate | 15.5 | 7.2 | 7.6 | 12.4 | insoluble |
| THF | 16.8 | 5.7 | 8 | 10.7 | insoluble |
| Hexane | 14.9 | 0 | 0 | 7.9 | soluble |
| Cyclohexane | 16.8 | 0 | 0.2 | 4.1 | soluble |
| Benzene | 18.4 | 0 | 2 | 2.2 | soluble |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, H.; Kim, S.; Gil, M.; Song, S.; Kim, T.-H.; Lee, K.J. Preparation of Poly-1-butene Nanofiber Mat and Its Application as Shutdown Layer of Next Generation Lithium Ion Battery. Polymers 2020, 12, 2267. https://doi.org/10.3390/polym12102267
Jeong H, Kim S, Gil M, Song S, Kim T-H, Lee KJ. Preparation of Poly-1-butene Nanofiber Mat and Its Application as Shutdown Layer of Next Generation Lithium Ion Battery. Polymers. 2020; 12(10):2267. https://doi.org/10.3390/polym12102267
Chicago/Turabian StyleJeong, Hanjin, Sohee Kim, Manjae Gil, Sanghoon Song, Tae-Ho Kim, and Kyung Jin Lee. 2020. "Preparation of Poly-1-butene Nanofiber Mat and Its Application as Shutdown Layer of Next Generation Lithium Ion Battery" Polymers 12, no. 10: 2267. https://doi.org/10.3390/polym12102267
APA StyleJeong, H., Kim, S., Gil, M., Song, S., Kim, T.-H., & Lee, K. J. (2020). Preparation of Poly-1-butene Nanofiber Mat and Its Application as Shutdown Layer of Next Generation Lithium Ion Battery. Polymers, 12(10), 2267. https://doi.org/10.3390/polym12102267






