Multi-Fold Enhancement in Compressive Properties of Polystyrene Foam Using Pre-delaminated Stearate Functionalized Layer Double Hydroxides
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
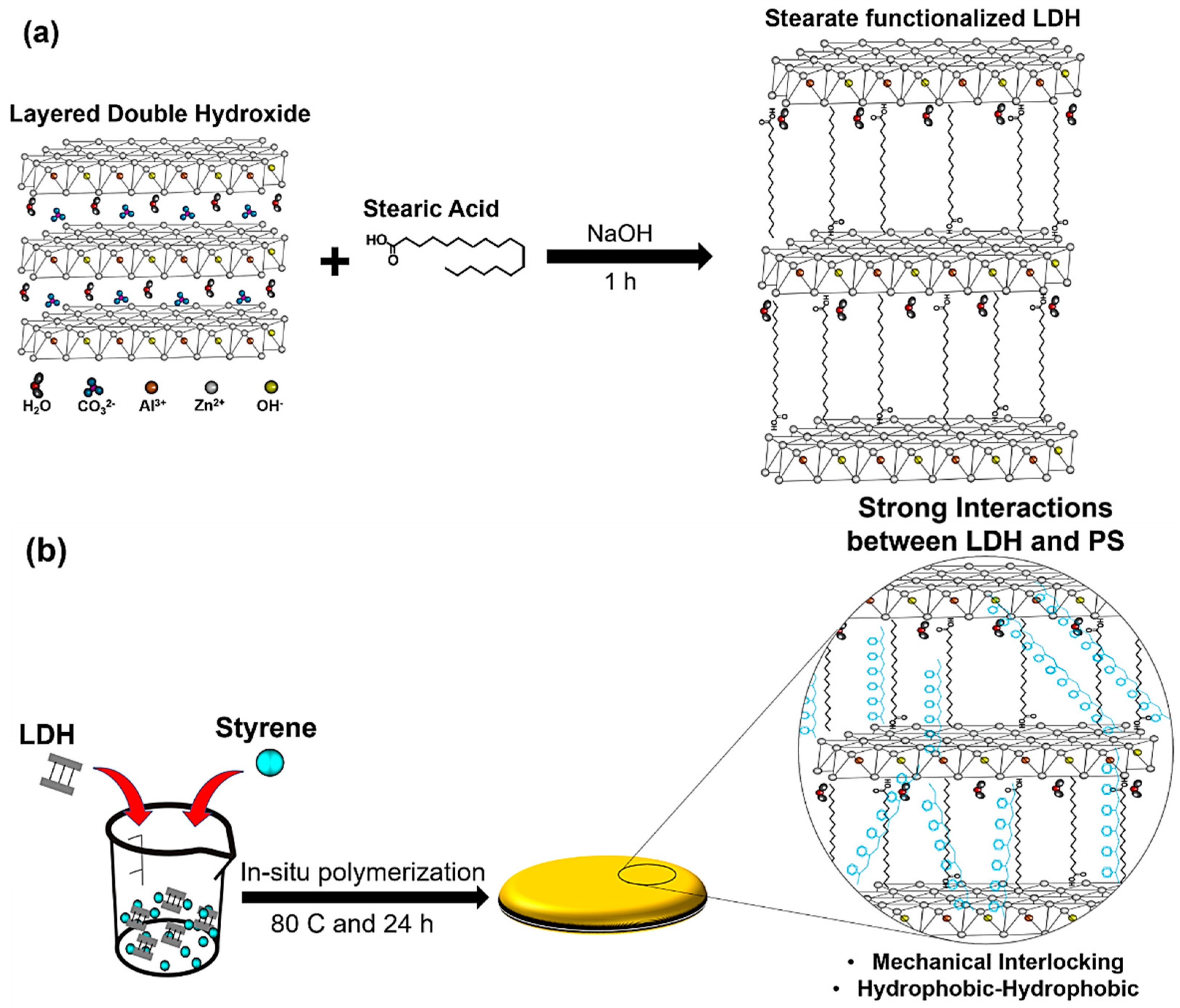
2.2. Synthesis of Stearate Functionalized Layered Double Hydroxide
2.3. Preparation of PS and PS-LDH Nanocomposites
2.4. Supercritical Carbon Dioxide Foaming of PS and Nanocomposites
2.5. Thermal Analysis of PS and PS-LDH Nanocomposites
2.6. Characterization and Morphological Analysis of Foamed Nanocomposites
2.7. Mechanical Properties
3. Results and Discussions
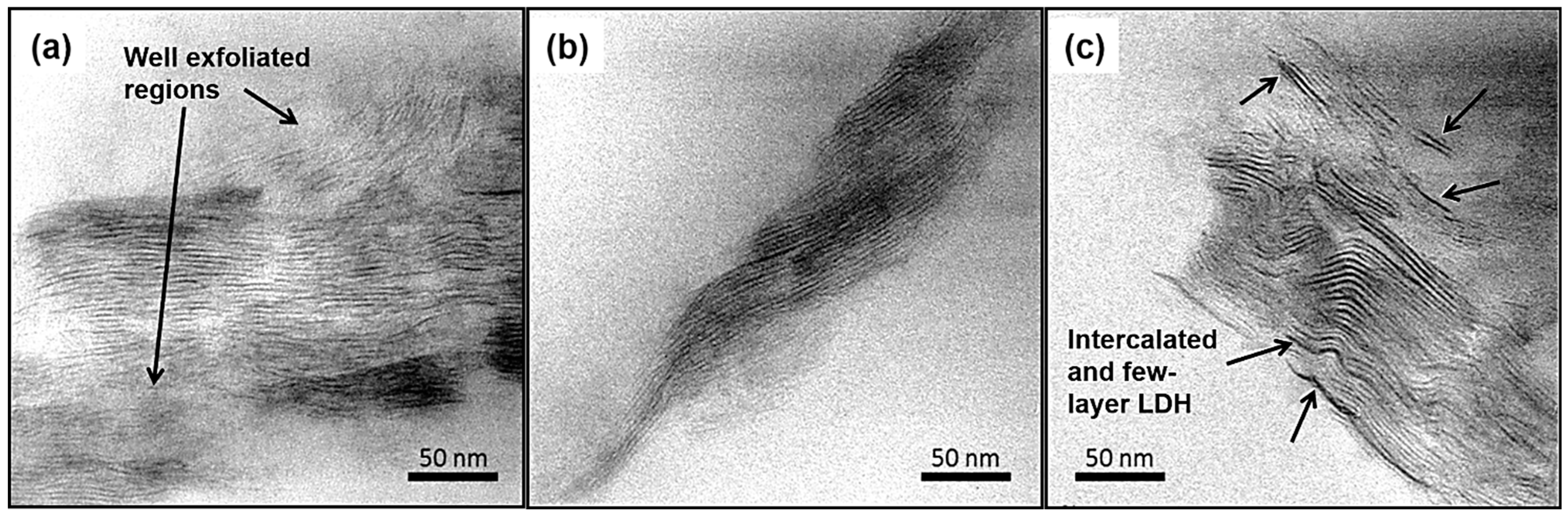
3.1. X-Ray Diffraction Analysis
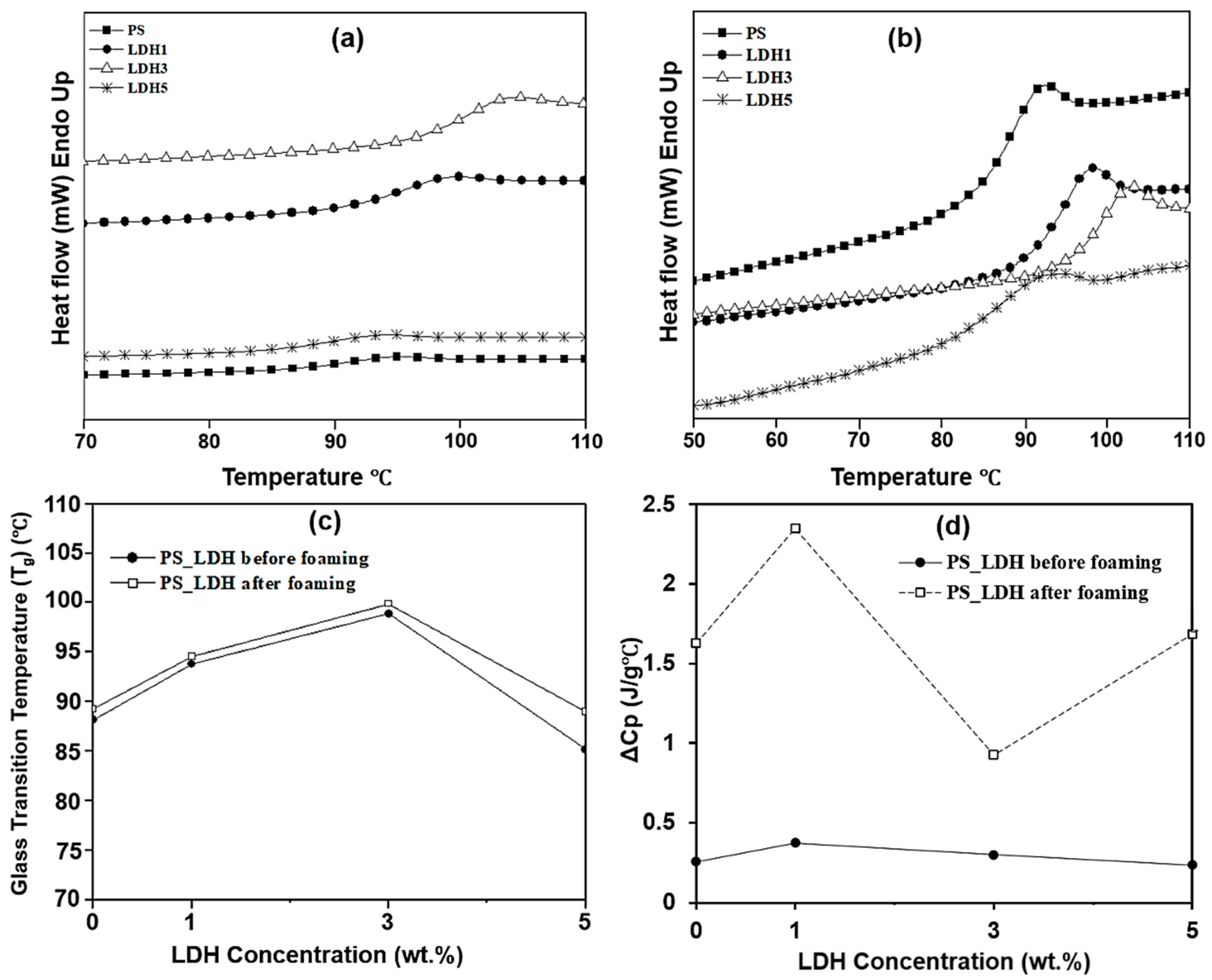
3.2. Effect of LDH and Foaming on the Thermal Properties of PS and PS-LDH Nanocomposites
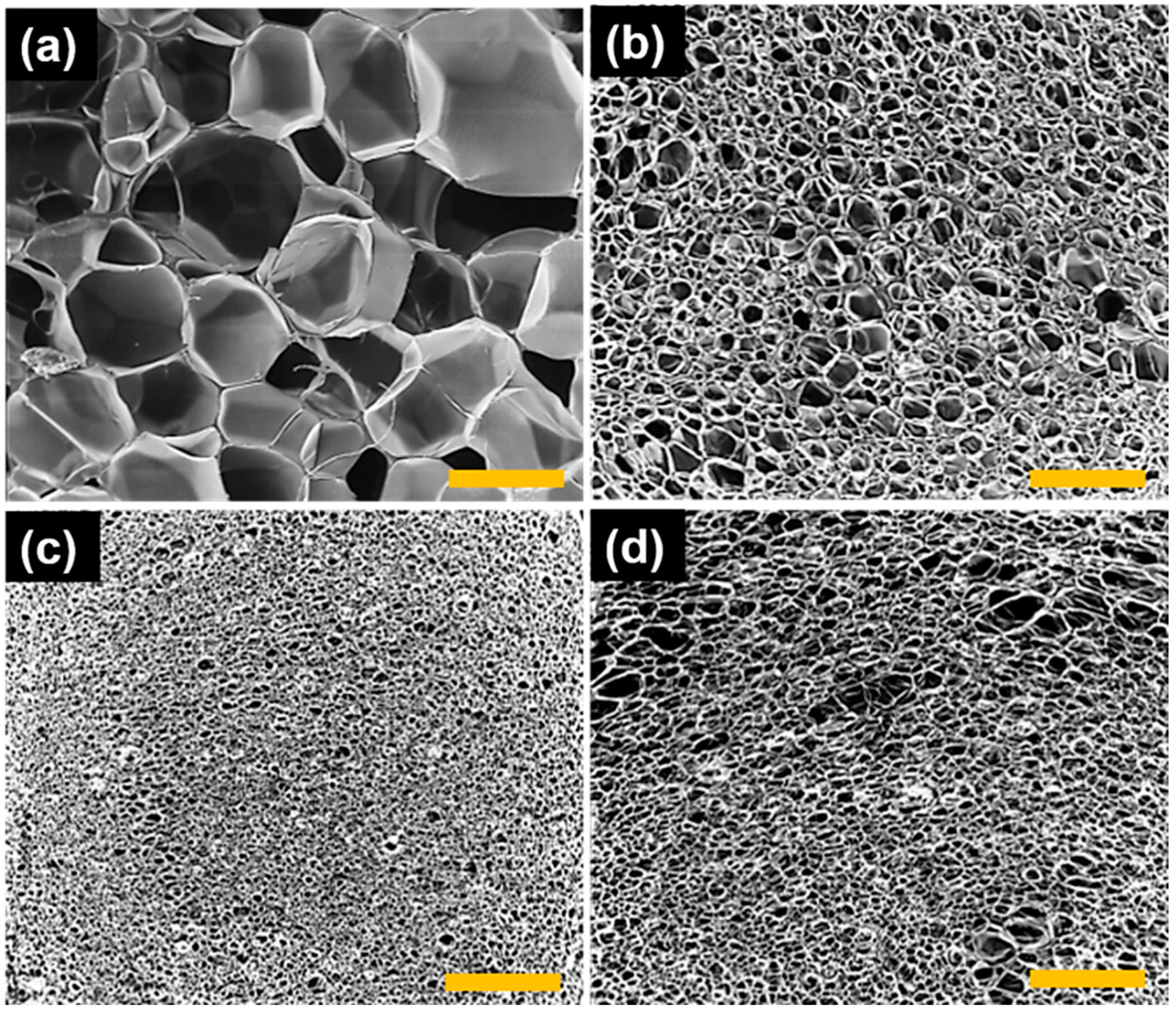
3.3. Characterization of PS and PS-LDH Nanocomposite Foam Properties
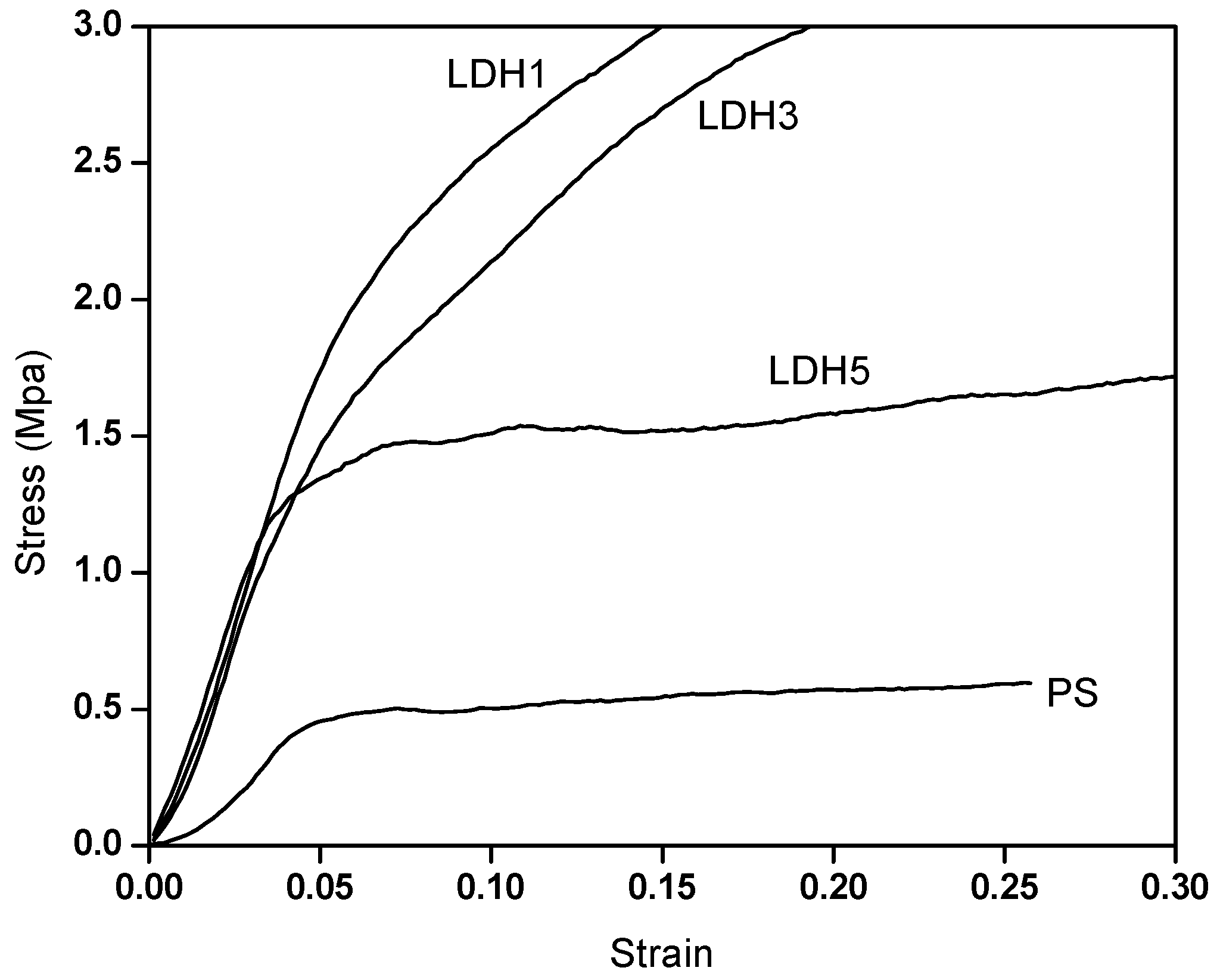
3.4. Mechanical Properties—Compression Test
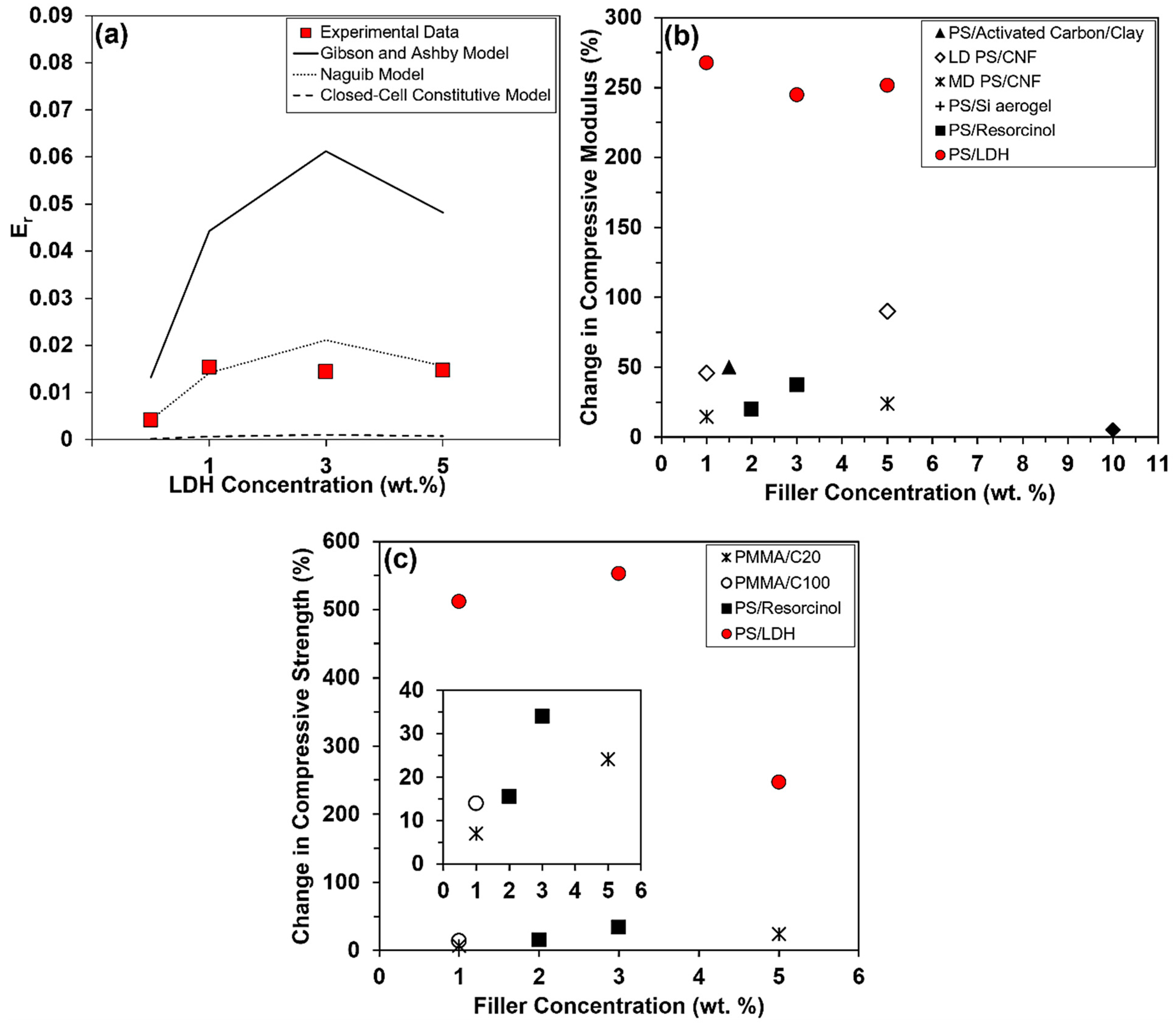
3.5. Theoretical Modeling of the Relative Compressive Moduli of PS and PS Composite Foams
3.6. Benchmarking the Compressive Properties of PS Nanocomposites Against Literature
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Soykeabkaew, N.; Thanomsilp, C.; Suwantong, O. A review: Starch-based composite foams. Compos. Part A Appl. Sci. Manuf. 2015, 78, 246–263. [Google Scholar] [CrossRef]
- Dement’ev, K.I.; Palankoev, T.A.; Alekseeva, O.A.; Babkin, I.A.; Maksimov, A.L. Thermal depolymerization of polystyrene in highly aromatic hydrocarbon medium. J. Anal. Appl. Pyrolysis 2019, 142, 104612. [Google Scholar] [CrossRef]
- Guyot, A. Recent developments in the thermal degradation of polystyrene—A review. Polym. Degrad. Stab. 1986, 15, 219–235. [Google Scholar] [CrossRef]
- Kunwar, B.; Cheng, H.N.; Chandrashekaran, S.R.; Sharma, B.K. Plastics to fuel: A review. Renew. Sustain. Energy Rev. 2016, 54, 421–428. [Google Scholar] [CrossRef]
- Jin, F.L.; Zhao, M.; Park, M.; Park, S.J. Recent Trends of Foaming in Polymer Processing: A Review. Polymers 2019, 11, 953. [Google Scholar] [CrossRef] [PubMed]
- Holl, M.R.; Ma, M.; Kumar, V. The Effect of Additives on Microcellular PVC Foams: Part I—Effect on Processing and Microstructure. Cell. Polym. 1998, 17, 271–283. [Google Scholar]
- Kumar, V.; Weller, J.E.; Ma, M. The Effect of Additives on Microcellular PVC Foams: Part II—Tensile Behavior. Cell. Polym. 1998, 17, 350–361. [Google Scholar]
- Chen, L.; Rende, D.; Schadler, L.S.; Ozisik, R. Polymer nanocomposite foams. J. Mater. Chem. A 2013, 1, 3837. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, J.; Ju, J.; Kuang, T. Fabrication of Poly(butylene succinate)/Carbon Black Nanocomposite Foams with Good Electrical Conductivity and High Strength by a Supercritical CO2 Foaming Process. Polymers 2019, 11, 1852. [Google Scholar] [CrossRef]
- Ju, J.; Peng, X.; Huang, K.; Li, L.; Liu, X.; Chitrakar, C.; Chang, L.; Gu, Z.; Kuang, T. High-performance porous PLLA-based scaffolds for bone tissue engineering: Preparation, characterization, and in vitro and in vivo evaluation. Polymer 2019, 180, 121707. [Google Scholar] [CrossRef]
- Min, Z.; Yang, H.; Chen, F.; Kuang, T. Scale-up production of lightweight high-strength polystyrene/carbonaceous filler composite foams with high-performance electromagnetic interference shielding. Mater. Lett. 2018, 230, 157–160. [Google Scholar] [CrossRef]
- Ogunsona, E.O.; Codou, A.; Misra, M.; Mohanty, A.K. Thermally Stable Pyrolytic Biocarbon as an Effective and Sustainable Reinforcing Filler for Polyamide Bio-composites Fabrication. J. Polym. Environ. 2018, 26, 3574–3589. [Google Scholar] [CrossRef]
- Fei, Y.; Fang, W.; Zhong, M.; Jin, J.; Fan, P.; Yang, J.; Fei, Z.; Xu, L.; Chen, F. Extrusion Foaming of Lightweight Polystyrene Composite Foams with Controllable Cellular Structure for Sound Absorption Application. Polymers 2019, 11, 106. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zeng, C.; Lee, L.J.; Koelling, K.W.; Tomasko, D.L. Extrusion of polystyrene nanocomposite foams with supercritical CO2. Polym. Eng. Sci. 2003, 43, 1261–1275. [Google Scholar] [CrossRef]
- Ogunsona, E.O.; Ogbomo, S.; Nar, M.; D’Souza, N.A. Thermal and Mechanical Effects in Polystyrene-Montmorillonite Nanocomposite Foams. Cell. Polym. 2010, 30, 79–93. [Google Scholar] [CrossRef]
- Zeng, C.; Han, X.; Lee, L.J.; Koelling, K.W.; Tomasko, D.L. Polymer–Clay Nanocomposite Foams Prepared Using Carbon Dioxide. Adv. Mater. 2003, 15, 1743–1747. [Google Scholar] [CrossRef]
- Saraeian, P.; Tavakoli, H.; Ghassemi, A. Production of polystyrene-nanoclay nanocomposite foam and effect of nanoclay particles on foam cell size. J. Compos. Mater. 2013, 47, 2211–2217. [Google Scholar] [CrossRef]
- Colton, J.S.; Suh, N.P. The nucleation of microcellular thermoplastic foam with additives: Part I: Theoretical considerations. Polym. Eng. Sci. 1987, 27, 485–492. [Google Scholar] [CrossRef]
- Costa, F.R.; Leuteritz, A.; Wagenknecht, U.; Jehnichen, D.; Häußler, L.; Heinrich, G. Intercalation of Mg–Al layered double hydroxide by anionic surfactants: Preparation and characterization. Appl. Clay Sci. 2008, 38, 153–164. [Google Scholar] [CrossRef]
- Suresh, K.; Kumar, R.V.; Pugazhenthi, G. Processing and characterization of polystyrene nanocomposites based on CoAl layered double hydroxide. J. Sci. Adv. Mater. Devices 2016, 1, 351–361. [Google Scholar] [CrossRef][Green Version]
- Costantino, U.; Gallipoli, A.; Nocchetti, M.; Camino, G.; Bellucci, F.; Frache, A. New nanocomposites constituted of polyethylene and organically modified ZnAl-hydrotalcites. Polym. Degrad. Stab. 2005, 90, 586–590. [Google Scholar] [CrossRef]
- Dagnon, K.L.; Ambadapadi, S.; Shaito, A.; Ogbomo, S.M.; DeLeon, V.; Golden, T.D.; Rahimi, M.; Nguyen, K.; Braterman, P.S.; D’Souza, N.A. Poly(L-lactic acid) nanocomposites with layered double hydroxides functionalized with ibuprofen. J. Appl. Polym. Sci. 2009, 113, 1905–1915. [Google Scholar] [CrossRef]
- Strauss, W.C. Saturation and Foaming of Thermoplastic Nanocomposites Using Supercritical CO2; University of North Texas: Denton, TX, USA, 2005. [Google Scholar]
- Kumar, V.; Weller, J. Production of Microcellular Polycarbonate Using Carbon Dioxide for Bubble Nucleation. ASME J. Eng. Ind. 1994, 116, 413–420. [Google Scholar] [CrossRef]
- Lane, C.A.; Burton, D.E.; Crabb, C.C. Accurate molecular dimensions from stearic acid monolayers. J. Chem. Educ. 1984, 61, 815. [Google Scholar] [CrossRef]
- Badre, C.; Dubot, P.; Lincot, D.; Pauporte, T.; Turmine, M. Effects of nanorod structure and conformation of fatty acid self-assembled layers on superhydrophobicity of zinc oxide surface. J. Colloid Interface Sci. 2007, 316, 233–237. [Google Scholar] [CrossRef]
- Strauss, W.; D’Souza, N.A. Supercritical CO2 Processed Polystyrene Nanocomposite Foams. J. Cell. Plast. 2004, 40, 229–241. [Google Scholar] [CrossRef]
- De Graaf, R.A.; Karman, A.P.; Nobel, A. Material Properties and Glass Transition Temperatures of Different Thermoplastic Starches After Extrusion Processing Research Paper. Starch Stärke 2003, 55, 80–86. [Google Scholar] [CrossRef]
- Ogunsona, E.; D’Souza, N.A. Characterization and mechanical properties of foamed poly(caprolactone) and Mater-Bi blends using CO2 as blowing agent. J. Cell. Plast. 2014, 51, 245–268. [Google Scholar] [CrossRef]
- Gibson, L.J.; Ashby, M.F. Cellular Solids; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Chen, L.; Schadler, L.S.; Ozisik, R. An experimental and theoretical investigation of the compressive properties of multi-walled carbon nanotube/poly(methyl methacrylate) nanocomposite foams. Polymer 2011, 52, 2899–2909. [Google Scholar] [CrossRef]
- Jo, C.; Fu, J.; Naguib, H.E. Constitutive modeling for characterizing the compressive behavior of PMMA open-cell foams. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 436–443. [Google Scholar] [CrossRef]
- Doroudiani, S.; Kortschot, M.T. Polystyrene foams. III. Structure-tensile properties relationships. J. Appl. Polym. Sci. 2003, 90, 1427–1434. [Google Scholar] [CrossRef]
- Goods, S.H.; Neuschwanger, C.L.; Whinnery, L.L.; Nix, W.D. Mechanical properties of a particle-strengthened polyurethane foam. J. Appl. Polym. Sci. 1999, 74, 2724–2736. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, B.; Li, D.; Lee, L.J. Extruded polystyrene foams with bimodal cell morphology. Polymer 2012, 53, 2435–2442. [Google Scholar] [CrossRef]
- Shen, J.; Han, X.; Lee, L.J. Nanoscaled Reinforcement of Polystyrene Foams using Carbon Nanofibers. J. Cell. Plast. 2006, 42, 105–126. [Google Scholar] [CrossRef]
- Nyitray, A.M.; Williams, J.M. Microcellular Composite Foams. J. Cell. Plast. 1989, 25, 217–229. [Google Scholar] [CrossRef]
- Martínez, A.B.; Realinho, V.; Antunes, M.; Maspoch, M.L.; Velasco, J.I. Microcellular Foaming of Layered Double Hydroxide−Polymer Nanocomposites. Ind. Eng. Chem. Res. 2011, 50, 5239–5247. [Google Scholar] [CrossRef]



| LDH Sample | Zn (%) | Al (%) | C (%) | H (%) | Zn/Al ratio | C/Al ratio | Residue (%) |
|---|---|---|---|---|---|---|---|
| As-prepared Zn Al-stearate | 20.2 | 4.19 | 42.4 | 8.43 | 1.99 | 22.7 | 28.0 |
| Zn2Al(OH)6(stearate)1.1.2H2O | 22.6 | 4.66 | 41.0 | 8.37 | 2.00 | 19.8 | 38.1 |
| Sample | Before Foaming | After Foaming | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tg (°C) | Onset (°C) | End (°C) | Breadth (°C) | ΔCp (J/g·°C) | Tg (°C) | Onset (°C) | End (°C) | Breadth (°C) | ΔCp (J/g·°C) | |
| PS | 88.1 | 85.2 | 90.9 | 5.7 | 0.256 | 89.3 | 86.0 | 92.34 | 6.4 | 1.627 |
| LDH1 | 93.8 | 90.9 | 97.0 | 6.1 | 0.372 | 94.6 | 90.9 | 98.2 | 7.3 | 2.350 |
| LDH3 | 98.9 | 96.4 | 101.6 | 5.3 | 0.300 | 99.9 | 97.00 | 102.9 | 5.9 | 0.925 |
| LDH5 | 85.1 | 81.0 | 89.0 | 8 | 0.236 | 89.0 | 85.7 | 92.3 | 6.6 | 1.684 |
| Foam Properties | PS | LDH1 | LDH3 | LDH5 |
|---|---|---|---|---|
| Polymer Density, (ρf) (g/cm3) | 1.01 ± 0.04 | 0.97 ± 0.02 | 1.03 ± 0.01 | 1.08 ± 0.03 |
| Foam Density, (ρm) (g/cm3) | 0.08 ± 0.006 | 0.21 ± 0.05 | 0.19 ± 0.02 | 0.21 ± 0.003 |
| Relative Foam Density, (ρr) | 0.08 ± 0.001 | 0.22 ± 0.002 | 0.18 ± 0.001 | 0.19 ± 0.002 |
| Average Cell Diameter, (D) (mm) | 8.6 ± 0.33 | 2.3 ± 0.06 | 0.6 ± 0.02 | 1.7 ± 0.06 |
| Cell Density, (No) (cells/cm3) | 4.45 × 109 | 3.12 × 1013 | 5.21 × 1015 | 1.13 × 1014 |
| Void Fraction, (Vf) (%) | 92.36 | 78.07 | 81.85 | 97.16 |
| Samples | Modulus (MPa) | Strength (MPa) | Yield Strength (MPa) |
|---|---|---|---|
| PS | 11.25 ± 0.35 | 0.49 ± 0.049 | 0.40 ± 0.014 |
| LDH1 | 41.44 ± 0.57 | 3 ± 0.02 | 1.51 ± 0.587 |
| LDH3 | 38.86 ± 0.7 | 3.2 ± 0.07 | 1.07 ± 0.113 |
| LDH5 | 39.59 ± 0.4 | 1.7 ± 0.05 | 1.02 ± 0.037 |
| MLS1 | 19.84 ± 0.17 | 0.49 ± 0.017 | 0.40 ± 0.034 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogunsona, E.O.; Dagnon, K.L.; D'Souza, N.A. Multi-Fold Enhancement in Compressive Properties of Polystyrene Foam Using Pre-delaminated Stearate Functionalized Layer Double Hydroxides. Polymers 2020, 12, 8. https://doi.org/10.3390/polym12010008
Ogunsona EO, Dagnon KL, D'Souza NA. Multi-Fold Enhancement in Compressive Properties of Polystyrene Foam Using Pre-delaminated Stearate Functionalized Layer Double Hydroxides. Polymers. 2020; 12(1):8. https://doi.org/10.3390/polym12010008
Chicago/Turabian StyleOgunsona, Emmanuel O., Koffi L. Dagnon, and Nandika Anne D'Souza. 2020. "Multi-Fold Enhancement in Compressive Properties of Polystyrene Foam Using Pre-delaminated Stearate Functionalized Layer Double Hydroxides" Polymers 12, no. 1: 8. https://doi.org/10.3390/polym12010008
APA StyleOgunsona, E. O., Dagnon, K. L., & D'Souza, N. A. (2020). Multi-Fold Enhancement in Compressive Properties of Polystyrene Foam Using Pre-delaminated Stearate Functionalized Layer Double Hydroxides. Polymers, 12(1), 8. https://doi.org/10.3390/polym12010008





