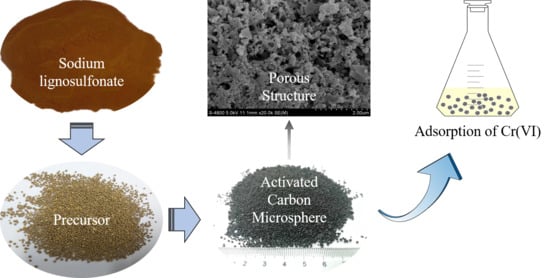
Activated Carbon Microsphere from Sodium Lignosulfonate for Cr(VI) Adsorption Evaluation in Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
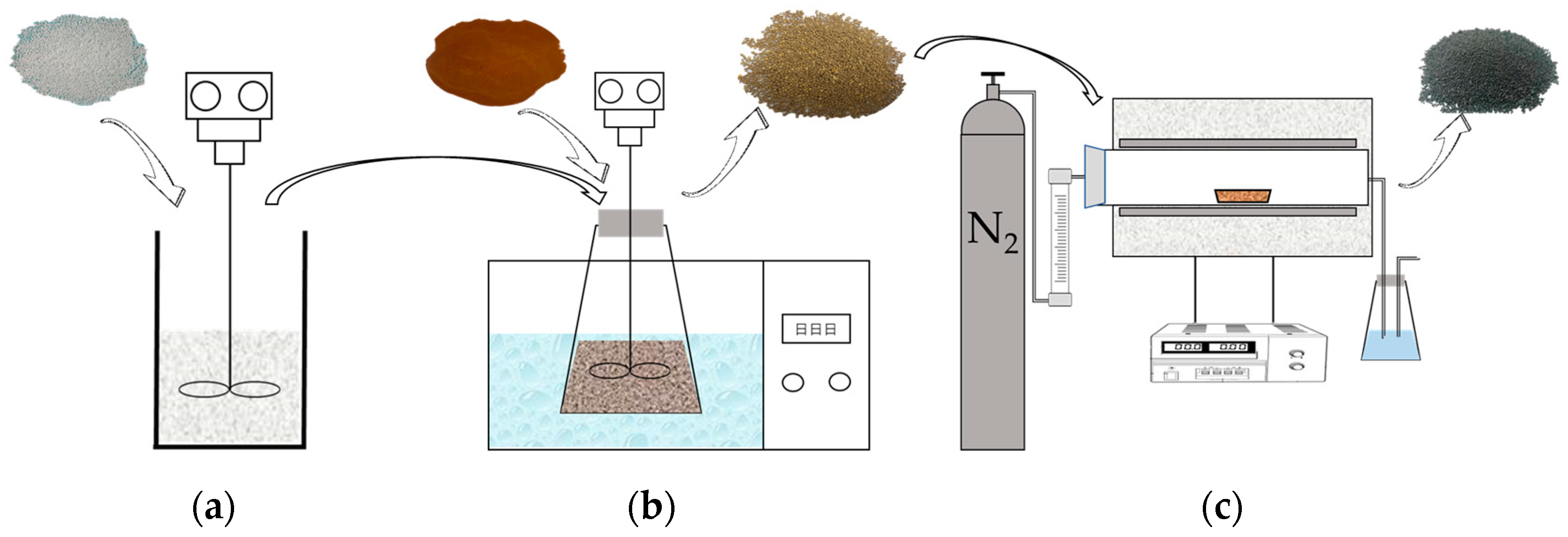
2.2. Preparation of SLACM
2.3. Characterizations of SLACM
2.4. Adsorption Experiment
2.4.1. Effects of Initial pH on Adsorption
2.4.2. Adsorption Kinetics
2.4.3. Adsorption Isotherm
3. Results and Discussion
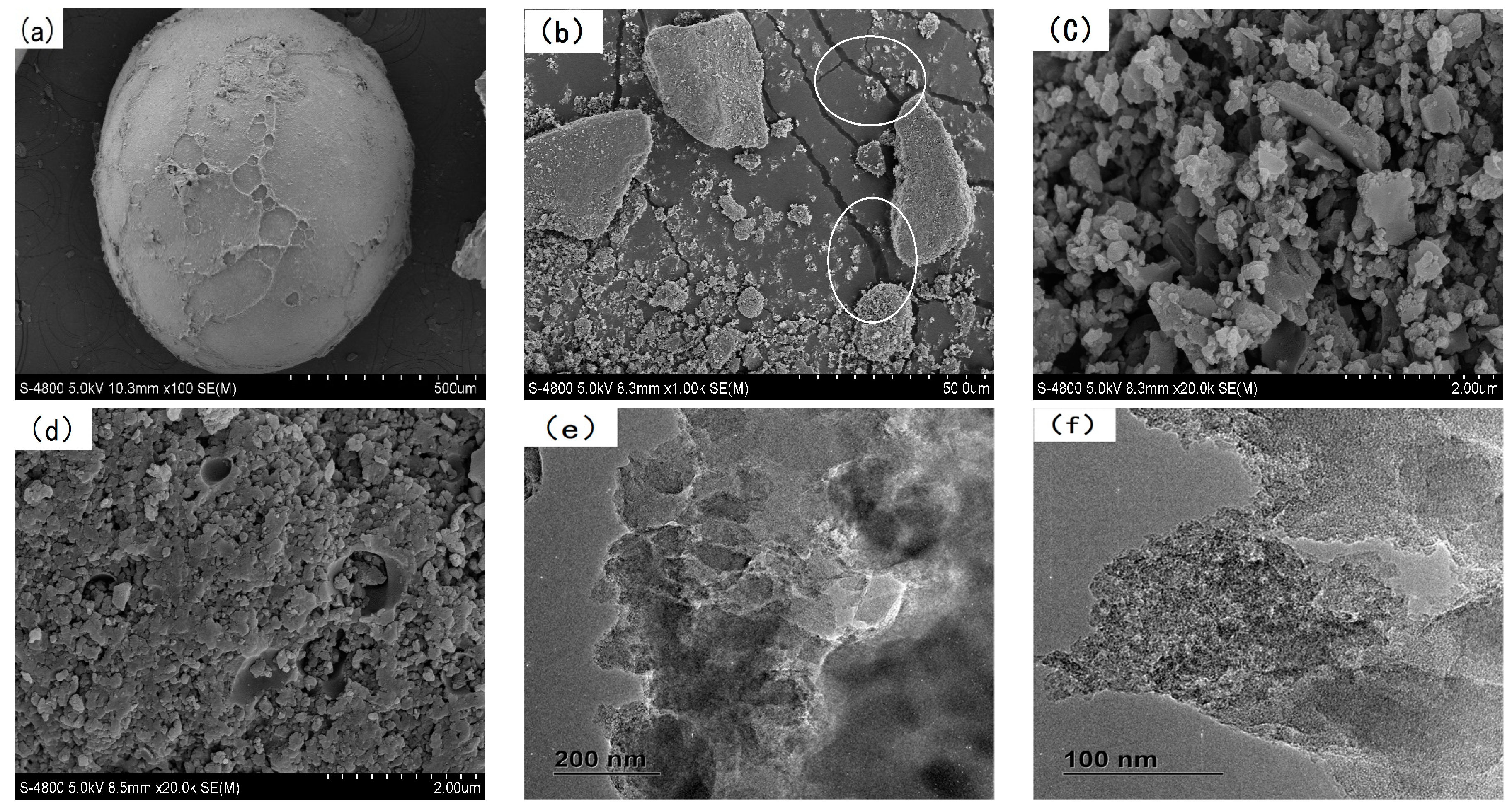
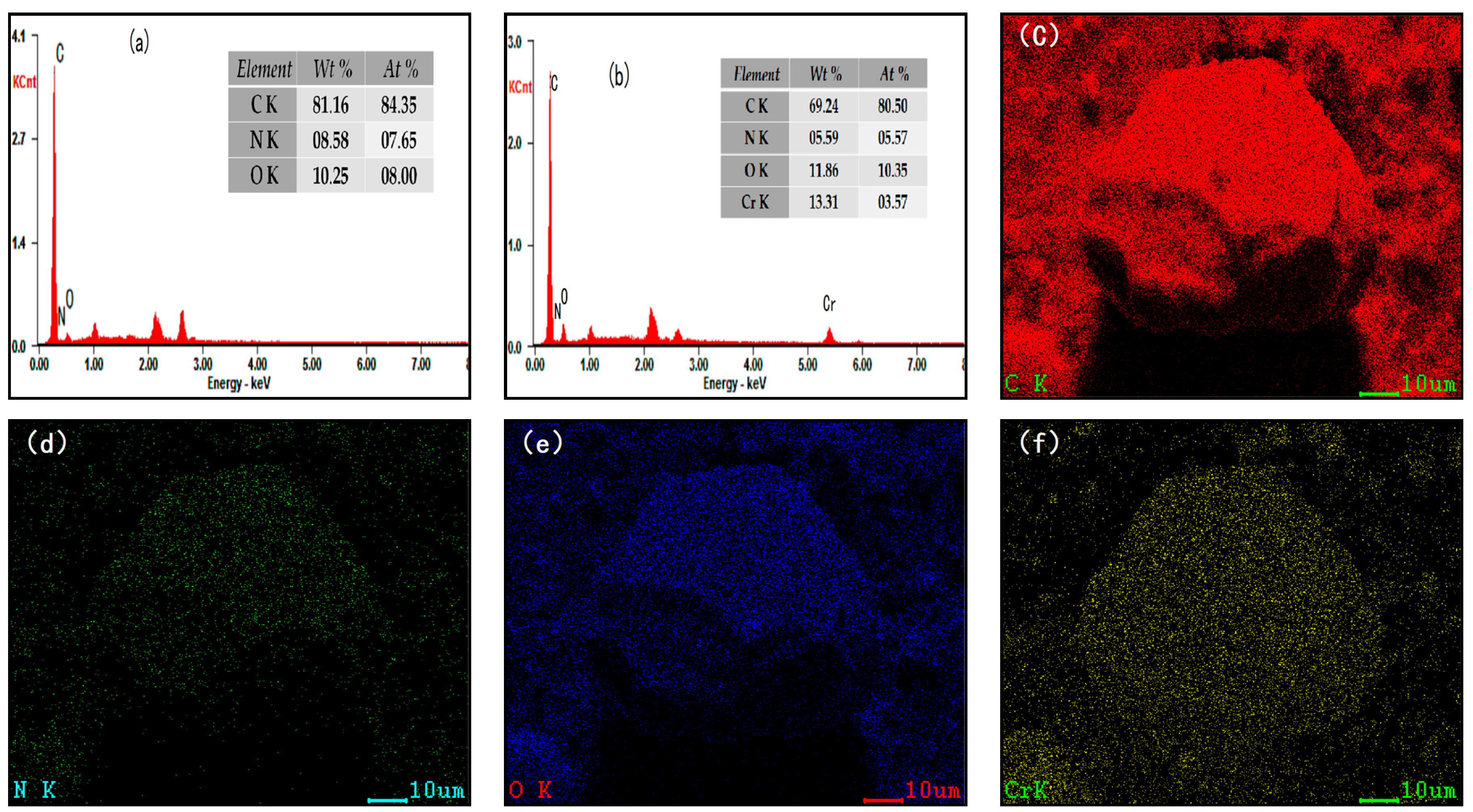
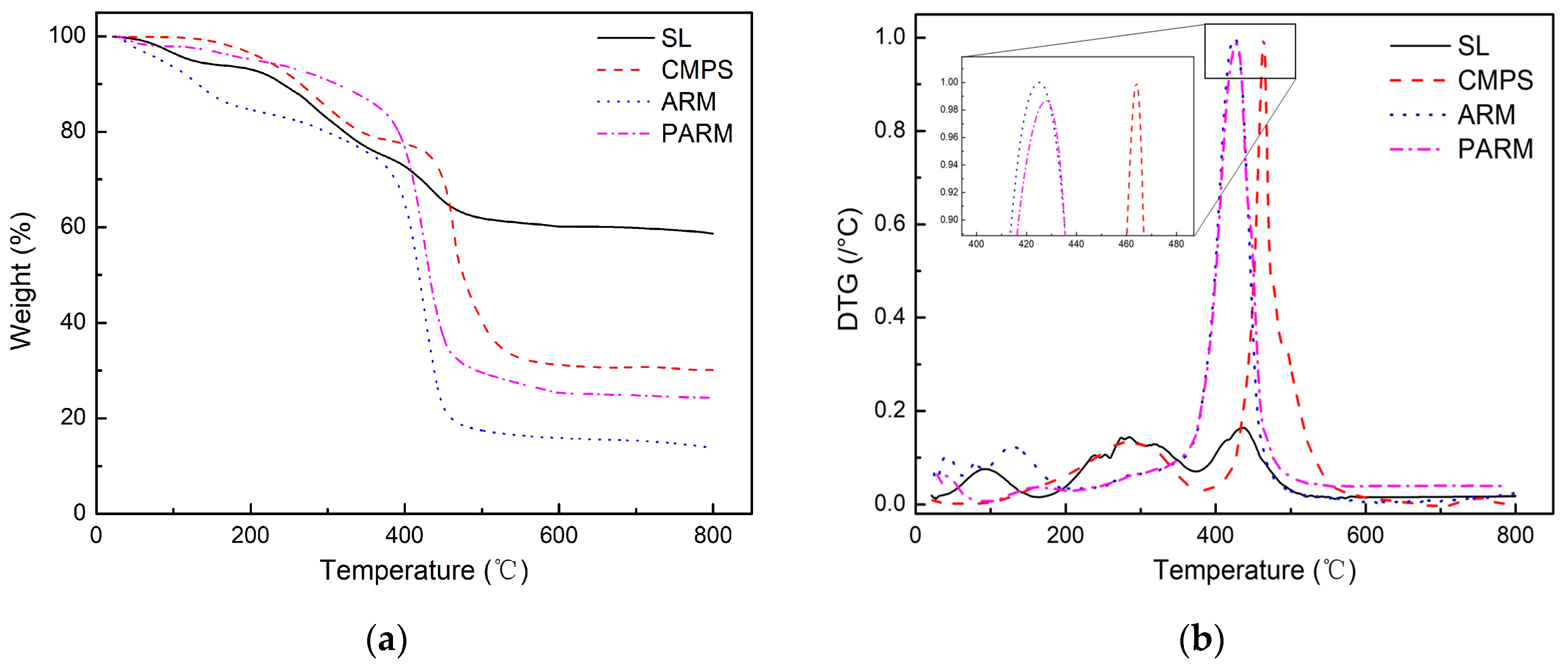
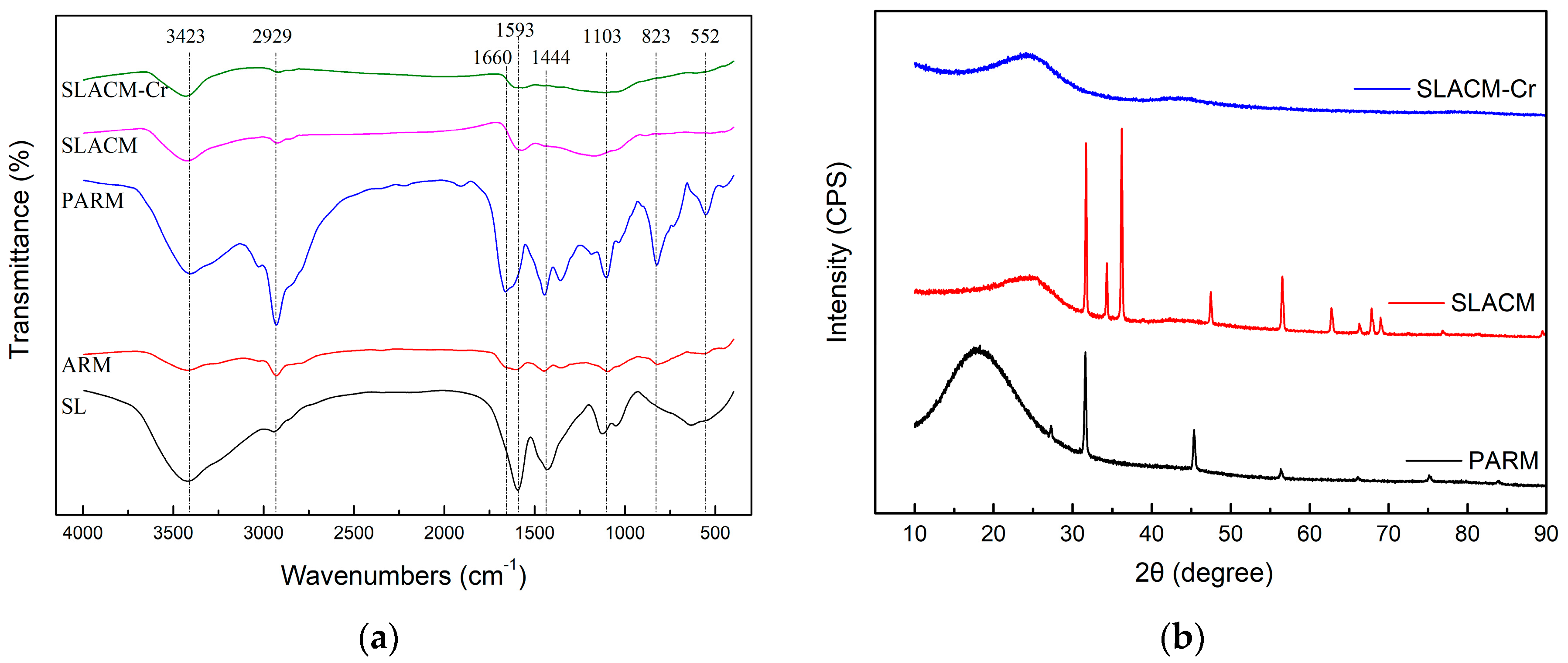
3.1. Characterization
3.2. Effect of Initial pH on Adsorption
3.3. Adsorption Kinetics
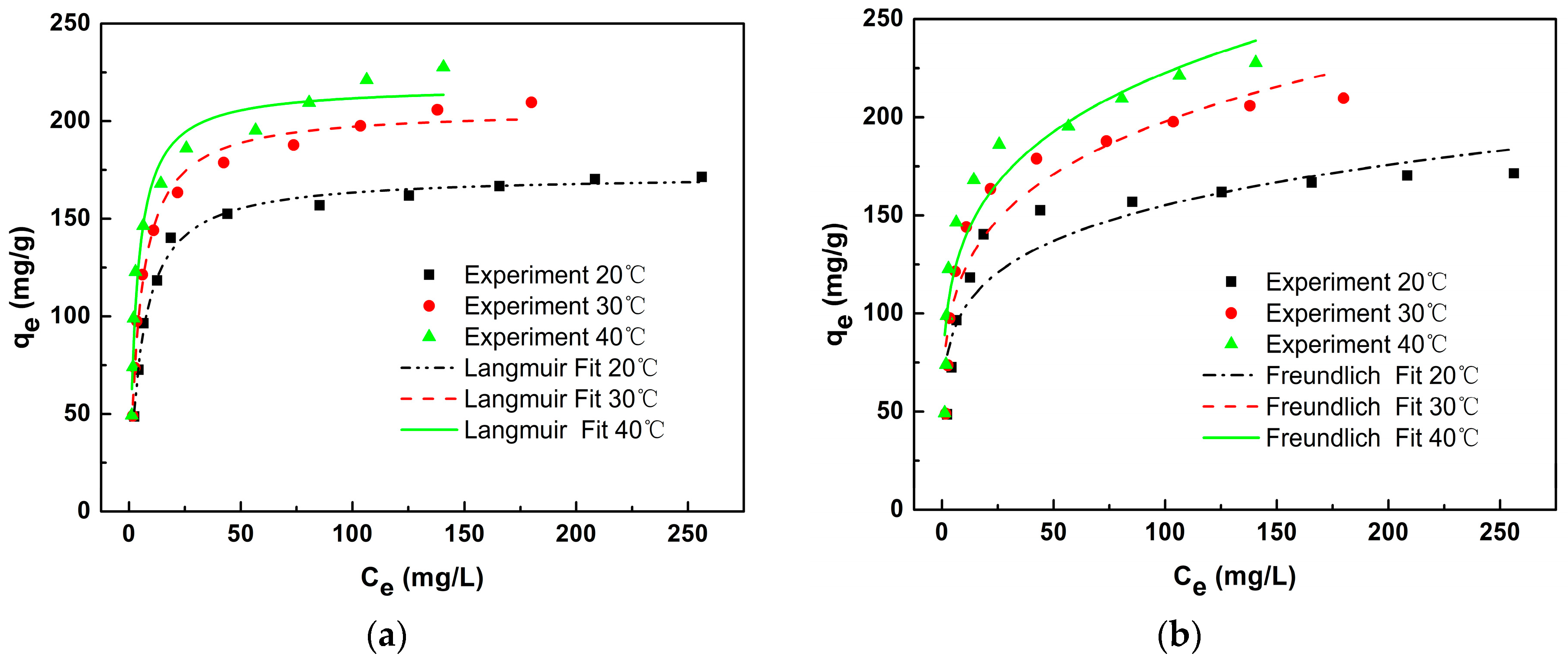
3.4. Adsorption Isotherm
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sessarego, S.; Rodrigues, S.C.G.; Xiao, Y.; Lu, Q.; Hill, J.M. Phosphonium-enhanced chitosan for Cr(VI) adsorption in wastewater treatment. Carbohydr. Polym. 2019, 211, 249–256. [Google Scholar] [CrossRef]
- Vilela, P.B.; Dalalibera, A.; Duminelli, E.C.; Becegato, V.A.; Paulino, A.T. Adsorption and removal of chromium (VI) contained in aqueous solutions using a chitosan-based hydrogel. Environ. Sci. Pollut. Res. 2018, 26, 28481–28489. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.; Ibrahim, S.F.; Kandeel, K.; Moawad, H. Biosorption of heavy metals from waste water using Pseudomonas sp. Electron. J. Biotechnol. 2004, 7, 38–46. [Google Scholar] [CrossRef]
- Yang, J.; Yu, M.; Chen, W. Adsorption of hexavalent chromium from aqueous solution by activated carbon prepared from longan seed: Kinetics, equilibrium and thermodynamics. J. Ind. Eng. Chem. 2015, 21, 414–422. [Google Scholar] [CrossRef]
- Fazlzadeh, M.; Rahmani, K.; Zarei, A.; Abdoallahzadeh, H.; Nasiri, F.; Khosravi, R. A novel green synthesis of zero valent iron nanoparticles (NZVI) using three plant extracts and their efficient application for removal of Cr(VI) from aqueous solutions. Adv. Powder Technol. 2017, 28, 122–130. [Google Scholar] [CrossRef]
- Miretzky, P.; Cirelli, A.F. Cr(VI) and Cr(III) removal from aqueous solution by raw and modified lignocellulosic materials: A review. J. Hazard. Mater. 2010, 180, 1–19. [Google Scholar] [CrossRef]
- Norouzi, S.; Heidari, M.; Alipour, V.; Rahmanian, O.; Fazlzadeh, M.; Mohammadi-moghadam, F.; Nourmoradi, H.; Goudarzi, B.; Dindarloo, K. Preparation, characterization and Cr(VI) adsorption evaluation of NaOH-activated carbon produced from Date Press Cake; an agro-industrial waste. Bioresour. Technol. 2018, 258, 48–56. [Google Scholar] [CrossRef]
- Tan, X.; Liu, S.; Liu, Y.; Gu, Y.; Zeng, G.; Hu, X.; Wang, X.; Liu, S.; Jiang, L. Biochar as potential sustainable precursors for activated carbon production: Multiple applications in environmental protection and energy storage. Bioresour. Technol. 2017, 227, 359–372. [Google Scholar] [CrossRef]
- Tripathi, A.; Rawat Ranjan, M. Heavy Metal Removal from Wastewater Using Low Cost Adsorbents. J. Bioremediat. Biodegrad. 2015, 6, 1–5. [Google Scholar] [CrossRef]
- Ilangovan, M.; Guna, V.; Olivera, S.; Ravi, A.; Muralidhara, H.B.; Santosh, M.S.; Reddy, N. Highly porous carbon from a natural cellulose fiber as high efficiency sorbent for lead in waste water. Bioresour. Technol. 2017, 245, 296–299. [Google Scholar] [CrossRef]
- Qiang, W.; Jiaoyan, S.; Mengzhu, S. Characteristic of adsorption, desorption and oxidation of Cr (III) on birnessite. Energy Procedia 2011, 5, 1104–1108. [Google Scholar] [CrossRef][Green Version]
- Parlayici, Ş.; Pehlivan, E. Comparative study of Cr(VI) removal by bio-waste adsorbents: Equilibrium, kinetics, and thermodynamic. J. Anal. Sci. Technol. 2019, 10, 15. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, S.; Zhang, H.; Wang, X. Removal behaviors and mechanisms of hexavalent chromium from aqueous solution by cephalosporin residue and derived chars. Bioresour. Technol. 2017, 238, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mu, C.; Zhong, L.; Xue, J.; Zhou, Y.; Han, X. Recycling of Cr (VI) from weak alkaline aqueous media using a chitosan/triethanolamine/Cu (II) composite adsorbent. Carbohydr. Polym. 2019, 205, 151–158. [Google Scholar] [CrossRef]
- Garg, V.K.; Gupta, R.; Kumar, R.; Gupta, R.K. Adsorption of chromium from aqueous solution on treated sawdust. Bioresour. Technol. 2004, 92, 79–81. [Google Scholar] [CrossRef]
- Aghababaei, A.; Ncibi, M.C.; Sillanpää, M. Optimized removal of oxytetracycline and cadmium from contaminated waters using chemically-activated and pyrolyzed biochars from forest and wood-processing residues. Bioresour. Technol. 2017, 239, 28–36. [Google Scholar] [CrossRef]
- Hernandez-Ramirez, O.; Holmes, S.M. Novel and modified materials for wastewater treatment applications. J. Mater. Chem. 2008, 18, 2751. [Google Scholar] [CrossRef]
- Pham, T.D.; Tran, T.T.; Le, V.A.; Pham, T.T.; Dao, T.H.; Le, T.S. Adsorption characteristics of molecular oxytetracycline onto alumina particles: The role of surface modification with an anionic surfactant. J. Mol. Liq. 2019, 287, 110900. [Google Scholar] [CrossRef]
- Pham, T.; Bui, T.; Nguyen, V.; Bui, T.; Tran, T.; Phan, Q.; Pham, T.; Hoang, T. Adsorption of Polyelectrolyte onto Nanosilica Synthesized from Rice Husk: Characteristics, Mechanisms, and Application for Antibiotic Removal. Polymers 2018, 10, 220. [Google Scholar] [CrossRef]
- Pham, T.D.; Do, T.T.; Ha, V.L.; Doan, T.H.Y.; Nguyen, T.A.H.; Mai, T.D.; Kobayashi, M.; Adachi, Y. Adsorptive removal of ammonium ion from aqueous solution using surfactant-modified alumina. Environ. Chem. 2017, 14, 327. [Google Scholar] [CrossRef]
- Qi, W.; Zhao, Y.; Zheng, X.; Ji, M.; Zhang, Z. Adsorption behavior and mechanism of Cr(VI) using Sakura waste from aqueous solution. Appl. Surf. Sci. 2016, 360, 470–476. [Google Scholar] [CrossRef]
- Kazakis, N.; Kantiranis, N.; Kalaitzidou, K.; Kaprara, E.; Mitrakas, M.; Frei, R.; Vargemezis, G.; Vogiatzis, D.; Zouboulis, A.; Filippidis, A. Environmentally available hexavalent chromium in soils and sediments impacted by dispersed fly ash in Sarigkiol basin (Northern Greece). Environ. Pollut. 2018, 235, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Rangabhashiyam, S.; Balasubramanian, P. The potential of lignocellulosic biomass precursors for biochar production: Performance, mechanism and wastewater application—A review. Ind. Crop. Prod. 2019, 128, 405–423. [Google Scholar]
- Li, W.; Zhang, L.; Peng, J.; Li, N.; Zhu, X. Preparation of high surface area activated carbons from tobacco stems with K2CO3 activation using microwave radiation. Ind. Crop. Prod. 2008, 27, 341–347. [Google Scholar] [CrossRef]
- Gao, H.; Liu, Y.; Zeng, G.; Xu, W.; Li, T.; Xia, W. Characterization of Cr(VI) removal from aqueous solutions by a surplus agricultural waste—Rice straw. J. Hazard. Mater. 2008, 150, 446–452. [Google Scholar] [CrossRef]
- Mekonnen, E.; Yitbarek, M.; Soreta, T.R. Kinetic and thermodynamic studies of the adsorption of Cr(VI) onto some selected local adsorbents. South Afr. J. Chem. 2015, 68, 45–52. [Google Scholar] [CrossRef]
- Malwade, K.; Lataye, D.; Mhaisalkar, V.; Kurwadkar, S.; Ramirez, D. Adsorption of hexavalent chromium onto activated carbon derived from Leucaena leucocephala waste sawdust: Kinetics, equilibrium and thermodynamics. Int. J. Environ. Sci. Technol. 2016, 13, 2107–2116. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; Selvaraju, N. Adsorptive remediation of hexavalent chromium from synthetic wastewater by a natural and ZnCl2 activated Sterculia guttata shell. J. Mol. Liq. 2015, 207, 39–49. [Google Scholar] [CrossRef]
- Doherty, W.O.S.; Mousavioun, P.; Fellows, C.M. Value-adding to cellulosic ethanol: Lignin polymers. Ind. Crop. Prod. 2011, 33, 259–276. [Google Scholar] [CrossRef]
- Xi, Y.; Yang, D.; Qiu, X.; Wang, H.; Huang, J.; Li, Q. Renewable lignin-based carbon with a remarkable electrochemical performance from potassium compound activation. Ind. Crop. Prod. 2018, 124, 747–754. [Google Scholar] [CrossRef]
- Zhang, N.; Shen, Y. One-step pyrolysis of lignin and polyvinyl chloride for synthesis of porous carbon and its application for toluene sorption. Bioresour. Technol. 2019, 284, 325–332. [Google Scholar] [CrossRef]
- Geng, J.; Gu, F.; Chang, J. Fabrication of magnetic lignosulfonate using ultrasonic-assisted in situ synthesis for efficient removal of Cr(Ⅵ) and Rhodamine B from wastewater. J. Hazard. Mater. 2019, 375, 174–181. [Google Scholar] [CrossRef]
- Ma, H.; Yang, J.; Gao, X.; Liu, Z.; Liu, X.; Xu, Z. Removal of chromium (VI) from water by porous carbon derived from corn straw: Influencing factors, regeneration and mechanism. J. Hazard. Mater. 2019, 369, 550–560. [Google Scholar] [CrossRef]
- Kumar, S.; Sivaranjanee, R.; Saravanan, A. Carbon sphere: Synthesis, characterization and elimination of toxic Cr(VI) ions from aquatic system. J. Ind. Eng. Chem. 2018, 60, 307–320. [Google Scholar]
- Fang, W.; Weisheng, N.; Andong, Z.; Weiming, Y. Enhanced anaerobic digestion of corn stover by thermo-chemical pretreatment. Int. J. Agric. Biol. Eng. 2015, 8, 84–90. [Google Scholar]
- Wang, F.; Zhang, D.; Wu, H.; Yi, W.; Fu, P.; Li, Y.; Li, Z. Enhancing biogas production of corn stover by fast pyrolysis pretreatment. Bioresour. Technol. 2016, 218, 731–736. [Google Scholar] [CrossRef]
- Xu, Z.; Yuan, Z.; Zhang, D.; Huang, Y.; Chen, W.; Sun, Z.; Zhou, Y. Cr(VI) removal with rapid and superior performance utilizing cost-efficient waste-polyester-textile-based mesoporous carbon: Behavior and mechanism. J. Mol. Liq. 2019, 278, 496–504. [Google Scholar] [CrossRef]
- Martins, A.C.; Pezoti, O.; Cazetta, A.L.; Bedin, K.C.; Yamazaki, D.A.S.; Bandoch, G.F.G.; Asefa, T.; Visentainer, J.V.; Almeida, V.C. Removal of tetracycline by NaOH-activated carbon produced from macadamia nut shells: Kinetic and equilibrium studies. Chem. Eng. J. 2015, 260, 291–299. [Google Scholar] [CrossRef]
- Cui, Y.; Masud, A.; Aich, N.; Atkinson, J.D. Phenol and Cr(VI) removal using materials derived from harmful algal bloom biomass: Characterization and performance assessment for a biosorbent, a porous carbon, and Fe/C composites. J. Hazard. Mater. 2019, 368, 477–486. [Google Scholar] [CrossRef]
- Pezoti, O.; Cazetta, A.L.; Bedin, K.C.; Souza, L.S.; Martins, A.C.; Silva, T.L.; Santos Júnior, O.O.; Visentainer, J.V.; Almeida, V.C. NaOH-activated carbon of high surface area produced from guava seeds as a high-efficiency adsorbent for amoxicillin removal: Kinetic, isotherm and thermodynamic studies. Chem. Eng. J. 2016, 288, 778–788. [Google Scholar] [CrossRef]
- Mondal, S.; Derebe, A.T.; Wang, K. Surface functionalized carbon microspheres for the recovery of copper ion from refinery wastewater. Korean J. Chem. Eng. 2018, 35, 147–152. [Google Scholar] [CrossRef]
- Jeon, C. Removal of Cr(VI) from aqueous solution using amine-impregnated crab shells in the batch process. J. Ind. Eng. Chem. 2019, 77, 111–117. [Google Scholar] [CrossRef]
- Zhang, X.; Lv, L.; Qin, Y.; Xu, M.; Jia, X.; Chen, Z. Removal of aqueous Cr(VI) by a magnetic biochar derived from Melia azedarach wood. Bioresour. Technol. 2018, 256, 1–10. [Google Scholar] [CrossRef]
- Cherdchoo, W.; Nithettham, S.; Charoenpanich, J. Removal of Cr(VI) from synthetic wastewater by adsorption onto coffee ground and mixed waste tea. Chemosphere 2019, 221, 758–767. [Google Scholar] [CrossRef]
- Liang, H.; Song, B.; Peng, P.; Jiao, G.; Yan, X.; She, D. Preparation of three-dimensional honeycomb carbon materials and their adsorption of Cr(VI). Chem. Eng. J. 2019, 367, 9–16. [Google Scholar] [CrossRef]


| Sample | SBET (m2/g) | Smic (m2/g) | Vtot (cm3/g) | Vmic (cm3/g) | Dp (nm) |
|---|---|---|---|---|---|
| SLACM | 769.37 | 639.28 | 0.47 | 0.26 | 2.46 |
| Model | Parameters | Value |
|---|---|---|
| PFO | 71.1 | |
| 106.75 | ||
| 38.83 | ||
| PSO | 72.03 | |
| 3.59 | ||
| 73.12 | ||
| Elovich | 5.81 | |
| 0.54 | ||
| 95.63 |
| Temp °C | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| 20 | 172.41 | 0.18 | 99.22 | 67.80 | 0.18 | 84.45 |
| 30 | 206.13 | 0.22 | 98.59 | 75.09 | 0.21 | 89.59 |
| 40 | 218.19 | 0.32 | 96.27 | 85.05 | 0.21 | 89.03 |
| Adsorbent Precursor | Activating Agend | qmax (mg/g) | pH | Temp °C | Reference |
|---|---|---|---|---|---|
| Mixed waste tea | / | 94.34 | 2.0 | 30 | [44] |
| Coffee ground | / | 87.72 | 2.0 | 30 | [44] |
| Algal bloom | CO2 | 96 | 1.0 | 20 | [39] |
| Glucose | KOH | 332.53 | 2.0 | 25 | [45] |
| Corn straw | KOH | 175.44 | 3.0 | 25 | [33] |
| Glucose monohydrate | H2O | 117.2 | 2.0 | 30 | [34] |
| SL | ZnCl2 | 227.7 | 2.0 | 40 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.; Xing, J.; Xu, P.; Chang, J.; Zhang, Q.; Usman, K.M. Activated Carbon Microsphere from Sodium Lignosulfonate for Cr(VI) Adsorption Evaluation in Wastewater Treatment. Polymers 2020, 12, 236. https://doi.org/10.3390/polym12010236
Yang K, Xing J, Xu P, Chang J, Zhang Q, Usman KM. Activated Carbon Microsphere from Sodium Lignosulfonate for Cr(VI) Adsorption Evaluation in Wastewater Treatment. Polymers. 2020; 12(1):236. https://doi.org/10.3390/polym12010236
Chicago/Turabian StyleYang, Keyan, Jingchen Xing, Pingping Xu, Jianmin Chang, Qingfa Zhang, and Khan Muhammad Usman. 2020. "Activated Carbon Microsphere from Sodium Lignosulfonate for Cr(VI) Adsorption Evaluation in Wastewater Treatment" Polymers 12, no. 1: 236. https://doi.org/10.3390/polym12010236
APA StyleYang, K., Xing, J., Xu, P., Chang, J., Zhang, Q., & Usman, K. M. (2020). Activated Carbon Microsphere from Sodium Lignosulfonate for Cr(VI) Adsorption Evaluation in Wastewater Treatment. Polymers, 12(1), 236. https://doi.org/10.3390/polym12010236