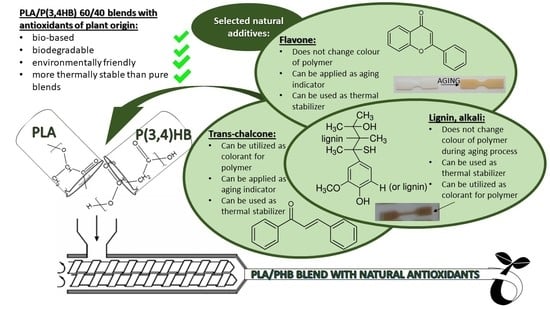
Thermal Analysis of Aliphatic Polyester Blends with Natural Antioxidants
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Sample Preparation
2.3. Differential Scanning Calorimetry (DSC)
2.4. Themogravimetric Analysis
2.5. Dynamic Mechanical Analysis
2.6. Solar Aging
2.7. Mechanical Tests
2.8. Fourier Transform Infrared Spectroscopy (FT-IR) Absorbance Spectra Analysis
2.9. Colour Measurement
3. Results and Discussion
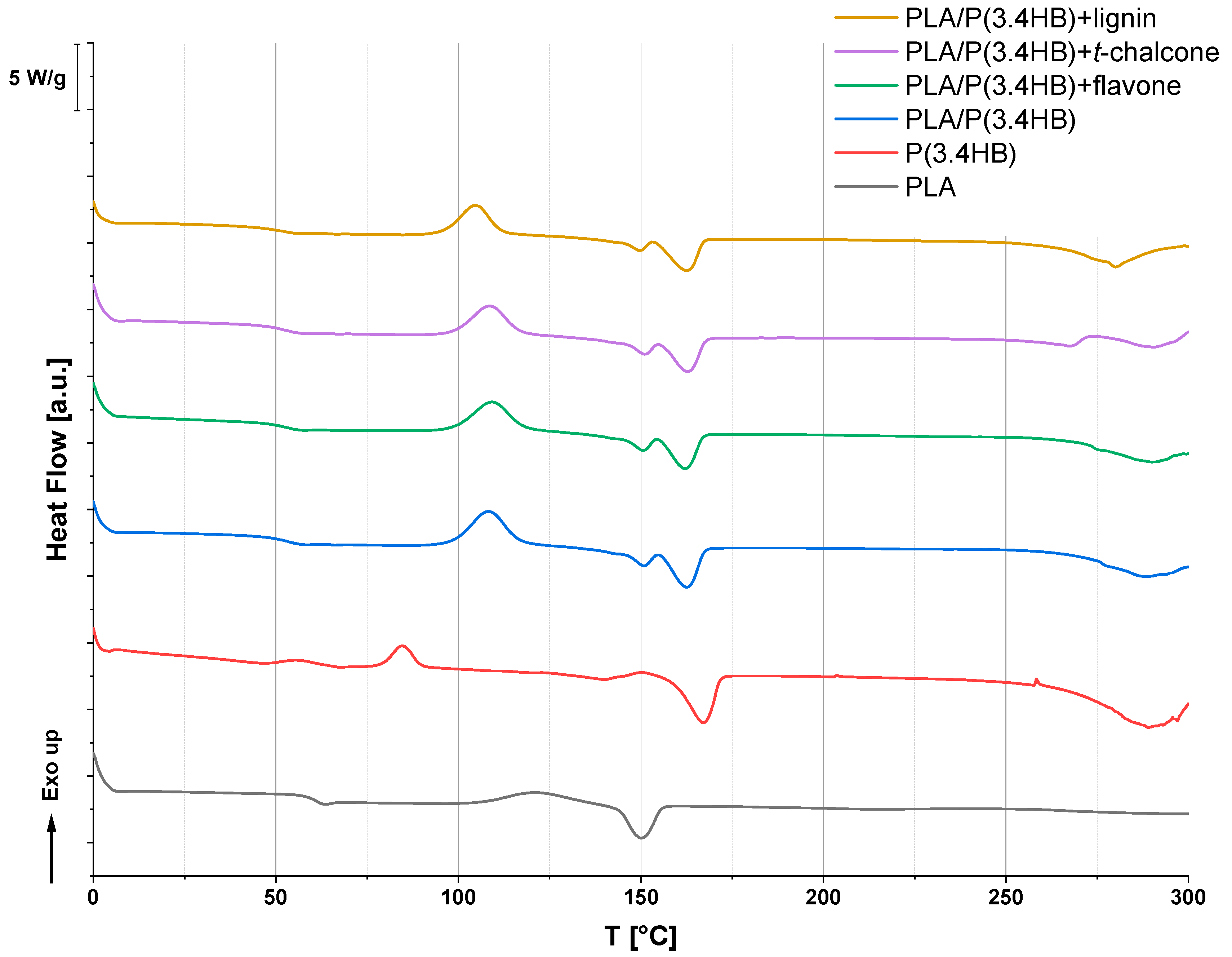
3.1. Differential Scanning Calorimetry (DSC)
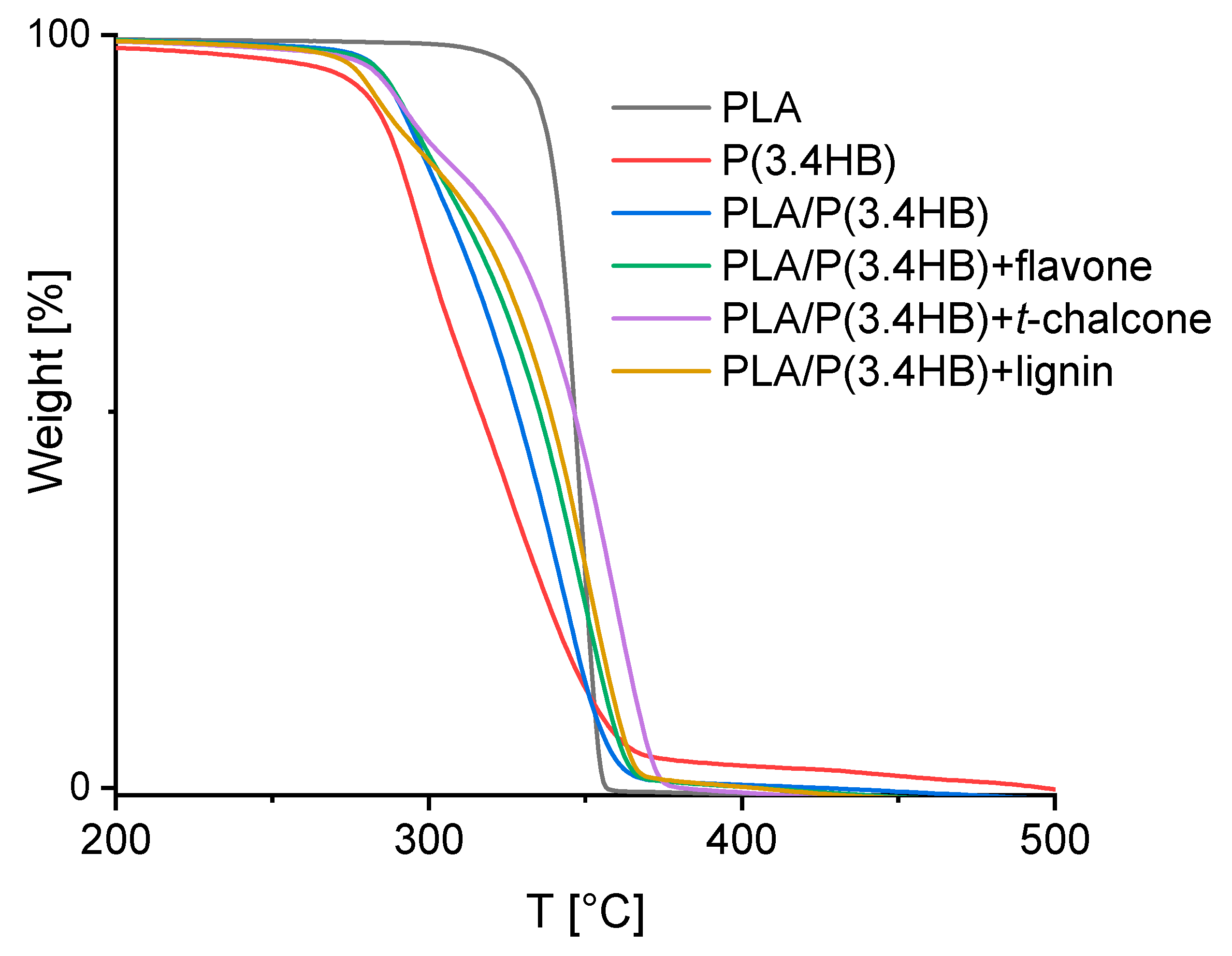
3.2. Thermogravimetric Analysis (TGA)
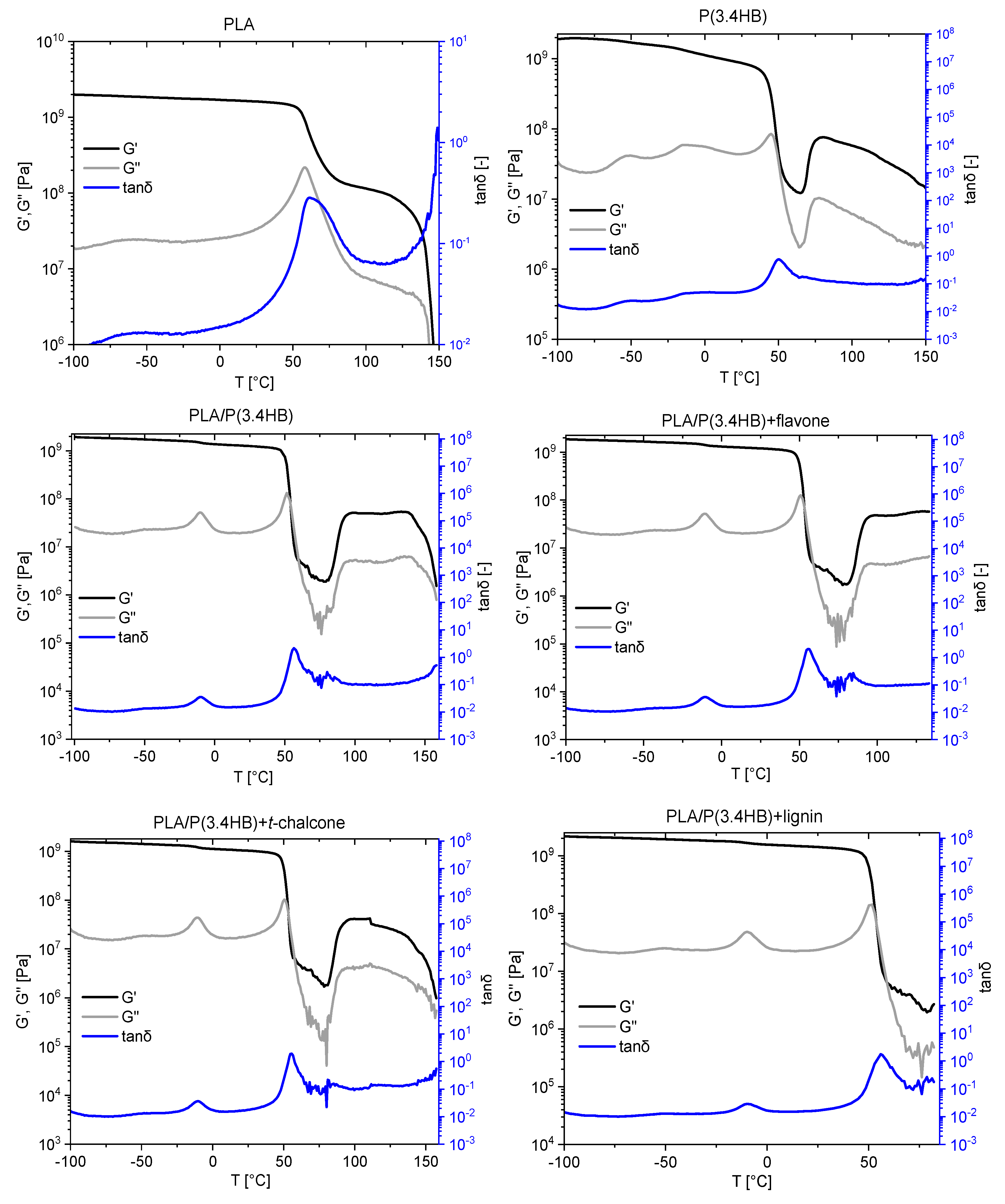
3.3. Dynamic Mechanical Analysis
3.4. Mechanical Tests
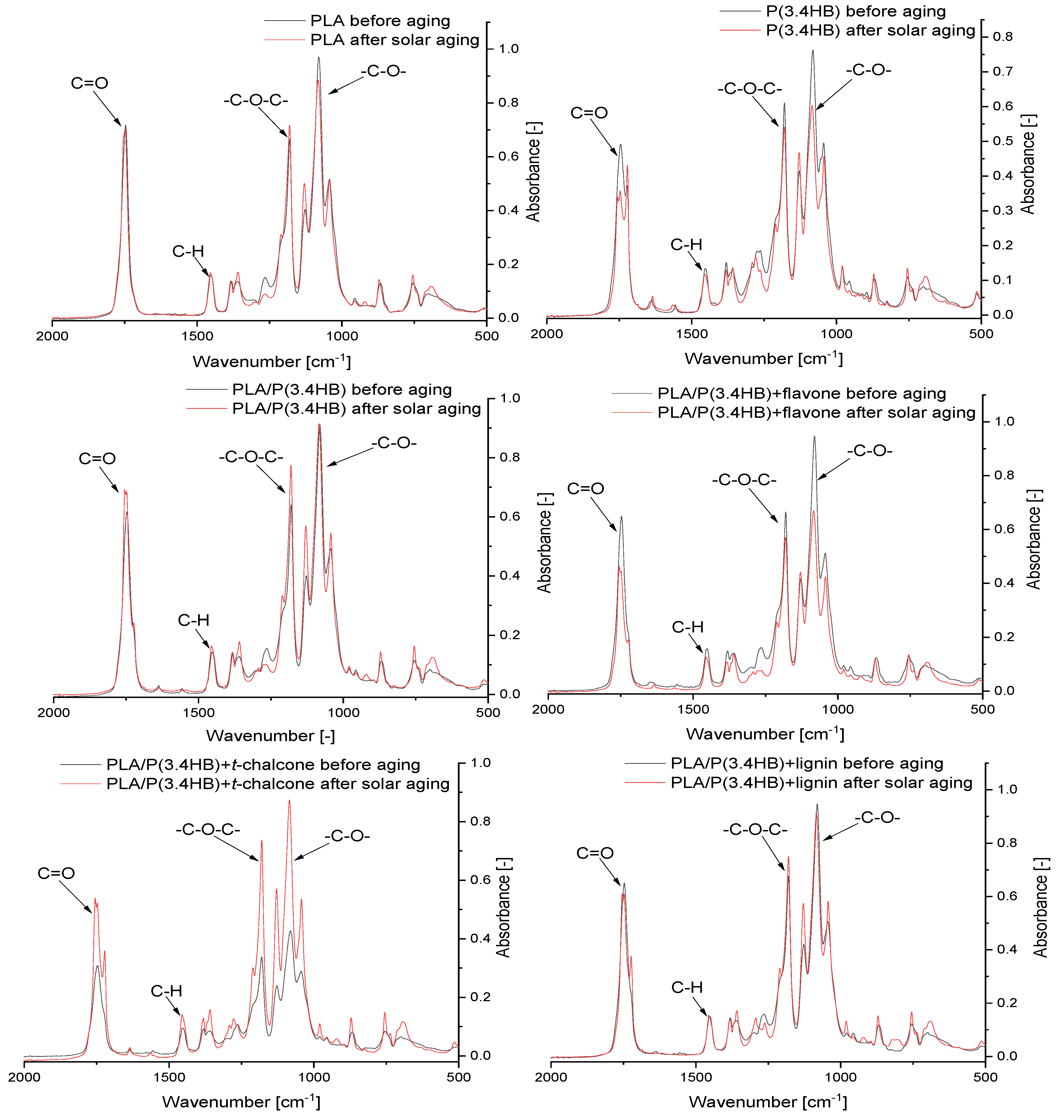
3.5. Fourier Transform Infrared Spectroscopy (FT-IR) Absorbance Spectra Analysis
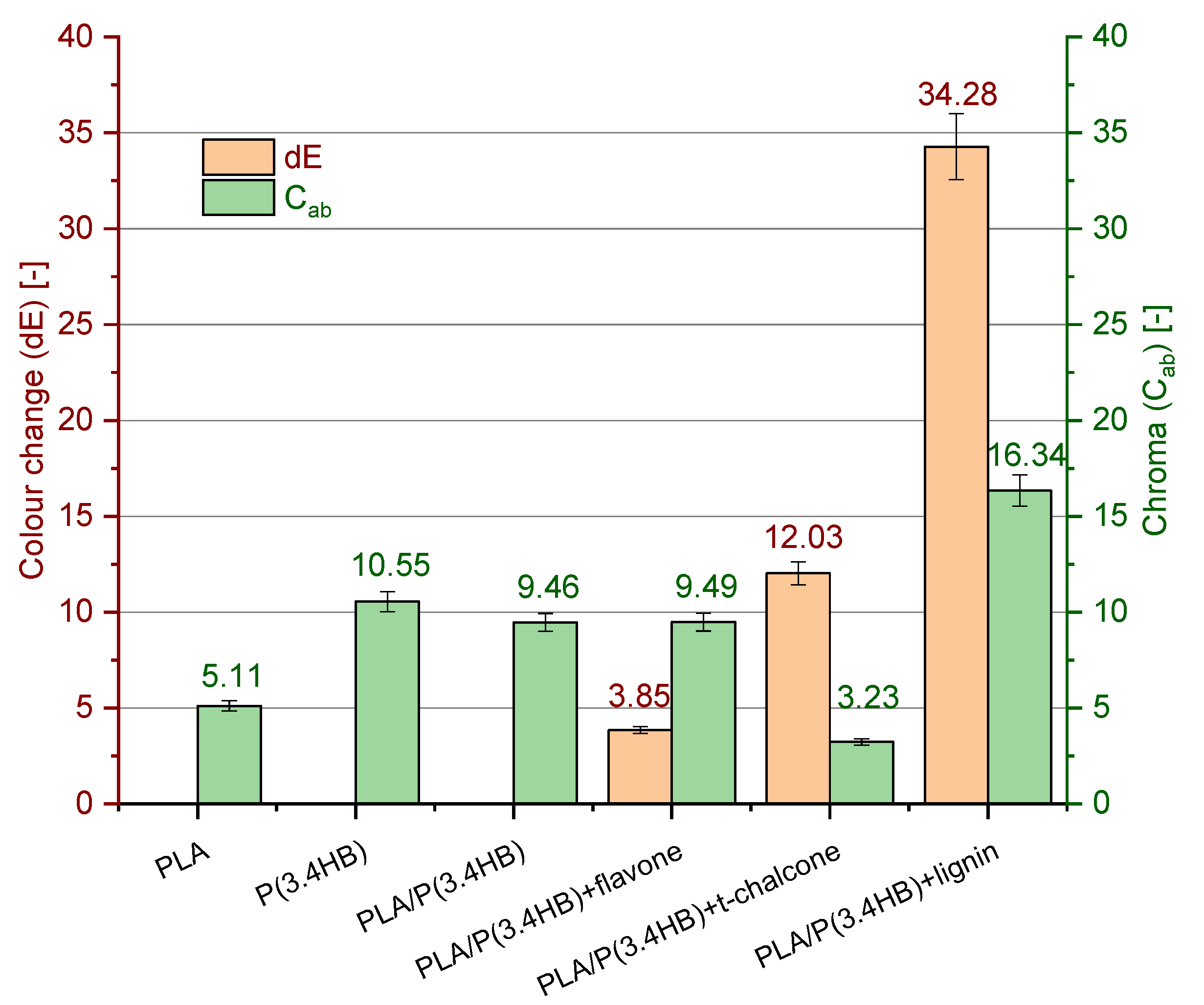
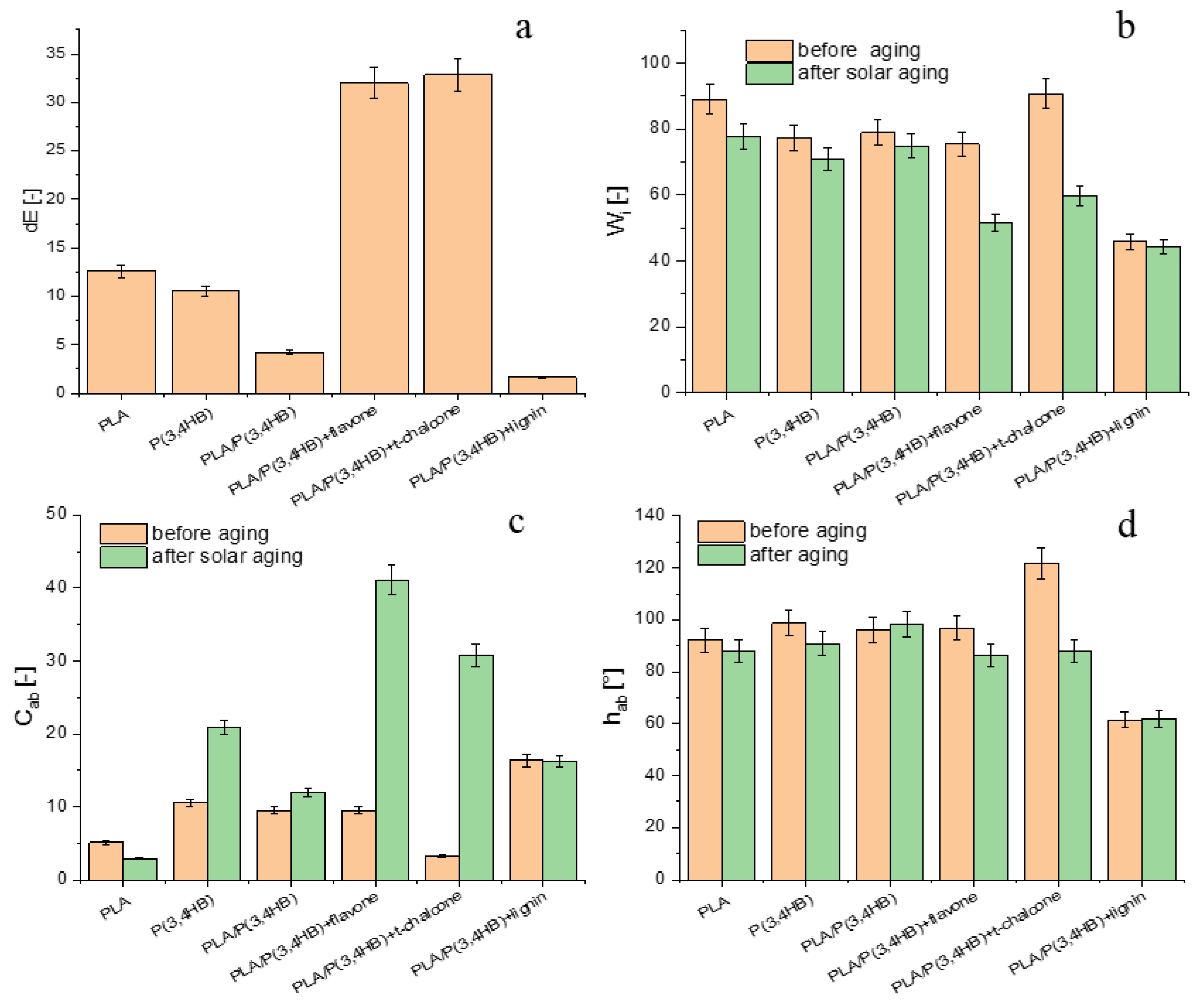
3.6. Colour Measurement
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nakajima, H.; Dijkstra, P.; Loos, K. The Recent Developments in Biobased Polymers toward General and Engineering Applications: Polymers that Are Upgraded from Biodegradable Polymers, Analogous to Petroleum-Derived Polymers, and Newly Developed. Polymers 2017, 9, 523. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Wang, X.; Wu, D. Development of Polyoxymethylene/Polylactide Blends for a Potentially Biodegradable Material: Crystallization Kinetics, Lifespan Prediction, and Enzymatic Degradation Behavior. Polymers 2019, 11, 1516. [Google Scholar] [CrossRef]
- Velázquez-Contreras, F.; Acevedo-Parra, H.; Nuño-Donlucas, S.M.; Núñez-Delicado, E.; Gabaldón, J.A. Development and Characterization of a Biodegradable PLA Food Packaging Hold Monoterpene–Cyclodextrin Complexes against Alternaria alternate. Polymers 2019, 11, 1720. [Google Scholar] [CrossRef]
- Olaiya, N.G.; Surya, I.; Oke, P.K.; Rizal, S.; Sadiku, E.R.; Ray, S.S.; Farayibi, P.K.; Hossain, M.S.; Abdul Khalil, H.P.S. Properties and Characterization of a PLA–Chitin–Starch Biodegradable Polymer Composite. Polymers 2019, 11, 1656. [Google Scholar] [CrossRef] [PubMed]
- Kirschweng, B.; Tátraaljai, D.; Földes, E.; Pukánszky, B. Natural antioxidants as stabilizers for polymers. Polym. Degrad. Stab. 2017, 145, 25–40. [Google Scholar] [CrossRef]
- Masek, A. Flavonoids as natural Stabilizers and Color Indicators of Ageing for Polymeric Materials. Polymers 2015, 7, 1125–1144. [Google Scholar] [CrossRef]
- Moraczewski, K.; Malinowski, R.; Sikorska, W.; Karasiewicz, T.; Stepczyńska, M.; Jagodziński, B.; Rytlewski, P. Composting of Polylactide Containing Natural Anti-Aging Compounds of Plant Origin. Polymers 2019, 11, 1582. [Google Scholar] [CrossRef]
- Moraczewski, K.; Malinowski, R.; Karasiewicz, T.; Stepczyńska, M.; Jagodziński, B.; Rytlewski, P. The Effect of Accelerated Aging on Polylactide Containing Plant Extracts. Polymers 2019, 11, 575. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed. 2006, 41, 1523–1542. [Google Scholar] [CrossRef]
- Peng, C.H.; Wang, X.; Chen, J.; Jiao, R.; Wang, L.; Li, Y.I.; Zuo, Y.; Liu, Y.; Lei, L.; Ma, K.Y.; et al. Biology of Ageing and Role of Dietary Antioxidants. Biomed. Res. Int. 2014, 2014, 831841. [Google Scholar] [CrossRef] [PubMed]
- Vieira da Silva, B.; Barreira, J.C.M.; Oliveira, M.B.P. Natural phytochemicals and probiotics as bioactive ingredients for functional foods: Extraction, biochemistry and protected-delivery technologies. Trends Food Sci. Tech. 2016, 50, 144–158. [Google Scholar] [CrossRef]
- Barbulova, A.; Colucci, G.; Apone, F. New Trends in Cosmetics: By-Products of Plant Origin and Their Potential Use as Cosmetic Active Ingredients. Cosmetics 2015, 2, 82–92. [Google Scholar] [CrossRef]
- Yoo, S.; Kim, K.; Nam, H.; Lee, D. Discovering Health Benefits of Phytochemicals with Integrated Analysis of the Molecular Network, Chemical Properties and Ethnopharmacological Evidence. Nutrients 2018, 10, 1042. [Google Scholar] [CrossRef] [PubMed]
- Chikara, S.; Nagaprashantha, L.D.; Singhal, J.; Horne, D.; Awasthi, S.; Singhal, S.S. Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Lett. 2018, 413, 122–134. [Google Scholar] [CrossRef]
- Skotnicka, M.; Golan, M.; Szmukała, N. Rola naturalnych przeciwutleniaczy pochodzenia roślinnego w profilaktyce nowotworowej. Ann. Acad. Med. Gedan 2017, 47, 119–127. [Google Scholar]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Gryszczyńska, A.; Gryszczyńska, B.; Opala, B. Karotenoidy. Naturalne źródła, biosynteza, wpływ na organizm ludzki. Post. Fitoter. 2011, 12, 127–143. [Google Scholar]
- Drużyńska, B.; Klepacka, M. Właściwości przeciwutleniające preparatów polifenoli otrzymywanych z okrywy nasiennej fasoli czarnej, różowej i białej (Phaseoulus). Żywność Nauka Technologia Jakość 2004, 41, 69–78. [Google Scholar]
- Latos, M.; Masek, A.; Zaborski, M. Fotodegradacja materiałów polimerowych. Przetwórstwo Tworzyw 2017, 4, 358–363. [Google Scholar]
- Lucero, A.; Rebolledo, C.; Buono-Core, G.E. Effect of some natural UV-absorbers on the photostabilization of active ingredients in german chamomile floral extracts. Part I. J. Chil. Chem. Soc. 2012, 57, 1309–1312. [Google Scholar] [CrossRef][Green Version]
- Baghel, S.S.; Shrivastava, N.; Baghel, R.S.; Agrawal, P.; Rajput, S. A review of quercetin: Antioxidant and anticancer properties. World J. Pharm. Pharm. Sci. 2012, 1, 146–160. [Google Scholar]
- Samper, M.D.; Fages, E.; Fenollar, O.; Boronat, T.; Balart, R. The Potential of Flavonoids as Natural Antioxidants and UV Light Stabilizers for Polypropylene. J. Appl. Polym. Sci. 2013, 129, 1707–1716. [Google Scholar] [CrossRef]
- Arrigo, R.; Tzankova, D.N. Natural Anti-oxidants for Bio-Polymeric Materials. Arch. Chem. Res. 2017, 1, 1–4. [Google Scholar] [CrossRef]
- Masek, A.; Chrześcijańska, E.; Diakowska, K.; Zaborski, M. Controlled Degradation of Polymeric Composites Stabilized with Natural Antioxidants. Int. J. Electrochem. Sci. 2015, 10, 7200–7211. [Google Scholar]
- Iyer, K.A.; Zhang, L.; Torkelson, J.M. Direct Use of Natural Antioxidant-rich Agro-wastes as Thermal Stabilizer for Polymer: Processing and Recycling. ACS Sustain. Chem. Eng. 2016, 4, 881–889. [Google Scholar] [CrossRef]
- Cerruti, P.; Malinconico, M.; Rychly, J.; Matisova-Rychla, L.; Carfagna, C. Effect of natural antioxidants on the stability of polypropylene films. Polym. Degrad. Stab. 2009, 94, 2095–2100. [Google Scholar] [CrossRef]
- Dopico-García, M.S.; Castor-López, M.M.; López-Vilariño, J.M.; González-Rodrigúez, M.V.; Valentão, P.; Andrade, P.B.; García-Garabal, S.; Abad, M.J. Natural Extracts as Potential Source of Antioxidants to Stabilize Polyolefins. J. Appl. Polym. Sci. 2010, 119, 3553–3559. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A. Effect of Impregnation of Biodegradable Polyesters with Polyphenols from Cistus linnaeus and Juglans regia Linnaeus Walnut Green Husk. Polymers 2019, 11, 669. [Google Scholar] [CrossRef]
- Dziemidkiewicz, A.; Maciejewska, M.; Pingot, M. Thermal analysis of halogenated rubber cured with a new cross-linking system. J. Therm. Anal. Calorim. 2019, 1–11. [Google Scholar] [CrossRef]
- da Silva Gois, G.; de Andrade, M.F.; Silva Garcia, S.M.; Vinhas, G.M.; Santos, A.S.F.; Medeiros, E.S.; Oliveira, J.E.; Bastos de Almeida, Y.M. Soil Biodegradation of PLA/CNW Nanocomposites Modified with Ethylene Oxide Derivatives. Mater. Res. 2017, 20, 899–904. [Google Scholar] [CrossRef]
- Pop, M.A.; Croitoru, C.; Bedő, T.; Geamăn, V.; Radomir, I.; Coșniță, M.; Zaharia, S.M.; Chicoș, L.A.; Miloșan, I. Structural changes during 3D printing of bioderived and synthetic thermoplastic materials. J. Appl. Polym. Sci. 2018, 1–11. [Google Scholar] [CrossRef]
- Cichosz, S.; Masek, A.; Wolski, K. Innovative cellulose fibres reinforced ethylene-norbornene copolymer composites of an increased degradation potential. Polym. Degrad. Stab. 2019, 159, 174–183. [Google Scholar] [CrossRef]
- Zhang, M.; Thomas, N.L. Blending Polylactic Acid with Polyhydroxybutyrate: The Effect on Thermal, Mechanical, and Biodegradation Properties. Adv. Polym. Tech. 2011, 30, 67–79. [Google Scholar] [CrossRef]
- Gordobil, O.; Egüés, I.; Llano-Ponte, R.; Labidi, J. Physicochemical properties of PLA lignin blends. Polym. Degrad. Stab. 2014, 108, 330–338. [Google Scholar] [CrossRef]
- Abdelwahab, M.A.; Flynn, A.; Chiou, B.S.; Imamc, S.; Orts, W.; Chiellini, E. Thermal, mechanical and morphological characterization of plasticized PLA-PHB. Polym. Degrad. Stab. 2012, 97, 1822–1828. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Samper, M.D.; Aldas, M.; López, J. On the Use of PLA-PHB Blends for Sustainable Food Packaging Applications. Materials 2017, 10, 1008. [Google Scholar] [CrossRef]
- Lim, J.S.; Park, K.; Chung, G.S.; Kim, J.H. Effect of composition ratio on the thermal and physical properties of semicrystalline PLA/PHB-HHx composites. Mat. Sci. Eng. C Mater. 2013, 33, 2131–2137. [Google Scholar] [CrossRef]
- Masek, A.; Chrzescijanska, E.; Zaborski, M. Morin hydrate as pro-ecological antioxidant and pigment for polyolefin polymers. Chimie 2013, 16, 990–996. [Google Scholar] [CrossRef]

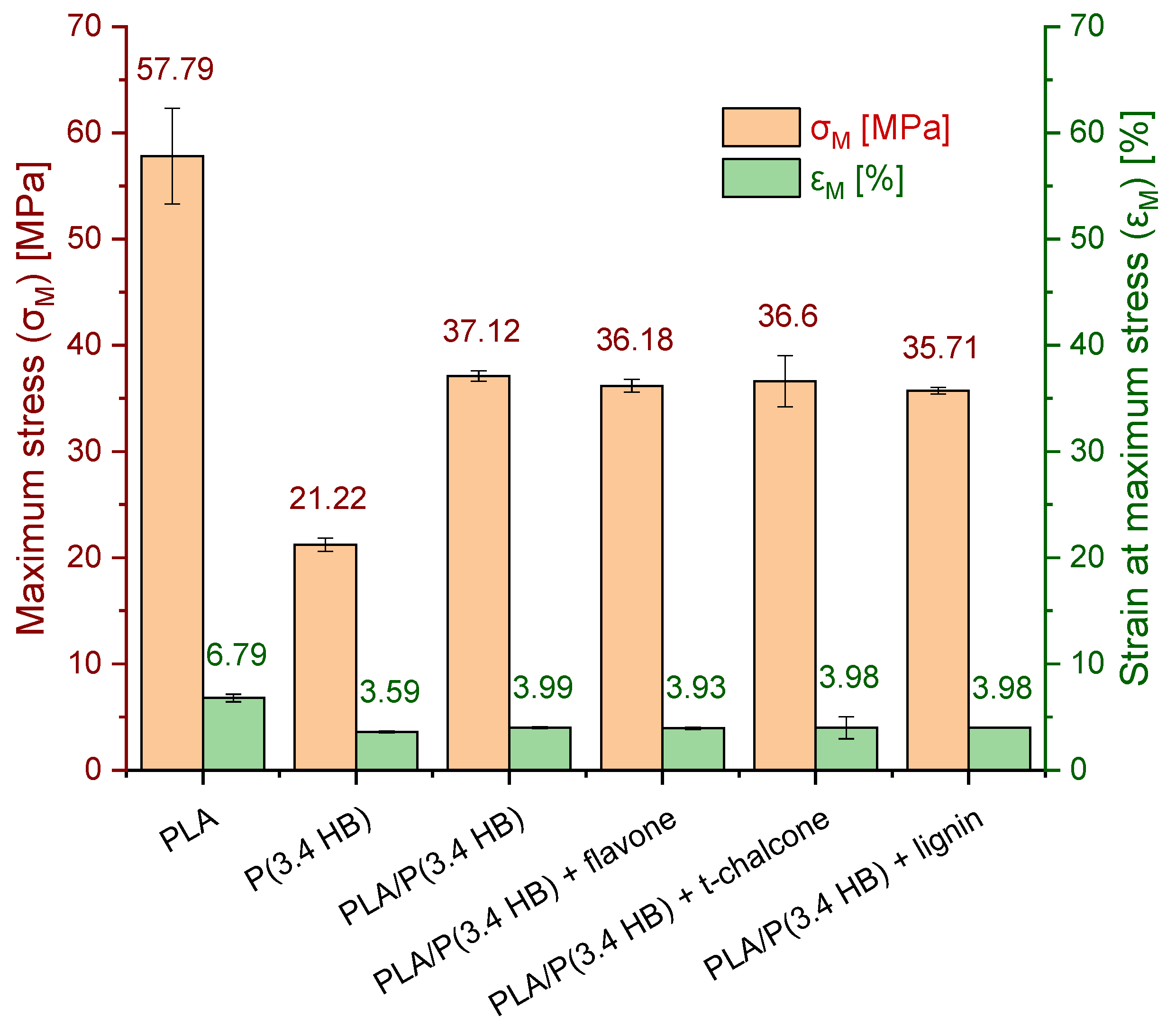

| Components | Weight Composition [phr] | |||||
|---|---|---|---|---|---|---|
| PLA | P(3.4 HB) | PLA/P(3.4 HB) | PLA/P(3.4 HB) + Flavone | PLA/P(3.4 HB) + t-Chalcone | PLA/P(3.4 HB) + Lignin | |
| Polylactic acid (PLA) | 100 | - | 60 | 60 | 60 | 60 |
| Polyhydroxybutyrate (P(3.4 HB)) | - | 100 | 40 | 40 | 40 | 40 |
| flavone | - | - | - | 1.25 | - | - |
| trans-chalcone | - | - | - | - | 1.25 | - |
| lignin | - | - | - | - | - | 1.25 |
| Sample | Tg (°C) | ΔHcc (°C) | Tcc (°C) | ΔHm (J/g) | Tm (°C) | ΔHo (J/g) | To (°C) |
|---|---|---|---|---|---|---|---|
| PLA | 60.9 | 18.0 | 121.4 | 14.8 | 150.1 | 4.9 | 226.0 |
| P(3.4 HB) | 47.4 | 12.3 | 84.6 | 6.8 | 139.8 | 0.7 | 205.7 |
| 31.6 | 166.7 | ||||||
| PLA/P(3.4 HB) | 54.8 | 24.4 | 108.4 | 30.0 | 162.2 | 5.5 | 232.7 |
| PLA/P(3.4 HB) + flavone | 53.5 | 24.6 | 109.2 | 29.1 | 161.9 | 3.3 | 241.1 |
| PLA/P(3.4 HB) + t-chalcone | 54.2 | 23.5 | 108.5 | 28.8 | 162.6 | 8.0 | 268.6 |
| PLA/P(3.4 HB) + lignin | 52.1 | 24.0 | 104.7 | 30.3 | 162.3 | 5.0 | 224.8 |
| Sample | T2% (°C) | T5% (°C) | T10% (°C) | T15% (°C) | T20% (°C) | T50% (°C) | T70% (°C) | T90% (°C) | T100% (°C) |
|---|---|---|---|---|---|---|---|---|---|
| PLA | 314.5 | 328.7 | 335.5 | 338.5 | 340.7 | 346.7 | 349.8 | 352.7 | 357.3 |
| P(3.4 HB) | 214.8 | 270.3 | 283.8 | 289.0 | 292.7 | 316.8 | 333.3 | 354.3 | 496.8 |
| PLA/P(3.4 HB) | 270.3 | 284.5 | 291.3 | 297.3 | 302.5 | 328.0 | 340.8 | 352.8 | 424.8 |
| PLA/P(3.4 HB) + flavone | 265.0 | 284.5 | 292.0 | 298.8 | 305.5 | 335.5 | 346.8 | 358.0 | 404.5 |
| PLA/P(3.4 HB) + t-chalcone | 256.0 | 283.0 | 292.8 | 301.8 | 313.8 | 346.0 | 357.3 | 367.0 | 380.5 |
| PLA/P(3.4 HB) + lignin | 259.8 | 277.8 | 286.8 | 296.5 | 307.0 | 338.5 | 349.8 | 360.3 | 403.8 |
| Sample | Tonset (°C) | Td (°C) |
|---|---|---|
| PLA | 352.7 | 357.3 |
| P(3.4 HB) | 283.3 | 308.6 |
| PLA/P(3.4 HB) | 284.8 | 353.3 |
| PLA/P(3.4 HB) + flavone | 311.4 | 360.9 |
| PLA/P(3.4 HB) + t-chalcone | 327.0 | 371.6 |
| PLA/P(3.4 HB) + lignin | 318.7 | 363.4 |
| Sample | Before Solar Aging | After Solar Aging | Af (−) | ||
|---|---|---|---|---|---|
| σB (MPa) | εB (%) | σB (MPa) | εB (%) | ||
| PLA | 53.24 | 8.28 | 13.76 | 2.77 | 0.086 |
| P(3.4 HB) | 14.21 | 10.53 | 1.74 | 0.47 | 0.005 |
| PLA/P(3.4 HB) | 19.71 | 61.57 | 2.23 | 2.59 | 0.005 |
| PLA/P(3.4 HB) + flavone | 21.79 | 47.02 | 2.95 | 0.99 | 0.003 |
| PLA/P(3.4 HB) + t-chalcone | 23.28 | 78.00 | 4.25 | 2.95 | 0.007 |
| PLA/P(3.4 HB) + lignin | 22.88 | 38.90 | 2.97 | 1.21 | 0.004 |
| flavone | does not change colour of polymer | can be applied as aging indicator | can be used as thermal stabilizer |
| trans-chalcone | can be utilized as colorant for polymer | can be applied as aging indicator | can be used as thermal stabilizer |
| lignin | can be utilized as colorant for polymer | does not change colour of polymer during aging process | can be used as thermal stabilizer |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olejnik, O.; Masek, A.; Kiersnowski, A. Thermal Analysis of Aliphatic Polyester Blends with Natural Antioxidants. Polymers 2020, 12, 74. https://doi.org/10.3390/polym12010074
Olejnik O, Masek A, Kiersnowski A. Thermal Analysis of Aliphatic Polyester Blends with Natural Antioxidants. Polymers. 2020; 12(1):74. https://doi.org/10.3390/polym12010074
Chicago/Turabian StyleOlejnik, Olga, Anna Masek, and Adam Kiersnowski. 2020. "Thermal Analysis of Aliphatic Polyester Blends with Natural Antioxidants" Polymers 12, no. 1: 74. https://doi.org/10.3390/polym12010074
APA StyleOlejnik, O., Masek, A., & Kiersnowski, A. (2020). Thermal Analysis of Aliphatic Polyester Blends with Natural Antioxidants. Polymers, 12(1), 74. https://doi.org/10.3390/polym12010074