Enhanced Intestinal Immune Response in Mice after Oral Administration of Korea Red Ginseng-Derived Polysaccharide
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Polysaccharide Fraction from Red Ginseng
2.3. Chemical Properties of KRG-P
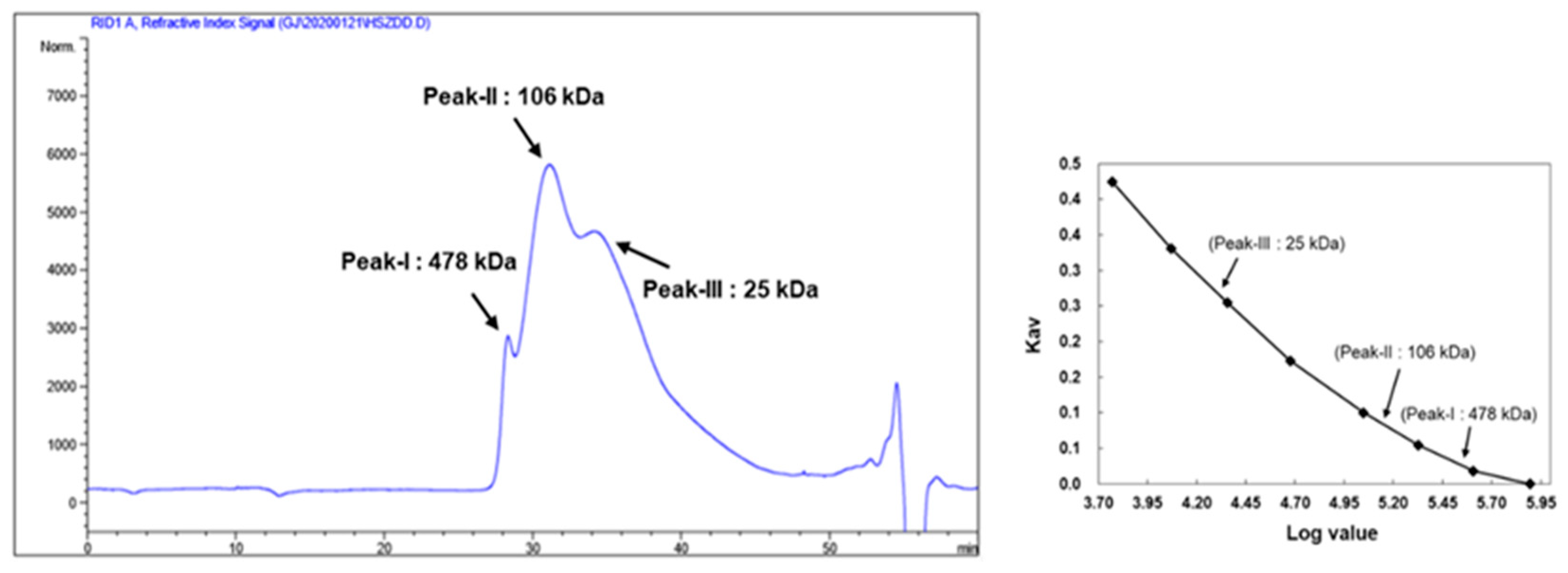
2.4. Determination of KRG-P Monosaccharide Composition and Molecular Weight
2.5. Animals
2.6. GM-CSF and IgA Production Analyses in Peyer’s Patch Cells
2.7. Bone Marrow Cell Proliferation Assay
2.8. Quantitative RT-PCR
2.9. Preparation of Tissue Lysate and Immuno-Blotting
2.10. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition and Molecular Weight of KRG-P Extracted from KRG
3.2. Effects of Intestinal Immunomodulatory Activity Mediated by Peyer’s Patch Cells
3.3. Effects of KRG and KRG-P Oral Administration on Mouse IgA Production
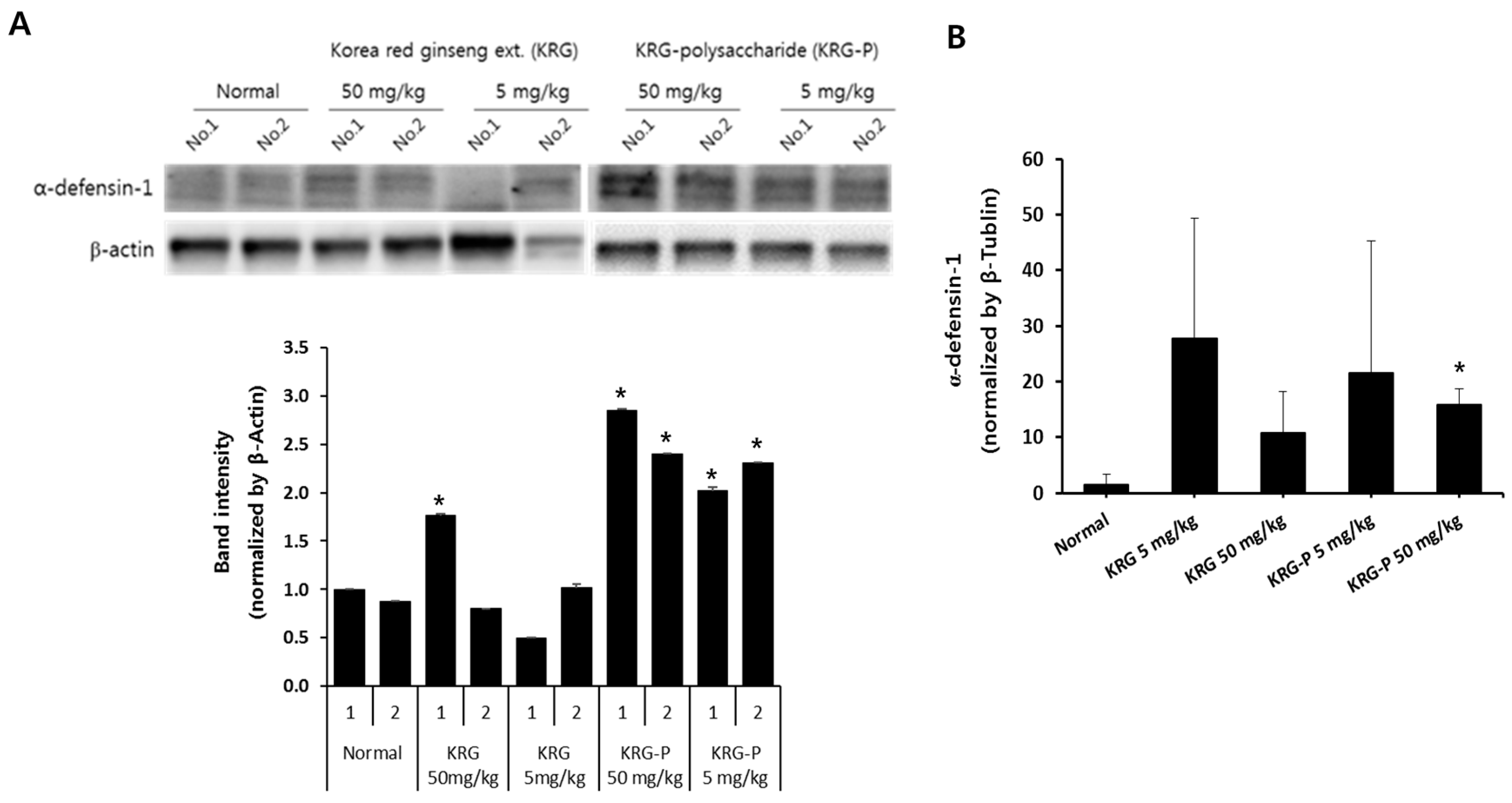
3.4. Effects of KRG and KRG-P Oral Administration on Intestinal Mouse α-Defensin-1 Expression
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Owen, J.A.; Punt, J.; Stranford, S.A. Kuby Immunology; WH Freeman: New York, NY, USA, 2013. [Google Scholar]
- Sminia, T.; Wilders, M.; Janse, E.; Hoefsmit, E. Characterization of non-lymphoid cells in Peyer’s patches of the rat. Immunobiology 1983, 164, 136–143. [Google Scholar] [CrossRef]
- Kagnoff, M.F. Mucosal immunology: New frontiers. Immunol. Today 1996, 17, 57–59. [Google Scholar] [CrossRef]
- Mowat, A.M.; Viney, J.L. The anatomical basis of intestinal immunity. Immunol. Rev. 1997, 156, 145–166. [Google Scholar] [CrossRef]
- Hara, S.; Sasaki, T.; Satoh-Takayama, N.; Kanaya, T.; Tachibana, N.; Kim, K.S.; Surh, C.D.; Ohno, H. Dietary antigens induce germinal center responses in Peyer’s patches and antigen-specific IgA production. Front. Immunol. 2019, 10, 2432. [Google Scholar] [CrossRef]
- Ayabe, T.; Satchell, D.P.; Wilson, C.L.; Parks, W.C.; Selsted, M.E.; Ouellette, A.J. Secretion of microbicidal α-defensins by intestinal Paneth cells in response to bacteria. Nat. Immunol. 2000, 1, 113–118. [Google Scholar] [CrossRef]
- Ayabe, T.; Satchell, D.P.; Pesendorfer, P.; Tanabe, H.; Wilson, C.L.; Hagen, S.J.; Ouellette, A.J. Activation of Paneth cell α-defensins in mouse small intestine. J. Biol. Chem. 2002, 277, 5219–5228. [Google Scholar] [CrossRef]
- Nakamura, K.; Sakuragi, N.; Takakuwa, A.; Ayabe, T. Paneth cell α-defensins and enteric microbiota in health and disease. Biosci. Microbiota Food Health 2015. [Google Scholar] [CrossRef]
- Wong, A.S.; Che, C.-M.; Leung, K.-W. Recent advances in ginseng as cancer therapeutics: A functional and mechanistic overview. Nat. Prod. Rep. 2015, 32, 256–272. [Google Scholar] [CrossRef]
- Irfan, M.; Kwak, Y.-S.; Han, C.-K.; Hyun, S.-H.; Rhee, M.H. Adaptogenic effects of Panax ginseng on modulation of cardiovascular functions. J. Ginseng Res. 2020, 44, 538–543. [Google Scholar] [CrossRef]
- Kim, J.-H. Cardiovascular diseases and Panax ginseng: A review on molecular mechanisms and medical applications. J. Ginseng Res. 2012, 36, 16. [Google Scholar] [CrossRef]
- Yuan, H.-D.; Kim, J.T.; Kim, S.H.; Chung, S.H. Ginseng and diabetes: The evidences from in vitro, animal and human studies. J. Ginseng Res. 2012, 36, 27. [Google Scholar] [CrossRef]
- Choi, K. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng CA Meyer. Acta Pharmacol. Sin. 2008, 29, 1109–1118. [Google Scholar] [CrossRef]
- Hyun, S.H.; Kim, S.W.; Seo, H.W.; Youn, S.H.; Kyung, J.S.; Lee, Y.Y.; In, G.; Park, C.-K.; Han, C.-K. Physiological and pharmacological features of the non-saponin components in Korean Red ginseng. J. Ginseng Res. 2020, 44, 527–537. [Google Scholar] [CrossRef]
- Lee, S.M.; Bae, B.-S.; Park, H.-W.; Ahn, N.-G.; Cho, B.-G.; Cho, Y.-L.; Kwak, Y.-S. Characterization of Korean Red Ginseng (Panax ginseng Meyer): History, preparation method, and chemical composition. J. Ginseng Res. 2015, 39, 384–391. [Google Scholar] [CrossRef]
- Lee, S.M. Thermal conversion pathways of ginsenoside in red ginseng processing. Nat. Prod. Sci. 2014, 20, 119–125. [Google Scholar]
- Kim, Y.-J.; Yamabe, N.; Choi, P.; Lee, J.W.; Ham, J.; Kang, K.S. Efficient thermal deglycosylation of ginsenoside Rd and its contribution to the improved anticancer activity of ginseng. J. Agric. Food Chem. 2013, 61, 9185–9191. [Google Scholar] [CrossRef]
- Choi, P.; Park, J.Y.; Kim, T.; Park, S.-H.; Kim, H.-k.; Kang, K.S.; Ham, J. Improved anticancer effect of ginseng extract by microwave-assisted processing through the generation of ginsenosides Rg3, Rg5 and Rk1. J. Funct. Foods 2015, 14, 613–622. [Google Scholar] [CrossRef]
- Shin, M.-S.; Song, J.H.; Choi, P.; Lee, J.H.; Kim, S.-Y.; Shin, K.-S.; Ham, J.; Kang, K.S. Stimulation of innate immune function by Panax ginseng after heat processing. J. Agric. Food Chem. 2018, 66, 4652–4659. [Google Scholar] [CrossRef]
- Byeon, S.E.; Lee, J.; Kim, J.H.; Yang, W.S.; Kwak, Y.-S.; Kim, S.Y.; Choung, E.S.; Rhee, M.H.; Cho, J.Y. Molecular mechanism of macrophage activation by red ginseng acidic polysaccharide from Korean red ginseng. Mediat. Inflamm. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.-W.; Yu, K.-W.; Suh, H.-J. Polysaccharides fractionated from enzyme digests of Korean red ginseng water extracts enhance the immunostimulatory activity. Int. J. Biol. Macromol. 2019, 121, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, N.; Feng, Q.; Li, H.; Wang, D.; Ma, L.; Liu, S.; Chen, C.; Wu, W.; Jiao, L. The core structure characterization and of ginseng neutral polysaccharide with the immune-enhancing activity. Int. J. Biol. Macromol. 2019, 123, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Youn, S.H.; Lee, S.M.; Han, C.-K.; In, G.; Park, C.-K.; Hyun, S.H. Immune activity of polysaccharide fractions isolated from Korean Red Ginseng. Molecules 2020, 25, 3569. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Food and Drug Safety of the Republic of Korea. Health Functional Food Code: Ministry of Food and Drug Safety Notification. 21 December 2016. Available online: https://www.khsa.or.kr/assets/extra/hfood/01.pdf (accessed on 6 August 2020).
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Dai, J.; Wu, Y.; Chen, S.-W.; Zhu, S.; Yin, H.-P.; Wang, M.; Tang, J. Sugar compositional determination of polysaccharides from Dunaliella salina by modified RP-HPLC method of precolumn derivatization with 1-phenyl-3-methyl-5-pyrazolone. Carbohydr. Polym. 2010, 82, 629–635. [Google Scholar] [CrossRef]
- Kim, H.W.; Shin, M.-S.; Lee, S.J.; Park, H.-R.; Jee, H.S.; Yoon, T.J.; Shin, K.-S. Signaling pathways associated with macrophage-activating polysaccharides purified from fermented barley. Int. J. Biol. Macromol. 2019, 131, 1084–1091. [Google Scholar] [CrossRef]
- Hong, T.; Matsumoto, T.; Kiyohara, H.; Yamada, H. Enhanced production of hematopoietic growth factors through T cell activation in Peyer’s patches by oral administration of Kampo (Japanese herbal) medicine,“Juzen-Taiho-To”. Phytomedicine 1998, 5, 353–360. [Google Scholar] [CrossRef]
- Yu, K.-W.; Kiyohara, H.; Matsumoto, T.; Yang, H.-C.; Yamada, H. Intestinal immune system modulating polysaccharides from rhizomes of Atractylodes lancea. Planta Med. 1998, 64, 714–719. [Google Scholar] [CrossRef]
- Shin, M.-S.; Yu, K.-W.; Shin, K.-S.; Lee, H. Enhancement of immunological activity in mice with oral administration of cell wall components of Bifidobacterium bifidum. Food Sci. Biotechnol. 2004, 13, 85–89. [Google Scholar]


| Apparatus | Liquid Chromatography LC-20AD (Shimadzu Corporation, Kyoto, Japan) |
| Detector | UV/VIS detector (Shimadzu Corporation, Kyoto, Japan) monitored at 245 nm |
| Column | AcclaimTM 120 C18 (Thermo Scientific, Sunnyvale, CA, USA) |
| Column size | 4.6 × 250 mm (5 μm particle size) |
| Column temperature | 30 °C |
| Flow rate | 1 mL/min, isocratic elution |
| Eluent | 0.1 M sodium phosphate buffer (pH 6.7): Acetonitrile (82: 18) |
| Injection volume | 10 μL |
| Integrator | Shimadzu data module (Shimadzu Corporation, Kyoto, Japan) |
| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| Mouse IgA | 5′-TGAGCGCTGGAACAGTGGCG-3′ | 5′-TCAGGGCCAGCTCCTCCGAC-3′ |
| Mouse α-defensin-1 | TaqMan Mm02524428 | |
| Mouse β-tubulin | 5′-CTCCCAGGTTAAAGTCCTTCAGTA-3′ | 5′-GCAACATAAATACAGAGGTGGCTA-3′ |
| Chemical Property (%) 1 | KRG-P |
|---|---|
| Neutral sugar | 74.3 ± 0.03 |
| Uronic acid | 24.6 ± 0.02 |
| Protein | 1.0 ± 0.05 |
| Component sugar | (Mole %) |
| Rhamnose | 1.9 ± 0.2 |
| Fucose | - |
| Arabinose | 6.8 ± 0.1 |
| Xylose | - |
| Mannose | - |
| Galactose | 11.0 ± 0.0 |
| Glucose | 60.5 ± 0.0 |
| Glucuronic acid | - |
| Galacturonic acid | 19.7 ± 0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, D.H.; Han, B.; Shin, M.-S.; Hwang, G.S. Enhanced Intestinal Immune Response in Mice after Oral Administration of Korea Red Ginseng-Derived Polysaccharide. Polymers 2020, 12, 2186. https://doi.org/10.3390/polym12102186
Park DH, Han B, Shin M-S, Hwang GS. Enhanced Intestinal Immune Response in Mice after Oral Administration of Korea Red Ginseng-Derived Polysaccharide. Polymers. 2020; 12(10):2186. https://doi.org/10.3390/polym12102186
Chicago/Turabian StylePark, Do Hwi, Byungcheol Han, Myoung-Sook Shin, and Gwi Seo Hwang. 2020. "Enhanced Intestinal Immune Response in Mice after Oral Administration of Korea Red Ginseng-Derived Polysaccharide" Polymers 12, no. 10: 2186. https://doi.org/10.3390/polym12102186
APA StylePark, D. H., Han, B., Shin, M.-S., & Hwang, G. S. (2020). Enhanced Intestinal Immune Response in Mice after Oral Administration of Korea Red Ginseng-Derived Polysaccharide. Polymers, 12(10), 2186. https://doi.org/10.3390/polym12102186






