Polycarbazole and Its Derivatives: Synthesis and Applications. A Review of the Last 10 Years
Abstract
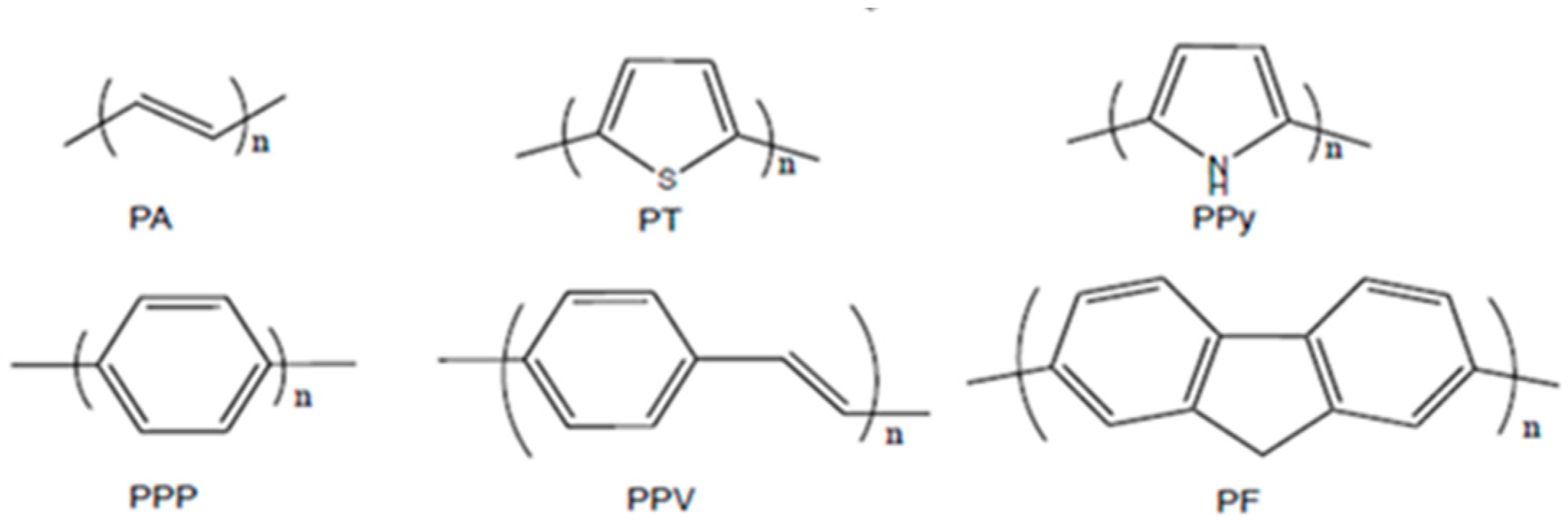
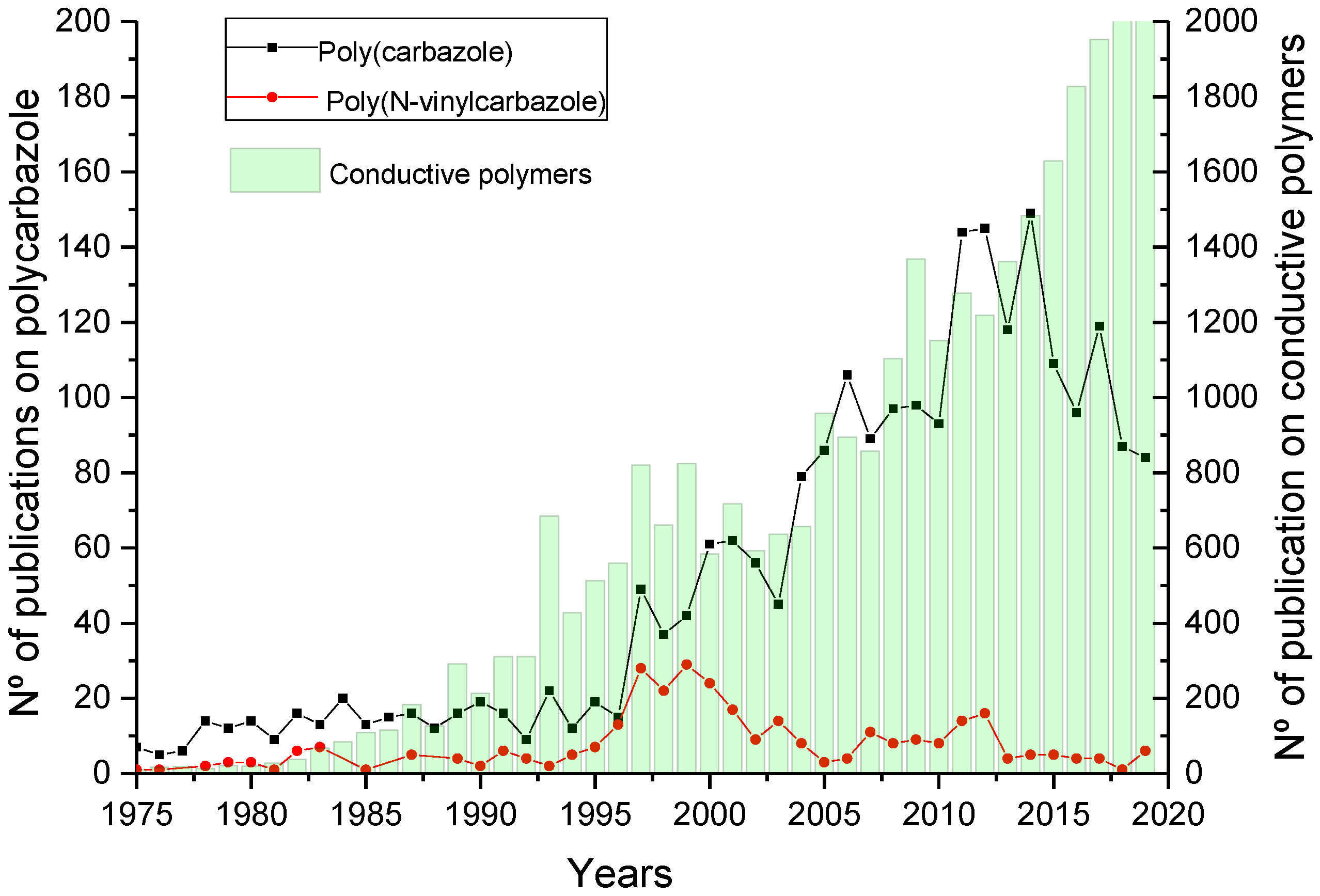
1. Introduction
2. Synthesis of Polymers
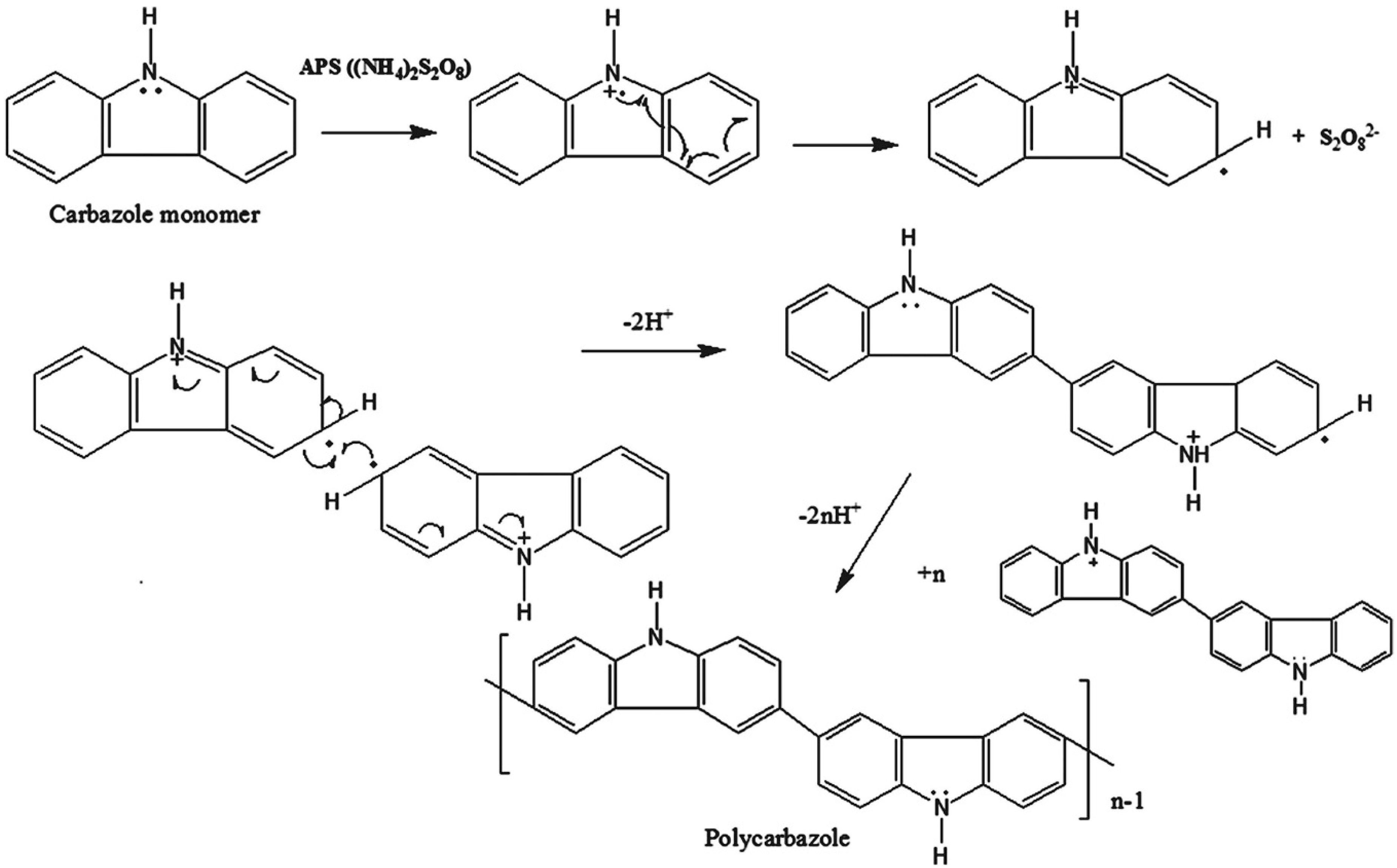
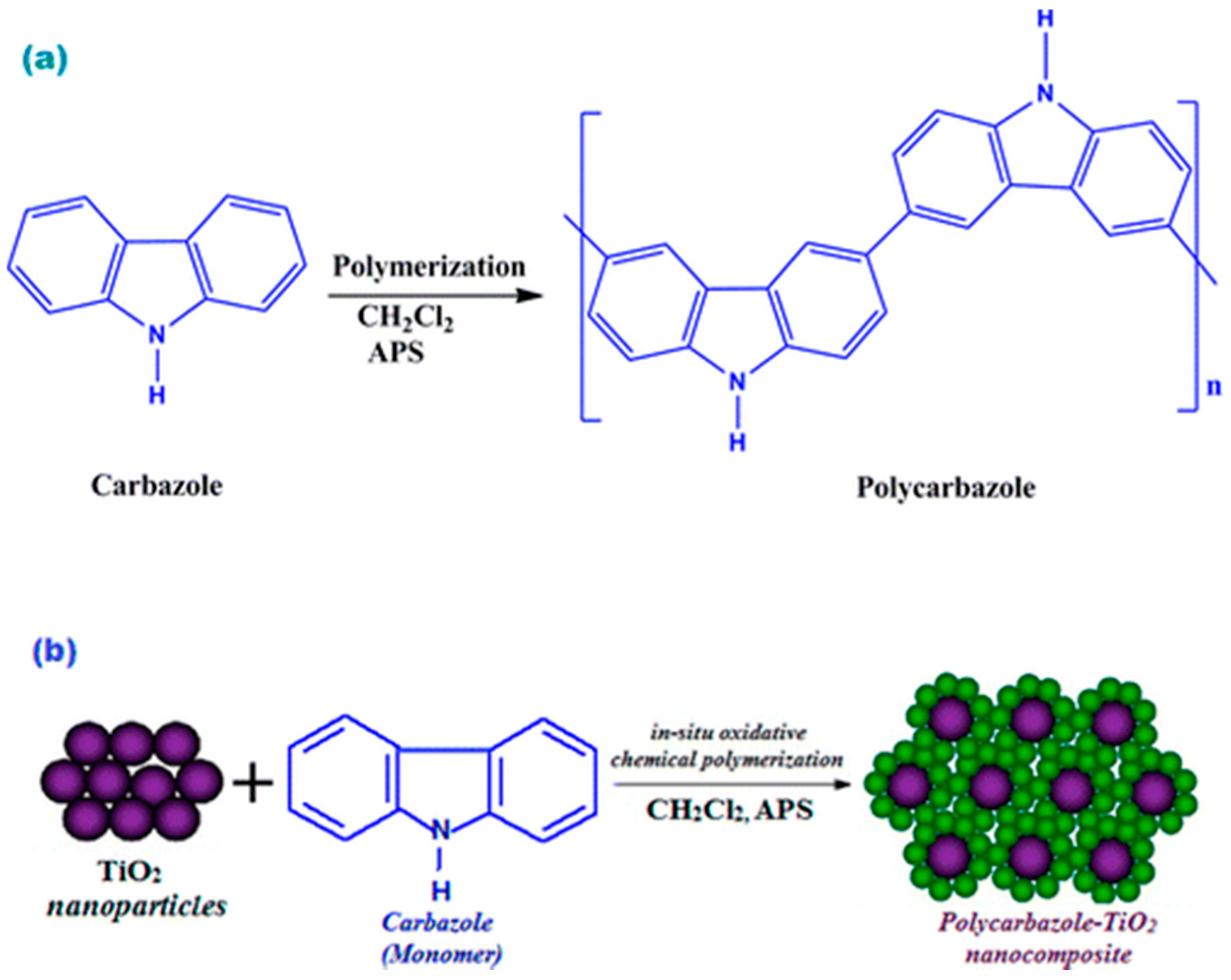
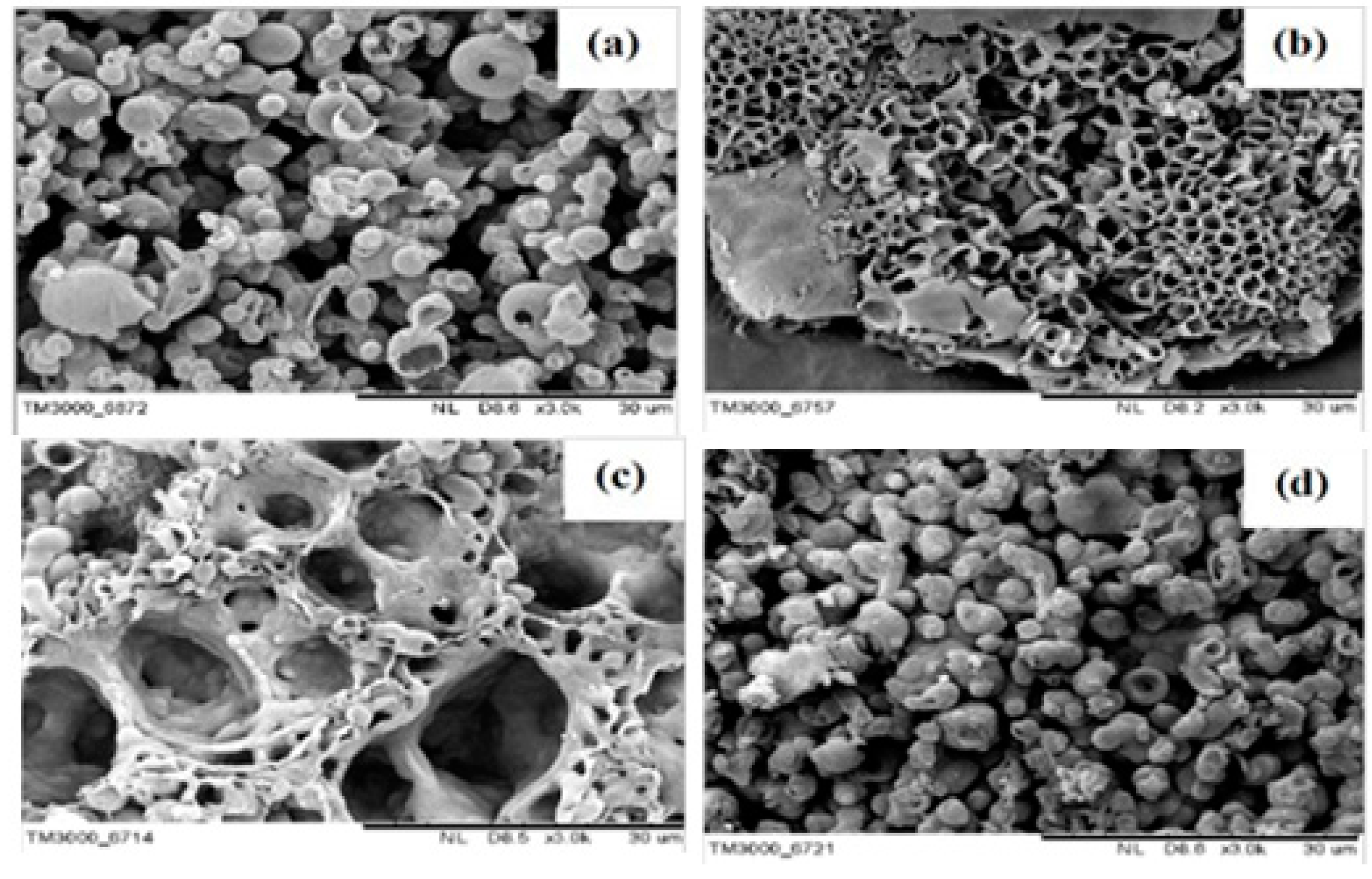
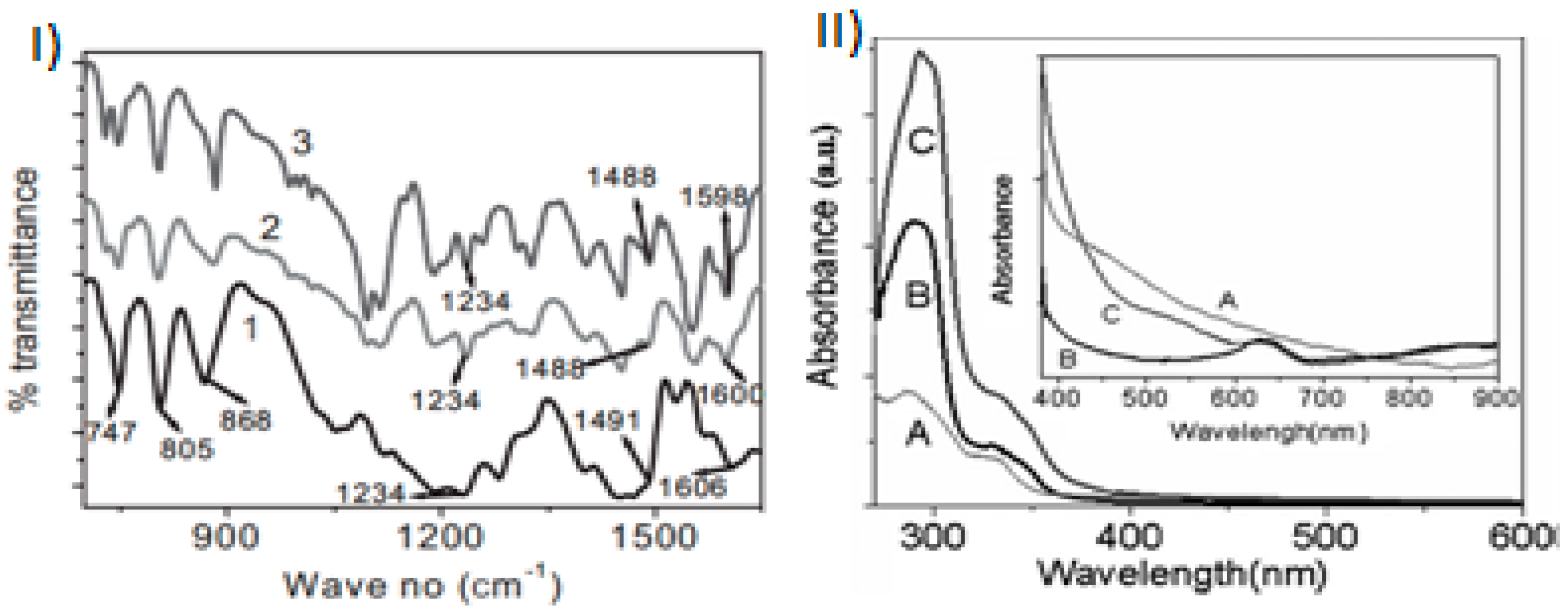
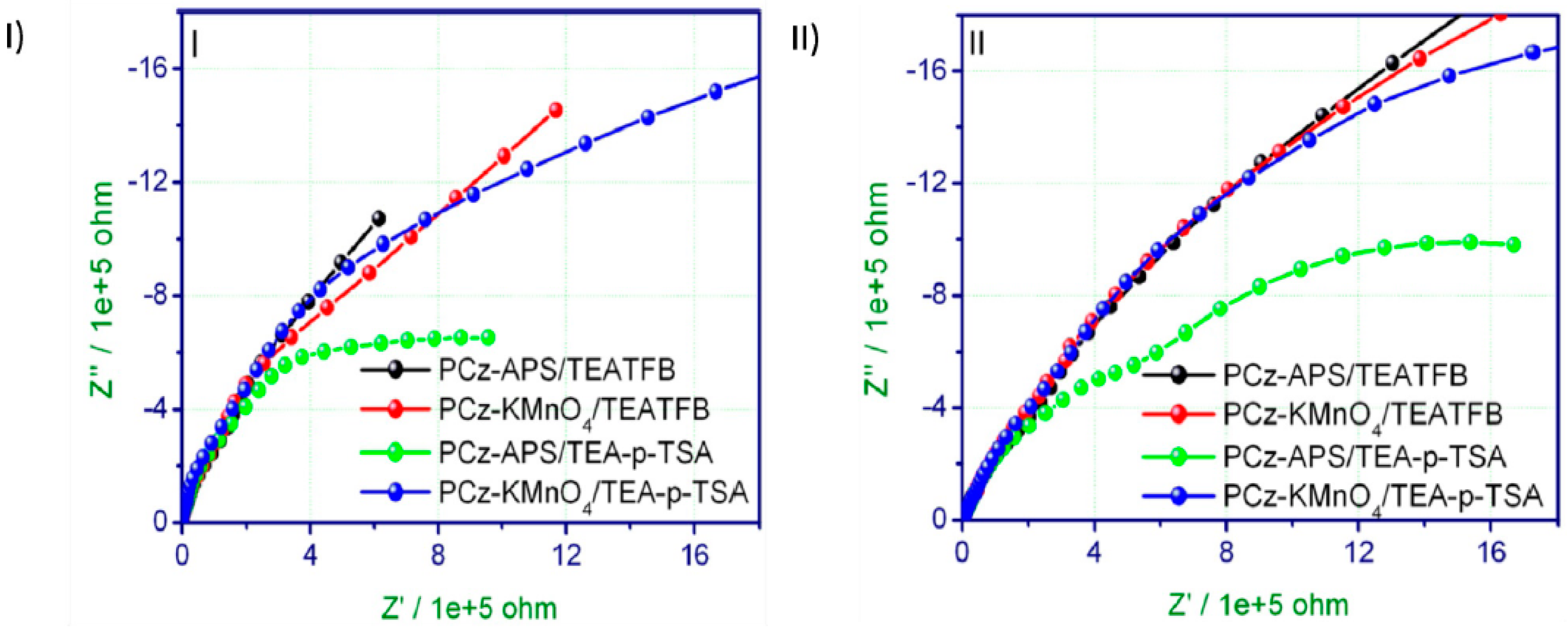
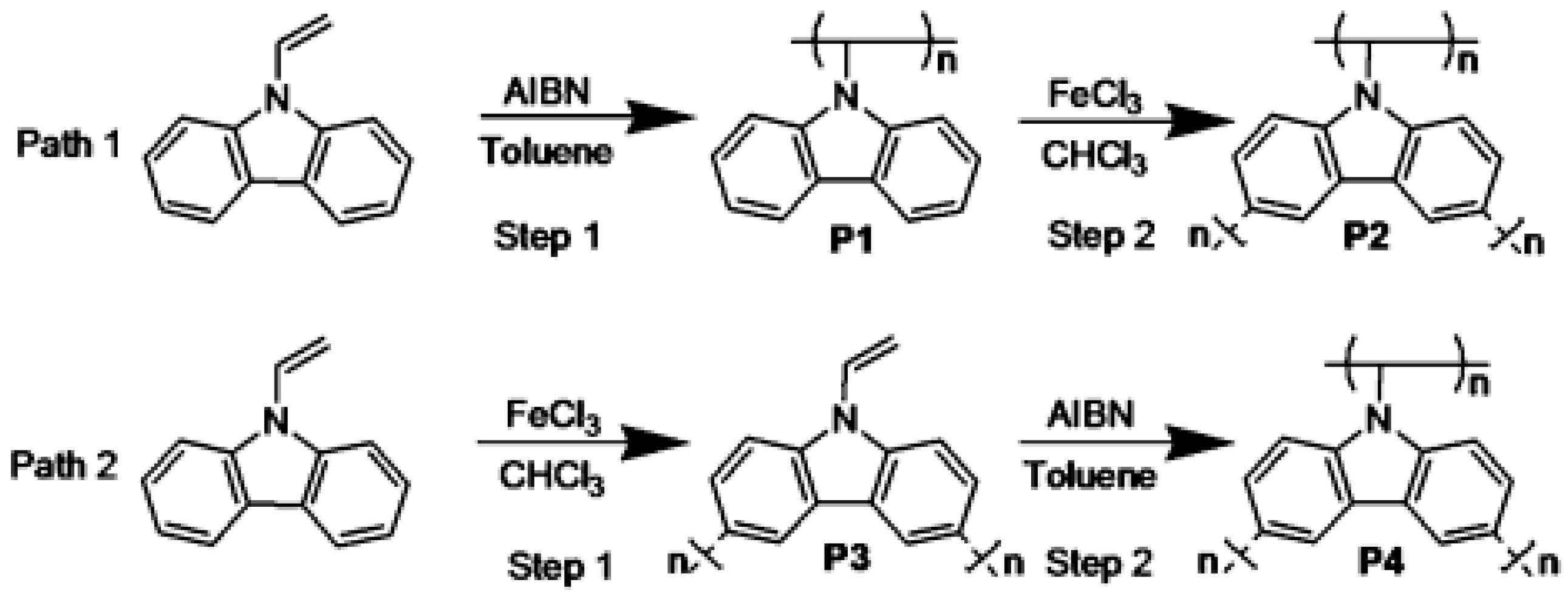
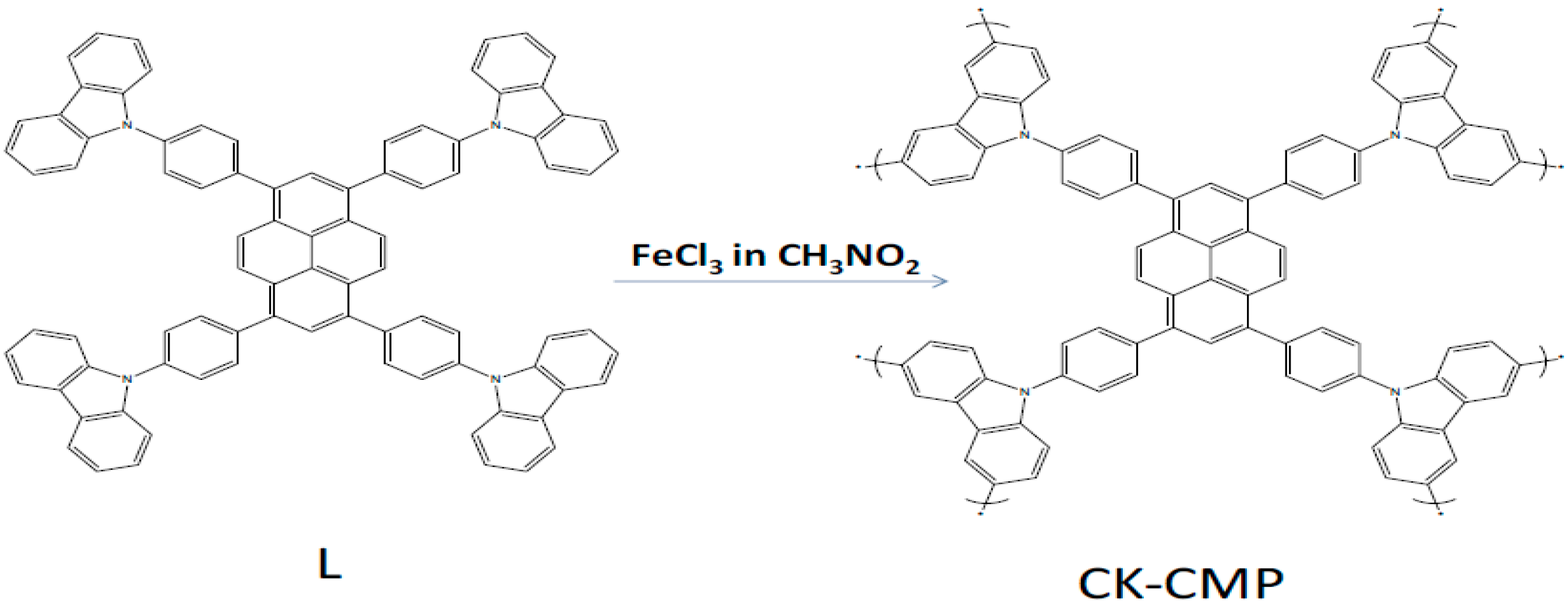
2.1. Chemical Polymerization of Carbazoles and Its Derivatives
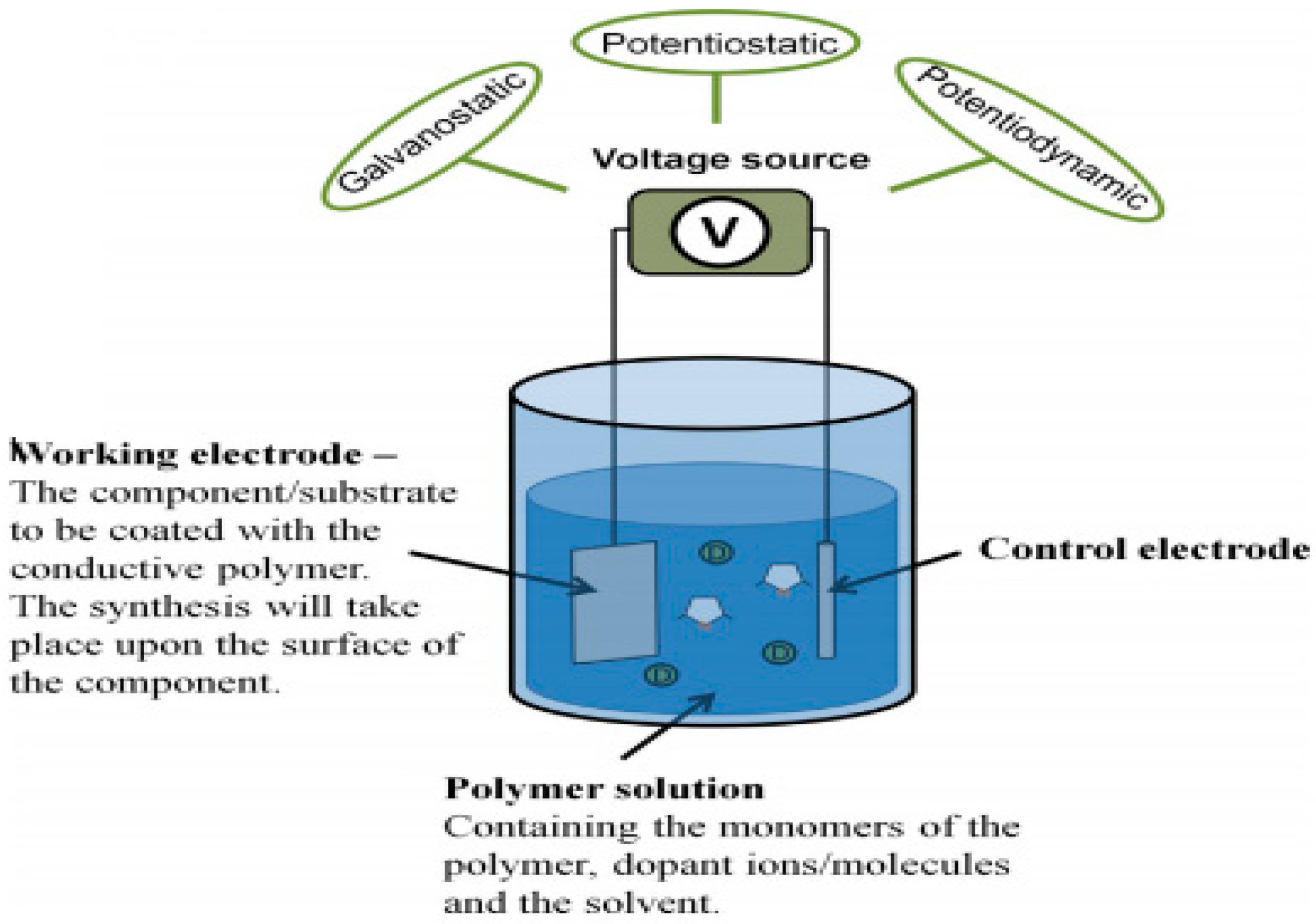
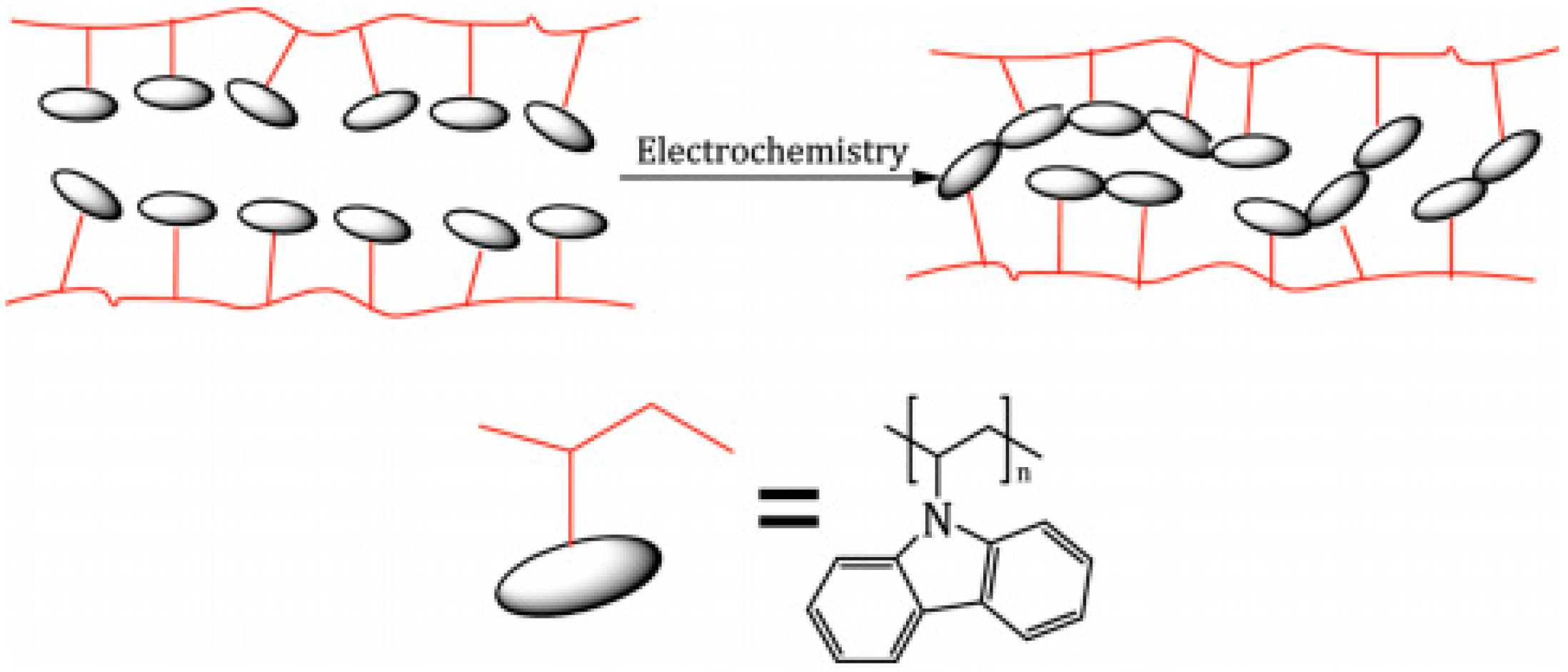
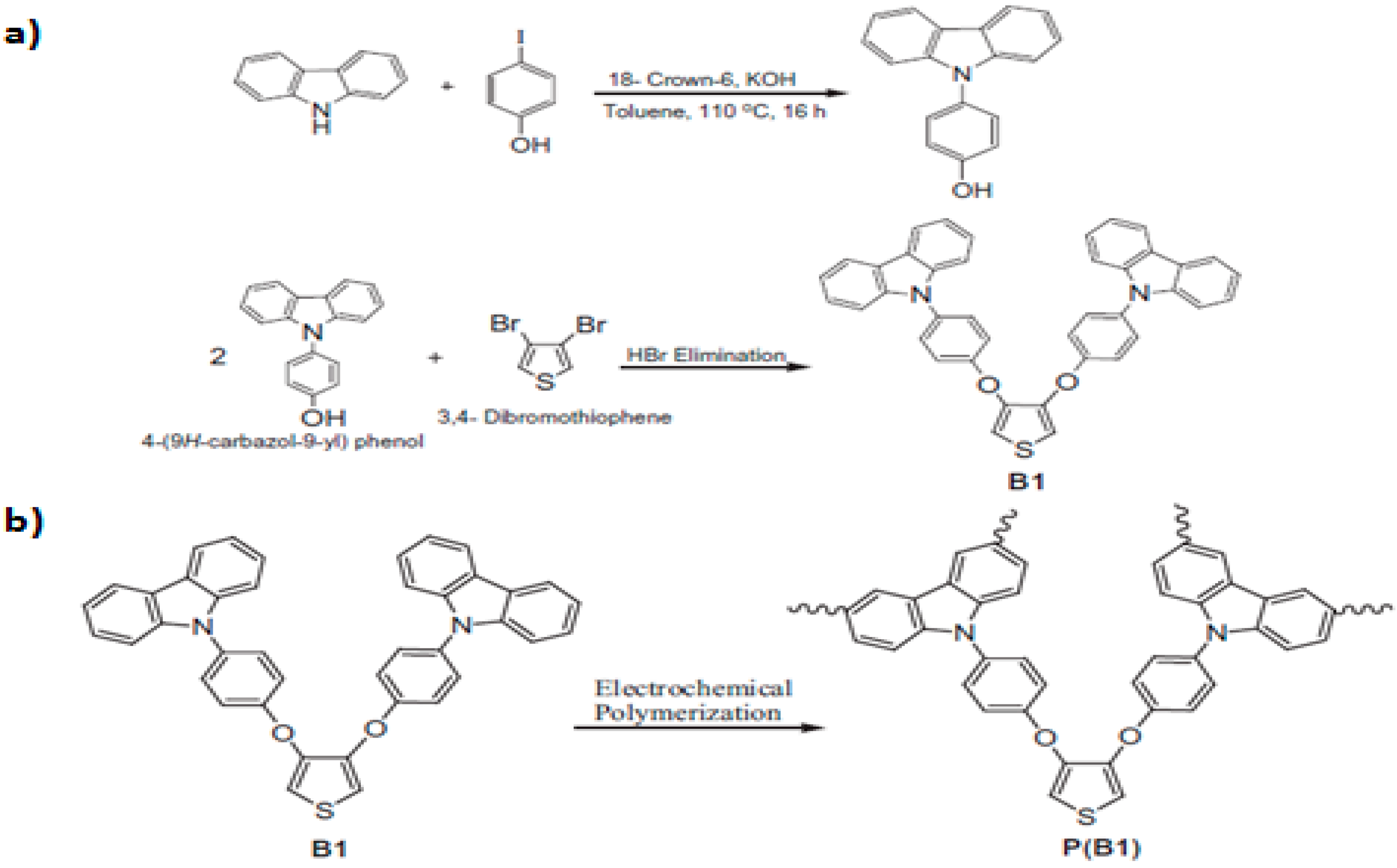
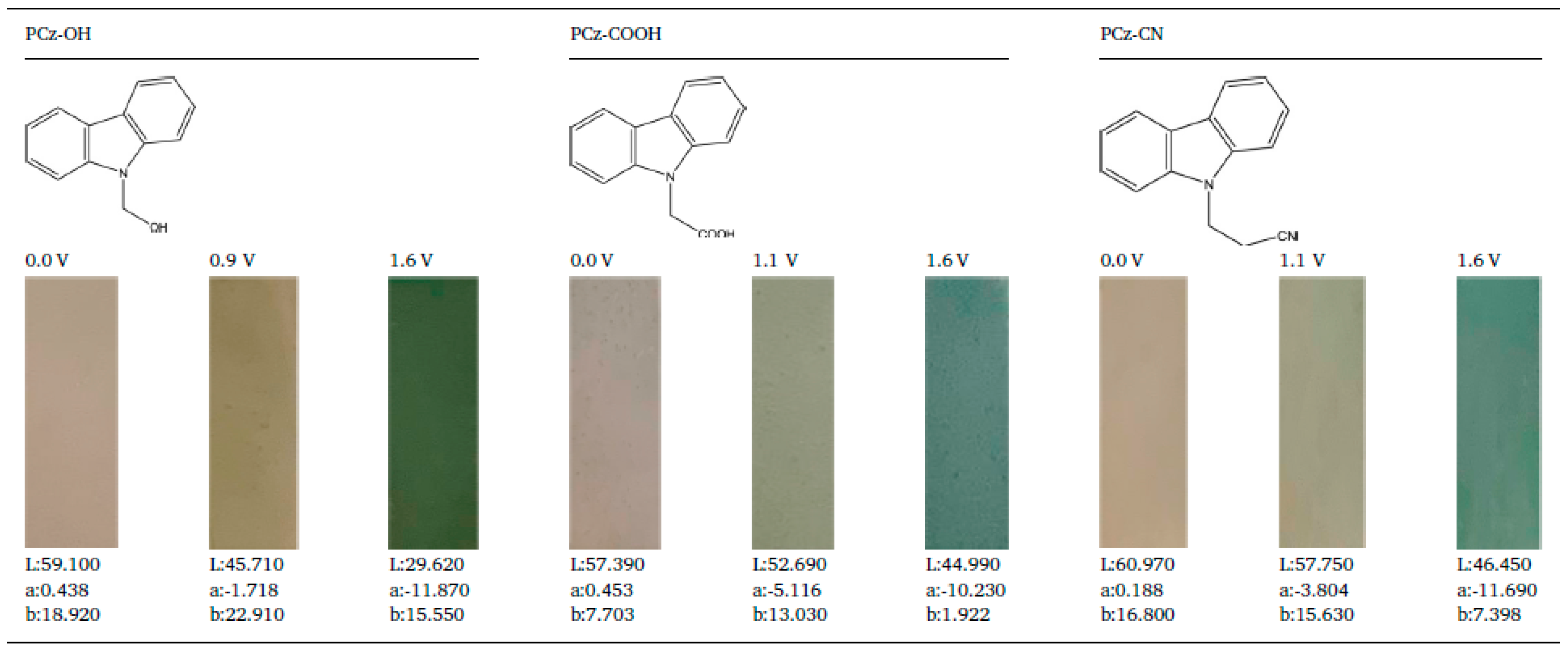
2.2. Electropolymerization of Carbazole and Its Derivatives
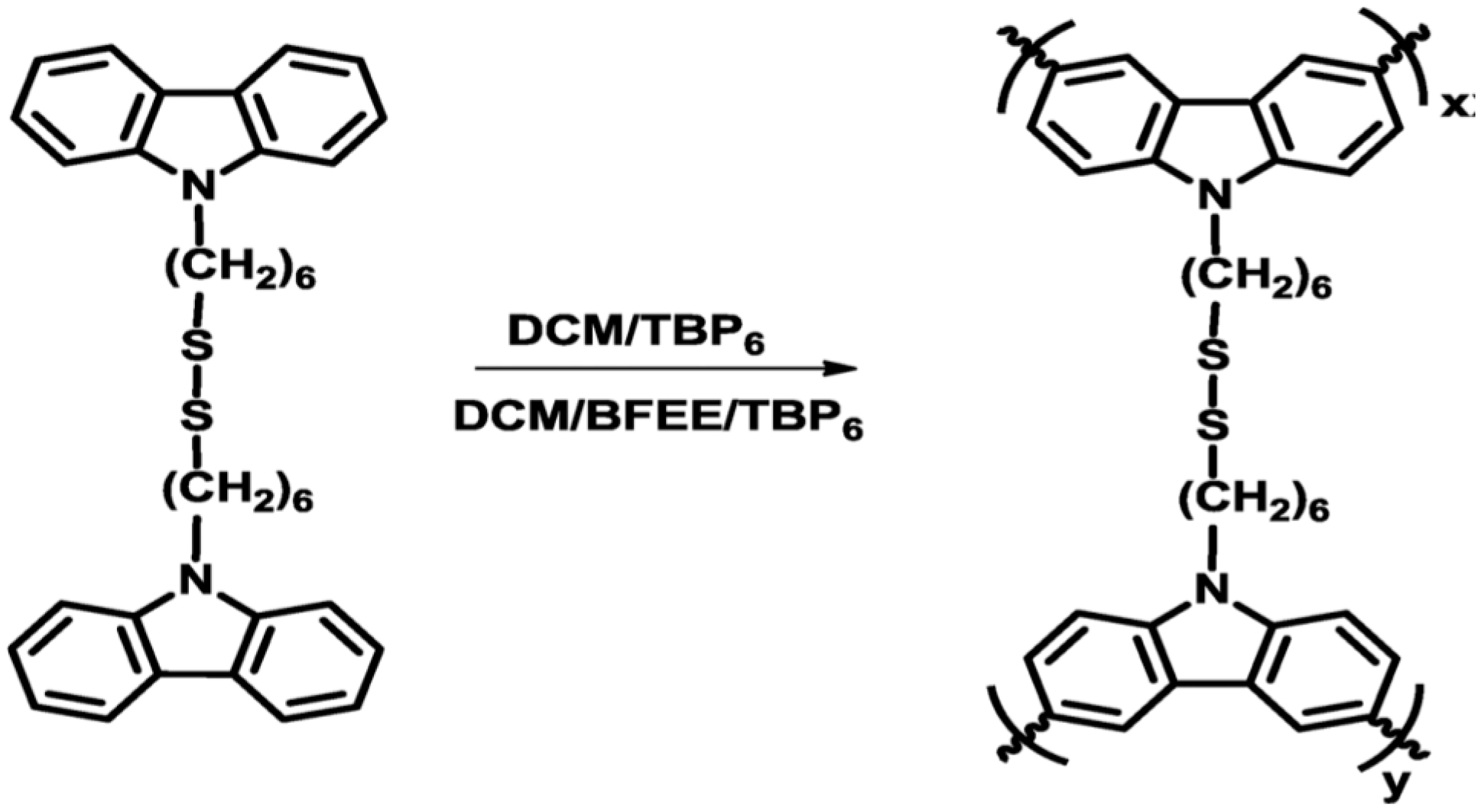
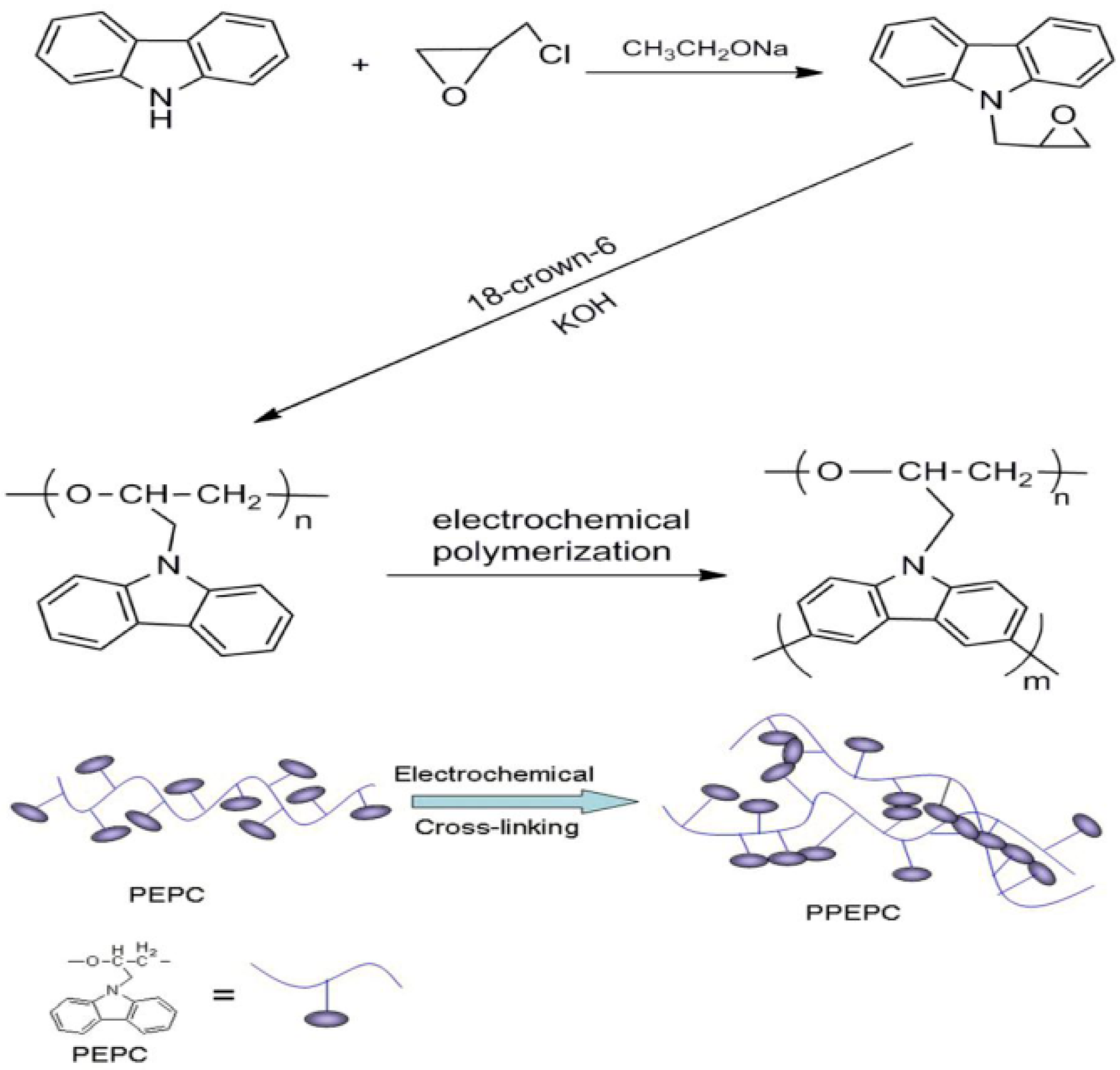
2.3. Polymerization of N-Substitution Carbazoles
3. Application
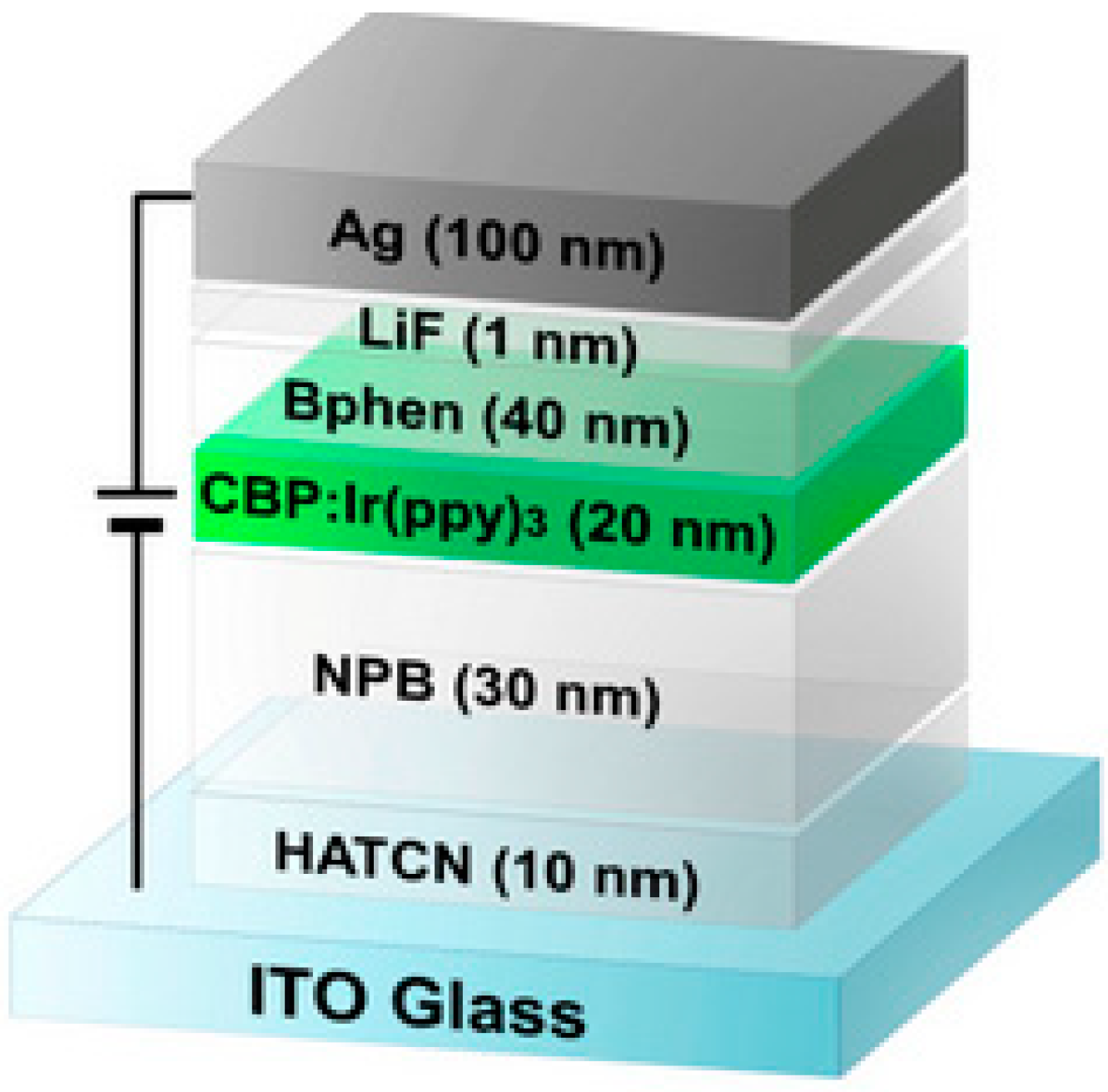
3.1. Light Emitting Diode Application
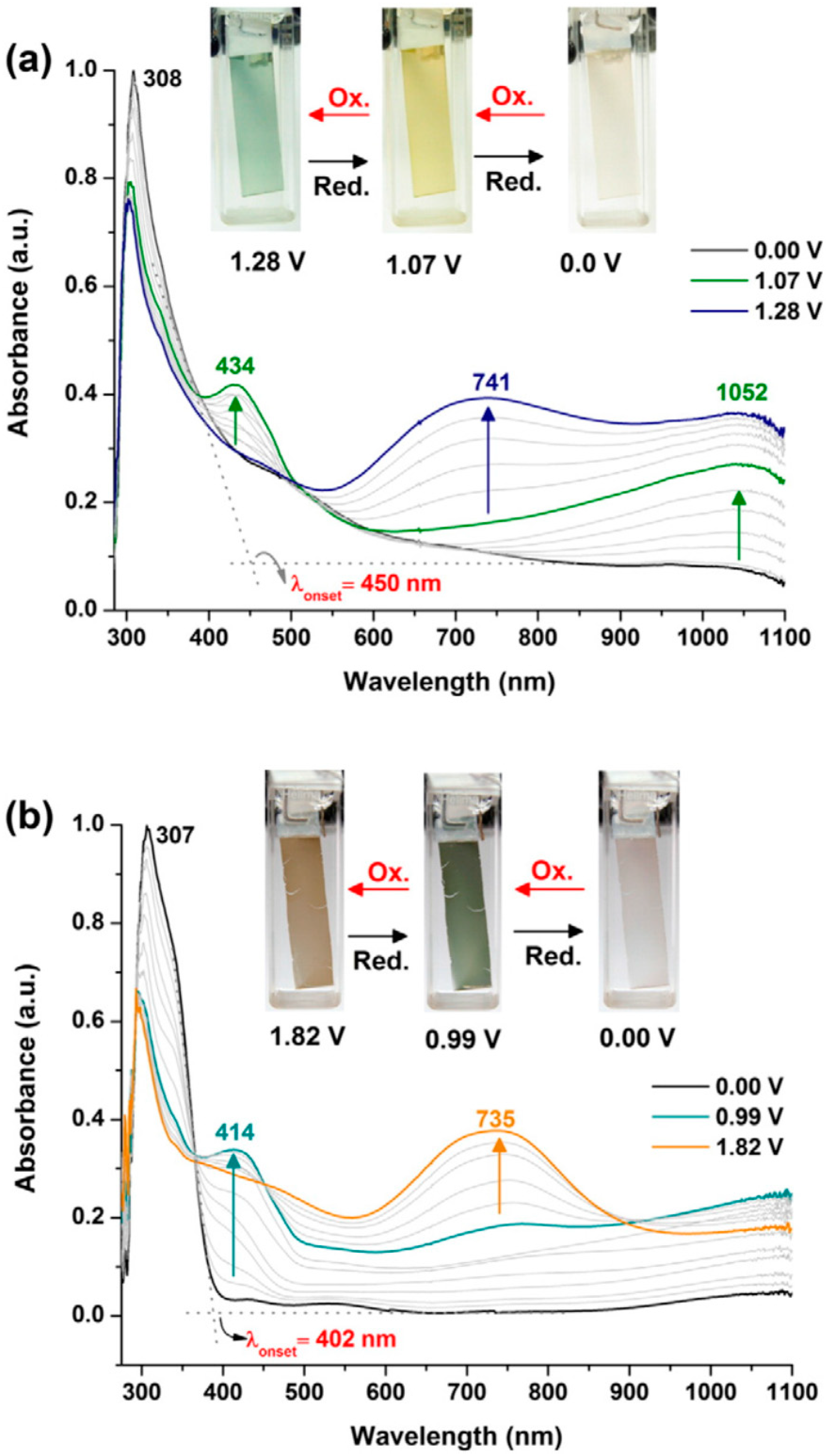
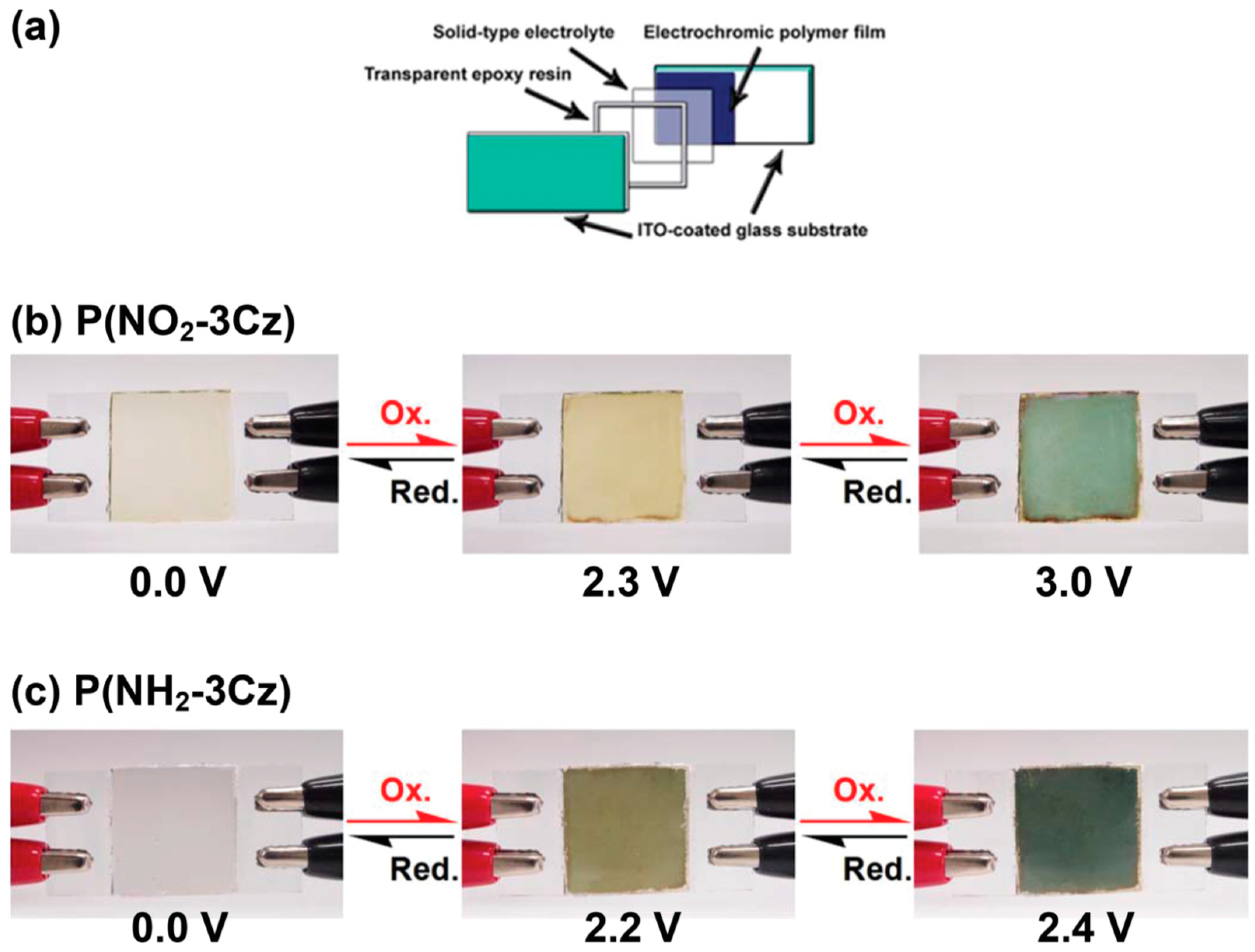
3.2. Electrochemical Capacitors
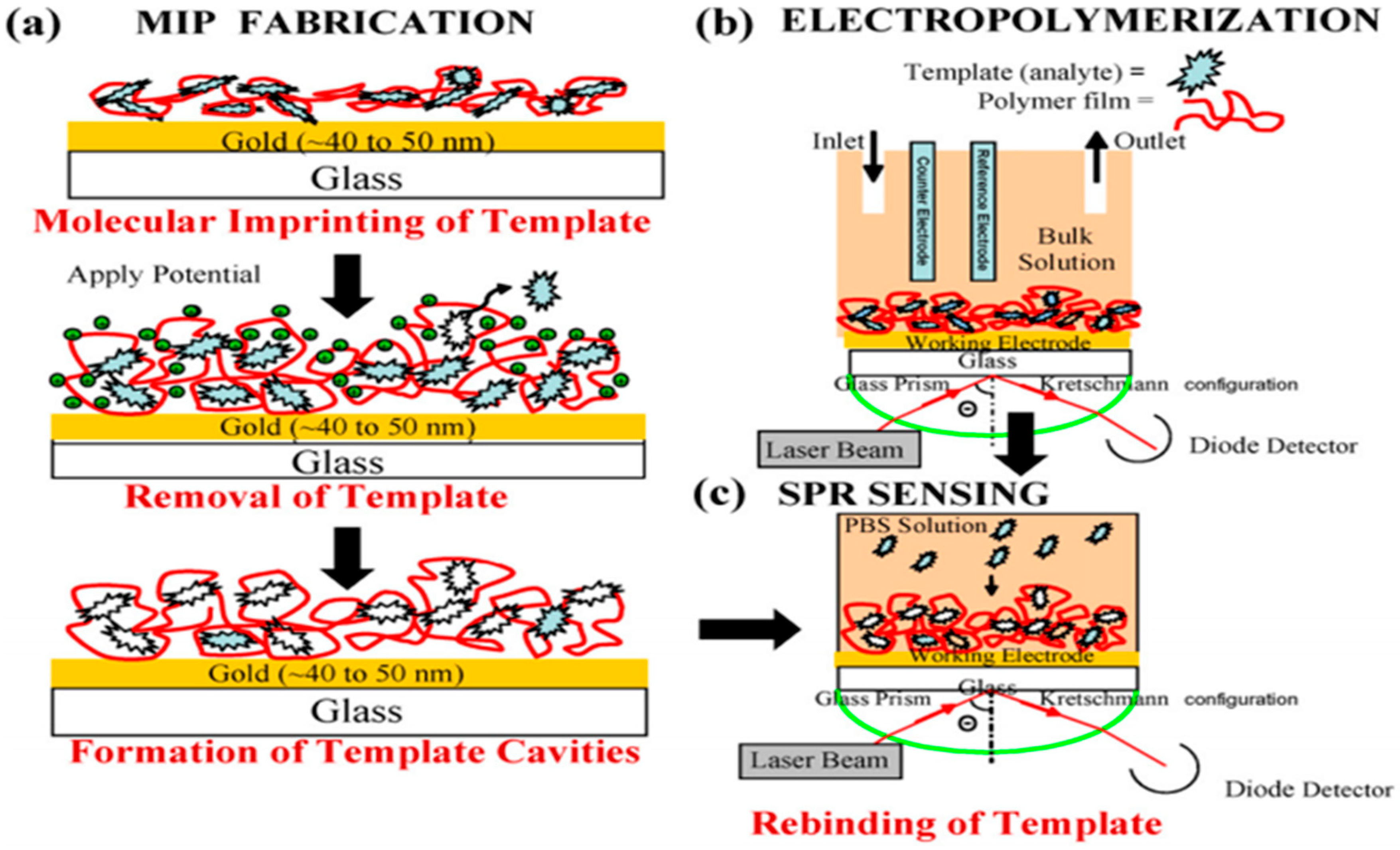
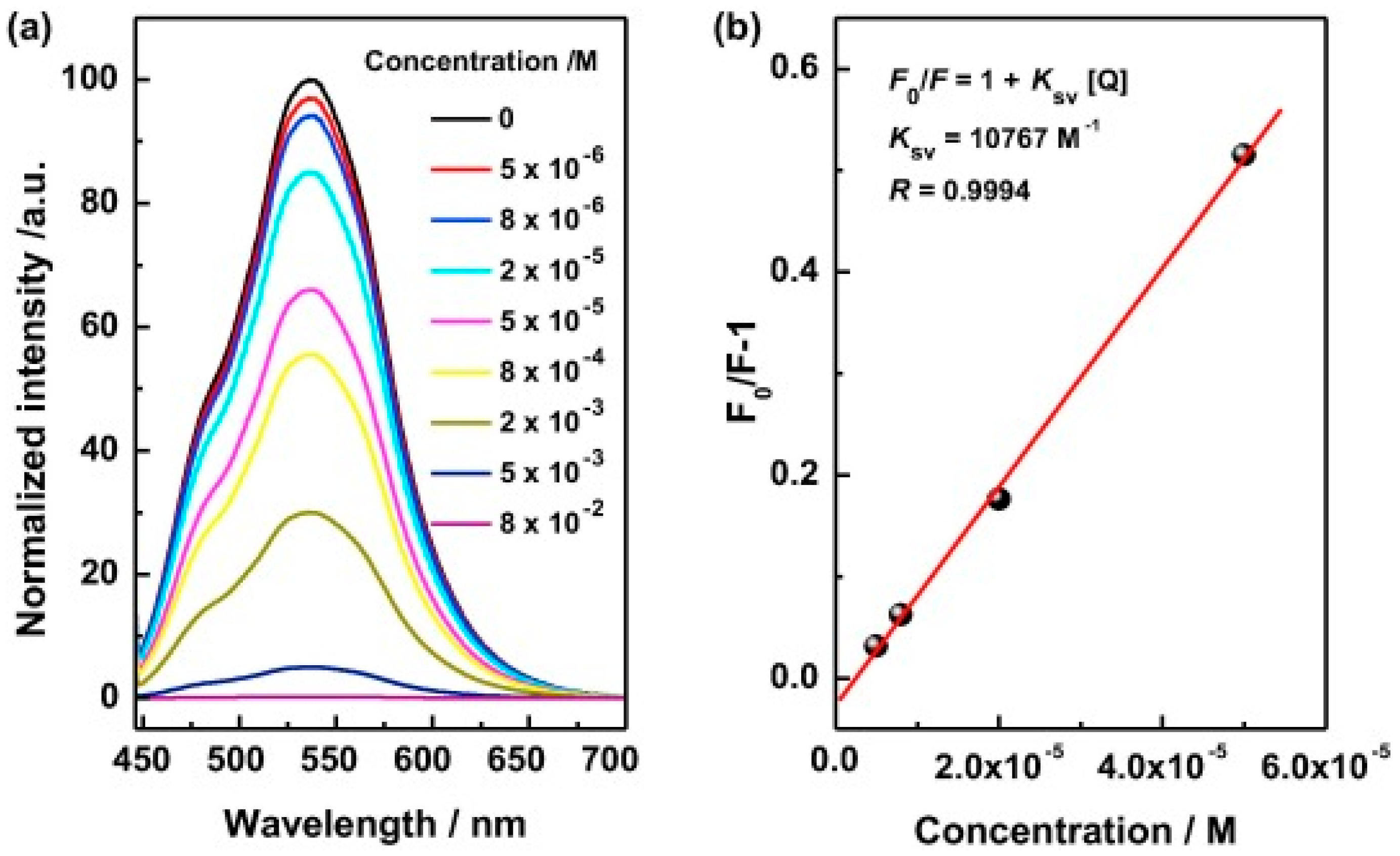
3.3. Biosensor Applications
3.4. Photovoltaic Devices Applications
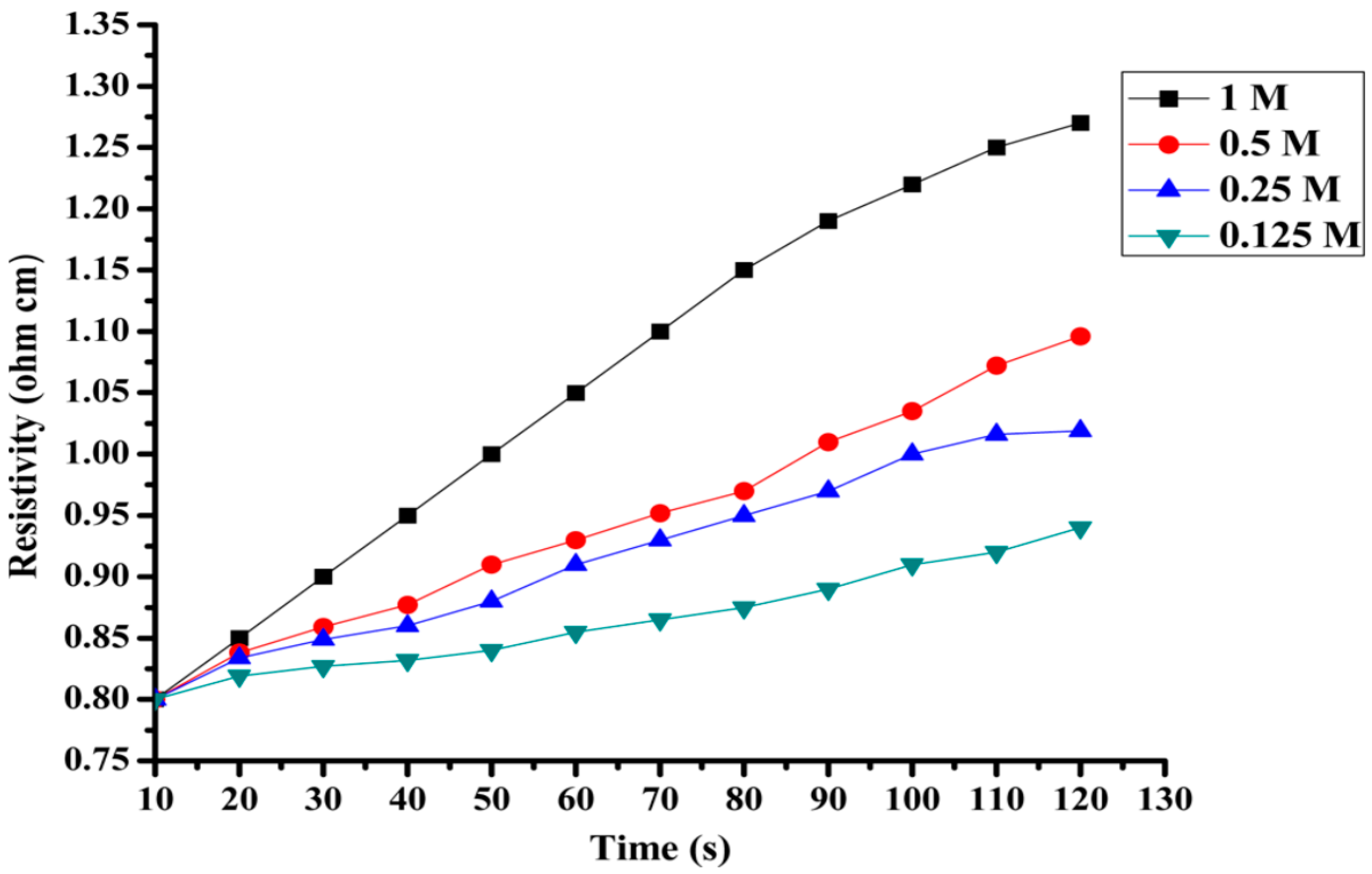
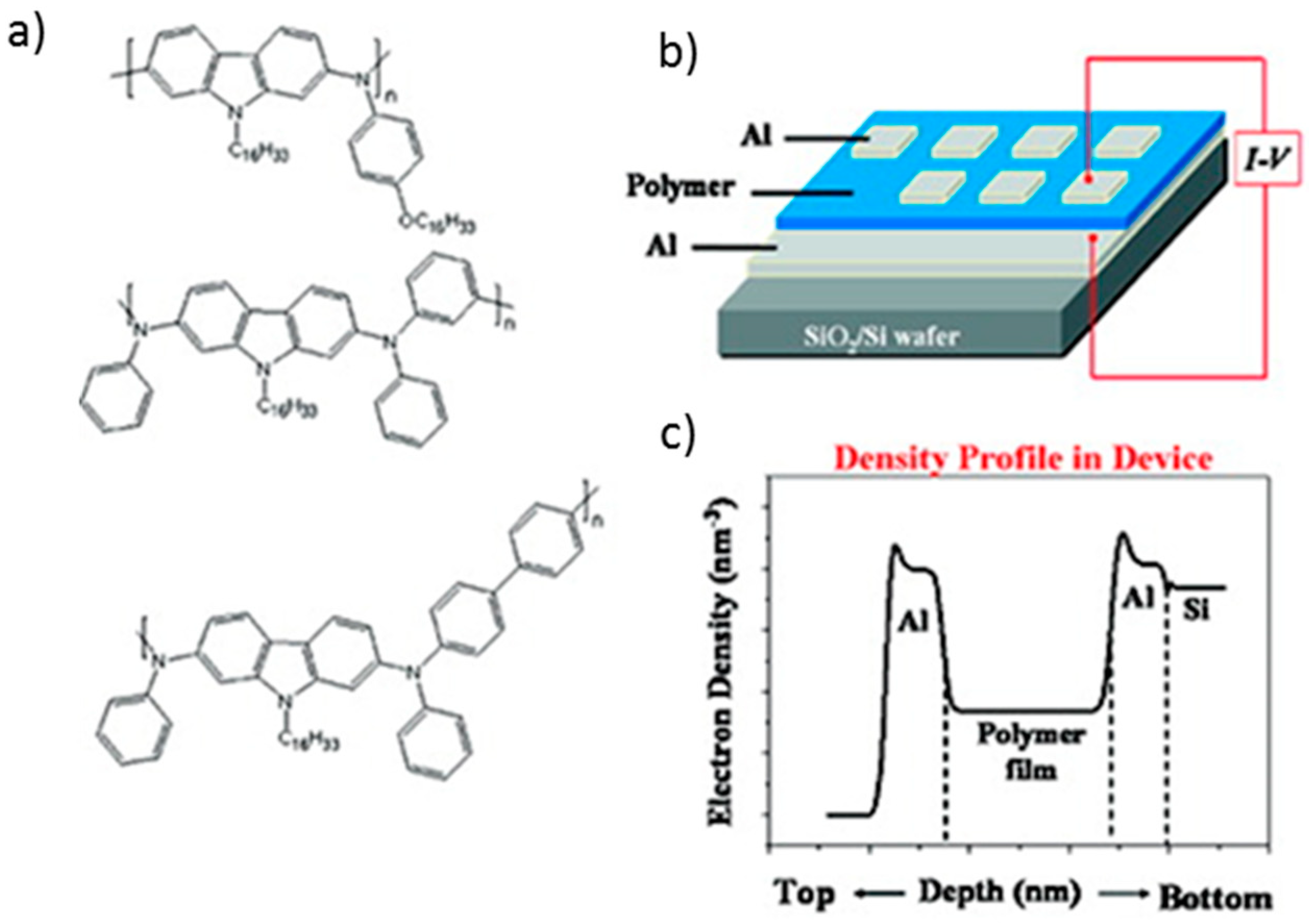
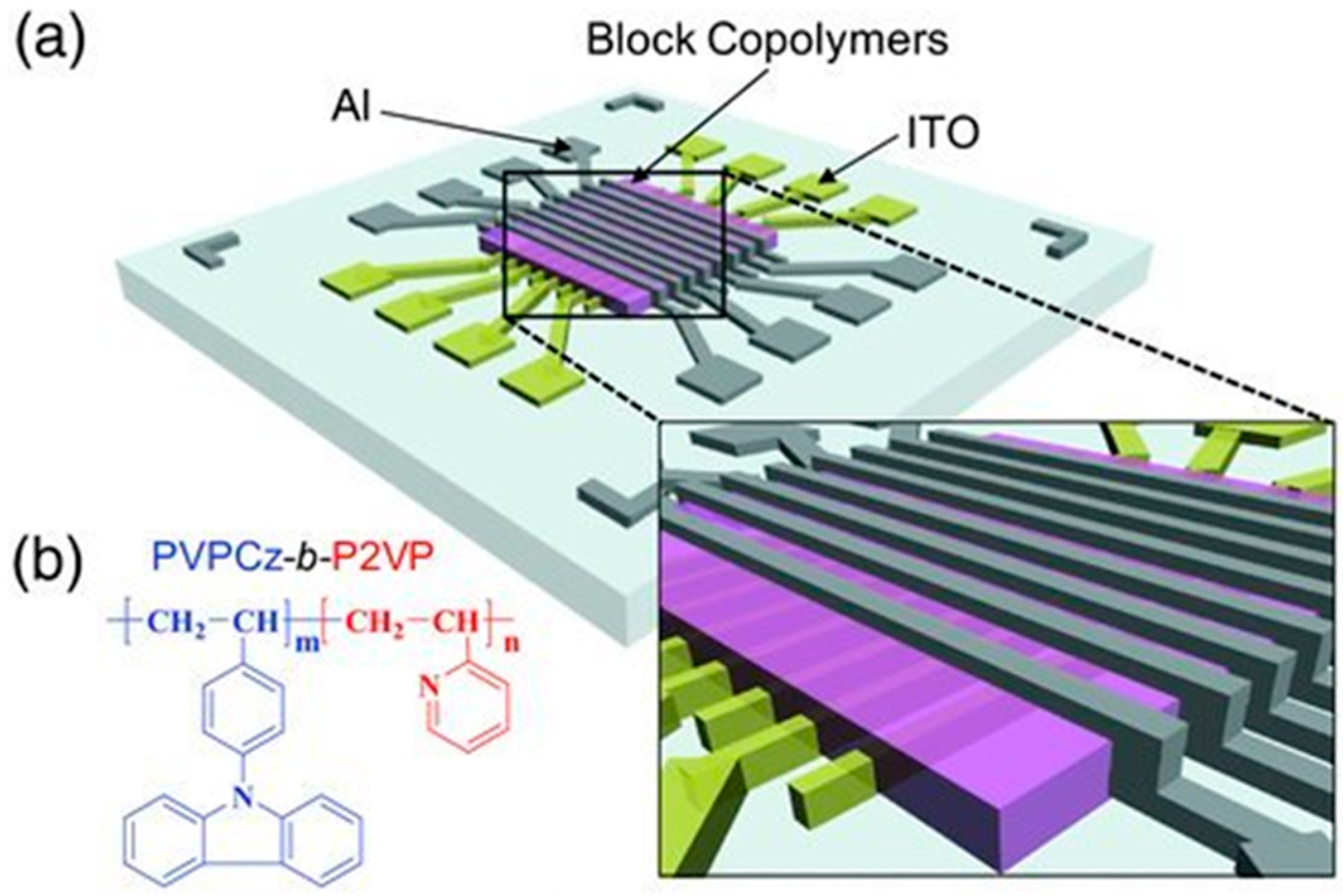
3.5. Memory Device Applications
4. Conclusions and Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chiang, C.K.; Fincher, C.R., Jr.; Park, Y.W.; Heeger, A.J.; Shirakawa, H.; Louis, E.J.; Gau, S.C.; MacDiarmid, A.G. Electrical conductivity in doped polyacetylene. Phys. Rev. Lett. 1977, 39, 1098. [Google Scholar] [CrossRef]
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene(CH)x. J. Chem. Soc. Chem. Commun. 1977, 578–580. [Google Scholar] [CrossRef]
- Zampetti, A.; Minotto, A.; Cacialli, F. Near-Infrared (NIR) Organic Light-Emitting Diodes (OLEDs): Challenges and Opportunities. Adv. Funct. Mater. 2019, 29, 1807623. [Google Scholar] [CrossRef]
- Izumi, S.; Higginbotham, H.F.; Nyga, A.; Stachelek, P.; Tohnai, N.; de Silva, P.; Data, P.; Takeda, Y.; Minakata, S. Thermally Activated Delayed Fluorescent Donor–Acceptor–Donor–Acceptor π-Conjugated Macrocycle for Organic Light-Emitting Diodes. J. Am. Chem. Soc. 2020, 142, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, C.; Godumala, M.; Choi, S.; Park, S.Y.; Cho, M.J.; Park, S.; Choi, D.H. Solution-processed thermally activated delayed fluorescence organic light-emitting diodes using a new polymeric emitter containing non-conjugated cyclohexane units. Polym. Chem. 2018, 9, 1318–1326. [Google Scholar] [CrossRef]
- AlSalhi, M.S.; Alam, J.; Dass, L.A.; Raja, M. Recent advances in conjugated polymers for light emitting devices. Int. J. Mol. Sci. 2011, 12, 2036–2054. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Guo, Y.; Liu, Y. Engineering of Amorphous Polymeric Insulators for Organic Field-Effect Transistors. Adv. Electron. Mater. 2017, 3, 1700157. [Google Scholar] [CrossRef]
- Kim, D.E.; Baeg, K.-J. Reduction Treatment of Molecular-Doped Polymer Semiconductors for High-Performance N-Channel Organic Field-Effect Transistors. J. Korean Phys. Soc. 2019, 75, 821–826. [Google Scholar] [CrossRef]
- Thuau, D.; Begley, K.; Dilmurat, R.; Ablat, A.; Wantz, G.; Ayela, C.; Abbas, M. Exploring the Critical Thickness of Organic Semiconductor Layer for Enhanced Piezoresistive Sensitivity in Field-Effect Transistor Sensors. Materials 2020, 13, 1583. [Google Scholar] [CrossRef]
- Baeg, K.-J.; Khim, D.; Kim, J.; Kim, D.-Y.; Sung, S.-W.; Yang, B.-D.; Noh, Y.-Y. Flexible complementary logic gates using inkjet-printed polymer field-effect transistors. IEEE Electron Device Lett. 2012, 34, 126–128. [Google Scholar] [CrossRef]
- Golmar, F.; Gobbi, M.; Llopis, R.; Stoliar, P.; Casanova, F.; Hueso, L.E. Non-conventional metallic electrodes for organic field-effect transistors. Org. Electron. 2012, 13, 2301–2306. [Google Scholar] [CrossRef]
- Keshtov, M.L.; Kuklin, S.A.; Konstantinov, I.O.; Khokhlov, A.R.; Dou, C.; Sharma, G.D. Synthesis and Characterization of Wide-Bandgap Conjugated Polymers Consisting of Same Electron Donor and Different Electron-Deficient Units and Their Application for Nonfullerene Polymer Solar Cells. Macromol. Chem. Phys. 2020, 221, 2000030. [Google Scholar] [CrossRef]
- Bildirir, H.; Di Carlo Rasi, D.; Wienk, M.M.; Janssen, R.A.J.; Avgeropoulos, A.; Gregoriou, V.G.; Allard, S.; Scherf, U.; Chochos, C.L. New n-Type Solution Processable All Conjugated Polymer Network: Synthesis, Optoelectronic Characterization, and Application in Organic Solar Cells. Macromol. Rapid Commun. 2018, 39, 1700629. [Google Scholar] [CrossRef] [PubMed]
- Scharber, M.C. On the efficiency limit of conjugated polymer: Fullerene-based bulk heterojunction solar cells. Adv. Mater. 2016, 28, 1994–2001. [Google Scholar] [CrossRef]
- Jung, E.H.; Ahn, H.; Jo, W.H.; Jo, J.W.; Jung, J.W. Isoindigo-based conjugated polymer for high-performance organic solar cell with a high VOC of 1.06 V as processed from non-halogenated solvent. Dye. Pigment. 2019, 161, 113–118. [Google Scholar] [CrossRef]
- Wong, Y.-T.; Lin, P.-C.; Tseng, C.-W.; Huang, Y.-W.; Su, Y.-A.; Chen, W.-C.; Chueh, C.-C. Biaxially-extended side-chain engineering of benzodithiophene-based conjugated polymers and their applications in polymer solar cells. Org. Electron. 2020, 79, 105630. [Google Scholar] [CrossRef]
- Facchetti, A. π-Conjugated polymers for organic electronics and photovoltaic cell applications. Chem. Mater. 2011, 23, 733–758. [Google Scholar] [CrossRef]
- Sun, L.; Xu, X.; Song, S.; Zhang, Y.; Miao, C.; Liu, X.; Xing, G.; Zhang, S. Medium-Bandgap Conjugated Polymer Donors for Organic Photovoltaics. Macromol. Rapid Commun. 2019, 40, 1900074. [Google Scholar] [CrossRef]
- An, C.; Zheng, Z.; Hou, J. Recent progress in wide bandgap conjugated polymer donors for high-performance nonfullerene organic photovoltaics. Chem. Commun. 2020, 56, 4750–4760. [Google Scholar] [CrossRef]
- Brebels, J.; Manca, J.V.; Lutsen, L.; Vanderzande, D.; Maes, W. High dielectric constant conjugated materials for organic photovoltaics. J. Mater. Chem. A 2017, 5, 24037–24050. [Google Scholar] [CrossRef]
- He, Y.; Hong, W.; Li, Y. New building blocks for π-conjugated polymer semiconductors for organic thin film transistors and photovoltaics. J. Mater. Chem. C 2014, 2, 8651–8661. [Google Scholar] [CrossRef]
- Jia, P.; Hu, T.; He, Q.; Cao, X.; Ma, J.; Fan, J.; Chen, Q.; Ding, Y.; Pyun, J.; Geng, J. Synthesis of a Macroporous Conjugated Polymer Framework: Iron Doping for Highly Stable, Highly Efficient Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2018, 11, 3087–3097. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, G.; Biswal, B.P.; Addicoat, M.; Paasch, S.; Sheng, W.; Zhuang, X.; Brunner, E.; Heine, T.; Berger, R.; et al. A Nitrogen-Rich 2D sp 2 -Carbon-Linked Conjugated Polymer Framework as a High-Performance Cathode for Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2019, 58, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Gu, P.; Zhang, Q. Nanostructured conjugated polymers: Toward high-performance organic electrodes for rechargeable batteries. ACS Energy Lett. 2017, 2, 1985–1996. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, C.; Gao, X.; Shu, C.; Yan, C.; Wang, F.; Chen, Y.; Zeng, J.H.; Jiang, J.-X. Structure evolution of azo-fused conjugated microporous polymers for high performance lithium-ion batteries anodes. J. Power Sources 2020, 453, 227868. [Google Scholar] [CrossRef]
- Gracia, R.; Mecerreyes, D. Polymers with redox properties: Materials for batteries, biosensors and more. Polym. Chem. 2013, 4, 2206–2214. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, L.; Liu, J.; Parvez, K.; Liang, H.; Shu, J.; Sachdev, H.; Graf, R.; Feng, X.; Müllen, K. High-performance electrocatalysts for oxygen reduction derived from cobalt porphyrin-based conjugated mesoporous polymers. Adv. Mater. 2014, 26, 1450–1455. [Google Scholar] [CrossRef]
- Pandey, R.K.; Lakshminarayanan, V. Ethanol electrocatalysis on gold and conducting polymer nanocomposites: A study of the kinetic parameters. Appl. Catal. B Environ. 2012, 125, 271–281. [Google Scholar] [CrossRef]
- Ramanavičius, A.; Ramanavičienė, A.; Malinauskas, A. Electrochemical sensors based on conducting polymer—Polypyrrole. Electrochim. Acta 2006, 51, 6025–6037. [Google Scholar] [CrossRef]
- Luo, J.-H.H.; Li, Q.; Chen, S.-H.H.; Yuan, R. Coreactant-Free Dual Amplified Electrochemiluminescent Biosensor Based on Conjugated Polymer Dots for the Ultrasensitive Detection of MicroRNA. ACS Appl. Mater. Interfaces 2019, 11, 27363–27370. [Google Scholar] [CrossRef]
- Mercante, L.A.; Scagion, V.P.; Migliorini, F.L.; Mattoso, L.H.C.; Correa, D.S. Electrospinning-based (bio) sensors for food and agricultural applications: A review. TrAC Trends Anal. Chem. 2017, 91, 91–103. [Google Scholar] [CrossRef]
- Ates, M. A review study of (bio)sensor systems based on conducting polymers. Mater. Sci. Eng. C 2013, 33, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- McCullough, R.D. The chemistry of conducting polythiophenes. Adv. Mater. 1998, 10, 93–116. [Google Scholar] [CrossRef]
- Leclerc, M. Optical and electrochemical transducers based on functionalized conjugated polymers. Adv. Mater. 1999, 11, 1491–1498. [Google Scholar] [CrossRef]
- Watanabe, A.; Murakami, S.; Mori, K.; Kashiwaba, Y. Electronic properties of polypyrrole/n-Si heterojunctions and polypyrrole/metal contacts. Macromolecules 1989, 22, 4231–4235. [Google Scholar] [CrossRef]
- Rehahn, M.; Schlüter, A.-D.; Wegner, G.; Feast, W.J. Soluble poly (para-phenylene) s. 1. Extension of the Yamamoto synthesis to dibromobenzenes substituted with flexible side chains. Polymer 1989, 30, 1054–1059. [Google Scholar] [CrossRef]
- Burroughes, J.H.; Bradley, D.D.C.; Brown, A.R.; Marks, R.N.; Mackay, K.; Friend, R.H.; Burns, P.L.; Holmes, A.B. Light-emitting diodes based on conjugated polymers. Nature 1990, 347, 539–541. [Google Scholar] [CrossRef]
- Leclerc, M. Polyfluorenes: Twenty years of progress. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 2867–2873. [Google Scholar] [CrossRef]
- Yang, Y.; Pei, Q.; Heeger, A.J. Efficient blue light-emitting diodes from a soluble poly (para-phenylene) internal field emission measurement of the energy gap in semiconducting polymers. Synth. Met. 1996, 78, 263–267. [Google Scholar] [CrossRef]
- Ranger, M.; Rondeau, D.; Leclerc, M. New well-defined poly (2, 7-fluorene) derivatives: Photoluminescence and base doping. Macromolecules 1997, 30, 7686–7691. [Google Scholar] [CrossRef]
- Neher, D. Polyfluorene homopolymers: Conjugated liquid-crystalline polymers for bright blue emission and polarized electroluminescence. Macromol. Rapid Commun. 2001, 22, 1365–1385. [Google Scholar] [CrossRef]
- Collin, G.; Höke, H.; Talbiersky, J. Carbazole. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; pp. 565–582. ISBN 9783527306732. [Google Scholar]
- Bashir, M.; Bano, A.; Ijaz, A.S.; Chaudhary, B.A. Recent developments and biological activities of n-substituted carbazole derivatives: A review. Molecules 2015, 20, 13496–13517. [Google Scholar] [CrossRef] [PubMed]
- Nandy, B.C.; Gupta, A.K.; Mittal, A.; Vyas, V. Carbazole: It’s biological activity. J. Biomed. Pharm. Res. 2014, 3, 42–48. [Google Scholar]
- Yavuz, Ö.; Sezer, E.; Saraç, A.S. Spectroelectrochemical study of N-ethyl-carbazole in the presence of acrylamide. Polym. Int. 2001, 50, 271–276. [Google Scholar] [CrossRef]
- Chen, C.-H.; Wang, Y.; Michinobu, T.; Chang, S.-W.; Chiu, Y.-C.; Ke, C.-Y.; Liou, G.-S. Donor–Acceptor Effect of Carbazole-Based Conjugated Polymer Electrets on Photoresponsive Flash Organic Field-Effect Transistor Memories. ACS Appl. Mater. Interfaces 2020, 12, 6144–6150. [Google Scholar] [CrossRef] [PubMed]
- Rice, N.A.; Bodnaryk, W.J.; Mirka, B.; Melville, O.A.; Adronov, A.; Lessard, B.H. Polycarbazole-Sorted Semiconducting Single-Walled Carbon Nanotubes for Incorporation into Organic Thin Film Transistors. Adv. Electron. Mater. 2019, 5, 1800539. [Google Scholar] [CrossRef]
- Soganci, T.; Baygu, Y.; Gök, Y.; Ak, M. Disulfide-linked symmetric N-alkyl carbazole derivative as a new electroactive monomer for electrochromic applications. Synth. Met. 2018, 244, 120–127. [Google Scholar] [CrossRef]
- Ates, M.; Özyilmaz, A.T.; Özyılmaz, A.T. The application of polycarbazole, polycarbazole/nanoclay and polycarbazole/Zn-nanoparticles as a corrosion inhibition for SS304 in saltwater. Prog. Org. Coat. 2015, 84, 50–58. [Google Scholar] [CrossRef]
- Sun, D.; Ren, Z.; Bryce, M.R.; Yan, S. Arylsilanes and siloxanes as optoelectronic materials for organic light-emitting diodes (OLEDs). J. Mater. Chem. C 2015, 3, 9496–9508. [Google Scholar] [CrossRef]
- Srivastava, A.; Chakrabarti, P. Experimental characterization of electrochemically polymerized polycarbazole film and study of its behavior with different metals contacts. Appl. Phys. A 2017, 123, 784. [Google Scholar] [CrossRef]
- Wu, C.-J.J.; Gaharwar, A.K.; Schexnailder, P.J.; Schmidt, G. Development of biomedical polymer-silicate nanocomposites: A materials science perspective. Materials 2010, 3, 2986–3005. [Google Scholar] [CrossRef]
- Pernites, R.; Ponnapati, R.; Felipe, M.J.; Advincula, R. Electropolymerization molecularly imprinted polymer (E-MIP) SPR sensing of drug molecules: Pre-polymerization complexed terthiophene and carbazole electroactive monomers. Biosens. Bioelectron. 2011, 26, 2766–2771. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Grimsdale, A.C. Carbazole-based polymers for organic photovoltaic devices. Chem. Soc. Rev. 2010, 39, 2399. [Google Scholar] [CrossRef] [PubMed]
- Ci, Z.; Yu, X.; Bao, M.; Wang, C.; Ma, T. Influence of the benzo [d] thiazole-derived π-bridges on the optical and photovoltaic performance of D–π–A dyes. Dye. Pigment. 2013, 96, 619–625. [Google Scholar] [CrossRef]
- El-Emary, T.I.; El-Aal, H.A.K.A.; Mohamed, S.K. Synthesis and Characterization of Assorted Heterocycles Based 3-(9Hcarbazol-9-yl) Propane Hydrazide. Chem. Sin. 2018, 9, 588–598. [Google Scholar]
- Zaia, E.W.; Gordon, M.P.; Yuan, P.; Urban, J.J. Progress and Perspective: Soft Thermoelectric Materials for Wearable and Internet-of-Things Applications. Adv. Electron. Mater. 2019, 5, 1800823. [Google Scholar] [CrossRef]
- Nitani, M.; Nakayama, K.; Maeda, K.; Omori, M.; Uno, M. Organic temperature sensors based on conductive polymers patterned by a selective-wetting method. Org. Electron. 2019, 71, 164–168. [Google Scholar] [CrossRef]
- Zhang, Y.; Park, S.-J. Flexible organic thermoelectric materials and devices for wearable green energy harvesting. Polymers 2019, 11, 909. [Google Scholar] [CrossRef]
- Gao, M.; Li, L.; Song, Y. Inkjet printing wearable electronic devices. J. Mater. Chem. C 2017, 5, 2971–2993. [Google Scholar] [CrossRef]
- Jeerapan, I.; Poorahong, S. Review—Flexible and Stretchable Electrochemical Sensing Systems: Materials, Energy Sources, and Integrations. J. Electrochem. Soc. 2020, 167, 037573. [Google Scholar] [CrossRef]
- Amjadi, M.; Kyung, K.; Park, I.; Sitti, M. Stretchable, skin-mountable, and wearable strain sensors and their potential applications: A review. Adv. Funct. Mater. 2016, 26, 1678–1698. [Google Scholar] [CrossRef]
- Zeng, W.; Shu, L.; Li, Q.; Chen, S.; Wang, F.; Tao, X. Fiber-based wearable electronics: A review of materials, fabrication, devices, and applications. Adv. Mater. 2014, 26, 5310–5336. [Google Scholar] [CrossRef] [PubMed]
- Di, J.; Zhang, X.; Yong, Z.; Zhang, Y.; Li, D.; Li, R.; Li, Q. Carbon-nanotube fibers for wearable devices and smart textiles. Adv. Mater. 2016, 28, 10529–10538. [Google Scholar] [CrossRef] [PubMed]
- Russ, B.; Glaudell, A.; Urban, J.J.; Chabinyc, M.L.; Segalman, R.A. Organic thermoelectric materials for energy harvesting and temperature control. Nat. Rev. Mater. 2016, 1, 1–14. [Google Scholar] [CrossRef]
- Khan, Y.; Ostfeld, A.E.; Lochner, C.M.; Pierre, A.; Arias, A.C. Monitoring of vital signs with flexible and wearable medical devices. Adv. Mater. 2016, 28, 4373–4395. [Google Scholar] [CrossRef]
- Dong, L.; Xu, C.; Li, Y.; Huang, Z.-H.; Kang, F.; Yang, Q.-H.; Zhao, X. Flexible electrodes and supercapacitors for wearable energy storage: A review by category. J. Mater. Chem. A 2016, 4, 4659–4685. [Google Scholar] [CrossRef]
- Kim, J.; Kumar, R.; Bandodkar, A.J.; Wang, J. Advanced materials for printed wearable electrochemical devices: A review. Adv. Electron. Mater. 2017, 3, 1600260. [Google Scholar] [CrossRef]
- Heo, J.S.; Eom, J.; Kim, Y.; Park, S.K. Recent progress of textile-based wearable electronics: A comprehensive review of materials, devices, and applications. Small 2018, 14, 1703034. [Google Scholar] [CrossRef]
- Boudreault, P.-L.T.; Beaupré, S.; Leclerc, M. Polycarbazoles for plastic electronics. Polym. Chem. 2010, 1, 127–136. [Google Scholar] [CrossRef]
- Boudreault, P.-L.T.; Blouin, N.; Leclerc, M. Poly(2,7-carbazole)s and Related Polymers. In Polyfluorenes; Springer: Berlin/Heidelberg, Germany, 2008; pp. 99–124. [Google Scholar]
- Blouin, N.; Michaud, A.; Gendron, D.; Wakim, S.; Blair, E.; Neagu-Plesu, R.; Belletête, M.; Durocher, G.; Tao, Y.; Leclerc, M. Toward a Rational Design of Poly(2,7-Carbazole) Derivatives for Solar Cells. J. Am. Chem. Soc. 2008, 130, 732–742. [Google Scholar] [CrossRef]
- Wakim, S.; Aïch, B.; Tao, Y.; Leclerc, M. Charge Transport, Photovoltaic, and Thermoelectric Properties of Poly(2,7-Carbazole) and Poly(Indolo[3,2-b]Carbazole) Derivatives. Polym. Rev. 2008, 48, 432–462. [Google Scholar] [CrossRef]
- Morin, J.-F.; Leclerc, M.; Adès, D.; Siove, A. Polycarbazoles: 25 Years of Progress. Macromol. Rapid Commun. 2005, 26, 761–778. [Google Scholar] [CrossRef]
- Houben, J.L.; Natucci, B.; Solaro, R.; Colella, O.; Chiellini, E.; Ledwith, A. Optically active vinyl polymers containing fluorescent groups: 5. Fluorescence properties of poly(9-vinyl carbazole) and optically active polymers containing carbazole units. Polymer 1978, 19, 811–818. [Google Scholar] [CrossRef]
- Murphy, S.M.; Hamilton, C.J.; Davies, M.L.; Tighe, B.J. Polymer membranes in clinical sensor applications. Biomaterials 1992, 13, 979–990. [Google Scholar] [CrossRef]
- Grazulevicius, J.V.V.; Strohriegl, P.; Pielichowski, J.; Pielichowski, K. Carbazole-containing polymers: Synthesis, properties and applications. Prog. Polym. Sci. 2003, 28, 1297–1353. [Google Scholar] [CrossRef]
- Ding, J.; Gao, J.; Cheng, Y.; Xie, Z.; Wang, L.; Ma, D.; Jing, X.; Wang, F. Highly Efficient Green-Emitting Phosphorescent Iridium Dendrimers Based on Carbazole Dendrons. Adv. Funct. Mater. 2006, 16, 575–581. [Google Scholar] [CrossRef]
- Faridbod, F.; Norouzi, P.; Dinarvand, R.; Ganjali, M. Developments in the Field of Conducting and Non-conducting Polymer Based Potentiometric Membrane Sensors for Ions Over the Past Decade. Sensors 2008, 8, 2331–2412. [Google Scholar] [CrossRef]
- Zou, Y.; Gendron, D.; Badrou-Aïch, R.; Najari, A.; Tao, Y.; Leclerc, M. A High-Mobility Low-Bandgap Poly(2,7-carbazole) Derivative for Photovoltaic Applications. Macromolecules 2009, 42, 2891–2894. [Google Scholar] [CrossRef]
- Ates, M.; Sarac, A.S. Conducting polymer coated carbon surfaces and biosensor applications. Prog. Org. Coat. 2009, 66, 337–358. [Google Scholar] [CrossRef]
- Beaupré, S.; Boudreault, P.-L.T.; Leclerc, M. Solar-Energy Production and Energy-Efficient Lighting: Photovoltaic Devices and White-Light-Emitting Diodes Using Poly(2,7-fluorene), Poly(2,7-carbazole), and Poly(2,7-dibenzosilole) Derivatives. Adv. Mater. 2010, 22, E6–E27. [Google Scholar] [CrossRef]
- Dubey, N.; Leclerc, M. Conducting polymers: Efficient thermoelectric materials. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 467–475. [Google Scholar] [CrossRef]
- Gendron, D.; Leclerc, M. New conjugated polymers for plastic solar cells. Energy Environ. Sci. 2011, 4, 1225. [Google Scholar] [CrossRef]
- Grigoras, A.G. A review on medical applications of poly(N-vinylcarbazole) and its derivatives. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 888–900. [Google Scholar] [CrossRef]
- Tan, S.E.; Sarjadi, M.S. The recent development of carbazole-, benzothiadiazole-, and isoindigo-based copolymers for solar cells application: A review. Polym. Sci. Ser. B 2017, 59, 479–496. [Google Scholar] [CrossRef]
- Liguori, R.; Botta, A.; Rubino, A.; Pragliola, S.; Venditto, V. Stereoregular polymers with pendant carbazolyl groups: Synthesis, properties and optoelectronic applications. Synth. Met. 2018, 246, 185–194. [Google Scholar] [CrossRef]
- Ghorbani Zamani, F.; Moulahoum, H.; Ak, M.; Odaci Demirkol, D.; Timur, S. Current trends in the development of conducting polymers-based biosensors. TrAC Trends Anal. Chem. 2019, 118, 264–276. [Google Scholar] [CrossRef]
- Perepichka, D.F.; Perepichka, I.F.; Meng, H.; Wudl, F. Light-emitting polymers. In Organic Light-Emitting Materials and Devices; CRC Press: Boca Raton, FL, USA, 2006; pp. 45–293. ISBN 9781420017069. [Google Scholar]
- Yemata, T.A.; Ye, Q.; Zhou, H.; Kyaw, A.K.K.; Chin, W.S.; Xu, J. Conducting polymer-based thermoelectric composites. In Hybrid Polymer Composite Materials; Elsevier: Amsterdam, The Netherlands, 2017; pp. 169–195. [Google Scholar]
- Li, Z.R. (Ed.) Organic Light-Emitting Materials and Devices; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781315216775. [Google Scholar]
- Gilbert, M. Miscellaneous Vinyl Thermoplastics. In Brydson’s Plastics Materials; Elsevier: Amsterdam, The Netherlands, 2017; pp. 427–440. [Google Scholar]
- Michinobu, T.; Osako, H.; Shigehara, K. Synthesis and Properties of 1,8-Carbazole-Based Conjugated Copolymers. Polymers 2010, 2, 159–173. [Google Scholar] [CrossRef]
- Michinobu, T.; Osako, H.; Shigehara, K. Synthesis and Properties of Conjugated Poly(1,8-carbazole)s. Macromolecules 2009, 42, 8172–8180. [Google Scholar] [CrossRef]
- Branch, G.E.K.; Smith, J.F. A bivalent nitrogen derivative of carbazole. J. Am. Chem. Soc. 1920, 42, 2405–2413. [Google Scholar] [CrossRef]
- Perkin, W.H.; Tucker, S.H. XXVI.—The oxidation of carbazole. J. Chem. Soc. Trans. 1921, 119, 216–225. [Google Scholar] [CrossRef]
- Branch, G.E.K.; Hall, W.W. Oxidation of carbazole by silver oxide. J. Am. Chem. Soc. 1924, 46, 438–445. [Google Scholar] [CrossRef]
- Aoai, T.; Nishio, R.; Hayashi, N.; Nomura, K. Photo Doping Process of Conductive Polymer with PAG and Application for Organic Thermoelectric Materials. J. Photopolym. Sci. Technol. 2016, 29, 335–341. [Google Scholar] [CrossRef]
- Sangwan, W.; Paradee, N.; Sirivat, A. Polycarbazole by chemical oxidative interfacial polymerization: Morphology and electrical conductivity based on synthesis conditions. Polym. Int. 2016, 65, 1232–1237. [Google Scholar] [CrossRef]
- Shakir, M.; Noor-e-Iram; Khan, M.S.; Al-Resayes, S.I.; Khan, A.A.; Baig, U. Electrical Conductivity, Isothermal Stability, and Ammonia-Sensing Performance of Newly Synthesized and Characterized Organic–Inorganic Polycarbazole–Titanium Dioxide Nanocomposite. Ind. Eng. Chem. Res. 2014, 53, 8035–8044. [Google Scholar] [CrossRef]
- Baig, U.; Wani, W.A.; Ting Hun, L. Facile synthesis of an electrically conductive polycarbazole–zirconium(IV)phosphate cation exchange nanocomposite and its room temperature ammonia sensing performance. New J. Chem. 2015, 39, 6882–6891. [Google Scholar] [CrossRef]
- Gupta, B.; Prakash, R. Interfacial polymerization of carbazole: Morphology controlled synthesis. Synth. Met. 2010, 160, 523–528. [Google Scholar] [CrossRef]
- Sun, Q.; Deng, Y. In situ synthesis of temperature-sensitive hollow microspheres via interfacial polymerization. J. Am. Chem. Soc. 2005, 127, 8274–8275. [Google Scholar] [CrossRef]
- Gupta, B.; Joshi, L.; Prakash, R. Novel Synthesis of Polycarbazole-Gold Nanocomposite. Macromol. Chem. Phys. 2011, 212, 1692–1699. [Google Scholar] [CrossRef]
- Gupta, B.; Prakash, R.; Melvin, A. Chemical Synthesis of Polycarbazole (PCz), modification and pH sensor application. Proc. Int. Conf. Sens. Technol. ICST 2012, 365–369. [Google Scholar] [CrossRef]
- Kumar, A.; Tiwari, M.; Prakash, R. Electrochemical Study of Interfacially Synthesized Polycarbazole with Different Oxidants. ChemElectroChem 2015, 2, 2001–2010. [Google Scholar] [CrossRef]
- Balun Kayan, D.; Polat, V. Improvement of electrochemical and structural properties of polycarbazole by simultaneous electrodeposition of chitosan. TURKISH J. Chem. 2017, 41, 233–242. [Google Scholar] [CrossRef]
- Li, C.; Xue, J.; Huang, A.; Ma, J.; Qing, F.; Zhou, A.; Wang, Z.; Wang, Y.; Li, J. Poly (N-vinylcarbazole) as an advanced organic cathode for potassium-ion-based dual-ion battery. Electrochim. Acta 2019, 297, 850–855. [Google Scholar] [CrossRef]
- Raies, A. Elaboration de Films Minces Électroluminescents à Base de Polymère Conducteur Électronique et de Nanotubes de Carbone. Ph.D. Thesis, Université Paris-Saclay, Saint-Aubin, France, 2015. [Google Scholar]
- Zotti, G.; Schiavon, G. Poly(2,5-thienylene)-coated electrodes formed by electroreduction of a nickel adduct with 2,5-dibromothiophene. J. Electroanal. Chem. Interfacial Electrochem. 1984, 163, 385–388. [Google Scholar] [CrossRef]
- Schiavon, G.; Zotti, G.; Bontempelli, G. An electroactive nickel containing polymeric film obtained by electrochemical reduction of an aryl-nickel derivative. J. Electroanal. Chem. Interfacial Electrochem. 1984, 161, 323–335. [Google Scholar] [CrossRef]
- Xu, Z.; Horowitz, G.; Garnier, F. Cathodic electropolymerization of polythiophene on platinum and various semiconducting electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1988, 246, 467–472. [Google Scholar] [CrossRef]
- Id, H.A.L. Synthèse Par Voie Électrochimique de Nanostructures de Polymères Conducteurs Sans Emploi d ’ une Matrice Support: Applications Aux (bio) Capteurs. Ph.D. Thesis, Université Pierre et Marie Curie—Paris VI, Paris, France, 2014. [Google Scholar]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef]
- Ambrose, J.F.; Nelson, R.F. Anodic Oxidation Pathways of Carbazoles. J. Electrochem. Soc. 1968, 115, 1159. [Google Scholar] [CrossRef]
- Lévesque, I.; Bertrand, P.O.; Blouin, N.; Leclerc, M.; Zecchin, S.; Zotti, G.; Ratcliffe, C.I.; Klug, D.D.; Gao, X.; Gao, F.; et al. Synthesis and thermoelectric properties of polycarbazole, polyindolocarbazole, and polydiindolocarbazole derivatives. Chem. Mater. 2007, 19, 2128–2138. [Google Scholar] [CrossRef]
- Zotti, G.; Schiavon, G.; Zecchin, S.; Morin, J.F.; Leclerc, M. Electrochemical, conductive, and magnetic properties of 2,7-carbazole-based conjugated polymers. Macromolecules 2002, 35, 2122–2128. [Google Scholar] [CrossRef]
- Ates, M.; Sarac, A.S. Electrochemical impedance spectroscopy of poly[carbazole-co-N-p-tolylsulfonyl pyrrole] on carbon fiber microelectrodes, equivalent circuits for modelling. Prog. Org. Coat. 2009, 65, 281–287. [Google Scholar] [CrossRef]
- Penwell, R.C. Poly(N-vinylcarbazole): A Selective Review of its Polymerization, Structure, Properties, and electrical characteristics. J. Polym. Sci. Macromol. Rev. 1978, 13, 63–160. [Google Scholar] [CrossRef]
- Tran-Van, F.; Henri, T.; Chevrot, C. Synthesis and electrochemical properties of mixed ionic and electronic modified polycarbazole. Electrochim. Acta 2002, 47, 2927–2936. [Google Scholar] [CrossRef]
- Sotzing, G.A.; Reddinger, J.L.; Katritzky, A.R.; Soloducho, J.; Musgrave, R.; Reynolds, J.R.; Steel, P.J. Multiply Colored Electrochromic Carbazole-Based Polymers. Chem. Mater. 1997, 9, 1578–1587. [Google Scholar] [CrossRef]
- Reppe, W.; Keyssner, E. N-Vinyl Compounds. DRP 1935, 618, 120. [Google Scholar]
- Baethge, H.; Butz, S.; Schmidt-Naake, G. “Living” free radical copolymerization of styrene and N-vinylcarbazole. Macromol. Rapid Commun. 1997, 18, 911–916. [Google Scholar] [CrossRef]
- Kim, W.; Nishikawa, Y.; Watanabe, H.; Kanazawa, A.; Aoshima, S.; Fujii, A.; Ozaki, M. Stereoregularity effect on hole mobility in poly (N-vinylcarbazole) thin film evaluated by MIS-CELIV method. Jpn. J. Appl. Phys. 2019, 59, SDDA01. [Google Scholar] [CrossRef]
- Natori, I. Anionic Polymerization of N-Vinylcarbazole with Alkyllithium as an Initiator. Macromolecules 2006, 39, 6017–6024. [Google Scholar] [CrossRef]
- Wang, T.; Yang, C.; Shieh, Y.; Yeh, A. Synthesis of CdSe–Poly (N-vinylcarbazole) Nanocomposite by Atom Transfer Radical Polymerization for Potential Optoelectronic Applications. Macromol. Rapid Commun. 2009, 30, 1679–1683. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, K.; Mori, H. Recent progress in controlled radical polymerization of N-vinyl monomers. Eur. Polym. J. 2013, 49, 2808–2838. [Google Scholar] [CrossRef]
- Hawker, C.J.; Bosman, A.W.; Harth, E. New polymer synthesis by nitroxide mediated living radical polymerizations. Chem. Rev. 2001, 101, 3661–3688. [Google Scholar] [CrossRef]
- Natsuume, T.; Akana, Y.; Tanabe, K.; Fujimatsu, M.; Shimizu, M.; Shirota, Y.; Hirata, H.; Kusabayashi, S.; Mikawa, H. Mechanism of charge-transfer polymerizations: Polymerization of N-vinylcarbazole with tetrachloro-p-benzoquinone in benzene. J. Chem. Soc. D Chem. Commun. 1969, 189a. [Google Scholar] [CrossRef]
- Hazra, D.K.; Chatterjee, R. In situ solid state polymerization and characterization of poly (N-vinylcarbazole) encapsulated Keggin type polyoxometalate nanocomposite. J. Mol. Struct. 2013, 1045, 139–144. [Google Scholar] [CrossRef]
- Hurtgen, M.; Detrembleur, C.; Jerome, C.; Debuigne, A. Insight into organometallic-mediated radical polymerization. Polym. Rev. 2011, 51, 188–213. [Google Scholar] [CrossRef]
- Marimuthu, E.; Murugesan, V. Influence of ultrasound on multi-site phase transfer catalyzed polymerization of N-vinyl carbazole in two phase system. SN Appl. Sci. 2019, 1, 1–11. [Google Scholar] [CrossRef]
- Frau, A.F.; Pernites, R.B.; Advincula, R.C. A Conjugated Polymer Network Approach to Anticorrosion Coatings: Poly(vinylcarbazole) Electrodeposition. Ind. Eng. Chem. Res. 2010, 49, 9789–9797. [Google Scholar] [CrossRef]
- Huang, W.; Gu, C.C.; Wang, T.; Gu, C.C.; Qiao, S.; Yang, R. Effect of two facile synthetic strategies with alterable polymerization sequence on the performance of N-vinyl carbazole-based conjugated porous materials. RSC Adv. 2014, 4, 62525–62531. [Google Scholar] [CrossRef]
- Liu, G.; Chen, Y.; Li, R.W.; Zhang, B.; Kang, E.T.; Wang, C.; Zhuang, X. Resistance-Switchable Graphene Oxide-Polymer Nanocomposites for Molecular Electronics. ChemElectroChem 2014, 1, 514–519. [Google Scholar] [CrossRef]
- Santos, C.M.; Tria, M.C.R.; Vergara, R.A.M.V.; Cui, K.M.; Pernites, R.; Advincula, R.C. Films of Highly Disperse Electrodeposited Poly(N-vinylcarbazole)–Graphene Oxide Nanocomposites. Macromol. Chem. Phys. 2011, 212, 2371–2377. [Google Scholar] [CrossRef]
- Wang, C.; Xin, G.; Lee, Y.; Hao, J.; Jiang, J.; Liu, H. Poly (9-vinylcarbazole)/silver composite nanotubes and networks formed at the air–water interface. J. Appl. Polym. Sci. 2010, 116, 252–257. [Google Scholar] [CrossRef]
- Aydın, A.; Kaya, İ. Syntheses of novel copolymers containing carbazole and their electrochromic properties. J. Electroanal. Chem. 2013, 691, 1–12. [Google Scholar] [CrossRef]
- Aydın, A.; Kaya, İ.I. Synthesis and characterization of yellow and green light emitting novel polymers containing carbazole and electroactive moieties. Electrochim. Acta 2012, 65, 104–114. [Google Scholar] [CrossRef]
- Kocaeren, A.A. Synthesis and characterization of novel polymers based on carbazole with NaOCl and FeCl3 oxidants. Iran. Polym. J. 2016, 25, 15–24. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Lin, S.W. Electrochemical synthesis of electrochromic polycarbazole films from N-phenyl-3,6-bis(N-carbazolyl)carbazoles. Polym. Chem. 2016, 7, 198–211. [Google Scholar] [CrossRef]
- Kocaeren, A.A. Electrochemical synthesis and electrochromic application of a novel polymer based on carbazole. Org. Electron. 2015, 24, 219–226. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Hsueh, J.-C. Electrochemical synthesis and electrochromic properties of new conjugated polycarbazoles from di(carbazol-9-yl)-substituted triphenylamine and N-phenylcarbazole derivatives. J. Electroanal. Chem. 2015, 758, 100–110. [Google Scholar] [CrossRef]
- Yang, X.-L.L.; Hu, D.-Y.Y.; Chen, Q.; Li, L.; Li, P.-X.X.; Ren, S.-B.B.; Bertuzzo, M.; Chen, K.; Han, D.-M.M.; Zhou, X.-H.H.; et al. A pyrene-cored conjugated microporous polycarbazole for sensitive and selective detection of hazardous explosives. Inorg. Chem. Commun. 2019, 107, 107453. [Google Scholar] [CrossRef]
- Duran, B.; Çakmakcı Ünver, İ.; Bereket, G.; Ünver, İ.Ç.; Bereket, G. Inhibition of steel corrosion by potentiodynamic deposition of poly(N-methyl carbazole). J. Adhes. Sci. Technol. 2017, 31, 1467–1479. [Google Scholar] [CrossRef]
- Elkhidr, H.E.; Ertekin, Z.; Udum, Y.A.; Pekmez, K. Electrosynthesis and characterizations of electrochromic and soluble polymer films based on N-substituted carbazole derivates. Synth. Met. 2020, 260, 116253. [Google Scholar] [CrossRef]
- Qin, L.; Zhang, S.; Xu, J.; Lu, B.; Duan, X.; Zhu, D.; Huang, Y. Novel poly(ethylene oxide) grafted polycarbazole conjugated freestanding network films via anionic and electrochemical polymerization. Int. J. Electrochem. Sci. 2013, 8, 5299–5313. [Google Scholar]
- Li, W.; Otsuka, M.; Kato, T.; Wang, Y.; Mori, T.; Michinobu, T. 3,6-Carbazole vs 2,7-carbazole: A comparative study of hole-transporting polymeric materials for inorganic-organic hybrid perovskite solar cells. Beilstein J. Org. Chem. 2016, 12, 1401–1408. [Google Scholar] [CrossRef]
- Tao, X.-T.; Zhang, Y.-D.; Wada, T.; Sasabe, H.; Suzuki, H.; Watanabe, T.; Miyata, S. Hyperbranched Polymers for Electroluminescence Applications. Adv. Mater. 1998, 10, 226–230. [Google Scholar] [CrossRef]
- Aïch, R.B.; Blouin, N.; Bouchard, A.; Leclerc, M. Electrical and Thermoelectric Properties of Poly(2,7-Carbazole) Derivatives. Chem. Mater. 2009, 21, 751–757. [Google Scholar] [CrossRef]
- Morin, J.-F.; Leclerc, M. Syntheses of Conjugated Polymers Derived from N -Alkyl-2,7-carbazoles. Macromolecules 2001, 34, 4680–4682. [Google Scholar] [CrossRef]
- Nguyen, Q.; Baek, S.; Kim, M.; Shin, N.; Kim, G.; Choe, D.; Kwon, J.; Chai, K. Novel Hole Transporting Materials Based on 4-(9H-Carbazol-9-yl)triphenylamine Derivatives for OLEDs. Molecules 2014, 19, 14247–14256. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.-J.; Shen, Y.; Xie, F.-M.; Chen, J.-D.; Li, Y.-Q.; Tang, J.-X. Recent advances in organic light-emitting diodes: Toward smart lighting and displays. Mater. Chem. Front. 2020, 4, 788–820. [Google Scholar] [CrossRef]
- Hebner, T.R.; Wu, C.C.; Marcy, D.; Lu, M.H.; Sturm, J.C. Ink-jet printing of doped polymers for organic light emitting devices. Appl. Phys. Lett. 1998, 72, 519–521. [Google Scholar] [CrossRef]
- Krucaite, G.; Grigalevicius, S.; Gupta, B.; Joshi, L.; Prakash, R. A review on low-molar-mass carbazole- based derivatives for organic light emitting diodes. Synth. Met. 2019, 247, 90–108. [Google Scholar] [CrossRef]
- Bhuvana, K.P.; Joseph Bensingh, R.; Abdul Kader, M.; Nayak, S.K. Polymer light emitting diodes: Materials, technology and device. Polym. Plast. Technol. Eng. 2018, 57, 1784–1800. [Google Scholar] [CrossRef]
- Dumur, F. Carbazole-based polymers as hosts for solution-processed organic light-emitting diodes: Simplicity, efficacy. Org. Electron. 2015, 25, 345–361. [Google Scholar] [CrossRef]
- Syutkin, R.V.; Abashev, G.G.; Shklyaeva, E.V.; Kudryavtsev, P.G. New carbazole-containing chalcones and pyrimidines based thereon: Synthesis and electrochemical study. Russ. J. Org. Chem. 2011, 47, 530–536. [Google Scholar] [CrossRef]
- Wang, H.; Lin, J.; Shen, Z.X. Polyaniline (PANi) based electrode materials for energy storage and conversion. J. Sci. Adv. Mater. Devices 2016, 1, 225–255. [Google Scholar] [CrossRef]
- Li, J.; Cheng, X.; Shashurin, A.; Keidar, M. Review of Electrochemical Capacitors Based on Carbon Nanotubes and Graphene. Graphene 2012, 1, 1–13. [Google Scholar] [CrossRef]
- Chen, Q.; Shen, Y.; Zhang, S.; Zhang, Q.M. Polymer-Based Dielectrics with High Energy Storage Density. Annu. Rev. Mater. Res. 2015, 45, 433–458. [Google Scholar] [CrossRef]
- An, K.H.H.; Jeon, K.K.K.; Heo, J.K.K.; Lim, S.C.C.; Bae, D.J.J.; Lee, Y.H.H. High-Capacitance Supercapacitor Using a Nanocomposite Electrode of Single-Walled Carbon Nanotube and Polypyrrole. J. Electrochem. Soc. 2002, 149, A1058. [Google Scholar] [CrossRef]
- Rudge, A.; Raistrick, I.; Gottesfeld, S.; Ferraris, J.P. A study of the electrochemical properties of conducting polymers for application in electrochemical capacitors. Electrochim. Acta 1994, 39, 273–287. [Google Scholar] [CrossRef]
- Ryu, K.S.; Kim, K.M.; Park, N.-G.; Park, Y.J.; Chang, S.H. Symmetric redox supercapacitor with conducting polyaniline electrodes. J. Power Sources 2002, 103, 305–309. [Google Scholar] [CrossRef]
- Ates, M. Graphene and its nanocomposites used as an active materials for supercapacitors. J. Solid State Electrochem. 2016, 20, 1509–1526. [Google Scholar] [CrossRef]
- Yiğit, D.; Güllü, M. Capacitive properties of novel N-alkyl substituted poly(3,6-dithienyl-9H-carbazole)s as redox electrode materials and their symmetric micro-supercapacitor applications. Electrochim. Acta 2018, 282, 64–80. [Google Scholar] [CrossRef]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Ates, M.; Uludag, N. Poly(9H-Carbazole-9-Carbothioic Dithioperoxyanhydride) Formation and Capacitor Study. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 755–761. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, Z.; Liao, Y.; Li, J.; Weber, J.; Thomas, A.; Faul, C.F.J. Conjugated Microporous Polycarbazole Networks as Precursors for Nitrogen-Enriched Microporous Carbons for CO 2 Storage and Electrochemical Capacitors. Chem. Mater. 2017, 29, 4885–4893. [Google Scholar] [CrossRef]
- Duran, B.; Ünver, İ.Ç.; Bereket, G. Investigation of Supporting Electrolyte Effect on Supercapacitor Properties of Poly (Carbazole) Films. J. Electrochem. Sci. Technol. 2020, 11, 41–49. [Google Scholar] [CrossRef]
- Zhang, B.; Li, B.; Wang, Z. Creation of Carbazole-Based Fluorescent Porous Polymers for Recognition and Detection of Various Pesticides in Water. ACS Sens. 2020, 5, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Dai, Z.; Li, J.; Wang, H.; Huang, M.-R.R.; Li, X.-G.G.; Liao, Y. Template-free synthesis of tunable hollow microspheres of aniline and aminocarbazole copolymers emitting colorful fluorescence for ultrasensitive sensors. Chem. Eng. J. 2019, 357, 776–786. [Google Scholar] [CrossRef]
- Sathiyan, G.; Sivakumar, E.K.T.K.T.; Ganesamoorthy, R.; Thangamuthu, R.; Sakthivel, P. Review of carbazole based conjugated molecules for highly efficient organic solar cell application. Tetrahedron Lett. 2016, 57, 243–252. [Google Scholar] [CrossRef]
- Zafer, C.; Gultekin, B.; Ozsoy, C.; Tozlu, C.; Aydin, B.; Icli, S. Carbazole-based organic dye sensitizers for efficient molecular photovoltaics. Sol. Energy Mater. Sol. Cells 2010, 94, 655–661. [Google Scholar] [CrossRef]
- Akhundi, A.; Habibi-Yangjeh, A.; Abitorabi, M.; Rahim Pouran, S. Review on photocatalytic conversion of carbon dioxide to value-added compounds and renewable fuels by graphitic carbon nitride-based photocatalysts. Catal. Rev. 2019, 61, 595–628. [Google Scholar] [CrossRef]
- Zhang, Z.; Liao, M.; Lou, H.; Hu, Y.; Sun, X.; Peng, H. Conjugated Polymers for Flexible Energy Harvesting and Storage. Adv. Mater. 2018, 30, 1–19. [Google Scholar] [CrossRef]
- Islam, G.M.N.; Ali, A.; Collie, S. Textile sensors for wearable applications: A comprehensive review. Cellulose 2020, 27, 6103–6131. [Google Scholar] [CrossRef]
- Fujita, H.; Michinobu, T. Synthesis and photovoltaic properties of 1,8-carbazole-based donor-acceptor type conjugated polymers. Macromol. Chem. Phys. 2012, 213, 447–457. [Google Scholar] [CrossRef]
- Qin, R.; Yang, J.; Li, P.; Wu, Q.; Zhou, Y.; Luo, H.; Chang, F. Structure property relationship for carbazole and benzothiadiazole based conjugated polymers. Sol. Energy Mater. Sol. Cells 2016, 145, 412–417. [Google Scholar] [CrossRef]
- Yen, H.-J.J.; Shan, C.; Wang, L.; Xu, P.; Zhou, M.; Wang, H.-L.L. Development of conjugated polymers for memory device applications. Polymers 2017, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-L.; Chen, W.-C. Donor–acceptor polymers for advanced memory device applications. Polym. Chem. 2011, 2, 2169. [Google Scholar] [CrossRef]
- Wu, H.-C.; Yu, A.-D.; Lee, W.-Y.; Liu, C.-L.; Chen, W.-C. A poly (fluorene-thiophene) donor with a tethered phenanthro [9, 10-d] imidazole acceptor for flexible nonvolatile flash resistive memory devices. Chem. Commun. 2012, 48, 9135–9137. [Google Scholar] [CrossRef]
- Elsawy, W.; Son, M.; Jang, J.; Kim, M.J.; Ji, Y.; Kim, T.W.; Ko, H.C.; Elbarbary, A.; Ham, M.H.; Lee, J.S. Isoindigo-Based Donor-Acceptor Conjugated Polymers for Air-Stable Nonvolatile Memory Devices. ACS Macro Lett. 2015, 4, 322–326. [Google Scholar] [CrossRef]
- Zha, D.; Chen, L.; Wu, F.; Wang, H.; Chen, Y. Donor-acceptor-integrated conjugated polymers based on carbazole[3,4-c:5,6- c]bis[1,2,5]thiadiazole with tight π-π Stacking for photovoltaics. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 565–574. [Google Scholar] [CrossRef]
- Chem, P.; Liu, C.; Chen, W. Polymer Chemistry MINIREVIEW Donor—Acceptor polymers for advanced memory device applications. Polym. Chem. 2011, 2169–2174. [Google Scholar] [CrossRef]
- Lin, W.P.; Liu, S.J.; Gong, T.; Zhao, Q.; Huang, W. Polymer-based resistive memory materials and devices. Adv. Mater. 2014, 26, 570–606. [Google Scholar] [CrossRef]
- Hahm, S.G.; Lee, T.J.; Kim, D.M.; Kwon, W.; Ko, Y.G.; Michinobu, T.; Ree, M. Electrical memory characteristics of nitrogen-linked poly(2,7-carbazole)s. J. Phys. Chem. C 2011, 115, 21954–21962. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, G.; Chen, Y.; Wang, C.; Neoh, K.G.; Bai, T.; Kang, E.T. Electrical bistability and WORM memory effects in donor-acceptor polymers based on poly(N-vinylcarbazole). Chempluschem 2012, 77, 74–81. [Google Scholar] [CrossRef]
- Kang, N.; Cho, B.; Kang, B.; Song, S.; Lee, T.; Lee, J. Structural and Electrical Characterization of a Block Copolymer-Based Unipolar Nonvolatile Memory Device. Adv. Mater. 2012, 24, 385–390. [Google Scholar] [CrossRef] [PubMed]
| Authors | Title | Year | Journal | Cited | Ref. |
|---|---|---|---|---|---|
| Houben J.L. et al. | Optically active vinyl polymers containing fluorescent groups: 5. Fluorescence properties of poly(9-vinyl carbazole) and optically active polymers containing carbazole units | 1978 | Polymer | 35 | [75] |
| Murphy S.M. et al. | Polymer membranes in clinical sensor applications. II. The design and fabrication of permselective hydrogels for electrochemical devices | 1992 | Biomaterials | 27 | [76] |
| J.V. Grazulevicius et al. | Carbazole-containing polymers: synthesis, properties and applications | 2003 | Progress in Polymer Science | 660 | [77] |
| Morin J.-F. et al. | Polycarbazoles: 25 years of progress | 2005 | Macromolecular Rapid Communications | 552 | [74] |
| Ding J. et al. | Highly efficient green-emitting phosphorescent iridium dendrimers based on carbazole dendrons | 2006 | Advanced Functional Materials | 290 | [78] |
| Faridbod F. et al. | Developments in the field of conducting and non-conducting polymer based potentiometric membrane sensors for ions over the past decade | 2008 | Sensors | 120 | [79] |
| Boudreault P.-L.T. et al. | Poly(2,7-carbazole)s and related polymers | 2008 | Advances in Polymer Science | 61 | [71] |
| Zou Y., Gendron D. et al. | A high-mobility low-bandgap poly(2,7-carbazole) derivative for photovoltaic applications | 2009 | Macromolecules | 220 | [80] |
| Ates M. et al. | Conducting polymer coated carbon surfaces and biosensor applications | 2009 | Progress in Organic Coatings | 106 | [81] |
| Beaupré S. et al. | Solar-energy production and energy-efficient lighting: Photovoltaic devices and white-light-emitting diodes using poly(2,7-fluorene), poly(2,7-carbazole), and poly(2,7-dibenzosilole) derivatives | 2010 | Advanced Materials | 187 | [82] |
| Boudreault P.-L.T. et al. | Polycarbazoles for plastic electronics | 2010 | Polymer Chemistry | 149 | [70] |
| Dubey N., Leclerc M. | Conducting polymers: Efficient thermoelectric materials | 2011 | Journal of Polymer Science, Part B: Polymer Physics | 252 | [83] |
| Gendron D., Leclerc M. | New conjugated polymers for plastic solar cells | 2011 | Energy and Environmental Science | 240 | [84] |
| Grigoras A.G. | A review on medical applications of poly(N-vinylcarbazole) and its derivatives | 2016 | International Journal of Polymeric Materials and Polymeric Biomaterials | 2 | [85] |
| Tan S.E., Sarjadi M.S. | The recent development of carbazole-, benzothiadiazole-, and isoindigo-based copolymers for solar cells application: A review | 2017 | Polymer Science—Series B | 7 | [86] |
| Liguori R. et al. | Stereoregular polymers with pendant carbazolyl groups: Synthesis, properties and optoelectronic applications | 2018 | Synthetic Metals | 2 | [87] |
| Ghorbani Zamani F. et al. | Current trends in the development of conducting polymers-based biosensors | 2019 | TrAC—Trends in Analytical Chemistry | 18 | [88] |
| Title | Author | Year | Book | Ref. |
|---|---|---|---|---|
| Light-emitting polymers | Perepichka, D.F., Perepichka, I.F., Meng, H., Wudl, F. | 2006 | Organic Light-Emitting Materials and Devices | [89] |
| Synthesis of Poly(2,7-carbazole)s and Derivatives | Boudreault, P.-L.T., Morin, J.-F., Leclerc, M. | 2010 | Design and Synthesis of Conjugated Polymers | [71] |
| Conducting polymer-based thermoelectric composites: Principles, processing, and applications | Yemata, T.A., Ye, Q., Zhou, H., (...), Chin, W.S., Xu, J. | 2017 | Hybrid Polymer Composite Materials: Applications | [90] |
| Light-emitting polymers | Xun, S., Perepichka, D.F., Perepichka, I.F., Meng, H., Wudl, F. | 2017 | Organic Light-Emitting Materials and Devices, Second Edition | [91] |
| Miscellaneous Vinyl Thermoplastics | Gilbert, M. | 2017 | Brydson’s Plastics Materials: Eighth Edition | [92] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekkar, F.; Bettahar, F.; Moreno, I.; Meghabar, R.; Hamadouche, M.; Hernáez, E.; Vilas-Vilela, J.L.; Ruiz-Rubio, L. Polycarbazole and Its Derivatives: Synthesis and Applications. A Review of the Last 10 Years. Polymers 2020, 12, 2227. https://doi.org/10.3390/polym12102227
Bekkar F, Bettahar F, Moreno I, Meghabar R, Hamadouche M, Hernáez E, Vilas-Vilela JL, Ruiz-Rubio L. Polycarbazole and Its Derivatives: Synthesis and Applications. A Review of the Last 10 Years. Polymers. 2020; 12(10):2227. https://doi.org/10.3390/polym12102227
Chicago/Turabian StyleBekkar, Fadila, Faiza Bettahar, Isabel Moreno, Rachid Meghabar, Mohammed Hamadouche, Estibaliz Hernáez, José Luis Vilas-Vilela, and Leire Ruiz-Rubio. 2020. "Polycarbazole and Its Derivatives: Synthesis and Applications. A Review of the Last 10 Years" Polymers 12, no. 10: 2227. https://doi.org/10.3390/polym12102227
APA StyleBekkar, F., Bettahar, F., Moreno, I., Meghabar, R., Hamadouche, M., Hernáez, E., Vilas-Vilela, J. L., & Ruiz-Rubio, L. (2020). Polycarbazole and Its Derivatives: Synthesis and Applications. A Review of the Last 10 Years. Polymers, 12(10), 2227. https://doi.org/10.3390/polym12102227






