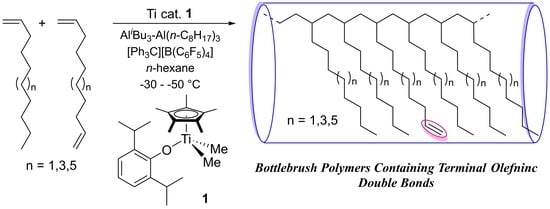
Synthesis of Ultrahigh Molecular Weight Polymers Containing Reactive Functionality with Low PDIs by Polymerizations of Long-Chain α-Olefins in the Presence of Their Nonconjugated Dienes by Cp*TiMe2(O-2,6-iPr2C6H3)–Borate Catalyst
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brintzinger, H.H.; Fischer, D.; Mülhaupt, R.; Rieger, B.; Waymouth, R.M. A Tailor-made metallocene for the copolymerization of ethene with bulky cycloalkenes. Angew. Chem. Int. Ed. Engl. 1995, 34, 1143–1170. [Google Scholar] [CrossRef]
- McKnight, A.L.; Waymouth, R.M. Group 4 ansa-cyclopentadienyl-amido catalysts for olefin polymerization. Chem. Rev. 1998, 98, 2587–2598. [Google Scholar] [CrossRef] [PubMed]
- Britovsek, G.J.P.; Gibson, V.C.; Wass, D.F. The search for new-generation olefin polymerization catalysts: Life beyond metallocenes. Angew. Chem. Int. Ed. Engl. 1999, 38, 428–447. [Google Scholar] [CrossRef]
- Gibson, V.C.; Spitzmesser, S.K. Advances in non-metallocene olefin polymerization catalysis. Chem. Rev. 2003, 103, 283–316. [Google Scholar] [CrossRef]
- Nomura, K.; Liu, J.; Sudhakar, P.; Kitiyanan, B. Nonbridged half-metallocenes containing anionic ancillary donor ligands: New promising candidates as catalysts for precise olefin polymerization. J. Mol. Catal. A 2007, 267, 1–29. [Google Scholar] [CrossRef]
- Nomura, K. Half-titanocenes containing anionic ancillary donor ligands as promising new catalysts for precise olefin polymerization. Dalton Trans. 2009, 8811–8823. [Google Scholar] [CrossRef]
- Nomura, K.; Liu, J. Half-titanocenes for precise olefin polymerisation: Effects of ligand substituents and some mechanistic aspects. Dalton Trans. 2011, 40, 7666–7682. [Google Scholar] [CrossRef]
- Makio, H.; Terao, H.; Iwashita, A.; Fujita, T. FI Catalysts for olefin polymerization—A comprehensive treatment. Chem. Rev. 2011, 111, 2363–2449. [Google Scholar] [CrossRef]
- Nomura, K.; Zhang, S. Design of vanadium complex catalysts for precise olefin polymerization. Chem. Rev. 2011, 111, 2342–2362. [Google Scholar] [CrossRef]
- Delferro, M.; Marks, T.J. Multinuclear olefin polymerization catalysts. Chem. Rev. 2011, 111, 2450–2485. [Google Scholar] [CrossRef]
- Redshaw, C.; Tang, Y. Tridentate ligands and beyond in group IV metal α-olefin homo-/co-polymerization catalysis. Chem. Soc. Rev. 2012, 41, 4484–4510. [Google Scholar] [CrossRef] [PubMed]
- Valente, A.; Mortreux, A.; Visseaux, M.; Zinck, P. Coordinative chain transfer polymerization. Chem. Rev. 2013, 113, 3836–3857. [Google Scholar] [CrossRef] [PubMed]
- Osakada, K. Organometallic Reactions and Polymerization; The Lecture Notes in Chemistry 85; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Hoff, R. Handbook of Transition Metal. Polymerization Catalysts, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018. [Google Scholar]
- López-Barrón, C.R.; Tsou, A.H.; Younker, J.M.; Norman, A.I.; Schaefer, J.J.; Hagadorn, J.R.; Throckmorton, J.A. Microstructure of crystallizable α-olefin molecular bottlebrushes: Isotactic and atactic poly(1-octadecene). Macromolecules 2018, 51, 872–883. [Google Scholar] [CrossRef]
- López-Barrón, C.R.; Tsou, A.H.; Hagadorn, J.R.; Throckmorton, J.A. Highly entangled α-olefin molecular bottlebrushes: Melt structure, linear rheology, and interchain friction mechanism. Macromolecules 2018, 51, 6958–6966. [Google Scholar] [CrossRef]
- Choi, H.J.; Jhon, M.S. Polymer-induced turbulent drag reduction. Ind. Eng. Chem. Res. 1996, 35, 2993–2998. [Google Scholar] [CrossRef]
- Brostow, W. Drag reduction in flow: Review of applications, mechanism and prediction. J. Ind. Eng. Chem. 2008, 14, 409–416. [Google Scholar] [CrossRef]
- Ivchenko, P.V.; Nifant’ev, I.E.; Tavtorkin, A.V. Polyolefin drag reducing agents. Petrol. Chem. 2016, 56, 775–787. [Google Scholar] [CrossRef]
- Cuenca, F.G.; Marín, M.G.; Folgueras Díaz, M.B. Energy-savings modeling of oil pipelines that use drag-reducing additives. Energy Fuels 2008, 22, 3293–3298. [Google Scholar] [CrossRef]
- Nomura, K.; Pengoubol, S.; Apisuk, W. Synthesis of ultrahigh molecular weight polymers by homopolymerisation of higher α-olefins catalysed by aryloxo-modified half-titanocenes. RSC Adv. 2016, 6, 16203–16207. [Google Scholar] [CrossRef]
- Nomura, K.; Pengoubol, S.; Apisuk, W. Synthesis of ultrahigh molecular weight polymers with low PDIs by polymerizations of 1-decene, 1-dodecene, and 1-tetradecene by Cp*TiMe2 (O-2,6-iPr2C6H3)–borate catalyst. Molecules 2019, 24, 1634. [Google Scholar] [CrossRef]
- Grumel, V.; Brüll, R.; Pasch, H.; Raubenheimer, H.G.; Sanderson, R.; Wahner, U.M. Homopolymerization of higher α-olefins with metallocene/MAO catalysts. Macromol. Mater. Eng. 2001, 286, 480–487. [Google Scholar] [CrossRef]
- Saito, J.; Suzuki, Y.; Fujita, T. Higher α-olefin polymerization behavior of a bis (phenoxy-imine)titanium complex/i-Bu3Al/Ph3CB(C6F5)4 catalyst system. Chem. Lett. 2003, 32, 236–237. [Google Scholar] [CrossRef]
- Fries, A.; Mise, T.; Matsumoto, A.; Ohmori, H.; Wakatsuki, Y. Polymerization of hex-1-ene by homogeneous zirconocene and hafnocene catalysts in compressed solution. Chem. Commun. 1996, 783–784. [Google Scholar] [CrossRef]
- Segal, S.; Goldberg, I.; Kol, M. Zirconium and titanium diamine bis (phenolate) catalysts for α-olefin polymerization: From atactic oligo(1-hexene) to ultrahigh-molecular-weight isotactic poly (1-hexene). Organometallics 2005, 24, 200–202. [Google Scholar] [CrossRef]
- Fujita, M.; Seki, Y.; Miyatake, T. Enhancement of productivity, molecular weight and stereoregularity of 1-butene polymerization by MAO modification. Macromol. Chem. Phys. 2004, 205, 884–887. [Google Scholar] [CrossRef]
- Fujita, M.; Seki, Y.; Miyatake, T. Synthesis of ultra-high-molecular-weight poly (α-olefin)s by thiobis(phenoxy)titanium/modified methylaluminoxane system. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 1107–1111. [Google Scholar] [CrossRef]
- Nomura, K.; Fudo, A. Efficient living polymerization of 1-hexene by Cp*TiMe2 (O-2,6-iPr2C6H3)-borate catalyst systems at low temperature. J. Mol. Catal. A Chem. 2004, 209, 9–17. [Google Scholar] [CrossRef]
- Jayaratne, K.C.; Sita, L.R. Stereospecific living Ziegler-Natta polymerization of 1-hexene. J. Am. Chem. Soc. 2000, 122, 958–959. [Google Scholar] [CrossRef]
- Domski, G.J.; Lobkovsky, E.B.; Coates, G.W. Polymerization of α-olefins with pridylamidohafnium catalysts: Living behavior and unexpected isoselectivity from a Cs-symmetric ctalyst precursor. Macromolecules 2007, 40, 3510–3513. [Google Scholar] [CrossRef]
- Cai, Z.; Ohmagari, M.; Nakayama, Y.; Shiono, T. Highly active syndiospecific living polymerization of higher 1-alkene with ansa-fluorenylamidodimethyltitanium complex. Macromol. Rapid Commun. 2009, 30, 1812–1816. [Google Scholar] [CrossRef]
- Kotzabasakis, V.; Mourmouris, S.; Pitsikalis, M.; Hadjichristidis, N.; Lohse, D.J. Synthesis and characterization of complex macromolecular architectures based on poly(α-olefins) utilizing a Cs-symmetry hafnium metallocene catalyst in combination with atom transfer radical polymerization (ATRP). Macromolecules 2011, 44, 1952–1968. [Google Scholar] [CrossRef]
- Kiesewetter, E.T.; Waymouth, R.M. Octahedral group IV bis (phenolate) catalysts for 1-hexene homopolymerization and ethylene/1-hexene copolymerization. Macromolecules 2013, 46, 2569–2575. [Google Scholar] [CrossRef]
- Nakata, N.; Toda, T.; Matsuo, T.; Ishii, A. Controlled isospecific polymerization of α-olefins by hafnium complex incorporating with a trans-cyclooctanediyl-bridged [OSSO]-type bis (phenolate) ligand. Macromolecules 2013, 46, 6758–6764. [Google Scholar] [CrossRef]
- Dai, S.; Zhou, S.; Zhang, W.; Chen, C. Systematic investigations of ligand steric effects on α-diimine palladium catalyzed olefin polymerization and copolymerization. Macromolecules 2016, 49, 8855–8862. [Google Scholar] [CrossRef]
- Nomura, K.; Fujita, K.; Fujiki, M. Effects of cyclopentadienyl fragment in ethylene, 1-hexene, and styrene polymerizations catalyzed by half-titanocenes containing ketimide ligand of the type, Cp’TiCl2 (N=CtBu2). Catal. Commun. 2004, 5, 413–417. [Google Scholar] [CrossRef]
- Nomura, K.; Liu, J.; Fujiki, M.; Takemoto, A. Facile, efficient functionalization of polyolefins via controlled incorporation of terminal olefins by repeated 1,7-octadiene insertion. J. Am. Chem. Soc. 2007, 129, 14170–14171. [Google Scholar] [CrossRef]
- Chung, T.C. Functionalization of Polyolefins; Academic Press: San Diego, CA, USA, 2002. [Google Scholar]
- Chung, T.C. Synthesis of functional polyolefin copolymers with graft and block structures. Prog. Polym. Sci. 2002, 27, 39. [Google Scholar] [CrossRef]
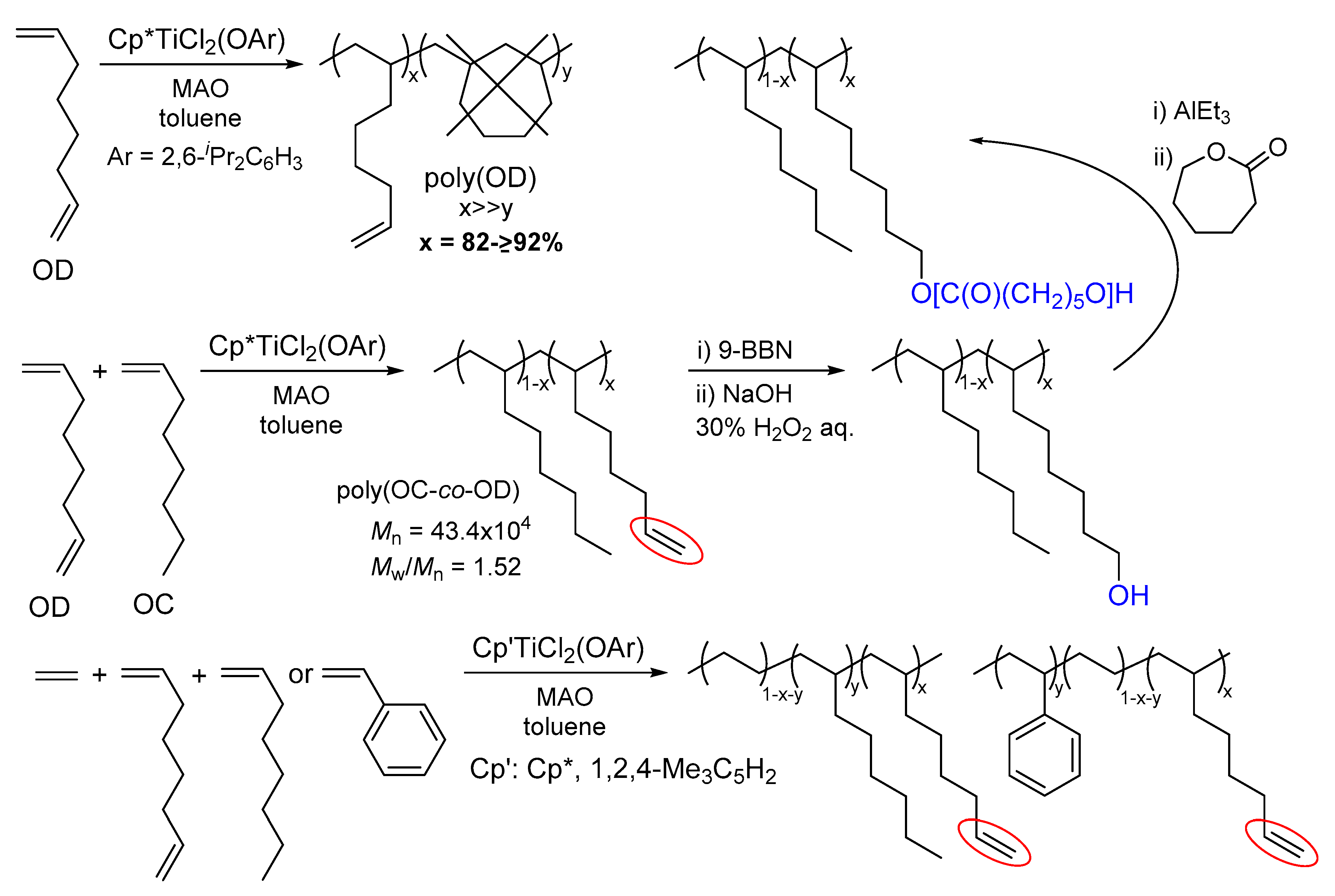
- Nomura, K. New approaches in precise synthesis of polyolefins containing polar functionalities by olefin copolymerizations using transition metal catalysts. J. Synth. Org. Chem. Jpn. 2010, 68, 1150–1158. [Google Scholar] [CrossRef]
- Franssen, N.M.G.; Reek, J.N.H.; de Bruin, B. Synthesis of functional ‘polyolefins’: State of the art and remaining challenges. Chem. Soc. Rev. 2013, 42, 5809–5832. [Google Scholar] [CrossRef]
- Mohanty, A.D.; Bae, C. Transition metal-catalyzed functionalization of polyolefins containing C–C, C=C, and C–H bonds. Adv. Organomet. Chem. 2015, 64, 1–39. [Google Scholar]
- Apisuk, W.; Nomura, K. Efficient terpolymerization of ethylene and styrene with 1,7-octadiene by aryloxo modified half-titanocenes—Cocatalyst systems: Efficient introduction of the reactive functionality. Macromol. Chem. Phys. 2014, 215, 1785–1791. [Google Scholar] [CrossRef]
- Zhao, W.; Nomura, K. Copolymerizations of norbornene, tetracyclododecene with α-olefins by half-titanocene catalysts: Efficient synthesis of highly transparent, thermal resistance polymers. Macromolecules 2016, 49, 59–70. [Google Scholar] [CrossRef]
- Nomura, K.; Naga, N.; Miki, M.; Yanagi, K. Olefin polymerization by (cyclopentadienyl) (aryloxy)—Titanium (IV) complexes-cocatalyst systems. Macromolecules 1998, 31, 7588–7597. [Google Scholar] [CrossRef]
- Nomura, K.; Fudo, A. Syndiospecific styrene polymerization by (tert-BuC5H4) TiCl2 (O-2,6-iPr2C6H3)—Borate catalyst system. Catal. Commun. 2003, 4, 269–274. [Google Scholar] [CrossRef]
- Nomura, K.; Suzuki, N.; Kim, D.-H.; Kim, H.J. Effect of cocatalyst in ethylene/styrene copolymerization by aryloxo-modified half-titanocene–cocatalyst systems for exclusive synthesis of copolymers at high styrene concentrations. Macromol. React. Eng. 2012, 6, 351–356. [Google Scholar] [CrossRef]
- Nomura, K.; Pracha, S.; Phomphrai, K.; Katao, S.; Kim, D.-H.; Kim, H.J.; Suzuki, N. Synthesis and structural analysis of phenoxy-substituted half-titanocenes with different anionic ligands, Cp*TiX(Y)(O-2,6-iPr2C6H3): Effect of anionic ligands (X,Y) in ethylene/styrene copolymerization. J. Mol. Catal. A Chem. 2012, 365, 136–145. [Google Scholar] [CrossRef]
- Hagihara, H.; Shiono, T.; Ikeda, T. Living polymerization of propene and 1-hexene with the [t-BuNSiMe2Flu]TiMe2/B(C6F5)3 catalyst. Macromolecules 1998, 31, 3184–3188. [Google Scholar] [CrossRef]
- Fukui, Y.; Murata, M.; Soga, K. Living polymerization of propylene and 1-hexene using bis-Cp type metallocene catalysts. Macromol. Rapid Commun. 1999, 20, 637. [Google Scholar] [CrossRef]
- Beckerle, K.; Manivannan, R.; Spaniol, T.P.; Okuda, J. Living isospecific styrene polymerization by chiral benzyl titanium complexes that contain a tetradentate [OSSO]-type bis(phenolato) ligand. Organometallics 2006, 25, 3019–3026. [Google Scholar] [CrossRef]
- As reported previously [21,22,29], the resultant polymers do not have stereo-regularity (atactic polymers), as observed in the spectra in poly(1-hexene) [29], poly(1-decene) and poly(1-dodecene) [22] using 1–borate catalyst. Resonances ascribed to 2,1- or other insertion units could not be found, strongly suggesting that the polymerizations proceeded with (in high certainty) 1,2-insertion manner.
- Nomura, K.; Hatanaka, Y.; Okumura, H.; Fujiki, M.; Hasegawa, K. Polymerization of 1,5-hexadiene by nonbridged half-titanocene complex—MAO catalyst system: Remarkable difference in the selectivity of repeated 1,2-insertion. Macromolecules 2004, 37, 1693–1695. [Google Scholar] [CrossRef]
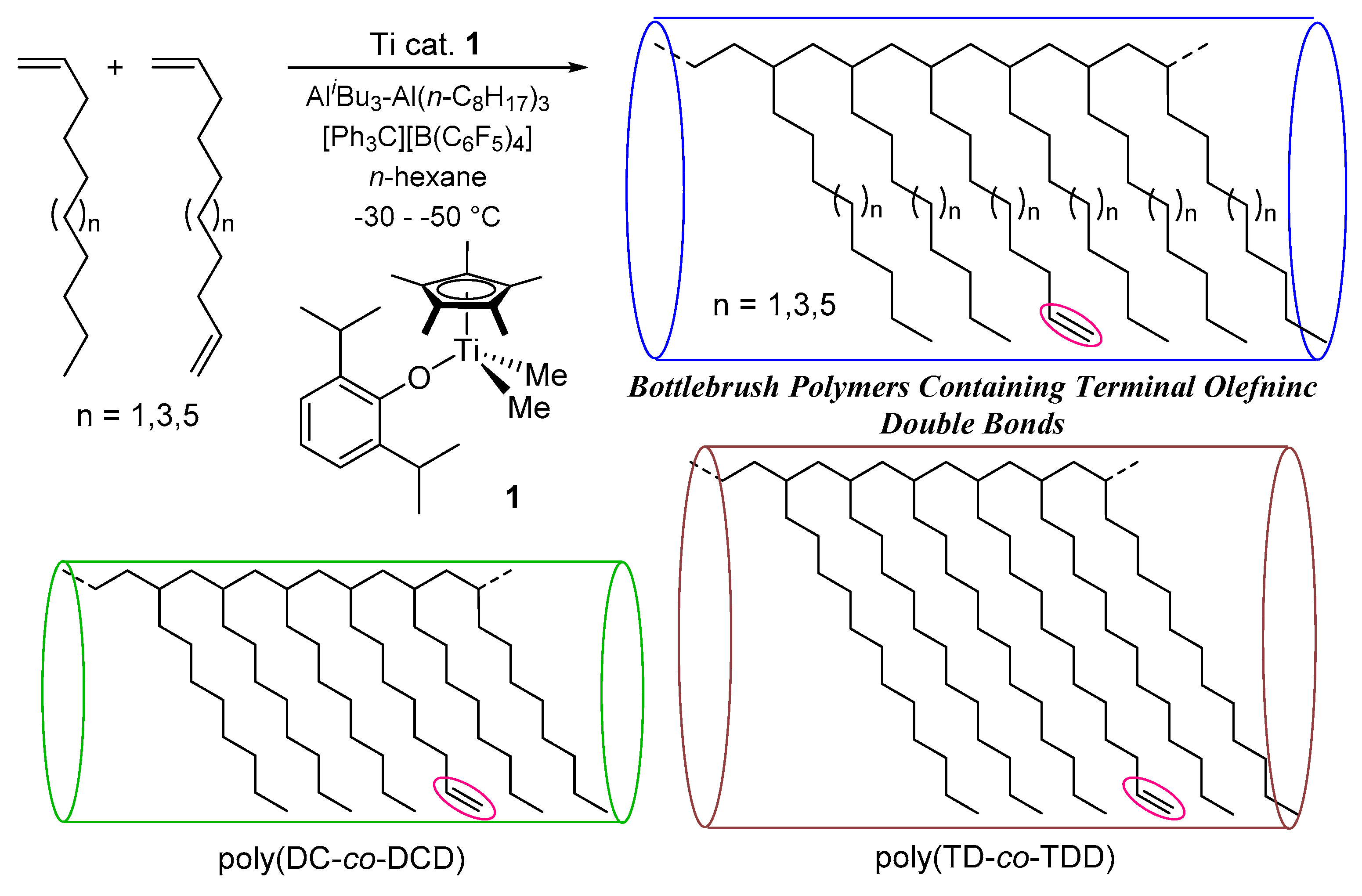


| Run | Al(n-C8H17)3/ AliBu3/Ti b | Temp. /°C | Time /min | Yield c /mg | Activity d | TON e | Mnf × 10−4 | Mw/ Mnf | DCD g /mol% | Conv. h /% |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 400/100/1.0 | −30 | 5 | 652 | 7820 | 4650 | 32.4 | 1.43 | 8.9 | 16 |
| 10 | 796 | 4780 | 5680 | 45.3 | 1.47 | 8.4 | 17 | |||
| 15 | 864 | 3460 | 6160 | 52.4 | 1.45 | 8.2 | 19 | |||
| 20 | 918 | 2750 | 6550 | 75.3 | 1.43 | 8.0 | 19 | |||
| 2 | 400/100/1.0 | −40 | 10 | 246 | 1480 | 1760 | 55.1 | 1.28 | 9.1 | 6 |
| 20 | 582 | 1750 | 4150 | 88.7 | 1.32 | 8.8 | 13 | |||
| 30 | 966 | 1930 | 6900 | 100.7 | 1.40 | 7.8 | 19 | |||
| 45 | 1400 | 1870 | 9990 | 140.1 | 1.39 | 5.6 | 20 | |||
| 3 | 300/200/1.0 | −50 | 60 | 396 | 400 | 2830 | 32.4 | 1.19 | 9.8 | 10 |
| 75 | 512 | 410 | 3660 | 38.9 | 1.20 | 9.2 | 12 | |||
| 90 | 574 | 380 | 4100 | 46.8 | 1.14 | 8.3 | 13 | |||
| 120 | 742 | 370 | 5300 | 53.8 | 1.18 | 7.4 | 15 |
| Run | Al(n-C8H17)3/ AliBu3/Ti b | Temp. /°C | Time /min | Yield c /mg | Activity d | TON e | Mnf × 10−4 | Mw/ Mnf | DDD g /mol% | Conv. h /% |
|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 400/100/1.0 | −40 | 20 | 828 | 2480 | 5900 | 42.6 | 1.34 | 7.9 | 17 |
| 30 | 897 | 1790 | 6390 | 48.3 | 1.39 | 7.7 | 18 | |||
| 40 | 932 | 1400 | 6640 | 51.4 | 1.38 | 6.6 | 18 | |||
| 5 | 250/250/1.0 | −50 | 75 | 402 | 320 | 2390 | 33.5 | 1.25 | ||
| 90 | 492 | 330 | 2920 | 42.6 | 1.25 | 7.7 | 10 | |||
| 105 | 582 | 330 | 3460 | 49.7 | 1.24 | 7.5 | 11 | |||
| 120 | 628 | 310 | 3730 | 54.6 | 1.28 | 7.5 | 12 |
| Run | Time/min | Yield b/mg | Activity c | TON d | Mne × 10−4 | Mw/Mne | TDD f/mol% |
|---|---|---|---|---|---|---|---|
| 6 | 10 | 184 | 1100 | 927 | 48.7 | 1.26 | |
| 30 | 516 | 1030 | 2600 | 52.8 | 1.38 | 4.5 | |
| 40 | 830 | 1110 | 4180 | 55.3 | 1.36 | 4.0 | |
| 60 | 1150 | 1150 | 5790 | 64.8 | 1.41 | 3.7 | |
| 75 | 1510 | 1210 | 7610 | 72.9 | 1.43 | 3.5 | |
| 90 | 1872 | 1250 | 9440 | 80.4 | 1.48 | 3.2 | |
| 120 | 2432 | 1220 | 12300 | 91.2 | 1.51 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nomura, K.; Pengoubol, S.; Apisuk, W. Synthesis of Ultrahigh Molecular Weight Polymers Containing Reactive Functionality with Low PDIs by Polymerizations of Long-Chain α-Olefins in the Presence of Their Nonconjugated Dienes by Cp*TiMe2(O-2,6-iPr2C6H3)–Borate Catalyst. Polymers 2020, 12, 3. https://doi.org/10.3390/polym12010003
Nomura K, Pengoubol S, Apisuk W. Synthesis of Ultrahigh Molecular Weight Polymers Containing Reactive Functionality with Low PDIs by Polymerizations of Long-Chain α-Olefins in the Presence of Their Nonconjugated Dienes by Cp*TiMe2(O-2,6-iPr2C6H3)–Borate Catalyst. Polymers. 2020; 12(1):3. https://doi.org/10.3390/polym12010003
Chicago/Turabian StyleNomura, Kotohiro, Sarntamon Pengoubol, and Wannida Apisuk. 2020. "Synthesis of Ultrahigh Molecular Weight Polymers Containing Reactive Functionality with Low PDIs by Polymerizations of Long-Chain α-Olefins in the Presence of Their Nonconjugated Dienes by Cp*TiMe2(O-2,6-iPr2C6H3)–Borate Catalyst" Polymers 12, no. 1: 3. https://doi.org/10.3390/polym12010003
APA StyleNomura, K., Pengoubol, S., & Apisuk, W. (2020). Synthesis of Ultrahigh Molecular Weight Polymers Containing Reactive Functionality with Low PDIs by Polymerizations of Long-Chain α-Olefins in the Presence of Their Nonconjugated Dienes by Cp*TiMe2(O-2,6-iPr2C6H3)–Borate Catalyst. Polymers, 12(1), 3. https://doi.org/10.3390/polym12010003