Fabrication of a Polycaprolactone/Alginate Bipartite Hybrid Scaffold for Osteochondral Tissue Using a Three-Dimensional Bioprinting System
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Terminology
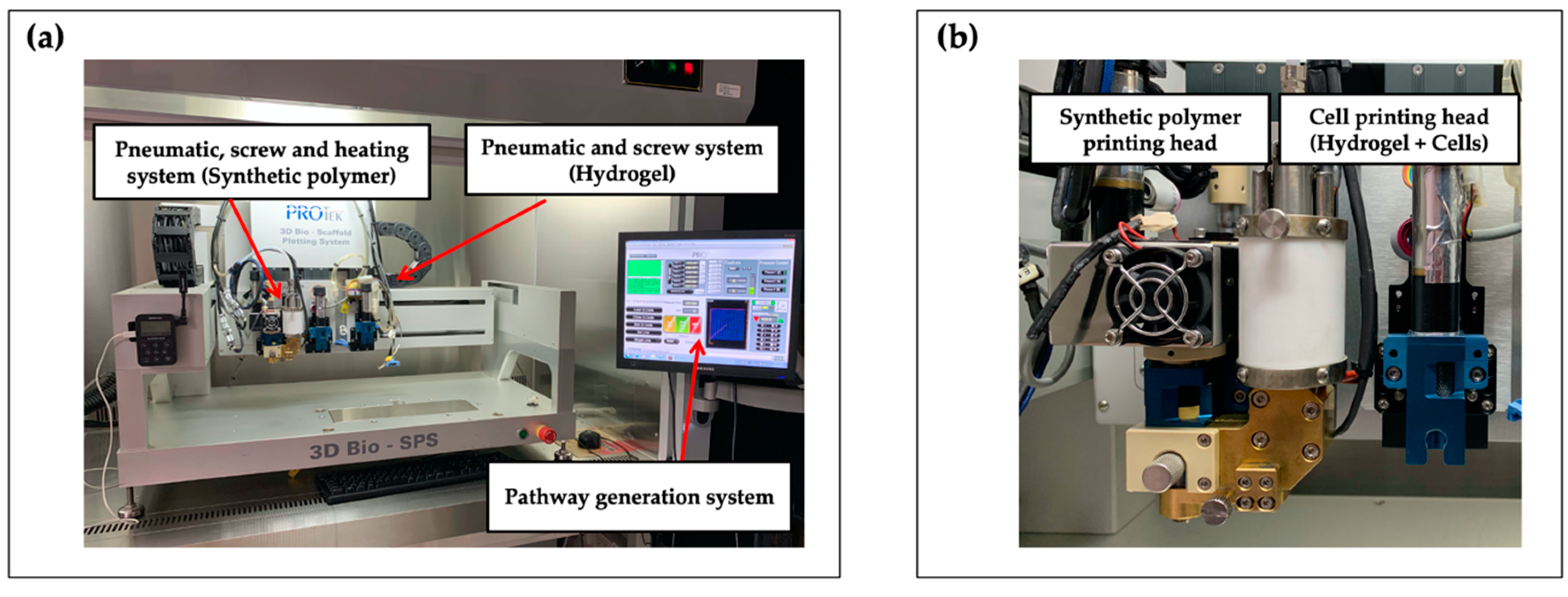
2.3. 3D Bioprinting System
2.4. Progenitor Cell Isolation and Culture
2.5. Printability and Cell Viability Assay with Different Concentrations of Alginate
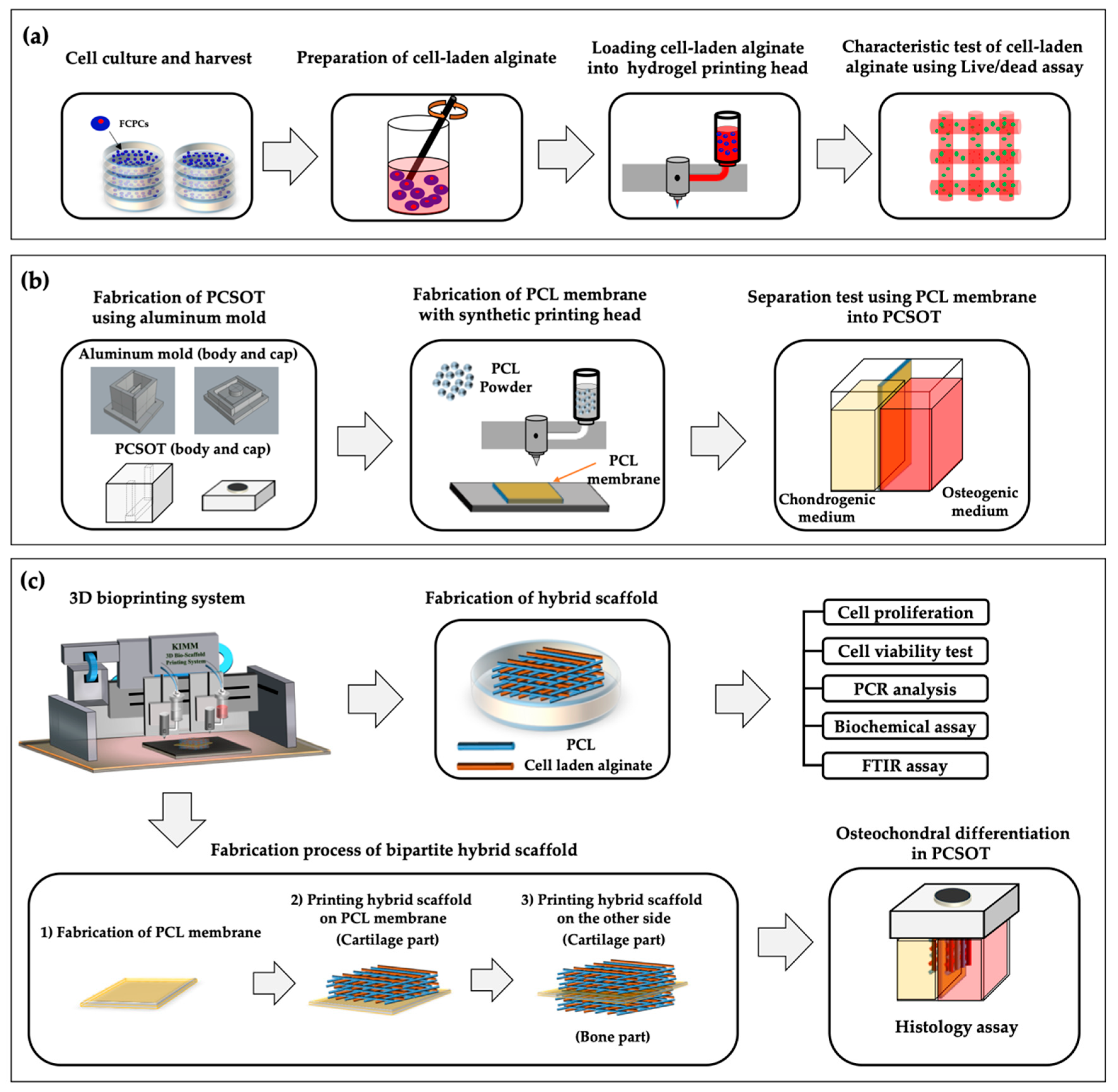
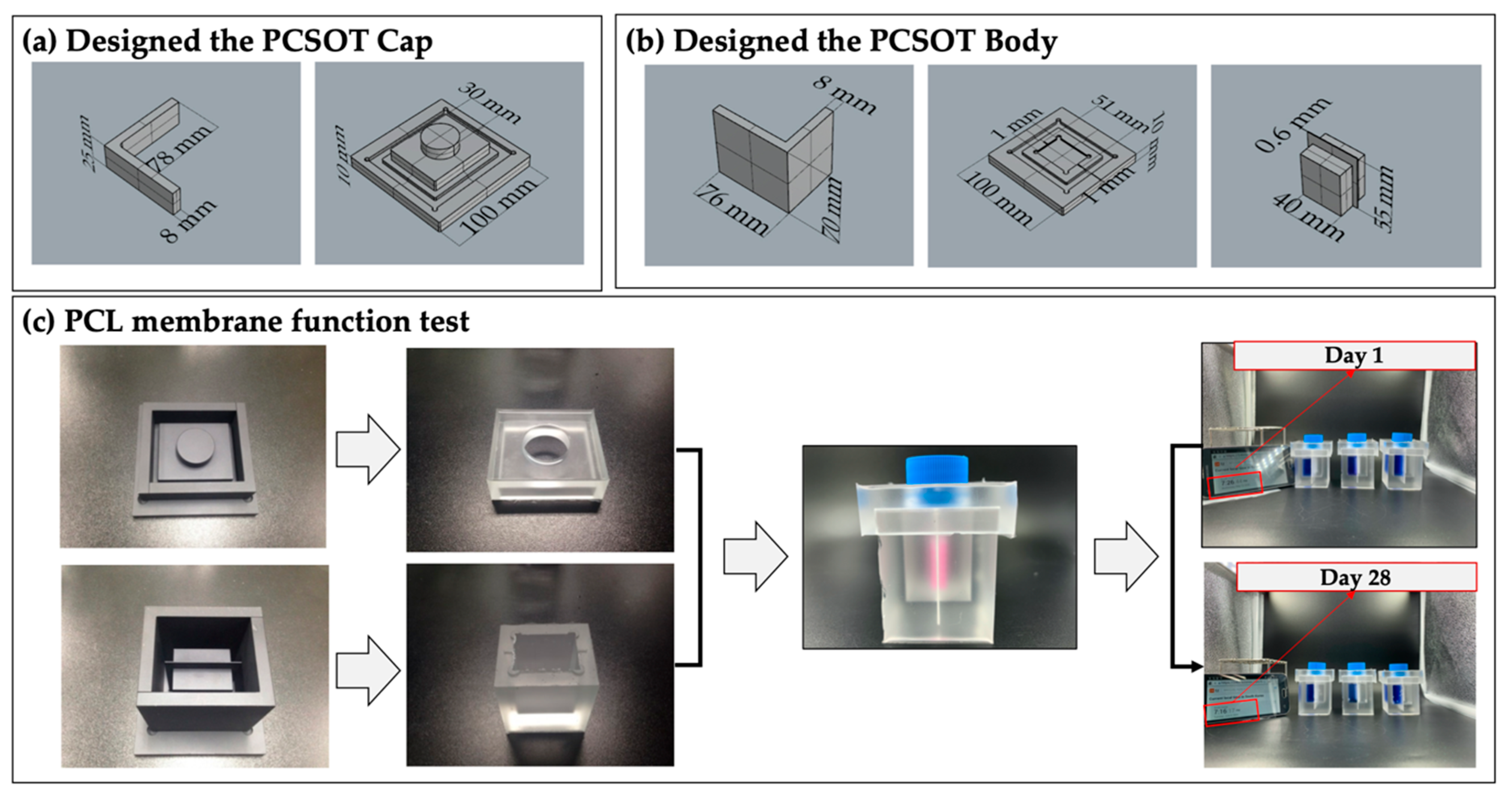
2.6. Design and Fabrication of the PCSOT
2.7. Fabrication Process of Hybrid and Bipartite Scaffold
2.8. Specific Induction Medium Conditions for the Hybrid Scaffold
2.9. Proliferation Assay
2.10. Cell Viability Assay
2.11. RNA Isolation and Quantitative Reverse Transcription PCR (RT-qPCR)
2.12. Biochemical and FT-IR Measurement
2.13. Histological Assays
2.14. Statics Analysis
3. Results and Discussion
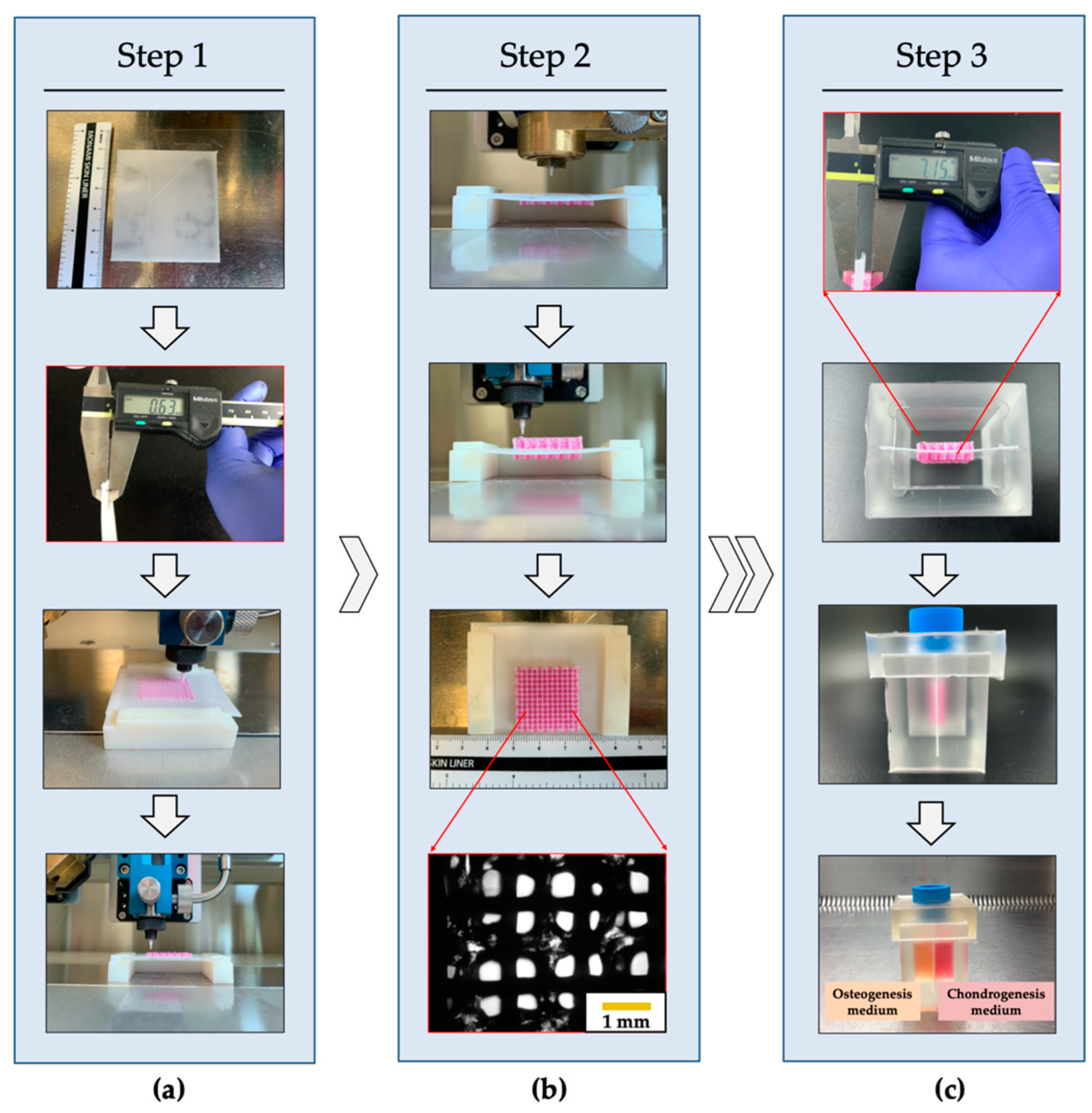
3.1. Fabrication of PCSOT and Separating Function Test
3.2. Fabrication of the PCL/Alginate Bipartite Scaffold
3.3. Evaluation of the Extrusion Performance and Cell Viabiltiy
3.4. Analysis of Biocompatibility
3.5. Ostechondral Gene Expression and Biochemical Assays Using the Hybrid Scaffolds
3.6. Histological Findings of Bipartite Hybrid Scaffolds in the PCSOT
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fai, L.K.; Cheah, C.; Chua, C. Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterials 2003, 24, 2363–2378. [Google Scholar] [CrossRef]
- Park, J.Y.; Choi, Y.-J.; Shim, J.-H.; Park, J.H.; Kim, Y. Development of a 3D cell printed structure as an alternative to autologs cartilage for auricular reconstruction. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 105, 1016–1028. [Google Scholar] [CrossRef] [PubMed]
- A Park, S.; Lee, J.B.; Kim, Y.E.; Kim, J.E.; Lee, J.H.; Shin, J.-W.; Kwon, I.K.; Kim, W. Fabrication of biomimetic PCL scaffold using rapid prototyping for bone tissue engineering. Macromol. Res. 2014, 22, 882–887. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Duan, B.; Hockaday, L.A.; Kang, K.H.; Butcher, J.T. 3D Bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. Part A 2012, 101, 1255–1264. [Google Scholar] [CrossRef]
- Rhee, S.; Puetzer, J.L.; Mason, B.N.; Reinhart-King, C.A.; Bonassar, L.J. 3D Bioprinting of Spatially Heterogeneous Collagen Constructs for Cartilage Tissue Engineering. ACS Biomater. Sci. Eng. 2016, 2, 1800–1805. [Google Scholar] [CrossRef]
- Ng, W.L.; Yeong, W.Y.; Naing, M.W. Polyelectrolyte gelatin-chitosan hydrogel optimized for 3D bioprinting in skin tissue engineering. Int. J. Bioprint. 2016, 2. [Google Scholar] [CrossRef]
- Axpe, E.; Oyen, M.L. Applications of Alginate-Based Bioinks in 3D Bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef]
- Freeman, F.E.; Kelly, D.J. Tuning Alginate Bioink Stiffness and Composition for Controlled Growth Factor Delivery and to Spatially Direct MSC Fate within Bioprinted Tissues. Sci. Rep. 2017, 7, 17042. [Google Scholar] [CrossRef]
- Izadifar, Z.; Chang, T.; Kulyk, W.M.; Chen, X.; Eames, B.F.; Chen, D. Analyzing Biological Performance of 3D-Printed, Cell-Impregnated Hybrid Constructs for Cartilage Tissue Engineering. Tissue Eng. Part C Methods 2016, 22, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.; Shim, J.-H.; Jang, J.; Kim, S.-W.; Kim, Y. An additive manufacturing-based PCL-alginate-chondrocyte bioprinted scaffold for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2013, 9, 1286–1297. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.-H.; Lee, J.-S.; Kim, J.-Y.; Kim, Y. Bioprinting of a mechanically enhanced three-dimensional dual cell-laden construct for osteochondral tissue engineering using a multi-head tissue/organ building system. J. Micromech. Microeng. 2012, 22. [Google Scholar] [CrossRef]
- Yeo, M.; Kim, G. Cell-printed hierarchical scaffolds consisting of micro-sized polycaprolactone (PCL) and electrospun PCL nanofibers/cell-laden alginate struts for tissue regeneration. J. Mater. Chem. B 2013, 2, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Ahn, S.; Bonassar, L.J.; Kim, G. Cell(MC3T3-E1)-Printed Poly(ϵ-caprolactone)/Alginate Hybrid Scaffolds for Tissue Regeneration. Macromol. Rapid Commun. 2012, 34, 142–149. [Google Scholar] [CrossRef]
- Shim, J.-H.; Jang, K.-M.; Hahn, S.K.; Park, J.Y.; Jung, H.; Oh, K.; Park, K.M.; Yeom, J.; Park, S.H.; Kim, S.W.; et al. Three-dimensional bioprinting of multilayered constructs containing human mesenchymal stromal cells for osteochondral tissue regeneration in the rabbit knee joint. Biofabrication 2016, 8, 014102. [Google Scholar] [CrossRef]
- Park, J.Y.; Choi, J.-C.; Shim, J.-H.; Lee, J.-S.; Park, H.; Kim, S.W.; Doh, J.; Kim, Y. A comparative study on collagen type I and hyaluronic acid dependent cell behavior for osteochondral tissue bioprinting. Biofabrication 2014, 6, 035004. [Google Scholar] [CrossRef]
- Mahmoudifar, N.; Doran, P.M. Osteogenic differentiation and osteochondral tissue engineering using human adipose-derived stem cells. Biotechnol. Prog. 2012, 29, 176–185. [Google Scholar] [CrossRef]
- Lin, H.; Lozito, T.P.; Alexander, P.G.; Gottardi, R.; Tuan, R.S. Stem Cell-Based Microphysiological Osteochondral System to Model Tissue Response to Interleukin-1β. Mol. Pharm. 2014, 11, 2203–2212. [Google Scholar] [CrossRef]
- Choi, W.H.; Kim, H.R.; Lee, S.J.; Jeong, N.; Park, S.R.; Choi, B.H.; Min, B.-H. Fetal Cartilage-Derived Cells Have Stem Cell Properties and Are a Highly Potent Cell Source for Cartilage Regeneration. Cell Transplant. 2016, 25, 449–461. [Google Scholar] [CrossRef]
- Daley, E.L.; Kuttig, J.; Stegemann, J.P. Development of Modular, Dual-Perfused Osteochondral Constructs for Cartilage Repair. Tissue Eng. Part C Methods 2019, 25, 127–136. [Google Scholar] [CrossRef]
- Jin, G.-Z.; Kim, J.-J.; Park, J.-H.; Seo, S.-J.; Kim, J.-H.; Lee, E.-J.; Kim, H.-W. Biphasic Nanofibrous Constructs with Seeded Cell Layers for Osteochondral Repair. Tissue Eng. Part C Methods 2014, 20, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, E.; Sakai, Y.; Fujii, T. Cell Culture in 3-Dimensional Microfluidic Structure of PDMS (polydimethylsiloxane). Biomed. Microdevices 2003, 5, 109–114. [Google Scholar] [CrossRef]
- Folch, A.; Toner, M. Microengineering of Cellular Interactions. Annu. Rev. Biomed. Eng. 2000, 2, 227–256. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, S.; Nakagawa, K.; Sah, R.L.; Wada, Y.; Ogawa, T.; Moriya, H.; Masuda, K. In VivoMaturation of Scaffold-free Engineered Articular Cartilage on Hydroxyapatite. Tissue Eng. Part A 2008, 14, 1905–1913. [Google Scholar] [CrossRef]
- Bhagat, A.A.S.; Jothimuthu, P.; Papautsky, I. Photodefinable polydimethylsiloxane (PDMS) for rapid lab-on-a-chip prototyping. Lab Chip 2007, 7, 1192. [Google Scholar] [CrossRef]
- Yao, H.; Kang, J.; Li, W.; Liu, J.; Xie, R.; Wang, D.-A.; Liu, S.; Wang, D.-A.; Ren, L. Novel β -TCP/PVA bilayered hydrogels with considerable physical and bio-functional properties for osteochondral repair. Biomed. Mater. 2017, 13, 015012. [Google Scholar] [CrossRef]
- Liao, J.; Tian, T.; Shi, S.; Xie, X.; Ma, Q.; Li, G.; Lin, Y. The fabrication of biomimetic biphasic CAN-PAC hydrogel with a seamless interfacial layer applied in osteochondral defect repair. Bone Res. 2017, 5, 17018. [Google Scholar] [CrossRef]
- Erickson, A.E.; Sun, J.; Levengood, S.K.L.; Swanson, S.; Chang, F.-C.; Tsao, C.T.; Zhang, M. Chitosan-based composite bilayer scaffold as an in vitro osteochondral defect regeneration model. Biomed. Microdevices 2019, 21, 34. [Google Scholar] [CrossRef]
- Keogh, M.B.; O’Brien, F.J.; Daly, J.S. A novel collagen scaffold supports human osteogenesis—Applications for bone tissue engineering. Cell Tissue Res. 2010, 340, 169–177. [Google Scholar] [CrossRef]
- Farea, M.; Husein, A.; Halim, A.S.; Abdullah, N.A.; Mokhtar, K.I.; Lim, C.K.; Berahim, Z.; Mokhtar, K. Synergistic effects of chitosan scaffold and TGFβ1 on the proliferation and osteogenic differentiation of dental pulp stem cells derived from human exfoliated deciduous teeth. Arch. Oral Boil. 2014, 59, 1400–1411. [Google Scholar] [CrossRef]
- Kato, H.; Taguchi, Y.; Yamawaki, I.; Ruan, Y.; Wu, Q.; Chen, Y.-C.; Tsumori, N.; Nakata, T.; Noguchi, M.; Umeda, M. Amelogenin Exon 5 Peptide Promotes Cell Proliferation and Osteogenic Differentiation in Human Dental Pulp Stem Cells. Appl. Sci. 2019, 9, 4425. [Google Scholar] [CrossRef]
- Yamada, T.; Matsukawa, N.; Matsumoto, M.; Morimoto, S.; Ogihara, T.; Ochi, T.; Yoshikawa, H.; Nampei, A.; Hashimoto, J.; Hayashida, K.; et al. Matrix extracellular phosphoglycoprotein (MEPE) is highly expressed in osteocytes in human bone. J. Bone Miner. Metab. 2004, 22, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Ruijtenberg, S.; Heuvel, S.V.D. Coordinating cell proliferation and differentiation: Antagonism between cell cycle regulators and cell type-specific gene expression. Cell Cycle 2016, 15, 196–212. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, S.D.; Buckley, C.T.; Vinardell, T.; O’Brien, F.J.; Campbell, V.A.; Kelly, D.J. The Response of Bone Marrow-Derived Mesenchymal Stem Cells to Dynamic Compression Following TGF-β3 Induced Chondrogenic Differentiation. Ann. Biomed. Eng. 2010, 38, 2896–2909. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Zhai, D.Y.; Tous, E.; Rai, R.; Mauck, R.L.; Burdick, J.A. Enhanced MSC chondrogenesis following delivery of TGF-β3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials 2011, 32, 6425–6434. [Google Scholar] [CrossRef]
- Gan, Y.; Li, S.; Li, P.; Xu, Y.; Wang, L.; Zhao, C.; Ouyang, B.; Tu, B.; Zhang, C.; Luo, L.; et al. A Controlled Release Codelivery System of MSCs Encapsulated in Dextran/Gelatin Hydrogel with TGF-β3-Loaded Nanoparticles for Nucleus Pulposus Regeneration. Stem Cells Int. 2016, 2016, 1–14. [Google Scholar] [CrossRef]
- Gheisari, H.; Karamian, E.; Abdellahi, M. A novel hydroxyapatite –Hardystonite nanocomposite ceramic. Ceram. Int. 2015, 41, 5967–5975. [Google Scholar] [CrossRef]
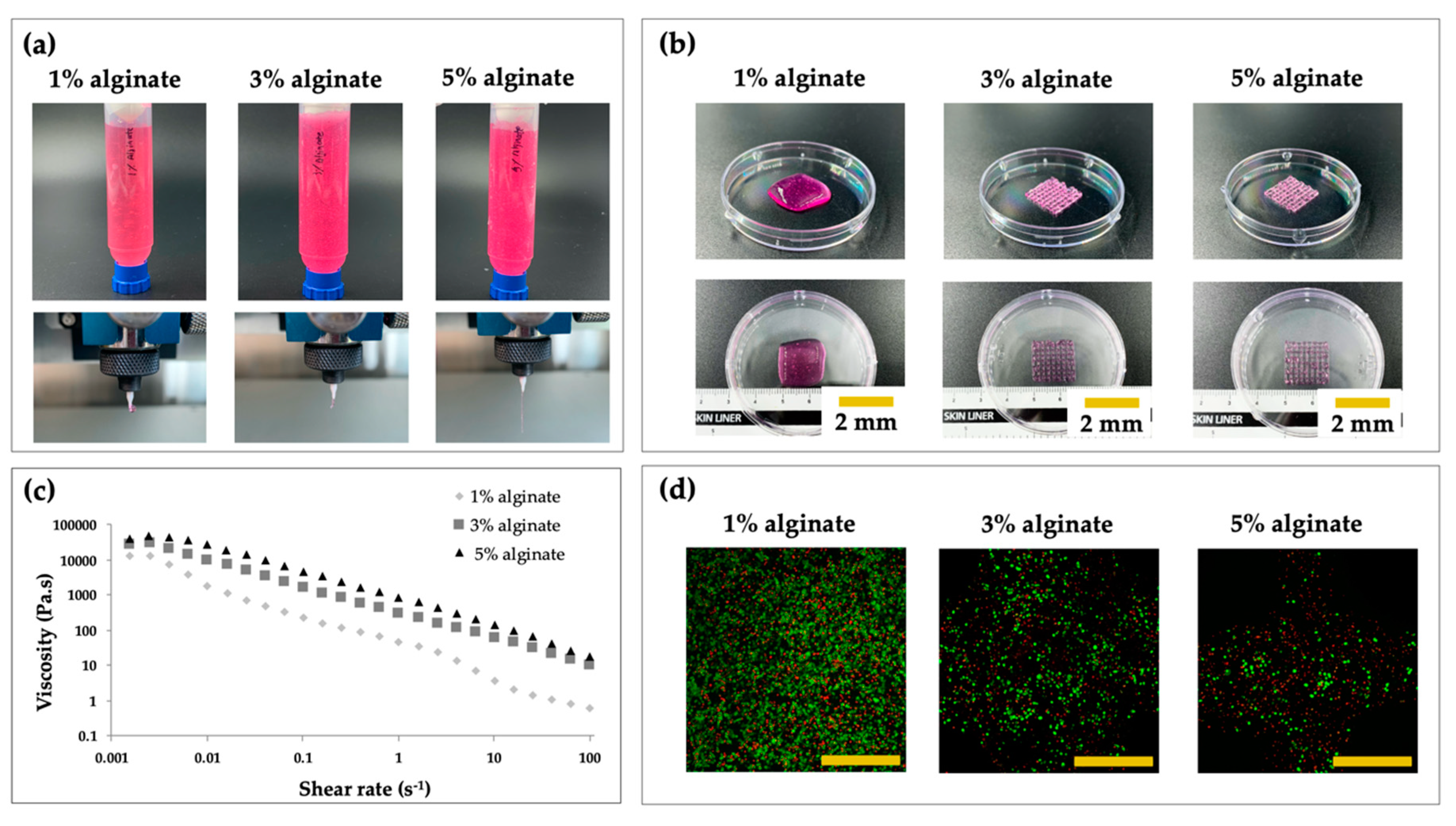


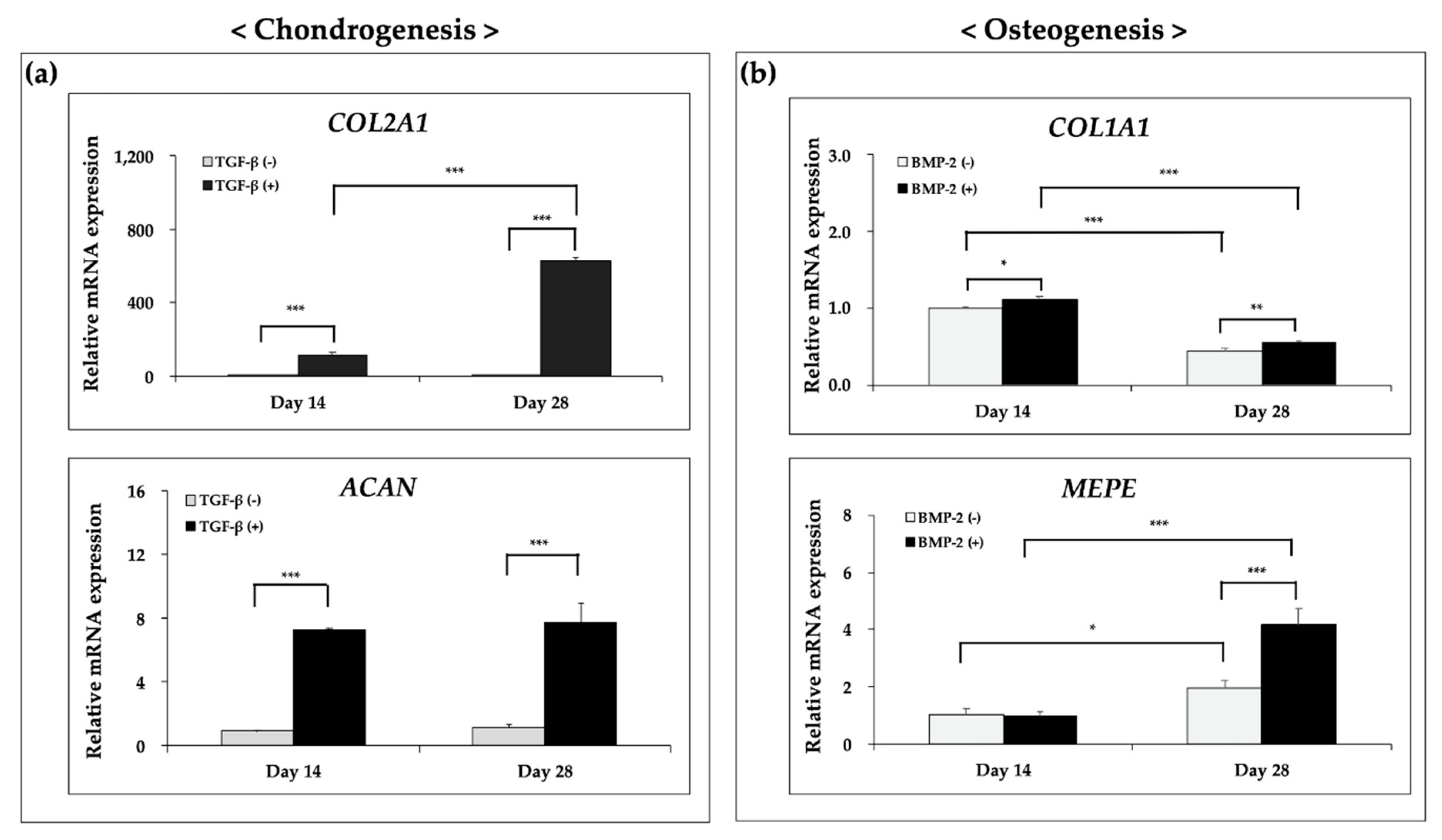
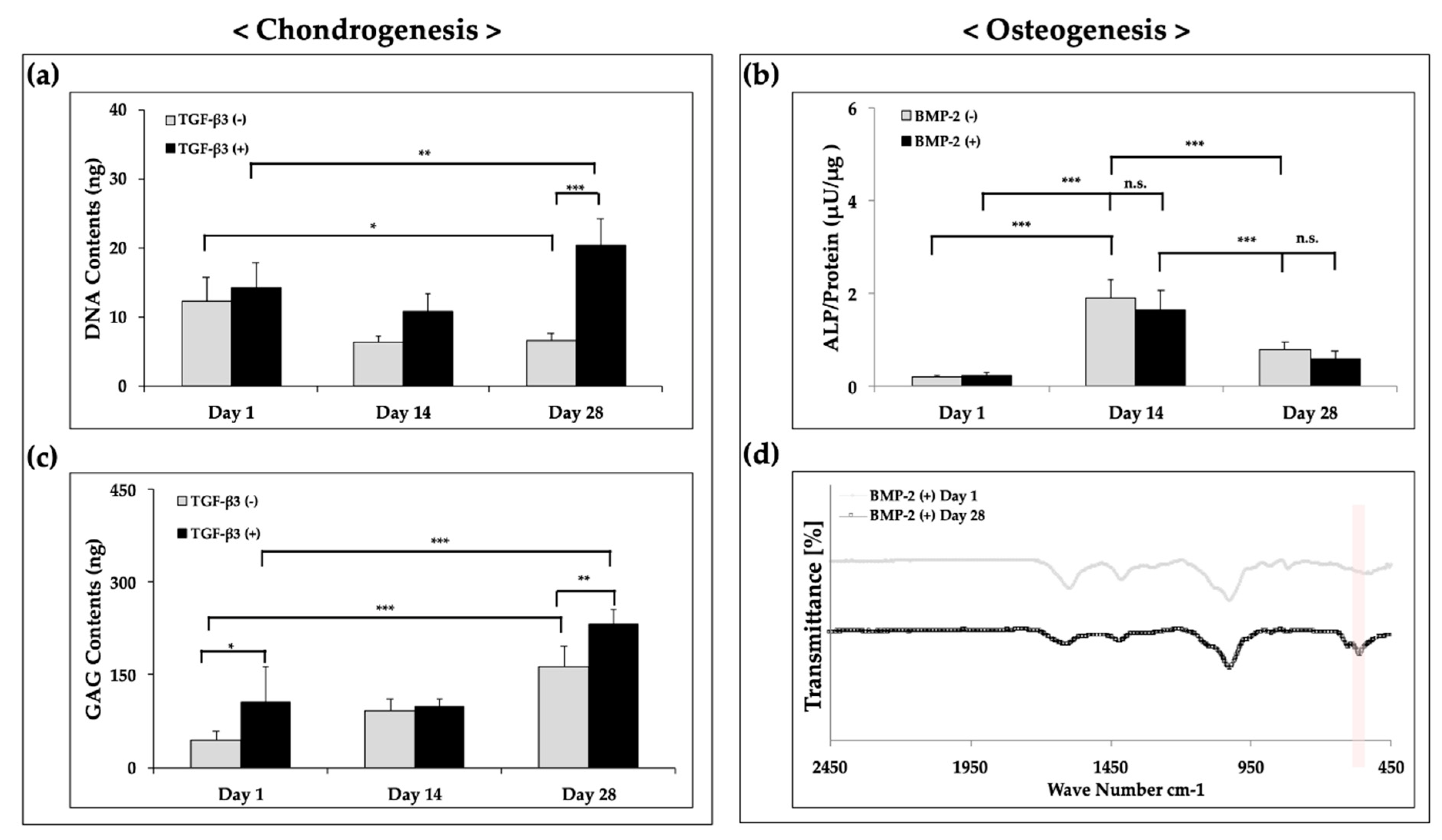
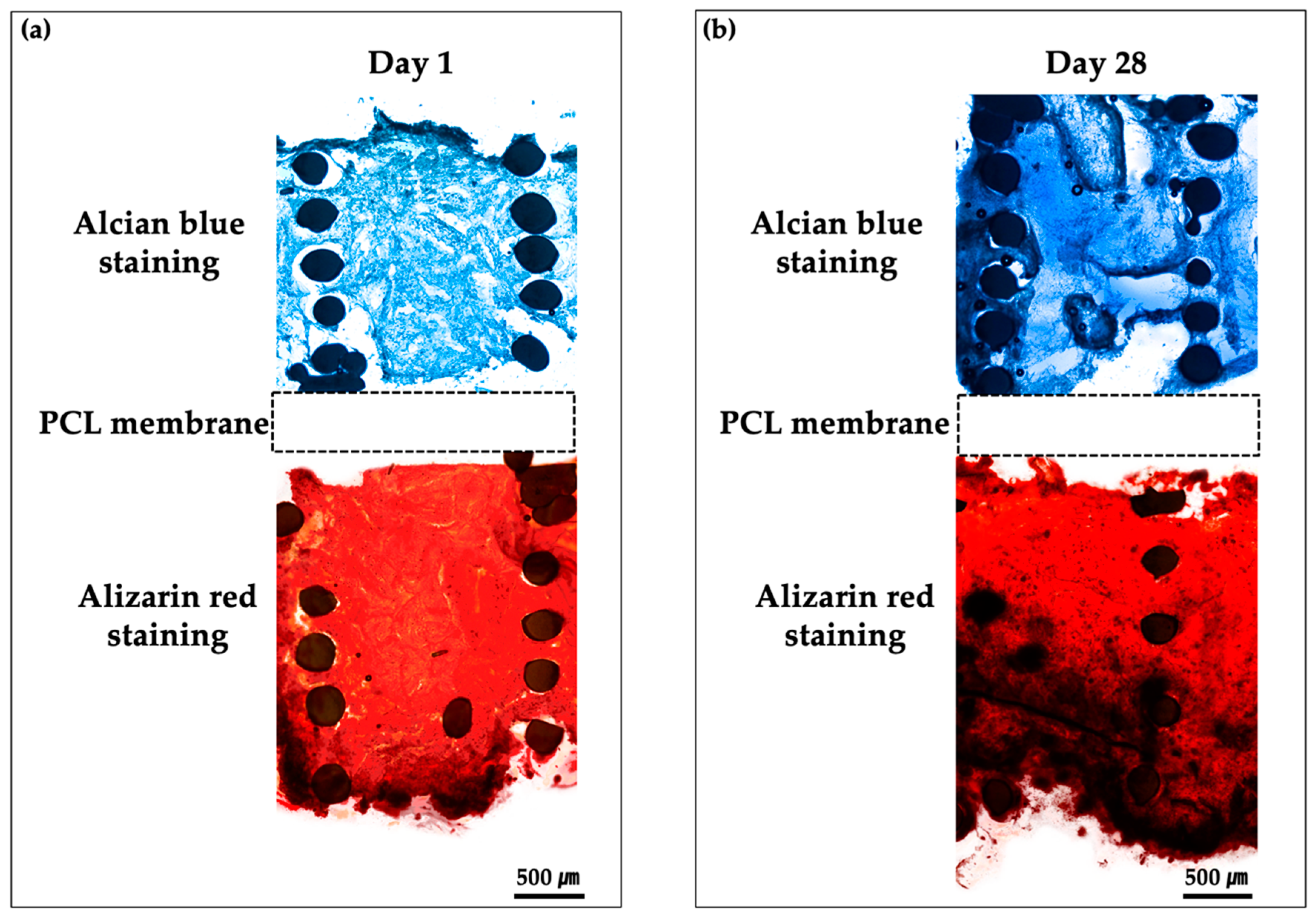
| Material | Pressure (kPa) | Temperature (°C) | Speed (mm/min) | Screw (rpm) | Nozzle (µm) |
|---|---|---|---|---|---|
| 1% Alginate | 60 | RT | 440 | 39 | 300 |
| 3% Alginate | 90 | RT | 440 | 39 | 300 |
| 5% Alginate | 125 | RT | 440 | 39 | 300 |
| Gene | Direction (5′-3′) | Primer Sequence | |
|---|---|---|---|
| Cartilage-specific genes | COL2A1 | Forward | GTTCACGTACACTGCCCTGA |
| Reverse | TCCACACCGAATTCCTGCTC | ||
| ACAN | Forward | CCTCTGCATTCCACGAAGCTAAC | |
| Reverse | TGCCTCTGTCCCCACATCAC | ||
| Bone-specific genes | COL1A1 | Forward | GTGTTCCTGGAGACCTTGGC |
| Reverse | CACCAGCATCACCCTTAGCA | ||
| MEPE | Forward | TGCGAGTTTTCTGTGTGGGAC | |
| Reverse | TCTTCCACACAGCTTTGCTTAG | ||
| Reference gene | GADPH | Forward | TTGAGGTCAATGAAGGGGTC |
| Reverse | GAAGGTGAAGGTCGGAGTCA | ||
| Material | Pressure (kPa) | Temperature (°C) | Speed (mm/min) | Screw (rpm) | Nozzle (μm) |
|---|---|---|---|---|---|
| PCL strand | 290 | 110 | 440 | 52 | 300 |
| Alginate strand | 90 | RT | 440 | 39 | 300 |
| PCL membrane | 290 | 110 | 800 | 52 | 400 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Lee, S.; Choi, S.; Kim, K.K.; Ryu, B.; Kim, C.-Y.; Jung, C.-R.; Min, B.-H.; Xin, Y.-Z.; Park, S.A.; et al. Fabrication of a Polycaprolactone/Alginate Bipartite Hybrid Scaffold for Osteochondral Tissue Using a Three-Dimensional Bioprinting System. Polymers 2020, 12, 2203. https://doi.org/10.3390/polym12102203
Yu J, Lee S, Choi S, Kim KK, Ryu B, Kim C-Y, Jung C-R, Min B-H, Xin Y-Z, Park SA, et al. Fabrication of a Polycaprolactone/Alginate Bipartite Hybrid Scaffold for Osteochondral Tissue Using a Three-Dimensional Bioprinting System. Polymers. 2020; 12(10):2203. https://doi.org/10.3390/polym12102203
Chicago/Turabian StyleYu, JunJie, SuJeong Lee, Sunkyung Choi, Kee K. Kim, Bokyeong Ryu, C-Yoon Kim, Cho-Rok Jung, Byoung-Hyun Min, Yuan-Zhu Xin, Su A Park, and et al. 2020. "Fabrication of a Polycaprolactone/Alginate Bipartite Hybrid Scaffold for Osteochondral Tissue Using a Three-Dimensional Bioprinting System" Polymers 12, no. 10: 2203. https://doi.org/10.3390/polym12102203
APA StyleYu, J., Lee, S., Choi, S., Kim, K. K., Ryu, B., Kim, C.-Y., Jung, C.-R., Min, B.-H., Xin, Y.-Z., Park, S. A., Kim, W., Lee, D., & Lee, J. (2020). Fabrication of a Polycaprolactone/Alginate Bipartite Hybrid Scaffold for Osteochondral Tissue Using a Three-Dimensional Bioprinting System. Polymers, 12(10), 2203. https://doi.org/10.3390/polym12102203






