Fluorinated Linear Copolyimide Physically Crosslinked with Novel Fluorinated Hyperbranched Polyimide Containing Large Space Volumes for Enhanced Mechanical Properties and UV-Shielding Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
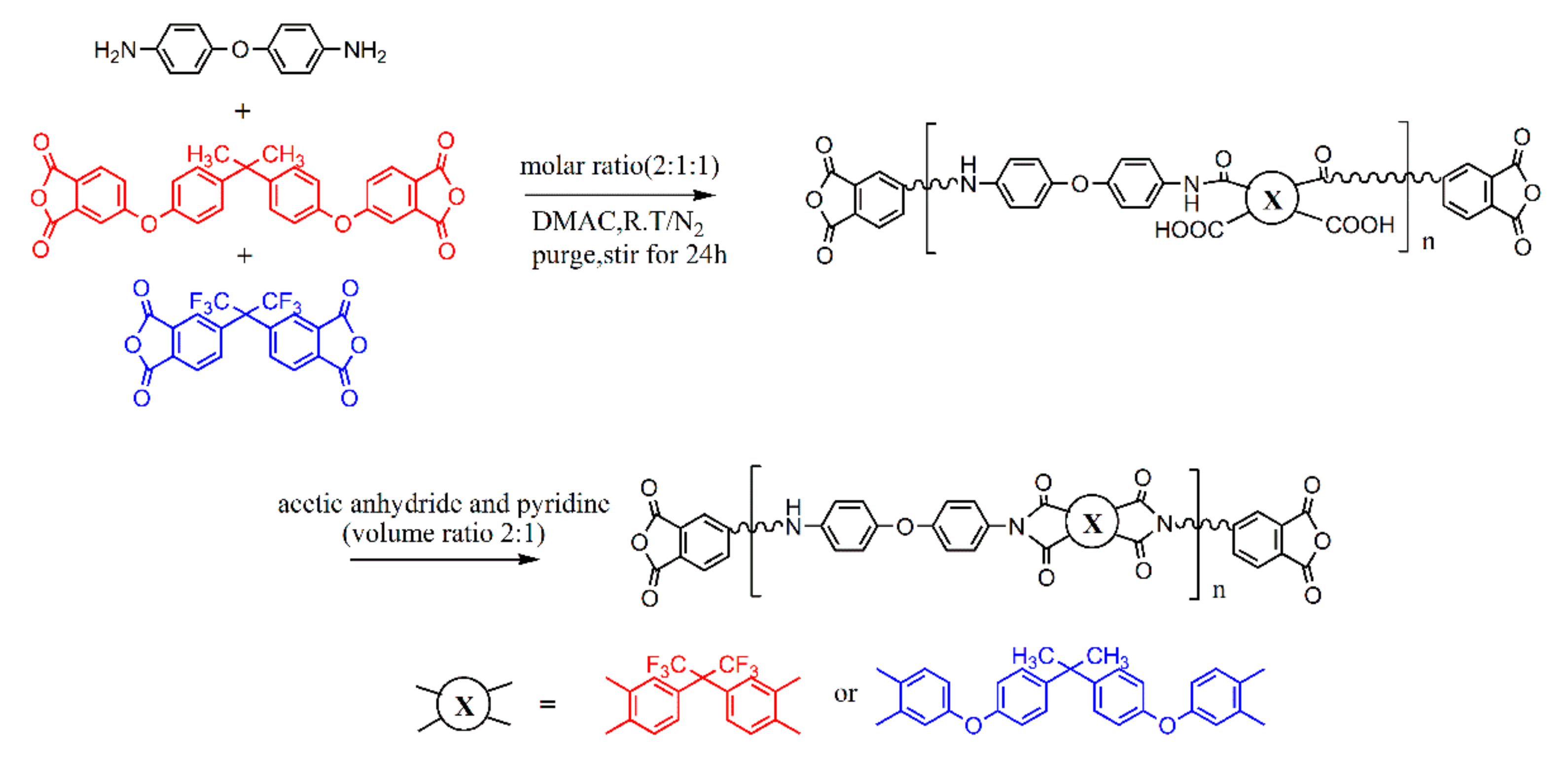
2.2. Synthesis of Anhydride-Terminated Fluorinated Copolyimide (FPI)
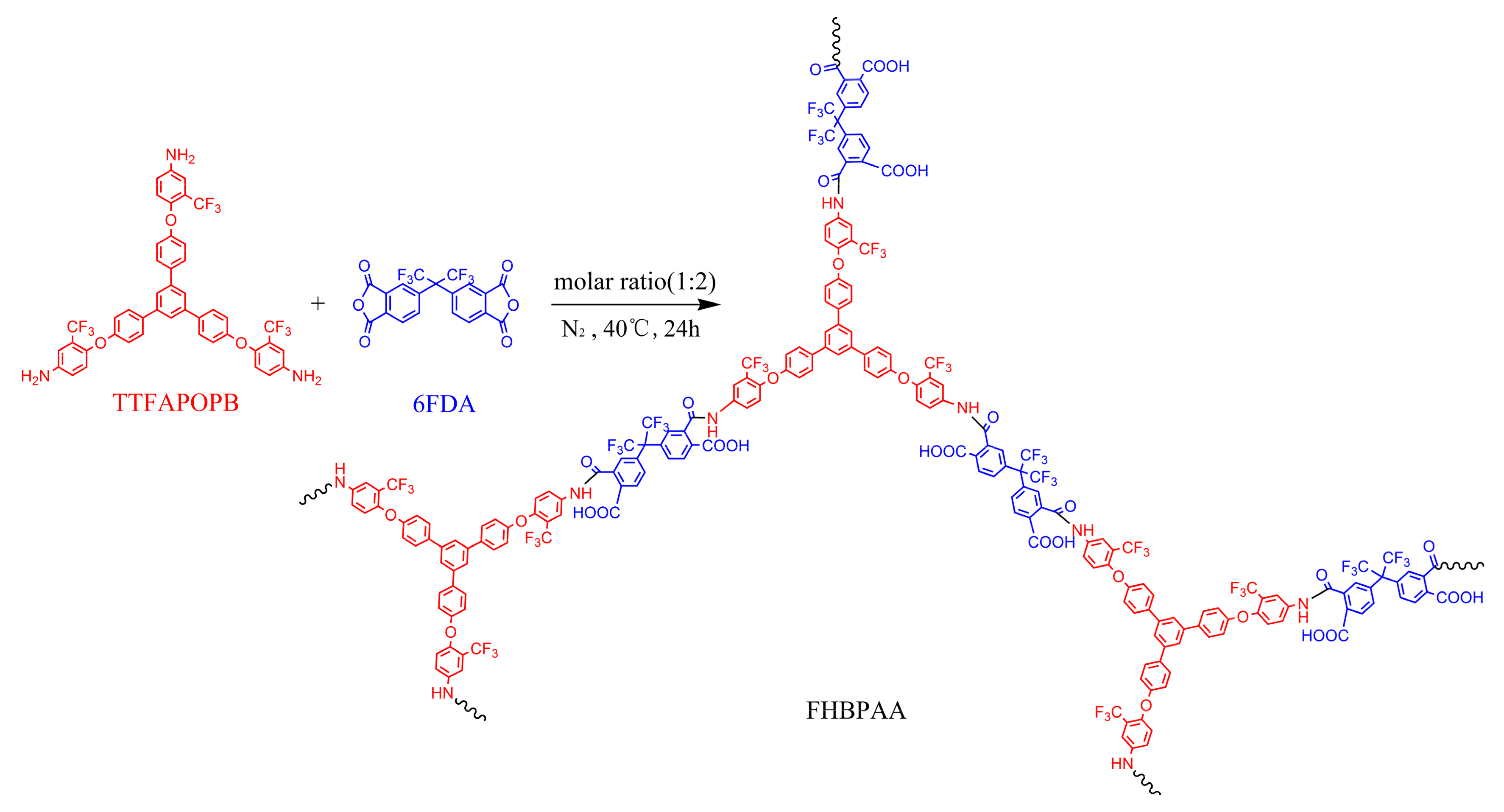
2.3. Preparation of Anhydride-Terminated FHBPAA
2.4. Preparation of Fluorinated Linear Copolyimide/Fluorinated Hyperbranched Polyimide (FPI/FHBPI) Composites
2.5. Characterization
3. Results and Discussion
3.1. Characterization of FPI, FPI/FHBPI Composites and FHBPI
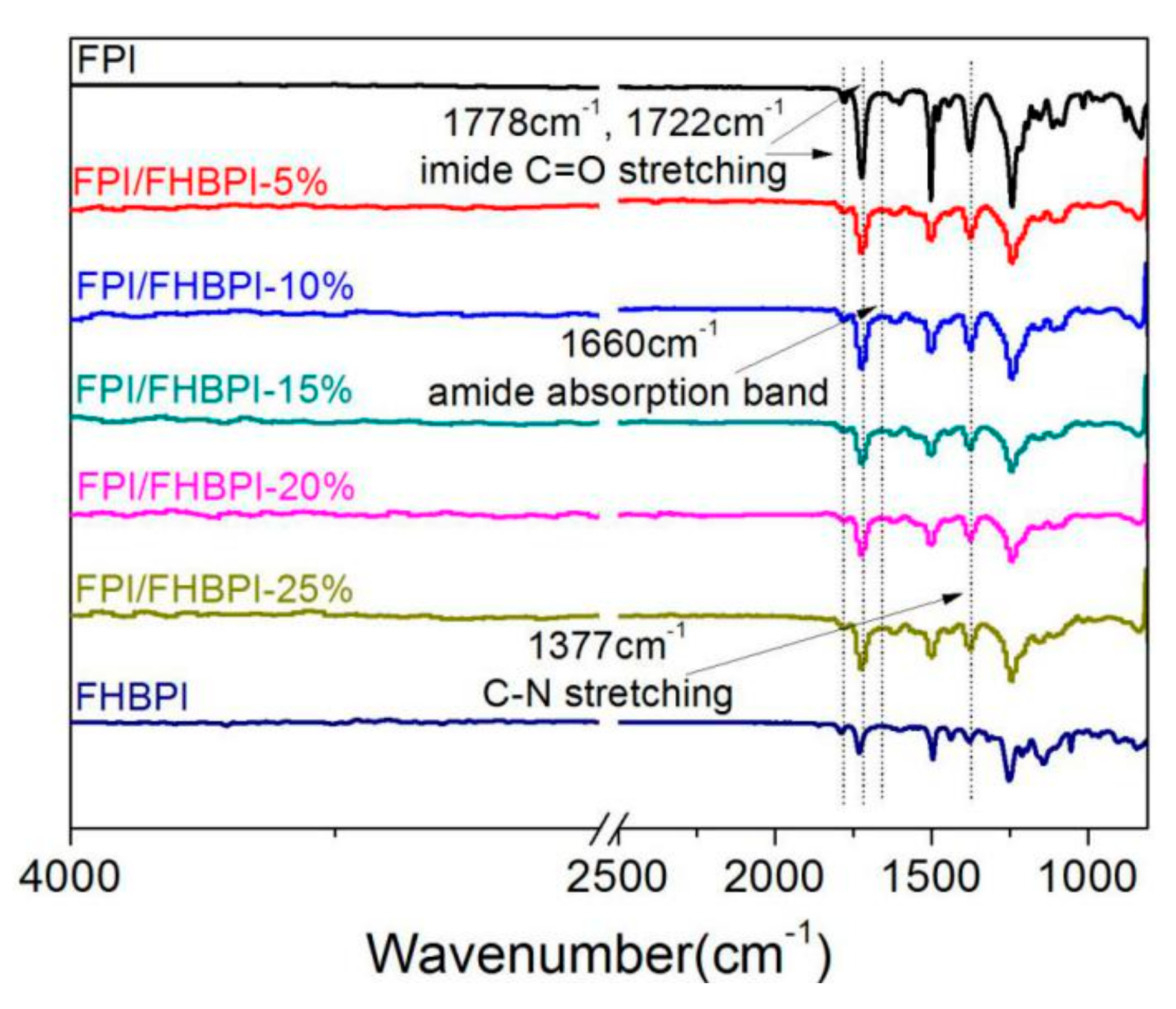
3.2. Structure Analysis
3.3. Thermal Properties
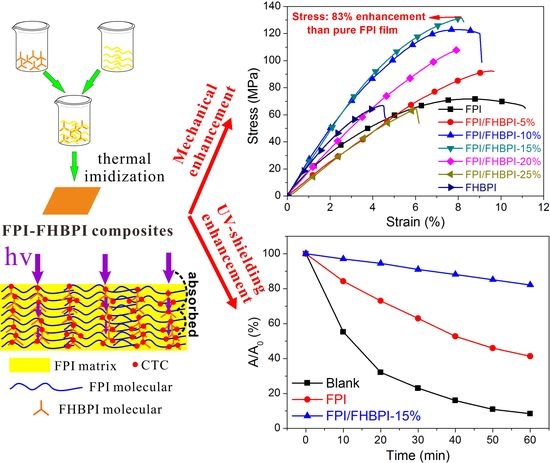
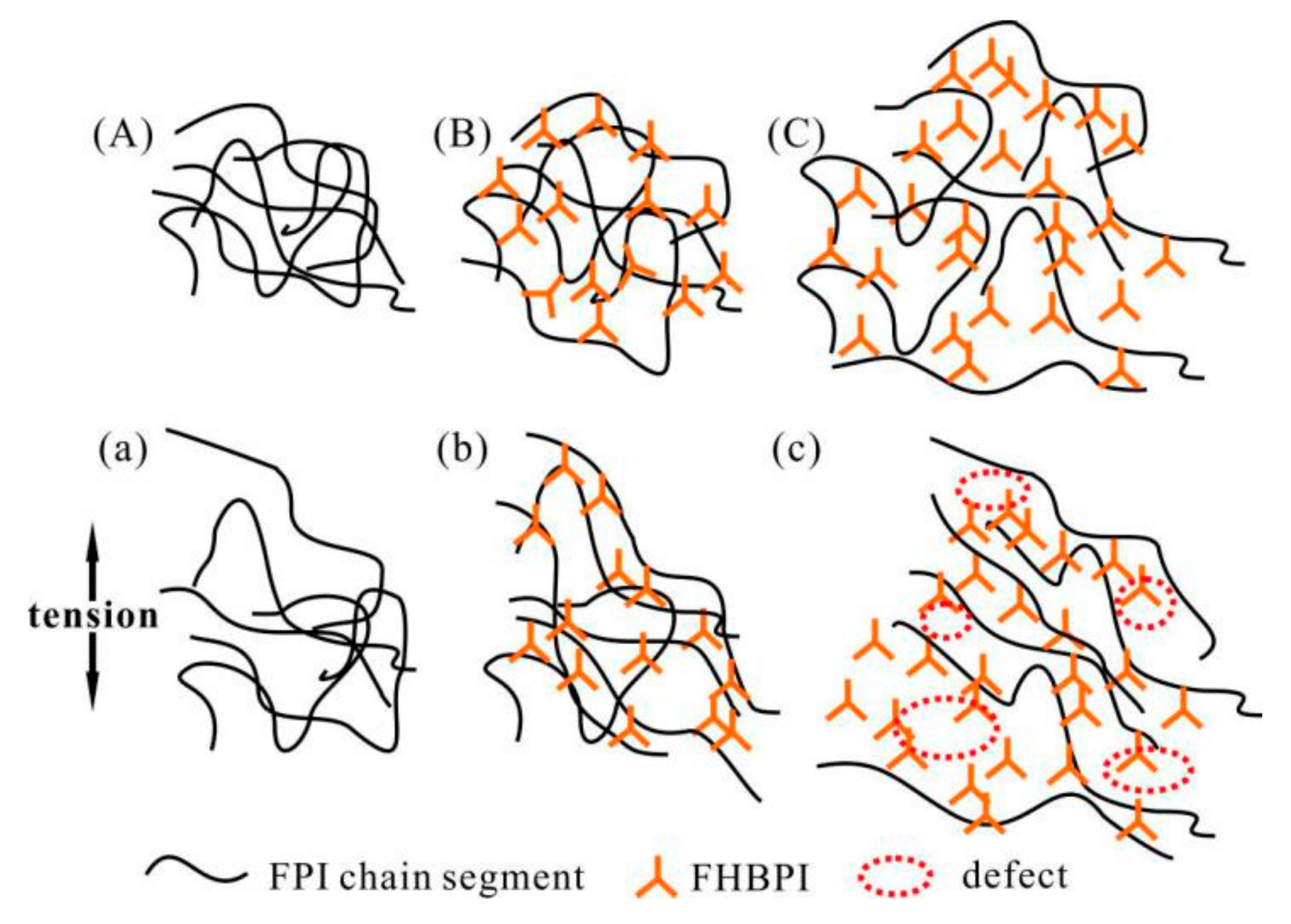
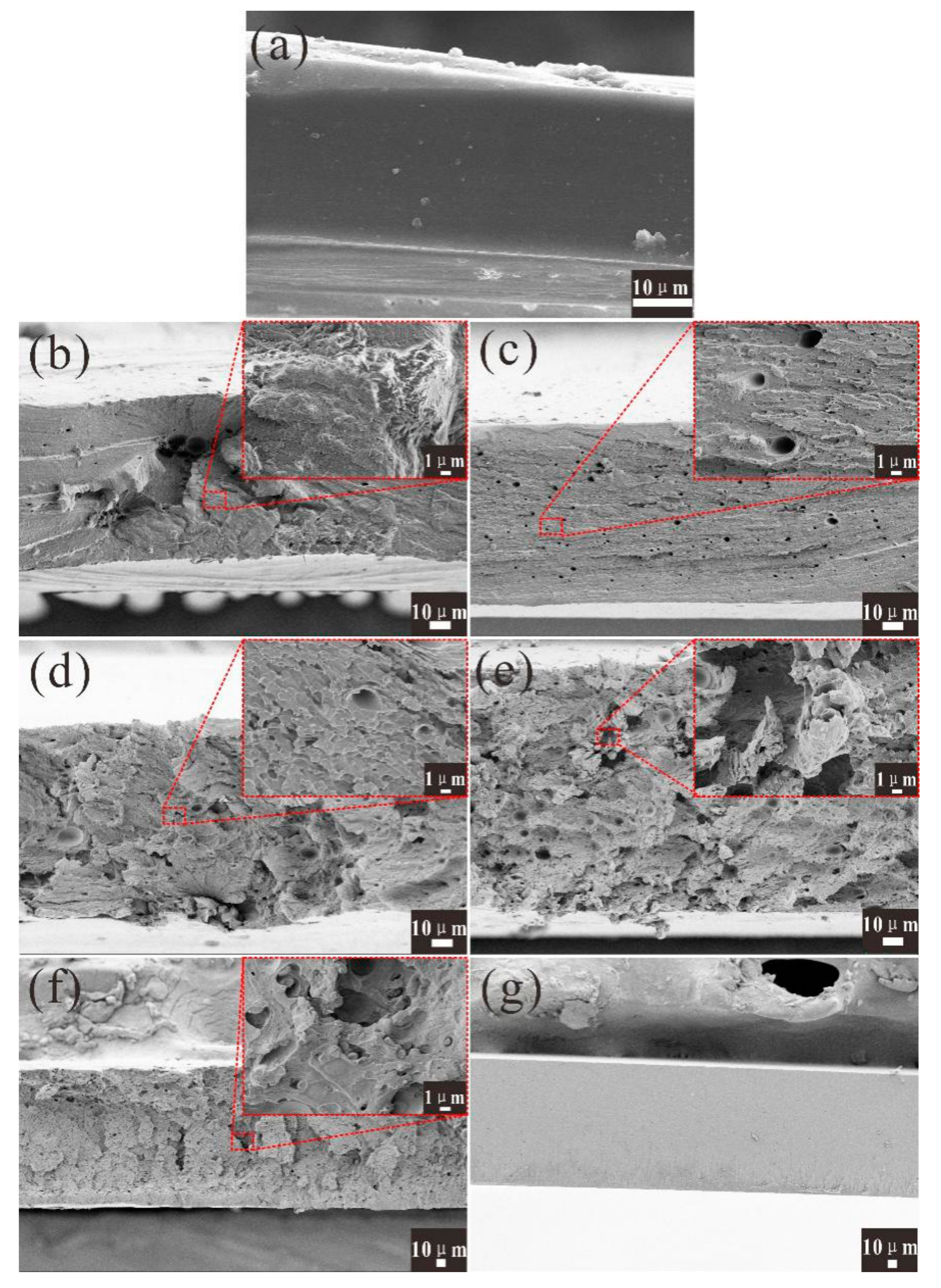
3.4. Strain-Stress Properties
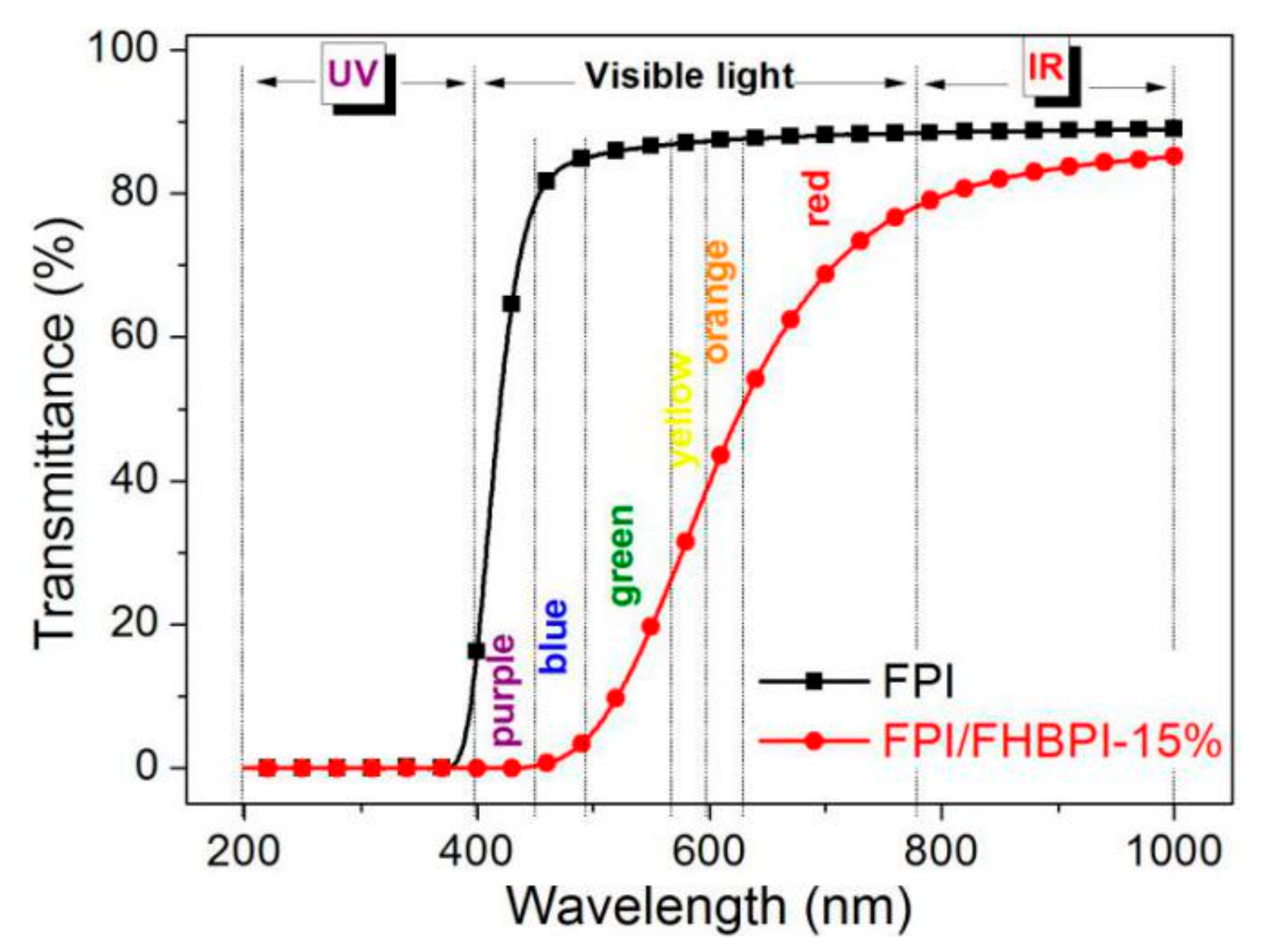
3.5. Optical Properties
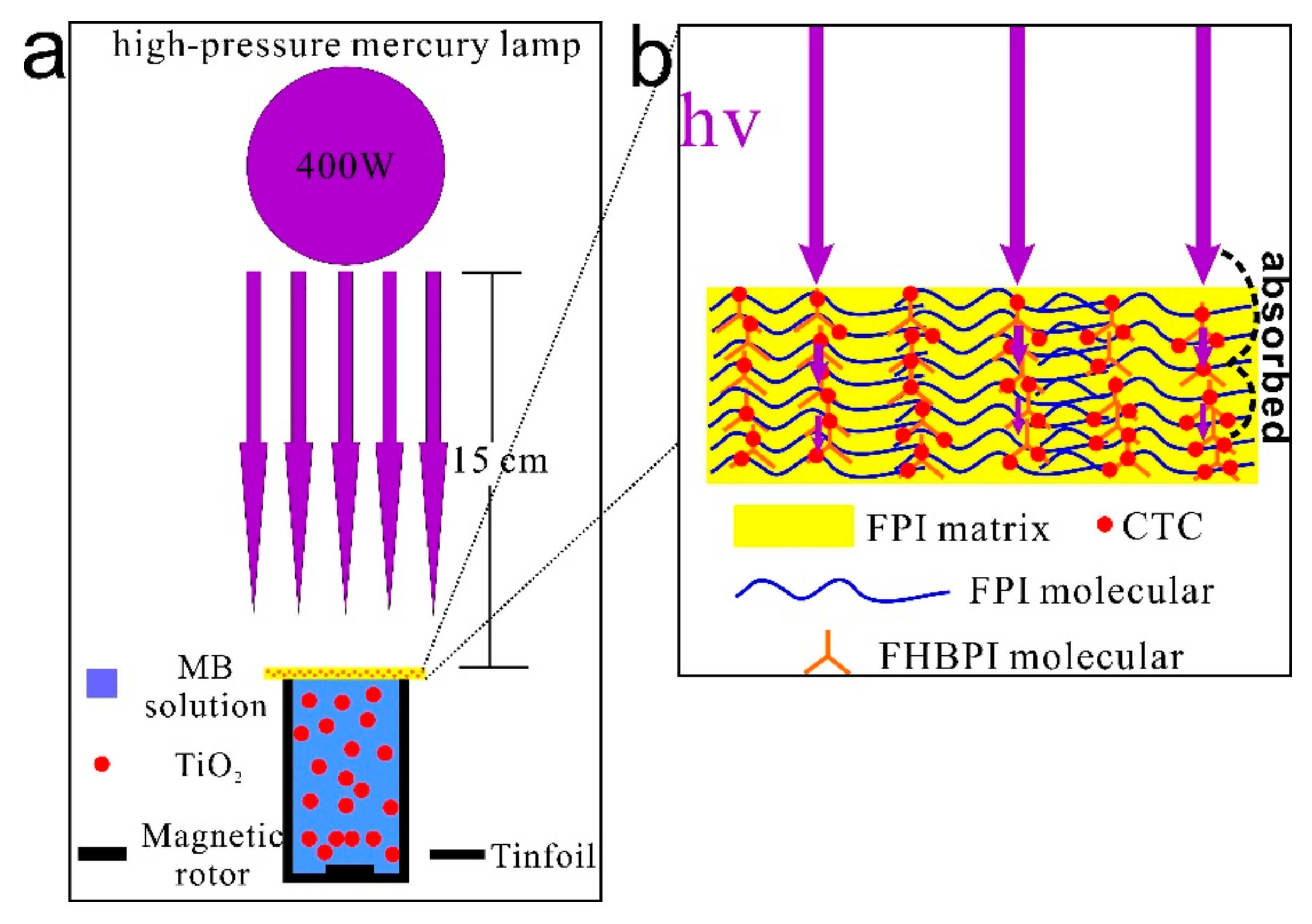
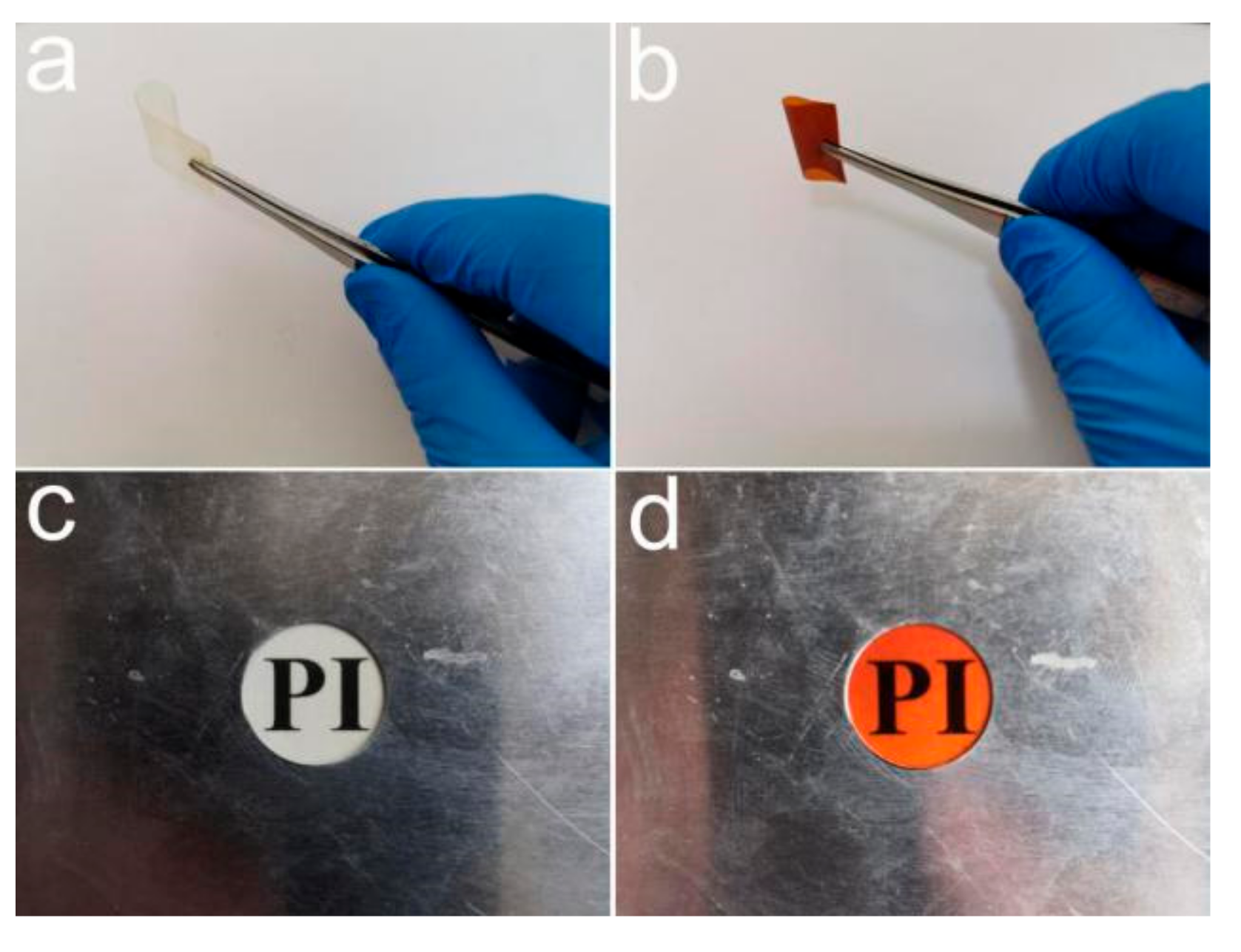
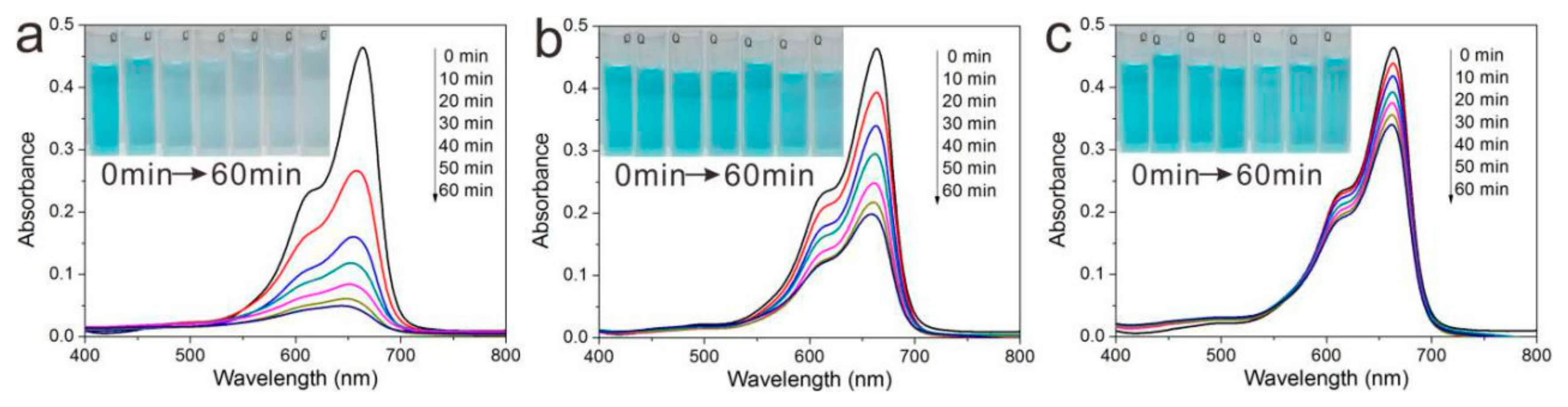
3.6. UV-Shielding Performance
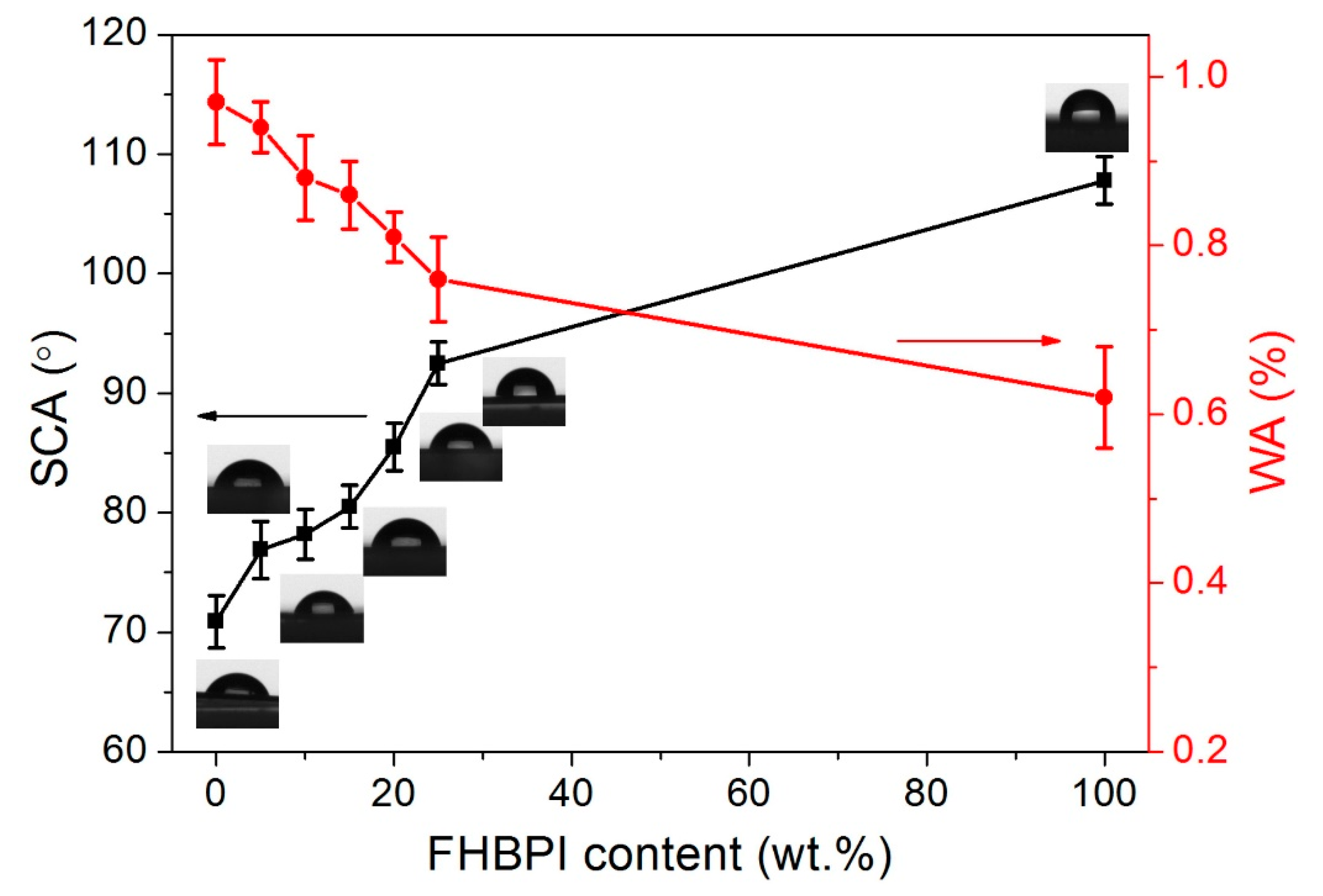
3.7. Surface Contact Angle (SCA), and Water Absorption (WA)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Duan, H.; Lyu, P.; Liu, J.; Zhao, Y.; Xu, Y. Semiconducting crystalline two-dimensional polyimide nanosheets with superior sodium storage properties. ACS Nano 2019, 13, 2473–2480. [Google Scholar] [CrossRef]
- Hao, Z.; Wu, J.; Wang, C.; Liu, J. Electrospun polyimide/metal-organic framework nanofibrous membrane with superior thermal stability for efficient PM2.5 capture. ACS Appl. Mater. Interfaces 2019, 11, 11904–11909. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, C.; Song, X.; Su, B.; Mandal, B.; Prasad, B.; Gao, X.; Gao, C. Graphene quantum dots-doped thin film nanocomposite polyimide membranes with enhanced solvent resistance for solvent-resistant nanofiltration. ACS Appl. Mater. Interfaces 2019, 11, 6527–6540. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Zheng, J.; Zhao, C.; Liu, C.; Su, C.; Tang, W.; Li, X.; Ning, G.-H. Carbonyl-based polyimide and polyquinoneimide for potassium-ion batteries. J. Mater. Chem. A 2019, 7, 9997–10003. [Google Scholar] [CrossRef]
- Wu, X.; Li, H.; Cheng, K.; Qiu, H.; Yang, J. Modified graphene/polyimide composite films with strongly enhanced thermal conductivity. Nanoscale 2019, 11, 8219–8225. [Google Scholar] [CrossRef]
- Zhu, Z.; Yao, H.; Dong, J.; Qian, Z.; Dong, W.; Long, D. High-mechanical-strength polyimide aerogels crosslinked with 4,4’-oxydianiline-functionalized carbon nanotubes. Carbon 2019, 144, 24–31. [Google Scholar] [CrossRef]
- Li, Q.; Liao, G.; Zhang, S.; Pang, L.; Tong, H.; Zhao, W.; Xu, Z. Effect of adjustable molecular chain structure and pure silica zeolite nanoparticles on thermal, mechanical, dielectric, UV-shielding and hydrophobic properties of fluorinated copolyimide composites. Appl. Surf. Sci. 2018, 427, 437–450. [Google Scholar] [CrossRef]
- Zhang, F.; Feng, Y.; Qin, M.; Gao, L.; Li, Z.; Zhao, F.; Zhang, Z.; Lv, F.; Feng, W. Stress controllability in thermal and electrical conductivity of 3D elastic graphene-crosslinked carbon nanotube sponge/polyimide nanocomposite. Adv. Funct. Mater. 2019, 29, 1901383. [Google Scholar] [CrossRef]
- Ji, D.; Li, T.; Hu, W.; Fuchs, H. Recent progress in aromatic polyimide dielectrics for organic electronic devices and circuits. Adv. Mater. 2019, 31, 1806070. [Google Scholar] [CrossRef]
- Padua, L.M.G.; Yeh, J.-M.; Santiago, K.S. A Novel Application of Electroactive Polyimide Doped with Gold Nanoparticles: As a Chemiresistor Sensor for Hydrogen Sulfide Gas. Polymers 2019, 11, 1918. [Google Scholar] [CrossRef]
- Kato, S.; Amat Yusof, F.A.; Harimoto, T.; Takada, K.; Kaneko, T.; Kawai, M.; Mitsumata, T. Electric Volume Resistivity for Biopolyimide Using 4, 4′-Diamino-α-truxillic acid and 1, 2, 3, 4-Cyclobutanetetracarboxylic dianhydride. Polymers 2019, 11, 1552. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Wu, W.; Hu, X.; Lin, G.; Guo, J.; Qu, H.; Zhao, J. 3D printing of carbon nanotubes reinforced thermoplastic polyimide composites with controllable mechanical and electrical performance. Compos. Sci. Technol. 2019, 182, 107671. [Google Scholar] [CrossRef]
- Yu, L.; Wang, L.; Yu, L.; Mu, D.; Wang, L.; Xi, J. Aliphatic/aromatic sulfonated polyimide membranes with cross-linked structures for vanadium flow batteries. J. Membr. Sci. 2019, 572, 119–127. [Google Scholar] [CrossRef]
- Gouzman, I.; Grossman, E.; Verker, R.; Atar, N.; Bolker, A.; Eliaz, N. Advances in polyimide-based materials for space applications. Adv. Mater. 2019, 31, 1807738. [Google Scholar] [CrossRef]
- Bae, Y.M.; Lee, Y.H.; Kim, H.S.; Lee, D.J.; Kim, S.Y.; Kim, H.D. Polyimide-polyurethane/urea block copolymers for highly sensitive humidity sensor with low hysteresis. J. Appl. Polym. Sci. 2017, 134, 44973. [Google Scholar] [CrossRef]
- Que, X.; Yan, Y.; Qiu, Z.; Wang, Y. Synthesis and characterization of trifluoromethyl-containing polyimide-modified epoxy resins. J. Mater. Sci. 2016, 51, 10833–10848. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, J.; Zhu, T.; Hua, Z.; Chen, Q.; Yu, X. Blends of thermoplastic polyurethane and polyether-polyimide: Preparation and properties. Polymer 2001, 42, 1493–1500. [Google Scholar] [CrossRef]
- Mekuria, T.D.; Zhang, C.; Liu, Y.; Fouad, D.E.D.; Lv, K.; Yang, M.; Zhou, Y. Surface modification of nano-silica by diisocyanates and their application in polyimide matrix for enhanced mechanical, thermal and water proof properties. Mater. Chem. Phys. 2019, 225, 358–364. [Google Scholar] [CrossRef]
- Hsu, S.-Y.; Lin, S.-C.; Wang, J.-A.; Cheng, T.-Y.; Lin, C.-W.; Chen, Y.-H.; Tsai, D.-H.; Ma, C.-C.M. Preparation and characterization of silsesquioxane-graphene oxide modified soluble polyimide nanocomposites with excellent dispersibility and enhanced tensile properties. Eur. Polym. J. 2019, 112, 95–103. [Google Scholar] [CrossRef]
- Chen, Y.; Li, D.; Yang, W.; Xiao, C.; Wei, M. Effects of different amine-functionalized graphene on the mechanical, thermal, and tribological properties of polyimide nanocomposites synthesized by in situ polymerization. Polymer 2018, 140, 56–72. [Google Scholar] [CrossRef]
- Dai, W.; Yu, J.; Wang, Y.; Song, Y.; Bai, H.; Nishimura, K.; Liao, H.; Jiang, N. Enhanced thermal and mechanical properties of polyimide/graphene composites. Macromol. Res. 2014, 22, 983–989. [Google Scholar] [CrossRef]
- Luong, N.D.; Hippi, U.; Korhonen, J.T.; Soininen, A.J.; Ruokolainen, J.; Johansson, L.-S.; Nam, J.-D.; Seppälä, J. Enhanced mechanical and electrical properties of polyimide film by graphene sheets via in situ polymerization. Polymer 2011, 52, 5237–5242. [Google Scholar] [CrossRef]
- Wang, L.; Tian, Y.; Ding, H.; Li, J. Microstructure and properties of organosoluble polyimide/silica hybrid films. Eur. Polym. J. 2006, 42, 2921–2930. [Google Scholar] [CrossRef]
- Li, Y.; Fu, S.-Y.; Li, Y.-Q.; Pan, Q.-Y.; Xu, G.; Yue, C.-Y. Improvements in transmittance, mechanical properties and thermal stability of silica-polyimide composite films by a novel sol-gel route. Compos. Sci. Technol. 2007, 67, 2408–2416. [Google Scholar] [CrossRef]
- Chao, T.-Y.; Chang, H.-L.; Su, W.-C.; Wu, J.-Y.; Jeng, R.-J. Nonlinear optical polyimide/montmorillonite nanocomposites consisting of azobenzene dyes. Dyes Pigments 2008, 77, 515–524. [Google Scholar] [CrossRef]
- Alias, A.; Ahmad, Z.; Ismail, A.B. Preparation of polyimide/Al2O3 composite films as improved solid dielectrics. Mater. Sci. Eng. B Adv. Funct. Solid-State Mater. 2011, 176, 799–804. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, G.; Yang, X.; Ruan, K.; Ma, T.; Zhang, Q.; Gu, J.; Wu, Y.; Liu, H.; Guo, Z. Significantly enhanced and precisely modeled thermal conductivity in polyimide nanocomposites with chemically modified graphene via in situ polymerization and electrospinning-hot press technology. J. Mater. Chem. C 2018, 6, 3004–3015. [Google Scholar] [CrossRef]
- Wu, L.-G.; Yang, C.-H.; Wang, T.; Zhang, X.-Y. Enhanced the performance of graphene oxide/polyimide hybrid membrane for CO2 separation by surface modification of graphene oxide using polyethylene glycol. Appl. Surf. Sci. 2018, 440, 1063–1072. [Google Scholar] [CrossRef]
- Kim, M.; Eo, K.; Lim, H.J.; Kwon, Y.K. Low shrinkage, mechanically strong polyimide hybrid aerogels containing hollow mesoporous silica nanospheres. Compos. Sci. Technol. 2018, 165, 355–361. [Google Scholar] [CrossRef]
- Kalchounaki, E.K.; Farhadi, A.; Zadehnazari, A. Preparation and properties evaluation of polyimide-matrix nanocomposites reinforced with glutamine functionalized multi-walled carbon nanotube. Polym. Bull. 2018, 75, 5731–5744. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Zhang, S.; Pang, L.; Tong, H.; Li, J.; Xu, Z. Novel fluorinated random co-polyimide/amine-functionalized zeolite MEL50 hybrid films with enhanced thermal and low dielectric properties. J. Mater. Sci. 2017, 52, 5283–5296. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, J.; Li, Q.; Pang, L.; Zhang, Q.; Xu, Z.; Yeung, K.W.; Yi, C. Synthesis and characterization of novel hyperbranched polyimides/attapulgite nanocomposites. Compos. Part A Appl. Sci. Manuf. 2013, 55, 161–168. [Google Scholar] [CrossRef]
- Li, Q.; Liao, G.; Tian, J.; Xu, Z. Preparation of novel fluorinated copolyimide/aminefunctionalized sepia eumelanin nanocomposites with enhanced mechanical, thermal, and UV-shielding properties. Macromol. Mater. Eng. 2018, 303, 1700407. [Google Scholar] [CrossRef]
- Flory, P.J. Molecular size distribution in three dimensional polymers. VI. branched polymers containing A-R-Bf−1 type units. J. Am. Chem. Soc. 1952, 74, 2718–2723. [Google Scholar] [CrossRef]
- Iqbal, A.; Lee, S.H.; Siddiqi, H.M.; Park, O.O.; Akhter, T. Correction to “Enhanced dielectric constant, ultralow dielectric loss, and high-strength imide-functionalized graphene oxide/hyperbranched polyimide nanocomposites”. J. Phys. Chem. C 2018, 122, 6555–6565. [Google Scholar] [CrossRef]
- Li, Q.; Li, J.; Liao, G.; Xu, Z. The preparation of heparin-like hyperbranched polyimides and their antithrombogenic, antibacterial applications. J. Mater. Sci.-Mater. Med. 2018, 29, 126. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Q.; Wang, T. Engineering a hyperbranched polyimide membrane for shape memory and CO2 capture. J. Mater. Chem. A 2017, 5, 13823–13833. [Google Scholar] [CrossRef]
- Suzuki, T.; Miki, M.; Yamada, Y. Gas transport properties of hyperbranched polyimide/hydroxy polyimide blend membranes. Eur. Polym. J. 2012, 48, 1504–1512. [Google Scholar] [CrossRef]
- Cao, Z.; Jin, L.; Liu, Y.; Jiang, Z.; Zhang, D. Crosslinkable fluorinated hyperbranched polyimide for thermo-optic switches with high thermal stability. J. Appl. Polym. Sci. 2013, 127, 607–611. [Google Scholar] [CrossRef]
- Scarpaci, A.; Blart, E.; Montembault, V.; Fontaine, L.; Rodriguez, V.; Odobel, F. Synthesis and nonlinear optical properties of a peripherally functionalized hyperbranched polymer by DR1 chromophores. ACS Appl. Mater. Interfaces 2009, 1, 1799–1806. [Google Scholar] [CrossRef]
- Peter, J.; Khalyavina, A.; Kříž, J.; Bleha, M. Synthesis and gas transport properties of ODPA-TAP-ODA hyperbranched polyimides with various comonomer ratios. Eur. Polym. J. 2009, 45, 1716–1727. [Google Scholar] [CrossRef]
- Bubniene, U.; Mazetyte, R.; Ramanaviciene, A.; Gulbinas, V.; Ramanavicius, A.; Karpicz, R. Fluorescence Quenching-Based Evaluation of Glucose Oxidase Composite with Conducting Polymer, Polypyrrole. J. Phys. Chem. C 2018, 122, 9491–9498. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, S.; Liao, G.; Yi, C.; Xu, Z. Novel fluorinated hyperbranched polyimides with excellent thermal stability, UV-shielding property, organosolubility, and low dielectric constants. High Perform. Polym. 2018, 30, 872–886. [Google Scholar] [CrossRef]
- Li, Q.; Xiong, H.; Pang, L.; Li, Q.; Zhang, Y.; Chen, W.; Xu, Z.; Yi, C. Synthesis and characterization of thermally stable, hydrophobic hyperbranched polyimides derived from a novel triamine. High Perform. Polym. 2015, 27, 426–438. [Google Scholar] [CrossRef]
- Zuiderduin, W.; Huetink, J.; Gaymans, R. Rigid particle toughening of aliphatic polyketone. Polymer 2006, 47, 5880–5887. [Google Scholar] [CrossRef]
- Wang, Y.; Li, T.; Ma, P.; Bai, H.; Xie, Y.; Chen, M.; Dong, W. Simultaneous enhancements of UV-shielding properties and photostability of poly(vinyl alcohol) via incorporation of sepia eumelanin. ACS Sustain. Chem. Eng. 2016, 4, 2252–2258. [Google Scholar] [CrossRef]
- Wang, Y.; Li, T.; Wang, X.; Ma, P.; Bai, H.; Dong, W.; Xie, Y.; Chen, M. Superior performance of polyurethane based on natural melanin nanoparticles. Biomacromolecules 2016, 17, 3782–3789. [Google Scholar] [CrossRef]
- Zhao, H.; She, W.; Shi, D.; Wu, W.; Zhang, Q.-C.; Li, R.K. Polyurethane/POSS nanocomposites for superior hydrophobicity and high ductility. Compos. Part B Eng. 2019, 177, 107441. [Google Scholar] [CrossRef]
- Liao, G.; Li, Q.; Zhao, W.; Pang, Q.; Gao, H.; Xu, Z. In-situ construction of novel silver nanoparticle decorated polymeric spheres as highly active and stable catalysts for reduction of methylene blue dye. Appl. Catal. A-Gen. 2018, 549, 102–111. [Google Scholar] [CrossRef]
- Liao, G.; Chen, J.; Zeng, W.; Yu, C.; Yi, C.; Xu, Z. Facile Preparation of Uniform Nanocomposite Spheres with Loading Silver Nanoparticles on Polystyrene-methyl Acrylic Acid Spheres for Catalytic Reduction of 4-Nitrophenol. J. Phys. Chem. C 2016, 120, 25935–25944. [Google Scholar] [CrossRef]
- Liao, G.; Fang, J.; Li, Q.; Li, S.; Xu, Z.; Fang, B. Ag-Based nanocomposites: Synthesis and applications in catalysis. Nanoscale 2019, 11, 7062–7096. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; Gong, Y.; Zhong, L.; Fang, J.; Zhang, L.; Xu, Z.; Gao, H.; Fang, B. Unlocking the door to highly efficient Ag-based nanoparticles catalysts for NaBH4-assisted nitrophenol reduction. Nano Res. 2019, 12, 2407–2436. [Google Scholar] [CrossRef]
- Liao, G.; Gong, Y.; Zhang, L.; Gao, H.; Yang, G.-J.; Fang, B. Semiconductor polymeric graphitic carbon nitride photocatalysts: The “holy grail” for the photocatalytic hydrogen evolution reaction under visible light. Energy Environ. Sci. 2019, 12, 2080–2147. [Google Scholar] [CrossRef]
- Zeng, W.; Chen, J.; Yang, H.; Deng, L.; Liao, G.; Xu, Z. Robust coating with superhydrophobic and self-cleaning properties in either air or oil based on natural zeolite. Surf. Coat. Technol. 2017, 309, 1045–1051. [Google Scholar] [CrossRef]
- Xiao, L.; Deng, M.; Zeng, W.; Zhang, B.; Xu, Z.; Yi, C.; Liao, G. Novel Robust Superhydrophobic Coating with Self-Cleaning Properties in Air and Oil Based on Rare Earth Metal Oxide. Ind. Eng. Chem. Res. 2017, 56, 12354–12361. [Google Scholar] [CrossRef]
- Xiao, L.; Zeng, W.; Liao, G.; Yi, C.; Xu, Z. Thermally and Chemically Stable Candle Soot Superhydrophobic Surface with Excellent Self-Cleaning Properties in Air and Oil. ACS Appl. Nano Mater. 2018, 1, 1204–1211. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, Q.; Yan, Z.; Liao, G.; Zhang, B.; Yu, Y.; Yi, C.; Xu, Z. Design and synthesis of novel aminosiloxane crosslinked linseed oil-based waterborne polyurethane composites and its physicochemical properties. Prog. Org. Coat. 2019, 127, 194–201. [Google Scholar] [CrossRef]


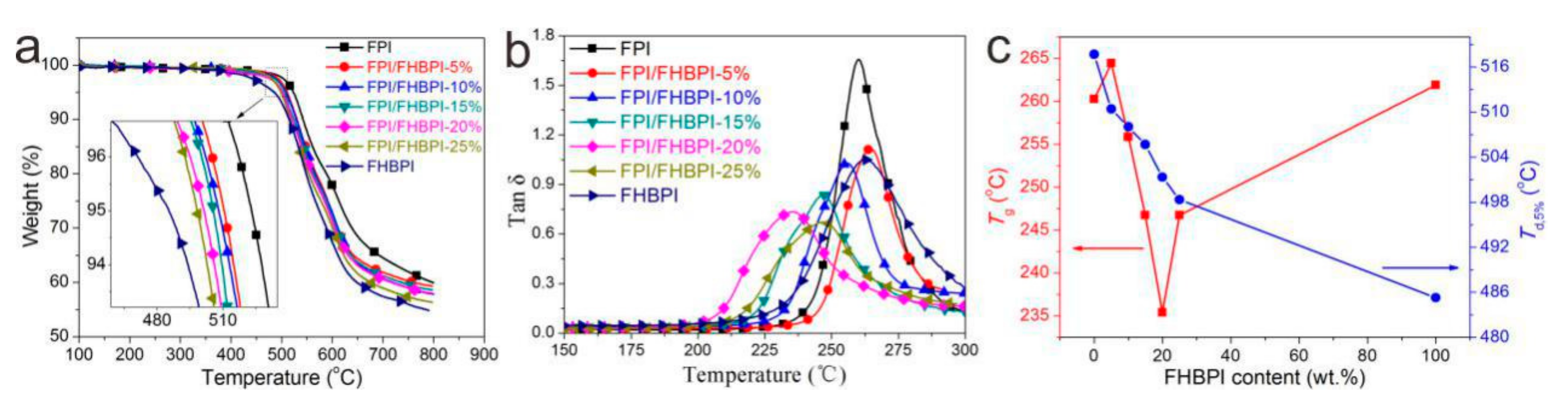
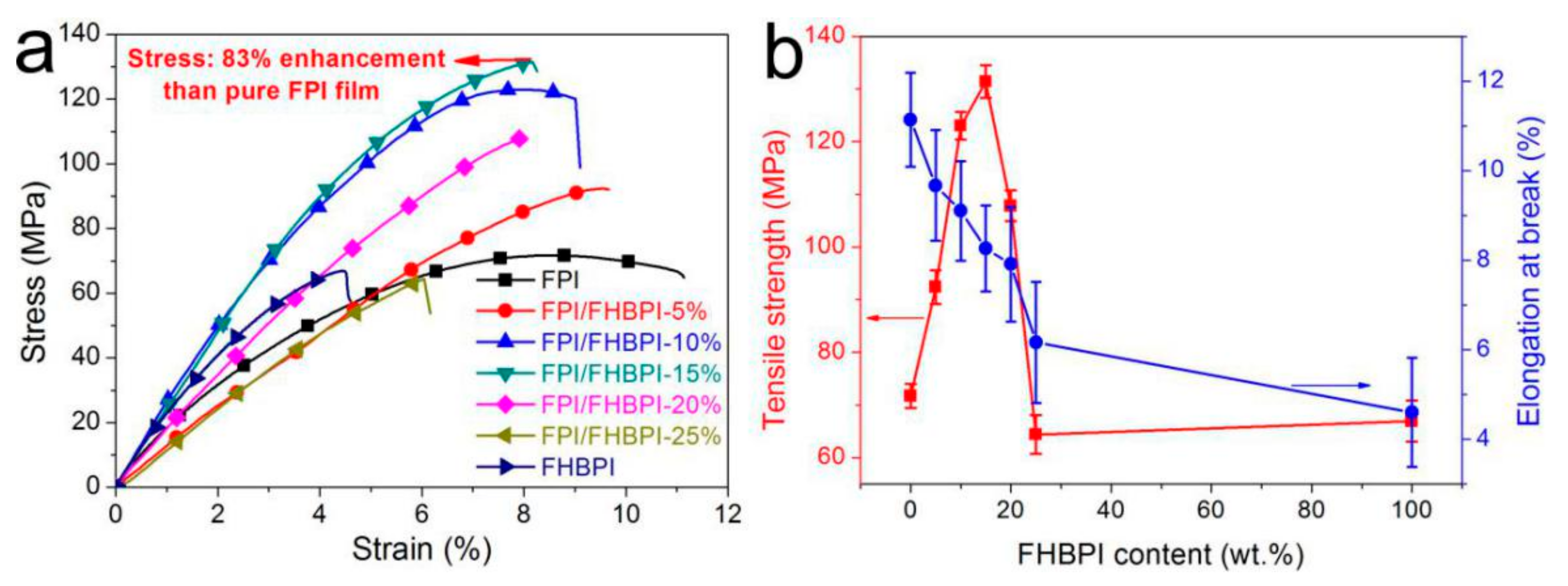


| Sample | Tg (°C) a | Td (°C) | Rw (wt %) b | |
|---|---|---|---|---|
| Td,5% | Td,10% | |||
| FPI | 260.3 | 523.2 | 542.1 | 59.9 |
| FPI/FHBPI-5% | 264.5 | 510.4 | 530.1 | 59.3 |
| FPI/FHBPI-10% | 255.8 | 508.1 | 528.7 | 57.8 |
| FPI/FHBPI-15% | 246.7 | 505.7 | 524.4 | 58.6 |
| FPI/FHBPI-20% | 235.4 | 501.3 | 519.7 | 58.0 |
| FPI/FHBPI-25% | 246.7 | 498.3 | 516.2 | 56.3 |
| FHBPI | 261.9 | 485.3 | 514.6 | 54.5 |
| Sample | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|
| FPI | 71.7 ± 2.3 | 11.14 ± 1.05 |
| FPI/FHBPI-5% | 92.4 ± 3.2 | 9.67 ± 1.24 |
| FPI/FHBPI-10% | 123.0 ± 2.6 | 9.10 ± 1.11 |
| FPI/FHBPI-15% | 131.4 ± 3.1 | 8.26 ± 0.96 |
| FPI/FHBPI-20% | 107.8 ± 2.9 | 7.91 ± 1.28 |
| FPI/FHBPI-25% | 64.38 ± 3.7 | 6.16 ± 1.35 |
| FHBPI | 66.9 ± 3.9 | 4.60 ± 1.22 |
| Sample | SCA (°) | WA (%) |
|---|---|---|
| FPI | 70.9 ± 2.2 | 0.97 ± 0.05 |
| FPI/FHBPI-5% | 76.9 ± 2.4 | 0.94 ± 0.03 |
| FPI/FHBPI-10% | 78.2 ± 2.1 | 0.88 ± 0.05 |
| FPI/FHBPI-15% | 80.5 ± 1.8 | 0.86 ± 0.04 |
| FPI/FHBPI-20% | 85.5 ± 2.0 | 0.81 ± 0.03 |
| FPI/FHBPI-25% | 92.5 ± 1.8 | 0.76 ± 0.05 |
| FHBPI | 107.8 ± 2.0 | 0.62 ± 0.06 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Chen, R.; Guo, Y.; Lei, F.; Xu, Z.; Zhao, H.; Liao, G. Fluorinated Linear Copolyimide Physically Crosslinked with Novel Fluorinated Hyperbranched Polyimide Containing Large Space Volumes for Enhanced Mechanical Properties and UV-Shielding Application. Polymers 2020, 12, 88. https://doi.org/10.3390/polym12010088
Li Q, Chen R, Guo Y, Lei F, Xu Z, Zhao H, Liao G. Fluorinated Linear Copolyimide Physically Crosslinked with Novel Fluorinated Hyperbranched Polyimide Containing Large Space Volumes for Enhanced Mechanical Properties and UV-Shielding Application. Polymers. 2020; 12(1):88. https://doi.org/10.3390/polym12010088
Chicago/Turabian StyleLi, Qing, Ronghua Chen, Yujuan Guo, Fuhou Lei, Zushun Xu, Hui Zhao, and Guangfu Liao. 2020. "Fluorinated Linear Copolyimide Physically Crosslinked with Novel Fluorinated Hyperbranched Polyimide Containing Large Space Volumes for Enhanced Mechanical Properties and UV-Shielding Application" Polymers 12, no. 1: 88. https://doi.org/10.3390/polym12010088
APA StyleLi, Q., Chen, R., Guo, Y., Lei, F., Xu, Z., Zhao, H., & Liao, G. (2020). Fluorinated Linear Copolyimide Physically Crosslinked with Novel Fluorinated Hyperbranched Polyimide Containing Large Space Volumes for Enhanced Mechanical Properties and UV-Shielding Application. Polymers, 12(1), 88. https://doi.org/10.3390/polym12010088